1. Introduction
Epilepsy is a common chronic brain disorders, with a prevalence of between 0.5 and 1%, and growing [
1]. The aetiology of epilepsy can be varied [
1]. Two large populational studies have shown the risk of epilepsy in children and adults with autoimmune diseases, compared to individuals without autoimmune diseases, to be significantly heightened, with myasthenia gravis the fourth most common autoimmune comorbid condition in the American cohort [
2,
3]
Myasthenia gravis (MG) is an immunomediated disease characterized by fatigable muscle weakness of the ocular, facial, bulbar, respiratory, and limb muscles. Nearly 80-85% of MG cases are associated with acetylcholine receptor antibodies (AChR-Abs) that destroy synaptic transmission across the neuromuscular junction [
4]. Moreover, other autoantibodies have been identified including Abs against muscle-specific tyrosine kinase (MuSK) and lipoprotein receptor-related protein 4 (LRP4). The thymus is affected in most patients with AChR-positive MG; approximately 70% of patients have thymic hyperplasia, 10% thymoma, and the remainder either a normal or atrophic thymus. MG incidence rate increases with age from 4.2 to 18.9 per million person-years [
4] and a prevalence between 5 and 24 cases per 100,000 people.
The first reported case of coexistent MG and epilepsy was published in 1958 [
5]; since then only a few studies have been published [
6,
7,
8].
The aim of the present study was to report the frequency of epilepsy and myasthenia gravis and to describe a case series of patients with myasthenia gravis and epilepsy.
2. Material and Methods
2.1. Patients
Retrospective, observational, single-adult-center study was conducted in 2022. Patients were recruited from the outpatient clinic of the MG and Epilepsy Units. Hospital Universitari de Bellvitge.
The study was approved by the ethical committee (PI10/00738). Written informed consent was obtained from all patients.
2.2. Definitions and Inclusion-Exclusion Criteria
MG definition [
9]: Documented myasthenia gravis symptoms plus one of these two criteria: a) Positivity for AChR ab or MuSK ab, and b) a pathological electrophysiological study: single fiber electromyography (SFEMG) or repetitive nerve stimulation (RepStim).
Epilepsy definition [
10]: Confirmed epilepsy based on clinical history and supportive electroencephalogram (EEG) and brain magnetic resonance imaging (MRI).
Seizures have been described according to the ILAE accepted criteria [
11].
Autoimmune-associated epilepsy [
1,
12]: Evidence of autoimmune-mediated central nervous system inflammation or the presence of neuronal autoantibodies that had already been related with epilepsy at high titres.
Patients with acute symptomatic seizures (ASS) secondary to autoimmune encephalitis (AE) were excluded from the study [
12]. To rule out the possibility of ASS a minimum follow-up of 2 years from the epilepsy debut was needed
.
2.3. Diagnostic Procedures
2.3.1. Immunological Analysis
An immunological battery test was carried out including onconeuronal antibodies (ab), ab against neuronal surface antigens (NSA), antibodies to glutamic acid decarboxylase (GAD ab), AchR ab, MusK ab, and LRP4 ab. Onconeuronal ab testing was done using immunoblot. NSA ab included LGI1/contactin-2 associated protein (CASPR2), N-methyl-D-aspartate receptor (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), γ-aminobutyric acid receptor (GABA
BR), and dipeptidyl-peptidase-like protein-6 (DPPX). NSA-abs were identified by indirect immunofluorescence in cells transfected with neuronal antigens. GAD65 ab was analyzed as described elsewhere [
13].
2.3.2. Electrophysiological Studies
A standard EEG or with sleep deprivation was done in all patients on a 64 channel digital Deltamed or 32 XLTEK equipment. In drug resistant epilepsy (DRE) patients prolonged video-EEG monitoring was done (a 128 XLTEK equipment).
An EMG with SFEMG and/or RepStim was done in all patients on a Nicolet EDX® EMG/NCS/EP/ IOM.
3. Results
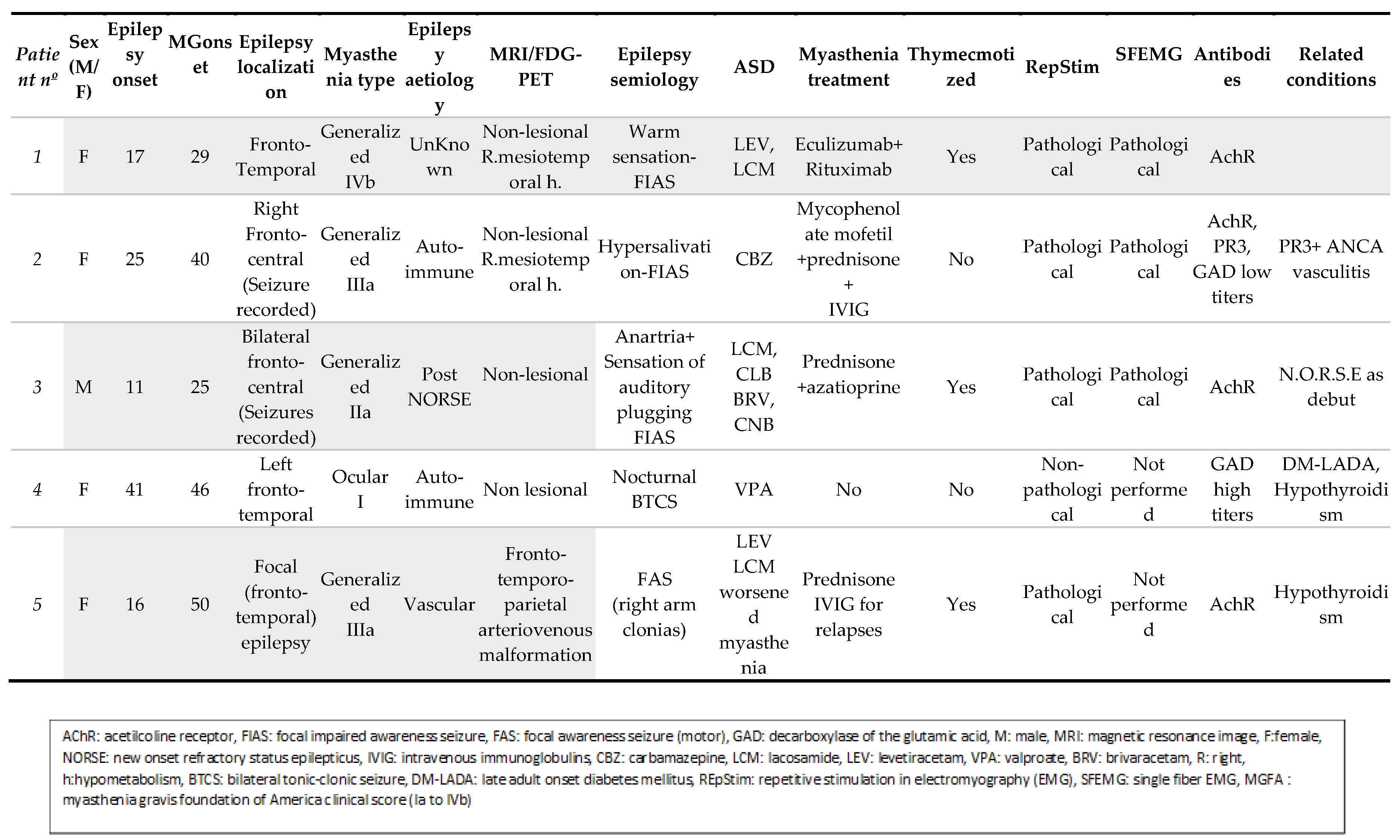
Of 469 patients with MG included in the MG database, 5 (1.1%) patients also suffered epilepsy. Of 1,432 patients with epilepsy included in the epilepsy database, 5 (0.35%) also suffered MG. The five identified are briefly described below (see also Table 1 and Image 1).
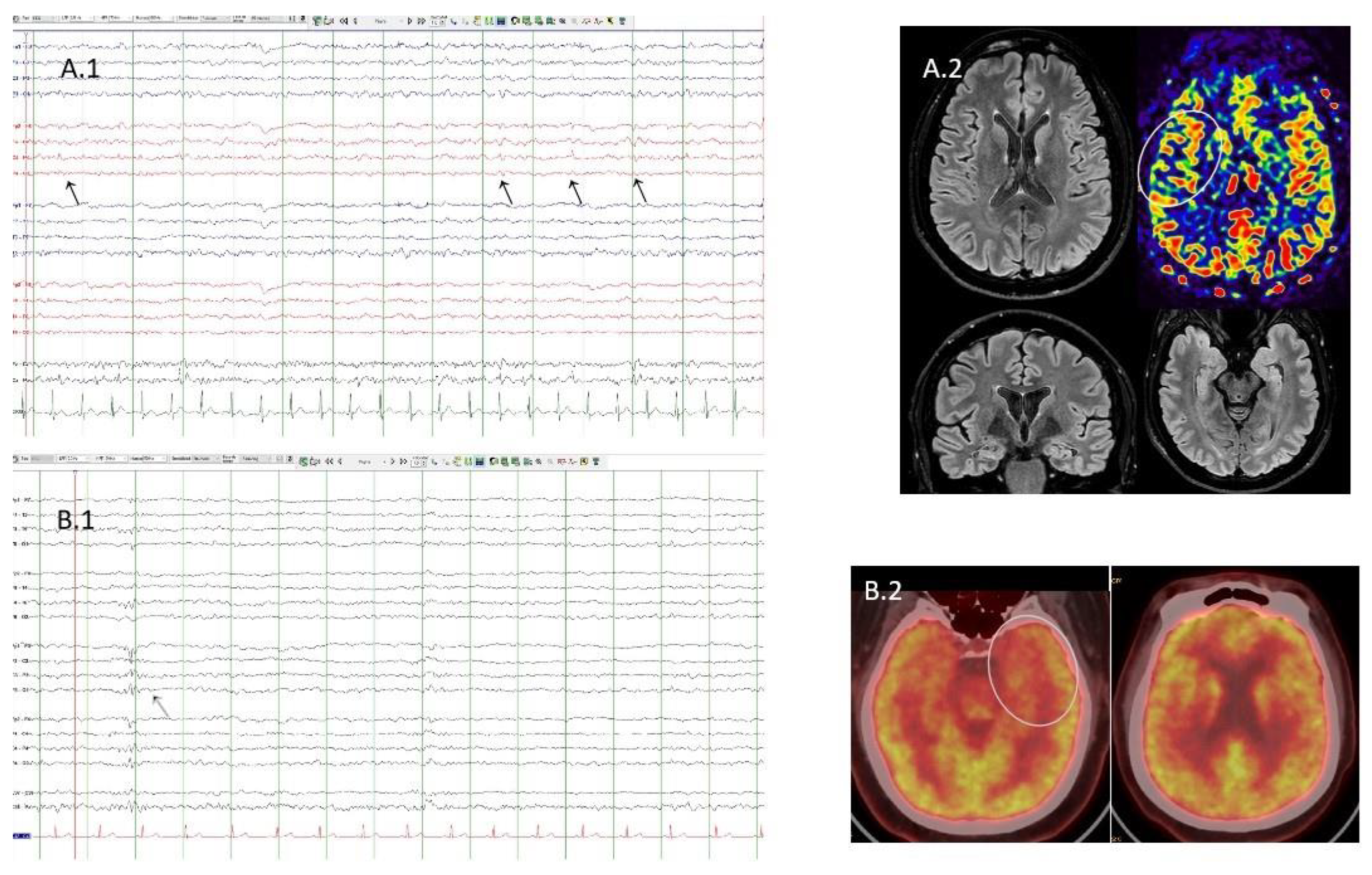
Figure 1.
A1. Case 4. EEG with partial sleep deprivation. Bipolar montage. Presence of interictal epileptiform discharges (black arrows) located in the right fronto-central area. A2. Case 4. Flair sequences showing normal structural MRI. Arterial spin labelling sequence shows discrete hypoperfusion in the right operculo-insular area (white circle). B1. Case 1. Sleep EEG. Bipolar montage. Presence of interictal epileptiform discharge (grey arrow) located in the left fronto-central area. B2. Case 1. FDG-PET/TC showing left temporopolar hypometabolism (white circle).
Figure 1.
A1. Case 4. EEG with partial sleep deprivation. Bipolar montage. Presence of interictal epileptiform discharges (black arrows) located in the right fronto-central area. A2. Case 4. Flair sequences showing normal structural MRI. Arterial spin labelling sequence shows discrete hypoperfusion in the right operculo-insular area (white circle). B1. Case 1. Sleep EEG. Bipolar montage. Presence of interictal epileptiform discharge (grey arrow) located in the left fronto-central area. B2. Case 1. FDG-PET/TC showing left temporopolar hypometabolism (white circle).
3.1. Case 1
A fifty-year-old woman with DRE from the age of 17. She presented focal impaired awareness seizures (FIAS) sometimes evolving to bilateral tonic-clonic (BTC). MRI was normal. She started on levetiracetam (LEV) and lacosamide (LCM) without complete seizure control. In addition, she debuted with generalized MG at the age of 29, requiring a thymectomy. She has also required immunosuppressive therapy and is currently on eculizumab and rytuximab with difficult control of the disease. Both MG relapses and epilepsy worsening coincided. The immunological battery found positive AchR antibodies.
3.2. Case 2
A forty-seven-year-old woman with a non-lesional fronto-temporal lobe epilepsy onset at 25 years of age, treated with carbamazepine (CBZ). At the age of 40 she reported seizure relapse, worsening of chronic headache, diplopia dyspnea, and muscle weakness, and was diagnosed with PR3+ ANCA vasculitis with renal, pulmonary, and cerebral involvement, and onset of generalized MG. Under treatment with mycophenolate mofetil and prednisone, symptoms were controlled. The immunological battery found positive AchR antibodies and low positive GAD ab, and positive PR3 ab.
3.3. Case 3
A thirty-two-year-old man who suffered a new onset of refractory status epilepticus (NORSE) at the age of 11. Immediately afterwards, he developed DRE. EEG recorded seizures starting independently from both frontocentral areas. Seizures started with a sensation of auditory plugging and anarthria. MRI and FDG-PET were normal. He is now on LCM-CBM and cenobamate, and still suffering weekly seizures. He debuted with MG at the age of 25. He was thymectomized and treated with prednisone and azathioprine with complete control of symptoms. The immunological battery found positive AchR ab.3.4
3.4. Case 4
A sixty-nine-year-old woman with well-controlled left temporal lobe epilepsy onset at 41 with a nocturnal BTC seizures. MRI was normal. Seronegative ocular MG was diagnosed at 46 and was completely controlled without treatment. She has also suffered diabetes mellitus since age 52. Apart from GAD ab (85.4000 UI titres) the rest of the immunological battery was negative.
3.5. Case 5
A fifty-six-year-old woman with focal epilepsy due to right fronto-temporo-parietal arteriovenous malformation requiring as many as 28 embolizations. Epilepsy began at the age of 16. She had been treated with CBZ and phenobarbital (PB) for years with good control, and afterwards with LCM. She suffered onset of generalized MG at the age of 50, just a few weeks of being treated with LCM. She was thymectomized and treated with prednisone and IVIG. The immunological battery found positive AchR ab.
4. Discussion
In the present study, the frequency of MG in the epilepsy cohort was 0.4%, higher than the prevalence of MG in the general population [
4] and similar to a populational study with more than 2 million people in which the prevalence of epilepsy in patients with MG was 0.4% [
2]. Other series such as Badurska [
6] have reported an even higher frequency (7%) of epilepsy in patients with MG. A possible reason for this difference is the included population; we included adult patients with epilepsy and excluded isolated seizures or ASS.
Focusing on clinical characteristics of epilepsy in our series, all patients suffered a focal epilepsy. Seizure semiology and EEG characteristics suggested a periopercular or temporo-insular origin in four patients, as reported previously [
7]. Furthermore, MG was generalized and with exacerbations in half of the patients and related to a thymic hyperplasia in all the thymectomized patients.
Considering the association of MG and epilepsy, epilepsy onset preceded MG onset in all patients. Other authors have found the same timeline association [
6,
8,
14]. In contrast to this, some patients with thymoma who developed a paraneoplastic AE suffered myasthenia and ASS, with seizure onset preceding the myasthenic symptoms [
15].
Many hypotheses have been proposed to explain the association of MG and epilepsy [
6,
14]. The one that we consider the strongest is that these diseases are a reflection of activation of a common autoimmune cascade, as in patients 2 and 4. Both patients suffer an autoimmune associated epilepsy, patient 2 a PR3+ ANCA vasculitis with cerebral involvement and patient 4 a GAD ab associated epilepsy.
Other hypotheses suggest that the MG and epilepsy association is a reflection of a previously damaged autoimmune system as in patient 3, who had suffered a NORSE. Another hypothesis that might explain the association, in patient 5, is that antiseizure drugs (ASD), specifically LCM, may alter the neuromuscular junction [
6] or trigger an autoimmune response in a susceptible individual, inducing MG [
16].However, LCM has been safely used in two patients with myasthenia[
17]. Finally, in patient 5, the association of the two diseases may have been produced by chance.
Interestingly, the trace element selenium (Se) could be a link between autoimmune disease predisposition and having suffered a NORSE or being treated with ASD. Se is essential for normal functioning of the immune system. A severe acute or chronic Se deficit disturbs the immune system, irrespective of the underlying reason (e.g., infection)[
17]. Furthermore ASD and immunomodulatory treatment may affect the homeostasis of several trace metal levels [
18]. Specifically, VPA can promote the clearance of copper, Se and zinc, reducing the synthesis of free radical-scavenging enzymes [
19]. There is no information about other ASDs.
This study has several limitations: it is a retrospective and chart review study so selection bias and less than accurate recordkeeping may be present. Also, we did not determine trace elements.
5. Conclusion
Epilepsy and MG, although infrequently associated, are comorbid condition. Epilepsy onset precedes MG onset in most cases. In some cases, epilepsy has an autoimmune aetiology and/or coexists with other autoimmune conditions.
Authors’ contribution statement
Iñigo Oyarzun: conceptualization, data curation, original draft preparation; Guillermo Hernández: investigation, writing-reviewing, and editing; Jacint Sala-Padró: investigation, writing-reviewing, and editing; Francisco Morandeira: data curation, writing-reviewing, and editing; Carlos Casasnovas: investigation, writing-reviewing, and editing; Mercè Falip: conceptualization, data curation, original draft preparation, writing-reviewing, and editing. All the authors reviewed the final draft of the paper and discussed the conclusions of the study.
Data availability
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all the patients for their collaboration. We are also grateful for the collaboration during the diagnosis work-up of the staff of the various services and departments of the Hospital Universitari de Bellvitge.
Conflicts of Interest statement
All the authors report no conflict of interest. .
Use of generative artificial intelligence (AI) statement
All the authors confirm that AI has not been used in the preparation of this manuscript.
Source of financial support
Thanks to the CERCA Programme/Generalitat de Catalunya and to the Hospital Universitari de Bellvitge Management Department for Institutional Support.
References
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.-S.; Kohane, I.S.; Cai, T.; Gorman, M.P.; Mandl, K.D. Population-Level Evidence for an Autoimmune Etiology of Epilepsy. JAMA Neurol. 2014, 71, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, A.W.; Engeland, A.; Gilhus, N.E. Epilepsy and autoimmune diseases: Comorbidity in a national patient cohort. Seizure 2019, 75, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Prim. 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Hoefer, P.F.A.; Aranow, H.; Rowland, L.P. Myasthenia Gravis and Epilepsy. Arch. Neurol. Psychiatry 1958, 80, 10–7. [Google Scholar] [CrossRef] [PubMed]
- Badurska, B.; Ryniewicz, B.; Kowalski, J. [Epileptic seizures in children with myasthenia gravis]. 1991, 25, 326–31. [Google Scholar] [PubMed]
- Sureshbabu, S.; Karunanidhi, S.; Peter, S.; Chindrippu, S.; Joseph, M.; Mittal, G.K. Myasthenia gravis and insular cortex epilepsy: more than a chance association? Acta Neurol. Belg. 2021, 122, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, P.J.; Ducci, R.D.-P.; Tensini, T.S.; Dalledone, G.; Kay, C.S.K.; de Paola, L.; Werneck, L.C.; Scola, R.H.; Silvado, C. Treatment of epilepsy in patients with myasthenia gravis: Is really harder than it looks? J. Clin. Neurosci. 2017, 44, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Hehir, M.K.; Li, Y. Diagnosis and Management of Myasthenia Gravis. Contin. Lifelong Learn. Neurol. 2022, 28, 1615–1642. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Steriade, C.; Britton, J.; Dale, R.C.; Gadoth, A.; Irani, S.R.; Linnoila, J.; McKeon, A.; Shao, X.; Venegas, V.; Bien, C.G. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: Conceptual definitions. Epilepsia 2020, 61, 1341–1351. [Google Scholar] [CrossRef]
- Ances, B.M.; Vitaliani, R.; Taylor, R.A.; Liebeskind, D.S.; Voloschin, A.; Houghton, D.J.; Galetta, S.L.; Dichter, M.; Alavi, A.; Rosenfeld, M.R.; et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005, 128, 1764–1777. [Google Scholar] [CrossRef]
- Lai, C.-W.; Leppik, I.E.; Jenkins, D.C.; Sood, P. Epilepsy, Myasthenia Gravis, and Effect of Plasmapheresis on Antiepileptic Drug Concentrations. Arch. Neurol. 1990, 47, 66–68. [Google Scholar] [CrossRef]
- Wang, B.; Hao, Y.; Zhu, R. Clinical features of myasthenia gravis with neurological and systemic autoimmune diseases. Front. Immunol. 2023, 14, 1223322. [Google Scholar] [CrossRef]
- Kurian, M.A.; King, M.D. Antibody Positive Myasthenia Gravis Following Treatment with Carbamazepine. Neuropediatrics 2003, 34, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Sánchez- Villalobos JM, Villegas-Martínez I, Pérez-Vicente JA. A well-tolerated and effective antiepileptic drug for patients with myasthenia gravis at last? Clinical Neuropharmacology 41(2): p 80-81, 3/4 2018. [CrossRef]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19—A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.; Zhang, L.; Li, F.; Zhang, L.; Tai, Z.; Yang, J.; Zhang, H.; Tuo, J.; Yu, C.; et al. Research progress on correlations between trace element levels and epilepsy. Front. Cell Dev. Biol. 2023, 11, 1167626. [Google Scholar] [CrossRef] [PubMed]
- Kürekçi, A.E.; Alpay, F.; Tanindi, .; Gokçay, E.; Ozcan, O.; Akin, R.; Işimer, A.; Sayal, A. Plasma Trace Element, Plasma Glutathione Peroxidase, and Superoxide Dismutase Levels in Epileptic Children Receiving Antiepileptic Drug Therapy. Epilepsia 1995, 36, 600–604. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).