1. Introduction
Monkeypox (mpox) is a zoonotic disease caused by the monkeypox virus (MPXV). MPXV represents a large double-stranded DNA virus belonging to the genus Orthopoxvirus (OPXV), family Poxviridae. Mpox is currently the most prevalent orthopoxvirus zoonosis in humans since the eradication variola virus [
1,
2,
3].
MPXV was first isolated from monkeys and was identified as a human pathogen in the Democratic Republic of the Congo (DRC, formerly Zaire) in 1970 [
4]. Over the past almost 50 years, human cases of mpox have been identified in 11 African countries, and MPXV is considered endemic in the DRC [
5,
6]. Based on serological data, MPXV is maintained by various mammalian species, with periodic introduction into human populations, where relatively short chains (≤7) of human-to-human transmission can occur [
7,
8]. Genetically, there are 2 clades of MPXV: West African (covering Nigeria, Benin, Côte d'Ivoire, Liberia and Sierra Leone) and Central African (covering the Congo Basin - Democratic Republic of Congo, Central African Republic, Republic of Congo, Gabon and Cameroon) [
9].
Since 2022, a multi-country outbreak of mpox has been reported and the number of cases has continued to increase worldwide (incl. in non-endemic countries). Up to 10 July 2024, 98,001 mpox cases have been identified in 118 countries and areas globally, of which only 7 countries historically reported mpox [
10]. The first cases occurred in people who had attended an international LGBT+ Pride event held on the Spanish island of Gran Canaria, which linked to transmission chains in several European countries [
11,
12]
However, locally acquired infections and community transmission became predominant by the end of May 2022 in all affected countries [
12]. The current global outbreak is characterized by atypically rapid human-to-human transmission, with no reported animal reservoir, which is probably determined by the accumulation of genetic mutations in the viral genome, favoring the circulation of the virus in the public, especially among highly affected groups (men who have sex with men), as well as non-immune household members and healthcare workers [
11]. Human-to-human transmission of MPXV can occur through respiratory secretions, direct contact, vertical transmission, percutaneous transmission, or indirect contact with fomites. People with mpox are considered infectious until all their lesions have crusted over, the scabs have fallen off and a new layer of skin has formed underneath, usually taking 2 to 4 weeks [
13]. It is also possible for the MPXV to persist for some time on clothing, bedding, towels, objects, electronics, and surfaces that a person with mpox has touched [
14].
In the laboratory diagnosis of the virus, mainly nucleic acid amplification tests (NAATs) - PCR identification of specific DNA sequences from the viral genome are used. The critical step in a reliable and accurate mpox diagnosis is choosing the correct patient's clinical specimens for testing [
15]. According to the WHO criteria, the recommended sample type for laboratory investigation is skin lesion material, including roofs from more than one lesion roofs (e.g., lesion crusts) and swabs of lesion surface and/or exudate [
16]. In addition to lesion samples, the collection of an oropharyngeal swab is also encouraged. Importantly, data on the accuracy of this type of sample for mpox diagnosis is scarce, and therefore a negative throat swab sample should be interpreted with caution. The literature data regarding the use of other types (alternative) samples for MPXV laboratory diagnosis are limited, which is the subject of investigation and discussion in the present study.
According to literature data, the variola vaccine can provide cross-protection against mpox in 85% of those immunized. Although post-vaccination immunity declines with time, it is suggested that the variola vaccine should provide some degree of protection in adults over 50 years of age [
17,
18]. Given the official announcement by the WHO of the eradication of smallpox in the world in 1980, vaccination in Bulgaria and other countries was not carried out after that year. According to the available data, the last vaccinated persons in Bulgaria were those born in 1976-78, and the vaccine was not administered to all children. The presence of a scarification scar on the right hand (in the area of the deltoid muscle) should be considered a sure sign of a variola vaccination [
17].
The present work described here aimed to investigate the frequency of MPXV and OPXV DNA detection in recommended and alternative clinical materials taken at acute and convalescent phases of the infection in Bulgarian patients.
2. Materials and Methods
2.1. Patients
From May 2022 to April 2024, a total of 181 clinical samples from 42 patients, 31 males, and 11 females, aged 6 to 76 years with possible mpox infections were tested. The tested clinical samples were: samples of vesicle contents (n=42), crust (n=38), nasal/oropharyngeal swab (n=42), urine (n=16), feces (n=18), ejaculate (n=16), and saliva (n=9) were taken in the first days of onset of clinical symptoms. The vesicle contents and nasal/oropharyngeal swabs were placed and transported in viral transport media (VTM). The collection and transportation of the clinical samples were to the requirements of the WHO (Laboratory Guidelines for the Detection and Diagnosis of Monkeypox Virus Infection – 2 September 2022) [
16]. The follow-up samples were requested from all patients confirmed for mpox one week after the first samples. Clinical specimens were tested at the National Reference Laboratory “Measles, Mumps, Rubella”, National Centre of Infectious and Parasitic Diseases, Sofia, Bulgaria.
2.2. PCR Assays
Viral DNA was extracted from all specimens using the PureLink Viral RNA/DNA Mini Kit (Thermo Fisherr Scientific Inc., Waltham, Massachusetts, USA). Screening for MPXV DNA (F3L gene) was performed by Monkeypox Virus Real-Time PCR kits (bioPerfectus Technologies Co., Shanghai, China) and for OPXV DNA rpo18 gene by SuperScript™ III Platinum™ One-Step qRT-PCR Kit (Thermo Fisher Scientific, Inc, Waltham, MA USA) with primers OPV rpo F1 and OPV rpo R1, and probe OPV rpo MGB [
19] (
Table 1). The protocols were designed to detect both Central and West African clades. The PCR machine used was the Real-time PCR System Gentier 96E, Tianlong Technology Co.
Positive and negative controls were included in each real-time PCR reaction. According to the Centers for Disease Control and Prevention (CDC), the detection of human DNA (e.g., RNase P) was used for extraction control [
20]. MPXV and OPXV DNA were detected on the FAM channel and the Ct value ≤40 was interpreted as positive (+). According to the kit manufacturer and literature, the protocols used do not cross-reactivity with the following microorganisms: measles, varicella-zoster, Epstein–Barr, Herpes simplex virus 1 and 2, rubella, cytomegalovirus, human herpesvirus 6, 7, and 8.
2.3. Statistical Analysis
For statistical processing of the obtained data, the following statistical approaches were used:
- Determination of indicators of relative share (%) and confidence interval (95%CI), utilizing which the dependence of applied diagnostic approaches and used clinical materials were evaluated.
- Standard deviation (SD).
3. Results
Of all tested patients six (6/42, 14%, 95%CI 3,51÷24,49) were confirmed with mpox infection. MPXV DNA was detected in 23/181 (12,71%, 95%CI 7,88÷17,54) clinical samples and OPXV DNA in 20/181 (11,05%, 95%CI 6,48÷15,62) specimens (
Table 1). The calculated percentage of coincidence between the obtained results (positive or negative) in the detection of MPXV and OPXV DNA, by the PCR protocols used, in our group of patients was 98%. The statistical analysis between the two groups MPXV DNA (+) and OPXV DNA (+) found p=0.459 and a statistical significance level of 54%. All confirmed patients were men who have sex with men, aged 23 to 44 (at an average age of 35,17±7,91 years) and two of them were HIV positive on antiretroviral therapy. The commonest clinical features were fever (5/6, 83%), vesicular-pustular rash: perianal (6/6, 100%), penile (4/6, 67%), and limbs (4/6, 67%). One of the patients was hospitalized. Regarding epidemiological data, three reported traveling abroad to Spain and the UK.
Among the examined clinical materials, MPXV DNA was found with the greatest frequency in specimens of vesicle contents and nasal/oropharyngeal swabs in 6/6 and 5/6 confirmed patients, respectively. OPXV DNA mainly was detected in vesicle contents (6/6), nasal/oropharyngeal swabs (4/6) and urine (4/6). On the other hand, MPXV is less likely to be demonstrated in samples from feces, only in one patient (with 33 Ct). The highest viral concentration of MPXV and OPXV DNA was demonstrated in specimens of vesicle contents and nasal/oropharyngeal swabs in the first three days of the rash (
Table 2).
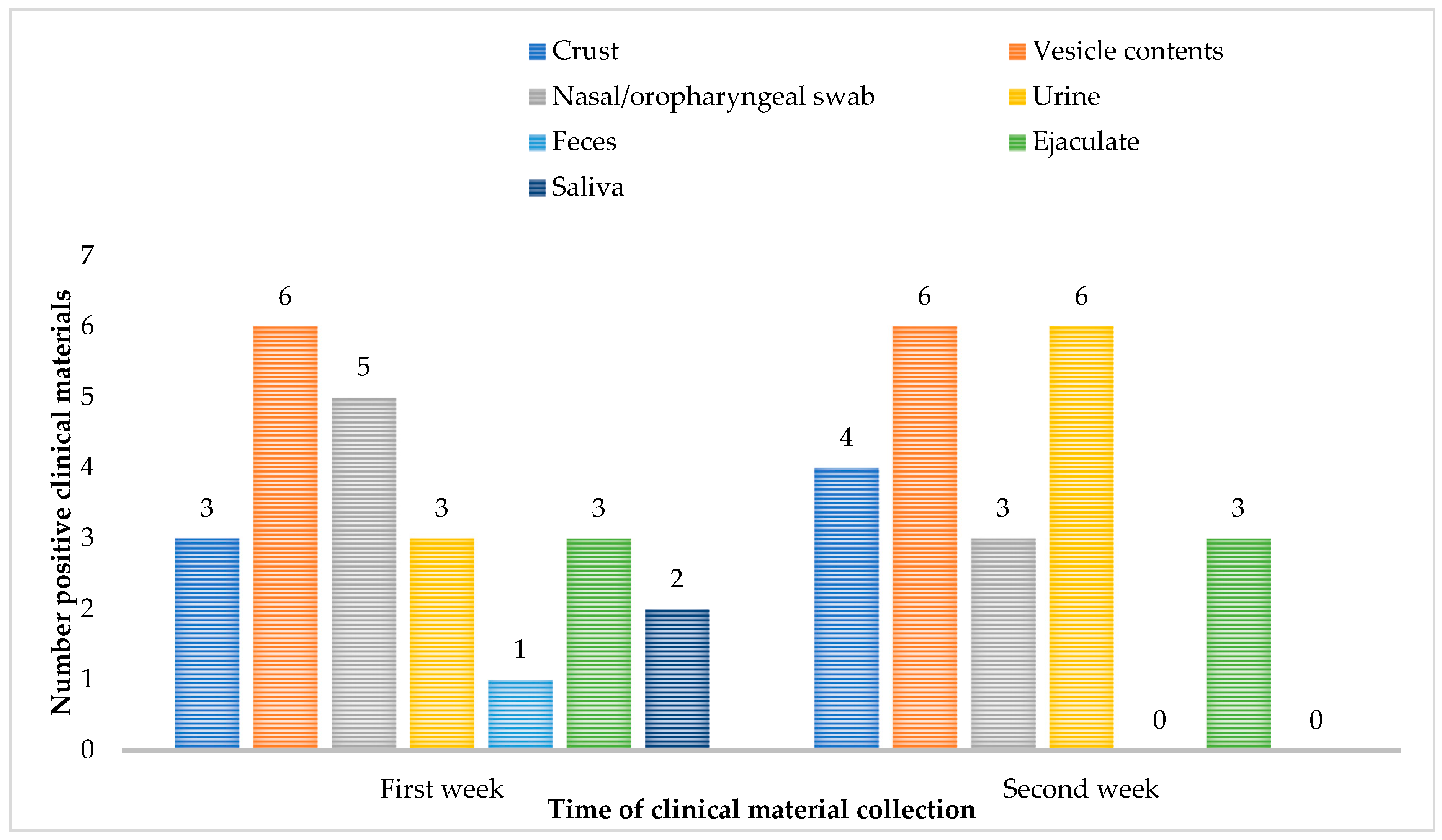
Follow-up samples were requested from all six confirmed patients and MPXV DNA in the repeat samples was detected in the highest percentage in the specimen’s vesicle contents (6/6, 100%) and urine (6/6, 100%). On the second week after confirmation of mpox infection, MPXV DNA is not demonstrated in samples of feces and saliva (
Figure 1).
4. Discussion
In the last multi-country mpox outbreak, MPXV was identified in non-endemic regions and persons with no direct travel history, confirming the person-to-person transmission pattern in the community countries. Many surveys reported the new clinical and epidemiological pattern of the 2022 outbreak, the high frequency of lesions in anogenital areas and oral mucosa may explain the transmission during sexual intercourse and not by animal reservoirs [
21]. Clusters associated with sex-on-site venues, festivals, pride parades, and saunas have been reported, suggesting a correlation between mpox transmission, and interconnected social networks [
22].
A total of 27,180 mpox cases have been identified in 46 countries and areas throughout the European Region up to 05 April 2024. Six of the cases were reported in TESSy from Bulgaria. The majority of cases were male (98%) between 31 and 40 years old and 96% of male cases with known sexual orientation, self-identified as men who have sex with men. Among cases with known HIV status, 38% were HIV-positive. Regarding clinical complications, 95% of cases presented with a rash and systemic symptoms such as fever, fatigue, muscle pain, chills, or headache (67%) [
23]. Seven percent of mpox cases were hospitalized and ten patients died. Globally, since 1 January 2022, cases of mpox have been reported to WHO from 118 Member States across all 6 WHO regions [
10].
All cases diagnosed in our cohort were young males, MSM, and some of them reported multiple risky sexual encounters. The transmission was overwhelmingly associated with person-to-person close contact and sexual activities, a recent history of traveling abroad (3/6, 50%), or contact with a confirmed mpox case (1/6, 17%). The proven epidemiological link determines infection with the virus outside the country's borders.
The WHO recommends MPXV PCR testing primarily from skin lesions, which have higher sensitivity than other specimen types [
24], in our study skin lesions also were the most common specimen type both during the first days of the onset of clinical symptoms and a week after. They were positive for MPXV and OPXV DNA between 26 and 30 Ct. In the first days of infection, the MPXV DNA was demonstrated with a high frequency in nasal/oropharyngeal swabs (5/6), and in the later stages in urine (6/6). This determines the passage of the virus through the host's body - primary viremia in the upper respiratory tract and subsequent excretion of virus particles through urine. Viral DNA was also shown in the ejaculate (3/6, 50%) and feces (1/6, 17%) samples from the patients, indicating a potential for spread of the virus through sexual contact and household contact. Of course, the question remains whether the detected virus nuclein acid has infectious potential, this can only be proven by virus isolation in Vero cell cultures [
25], which for the purposes of the present study was not carried out. Our results showed that more than two types of clinical samples with the potential to detect the virus are necessarily provided by one patient.
Since 2023, despite a decrease in the number of mpox cases in Europe, the main threat associated with the virus is the MPXV clade I outbreak in the Democratic Republic of Congo which is endemic to Central Africa and is generally considered to be more virulent than MPXV clade II [
26]. The previous mpox outbreak in the EU and worldwide was associated with widespread circulation of clade II and the population relies on cross-protection between the two clades. However, awareness among clinicians regarding the specificity of the virus and risk groups should be increased.
5. Conclusions
The main conclusions we can draw from the present study are:
In our practice, we successfully use different types of clinical material for MPXV and OPXV DNA detection. In addition to standard WHO-recommended samples (vesicle contents and nasal/oropharyngeal swab), we demonstrated MPXV and OPXV DNA in alternative samples, namely urine, feces, ejaculate, and saliva, including in follow-up patient samples in which the virus can persist for more than two weeks. This points to screening a larger set of materials from a single patient and its easier transmission from person to person, including through household contact.
The concordance in 98% of the PCR results in terms of proving MPXV and OPXV DNA shows the possibility of interchangeability between the two methods of mpox diagnosis.
As limitations of our study, we note the small number of mpox-confirmed patients that we studied in Bulgaria, which determines the possible hidden spread of the virus, without laboratory diagnosis and on the other hand the accumulation of mutations in the genetic regions which become undetectable from used primers and probe.
Despite the known epidemiology and clinical manifestation of mpox, the human-to-human spread of the virus after May 2022 surprised many scientists and health authorities. It imposed a more detailed investigation of virus transmission, genetics, and mutagenicity.
Author Contributions
Conceptualization, Stefka Krumova, and Iva Christova; Investigation, Stefka Krumova, and Petia Genova-Kalou; Methodology, Radostina Stefanova, Daniel Ivanov, Maria Pishmisheva and Stanislav Kotsev; Writing – original draft, Stefka Krumova and Iva Christova. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Fund, research project: "Monkeypox in Bulgaria – clinical manifestation, transmission, virological and molecular genetic analysis" (Contract No. КП-06-Н63/13 dated 03.07.2023).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the NATIONAL CENTRE OF INFECTIOUS AND PARASITIC DISEASES, Sofia, Bulgaria (ETHICS COMMITTEE IRB 00006384), protocol code 1/2024 on February 05, 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The study was supported by the National Science Fund, research project: "Monkeypox in Bulgaria – clinical manifestation, transmission, virological and molecular genetic analysis" (Contract No. КП-06-Н63/13 dated 03.07.2023).
Conflicts of Interest
All co-authors reported no conflict of interest.
References
- Simoes, P.; Bhagani, S. A viewpoint: The 2022 monkeypox outbreak. Journal of Virus Eradication 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses. 2022. https://ictv.global/taxonomy; [accessed 8 August 2022].
- WHO (2022). WHO Recommends New Name for Monkeypox Disease. https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease.
- Marennikova, S.S.; Seluhina, E.M.; Mal’ceva, N.N.; Cimiskjan, K.L.; Macevic, G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972, 46, 599–611. [Google Scholar] [PubMed]
- Simoes, P.; Bhagani, S. A viewpoint: The 2022 monkeypox outbreak. Journal of Virus Eradicatio 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, G.; Liszewski, M.K.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Doty, J.B.; Malekani, J.M.; Kalemba, L.N.; et al. Assessing Monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis 2016, 22, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; et al. A tale of two clades: monkeypox viruses. J Gen Virol 2005, 86, 2661–72. [Google Scholar] [CrossRef] [PubMed]
-
https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html.
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Selb, R.; Werber, D.; Falkenhorst, G.; et al. A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Euro Surveill. 2022, 27, 2200499. [Google Scholar] [CrossRef] [PubMed]
- Zaucha, G.M.; Jahrling, P.B.; Geisbert, T.W.; Swearengen, J.R.; Hensley, L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab Invest. 2001, 81, 1581–1600. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.J.R.D.; Kohl, A.; Pena, L.; Pardee, K. Clinical and laboratory diagnosis of monkeypox (mpox): Current status and future directions. iScience. 2023, 26, 106759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
-
https://www.paho.org/en/documents/laboratory-guidelines-detection-and-diagnosis-monkeypox-virus-infection-2-september-2022.
- Sanz-Muñoz, I.; Sánchez-dePrada, L.; Sánchez-Martínez, J.; Rojo-Rello, S.; Domínguez-Gil, M.; Hernán-García, C.; Fernández-Espinilla, V.; de Lejarazu-Leonardo, R.O.; Castrodeza-Sanz, J.; Eiros, J.M. Possible Mpox Protection from Smallpox Vaccine-Generated Antibodies among Older Adults. Emerg Infect Dis. 2023, 29, 656–658. [Google Scholar] [CrossRef]
- Monkeypox. 19 May 2022. Retrieved from, https://www.who.int/news-room/fact-sheets/det ail/monkeypox.
- Nitsche; et al. Detection of orthopoxvirus DNA by real-time PCR and identification of variola virus DNA by melting analysis. J Clin Microbiol. 2004, 42, 1207–1213. [Google Scholar] [CrossRef]
- Centers for Disease Control & Prevention Poxvirus & Rabies Branch (PRB). Test Procedure: Monkeypox virus Generic Real-Time PCR Test. https://www.cdc.gov/poxvirus/mpox/pdf/PCR-Diagnostic-Protocol-508.pdf.
- El Eid, R.; Allaw, F.; Haddad, S.F.; Kanj, S.S. Human monkeypox: A review of the literature. PLoS Pathog. 2022, 18, e1010768. [Google Scholar] [CrossRef] [PubMed]
- Ianache, I.; Skrzat-Klapaczynska, A.; Jilich, D.; Fleischhans, L.; Gmizic, I.; Ranin, J.; Papadopoulos, A.; Protopapas, K.; et al. Mpox across countries from Central and Eastern Europe - 2022 outbreak. Travel Medicine and Infectious Disease 2024, 59, 102719. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://monkeypoxreport.ecdc.europa.eu/.
- Edman-Wallér, J.; Jonsson, O.; Backlund, G.; Muradrasoli, S.; Sondén, K. Results of PCR Analysis of Mpox Clinical Samples, Sweden, 2022. Emerg Infect Dis. 2023, 29, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Moschese, D.; Pozza, G.; Mileto, D.; Giacomelli, A.; Cutrera, M.; Cossu, M.V.; Matone, M.; Beltrami, M.; Salari, F.; Antinori, S.; et al. Isolation of viable monkeypox virus from anal and urethral swabs, Italy, May to July 2022. Euro Surveill. 2022, 27, 2200675. [Google Scholar] [CrossRef] [PubMed]
- Masirika, L.M.; Udahemuka, J.C.; Ndishimye, P.; Martinez, G.S.; Kelvin, P.; Nadine, M.B.; et al. Epidemiology, clinical characteristics, and transmission patterns of a novel Mpox (Monkeypox) outbreak in eastern Democratic Republic of the Congo (DRC): an observational, cross-sectional cohort study. medRxiv 2024, 2024.03.05.24303395. Available at: https://www.medrxiv.org/content/medrxiv/early/2024/03/05/2024.03.05.24303395.full.pdf.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).