Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
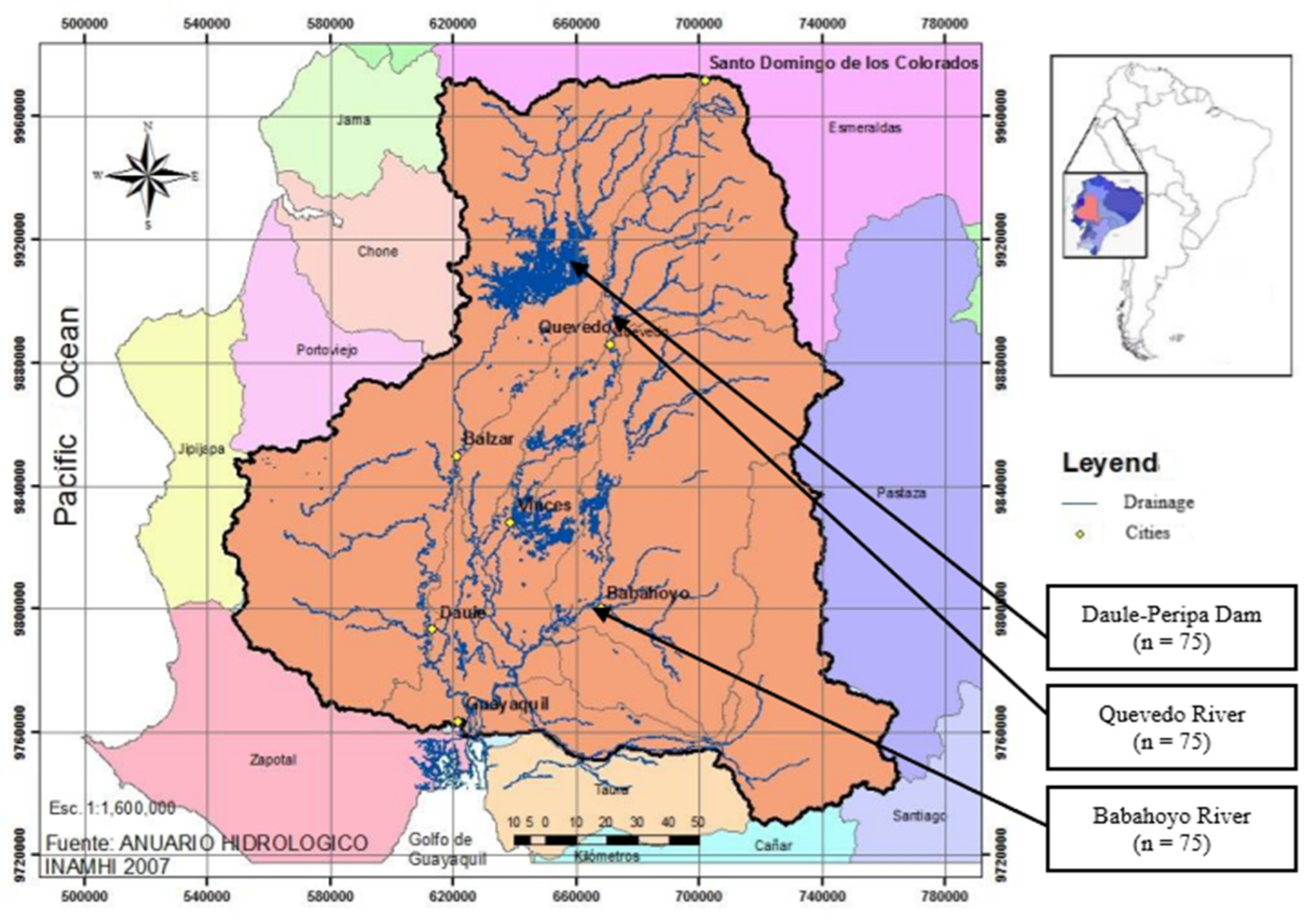
2.1. Study Area
2.2. Water Quality
2.3. Data Collection
2.4. Body Measurements
2.3. Fulton Condition Coefficient (K)
2.6. Statistical Analysis
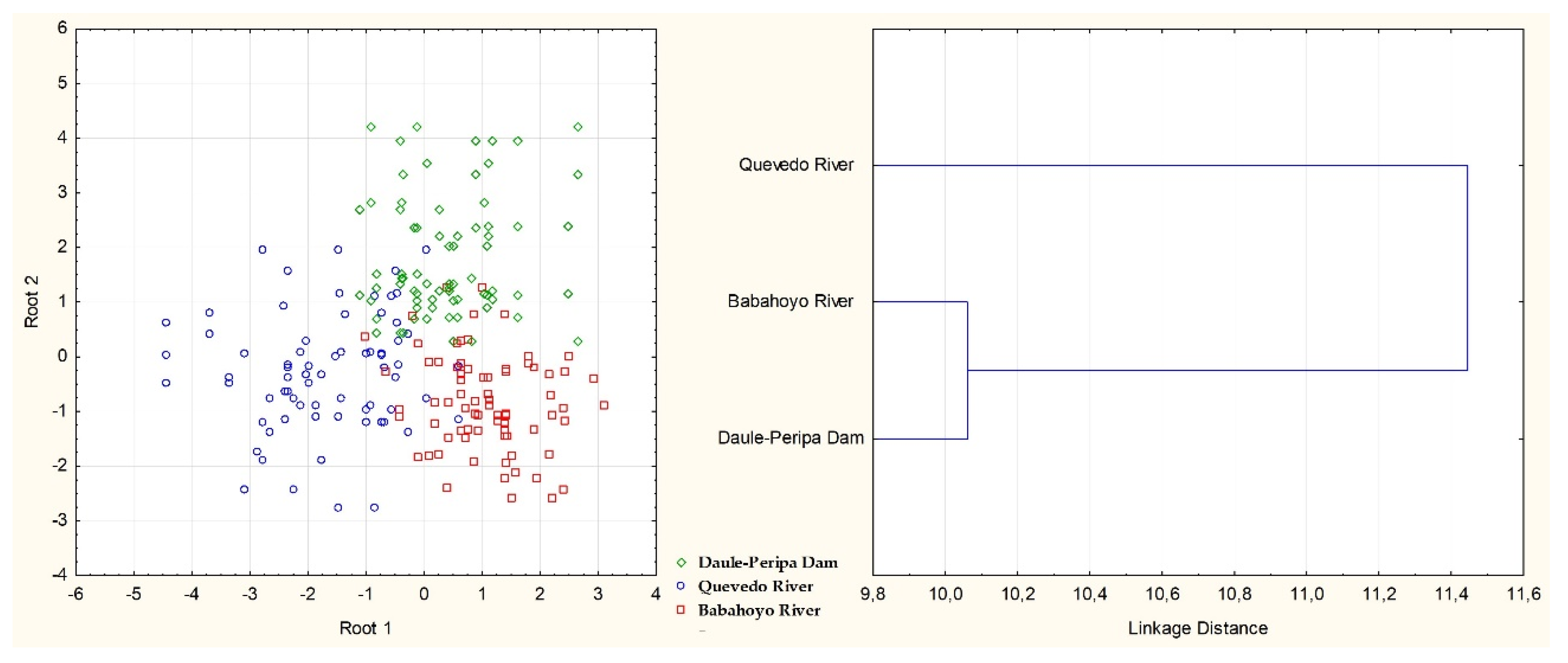
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barriga, R. Lista de peces de agua dulce e intermareales del Ecuador. Rev. Politec. 2012, 30, 83-119.
- Rodríguez, J.; González, A.; Angón, E.; Vivas, R.; Barba, C.; González, M.A.; Peña, F.; García, A. Efecto del tamaño de las reproductoras en la producción de alevines de Cichlasoma festae en condiciones semicontroladas en Ecuador. ITEA 2020, 116 (2), 93-105. [CrossRef]
- Jiménez-Prado, P.; Aguirre, W.; Laaz-Moncayo, E.; Navarrete-Amaya, R.; Nugra-Salazar, F.; Rebolledo-Monsalve, E.; Zárate-Hugo, E.; Torres-Noboa, A.; Valdiviezo-Rivera, J. Guía de peces para aguas continentales en la vertiente occidental del Ecuador. Pontificia Universidad Católica del Ecuador Sede Esmeraldas (PUCESE); Universidad del Azuay (UDA) y Museo Ecuatoriano de Ciencias Naturales (MECN) del Instituto Nacional de Biodiversidad. Esmeraldas, Ecuador. 2015, 416 pp.
- Olaya Carbó P. Estado ecológico del sistema estuarino del Río Guayas, Cantón Durán, Ecuador: Simulación numérica de su dinámica fluvial y principios ecológicos para el diseño de actuaciones de restauración y/o recuperación. MSc. Universidad de Alcalá, Universidad Complutense de Madrid, Universidad Rey Juan Carlos, Universidad Politécnica de Madrid. 2016.
- Alvarez-Mieles, G.; Irvine, K.; Griensven, A.V.; Arias-Hidalgo, M.; Torres, A. Mynett, A.E. Relationships between aquatic biotic communities and water quality in a tropical river–wetland system (Ecuador). Environ. Sci. Policy. 2013, 34, 115-127.
- Ansah, Y.B.; Frimpong, E.A.; Hallerman, E.M. Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts. Sustainability 2014, 6, 3697-3721. [CrossRef]
- Aguirre, W. E., Alvarez-Mieles, G., Anaguano-Yancha, F., Burgos Morán, R., Cucalón, R. V.; Escobar-Camacho, D.; ... & Zárate Hugo, E. Conservation threats and future prospects for the freshwater fishes of Ecuador: A hotspot of Neotropical fish diversity. J. Fish Biol. 2021, 99(4), 1158-1189.
- Soto, I.; Balzani, P.; Carneiro, L.; Cuthbert, R. N.; Macêdo, R.; Serhan Tarkan, A.; ... & Haubrock, P. J. Taming the terminological tempest in invasion science. Biol. Rev. 2024.
- Corlett, R. T. The Anthropocene concept in ecology and conservation. Trends Ecol. Evol. 2015, 30(1), 36-41. [CrossRef] [PubMed]
- Pievani, T. (2014). The sixth mass extinction: Anthropocene and the human impact on biodiversity. Rend. Fis. Acc. Lincei. 2014, 25, 85-93.
- Food and Agriculture Organization of the United Nations. Report on strategic priorities for action. For the sustainable use, development and conservation of animal genetic resources for food and agriculture. Second draft. Roma. Italia. 2005. Available online: (accessed on 25 July 2024).
- Choi, J-Y.; Kim, S-K. Effects of Aquatic Macrophytes on Spatial Distribution and Feeding Habits of Exotic Fish Species Lepomis macrochirus and Micropterus salmoides in Shallow Reservoirs in South Korea. Sustainability 2020, 12, 1447; doi:10.3390/su12041447. [CrossRef]
- Schluter, D. The Ecology of Adaptative Radiation, Oxford, UK: Oxford University Press. 2000.
- Travis, C.H.; Blum, M.J.; Heins, D.C. Morphological responses of a stream fish to water impoundment. Biol. Lett. 2010, 6, 803-806.
- Ndiwas, T.C.; Nyingi, D.W.; Claude, J.; Agnése, J.F. Morphological variation of wild populations of Nile tilapia (Oreochromis niloticus) living in extreme environmental conditions in the Kenyan Rift-Valley. Environ. Biol. Fish. 2016, 99, 473-485. [CrossRef]
- Escanta-Molina, R.; Jiménez-Prado, P. Uso de la morfometría geométrica para establecer contrastes biológicos y ambientales en poblaciones de peces del río Teaone. Revista Científica Hallazgos 2019, 4 (1), 55-69.
- Estrada Guagua, EP. Diferencias en la población de Eretmobrycon ecuadorensis del río Sálima, Cantón Atacames, a diferentes niveles altitudinales, como un bioindicador de la calidad ambiental. BSc. Pontificia Universidad Católica de Ecuador. 2019.
- Aguilar.; C.; González-Sansón, G.; Cabrera, Y.; Ruiz, A.; Allen Curry, R. Inter-habitat variation in density and size composition of reef fishes from the Cuban Northwestern shelf. Rev. Biol. Trop. 2014, 62 (2), 589-602.
- Kerezsy, A.; Arthington, A.H.; Balcombe, S.R. Fish Distribution in Far Western Queensland, Australia: The Importance of Habitat, Connectivity and Natural Flows. Diversity 2014, 6, 380-395. [CrossRef]
- Arceo-Carranza, Y.; Arceo-Carranzaa, D.; Gamboab, E.; Teutli-Hernández, C.; Badillo-Alemánay, M.; Herrera-Silveira, J.A. Conservación. Los peces como indicador de restauración de áreas de manglar en la costa norte de Yucatán. Rev. Mex. Biodiv. 2016, 87, 489-496.
- Cocha-Alulema, A.P. Análisis de la variación morfológica de Hoplias malabaricus (Bloch 1794), asociada al tipo de hábitat utilizando morfometría geométrica. Facultad de Ciencias Biológicas, Universidad Central del Ecuador, Quito, Ecuador. 2018.
- Kurtul, I.; Kaya, C.; Kaykaç, H.; Ilhan, A.; Düzbastilar, F. O.; Tosunoğlu, Z.; ... & Haubrock, P. J. How fish populations in Lake Bafa (Western Anatolia) respond to ecological shifts. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2024, 34(5), e4154.
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S. A.; ... Lazzaro, X. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia, 2010, 646, 73-90.
- Le Hen, G.; Balzani, P.; Haase, P.; Kouba, A.; Liu, C.; Nagelkerke, L. A.; ... Haubrock, P. J. Alien species and climate change drive shifts in a riverine fish community and trait compositions over 35 years. Sci. Total Environ. 2023, 867, 161486. [CrossRef]
- Gonzalez-Martinez, A.; De-Pablos-Heredero, C.; González, M.; Rodriguez, J.; Barba, C.; García, A. Morphological Variations of Wild Populations of Brycon dentex (Characidae, Teleostei) in the Guayas Hydrographic Basin (Ecuador). The Impact of Fishing Policies and Environmental Conditions. Animals 2021, 11(7), 1901. [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. El estado de la biodiversidad para la alimentación y la agricultura; comisión de recursos genéticos para la alimentación y la agricultura. Evaluaciones: Rome, Italy. 2019. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/1f51259a-7584-4cfc-bab7-d9109361199c/content (accessed on 25 July 2024).
- Food and Agriculture Organization of the United Nations. Phenotypic characterization of animal genetic resources. FAO Animal Production and Health Guidelines. No.11. 2012. Available online: http://14.139.252.116/agrportal/phenochar.htm (accessed on 25 July 2024).
- Caez, J.; González, A.; González, M.A.; Angón, E.; Rodríguez, J.M.; Peña, F.; Barca, C. García, A. Application of multifactorial discriminant analysis in the morphostructural differentiation of wild and cultured populations of Vieja Azul (Andinoacara rivulatus). Turk. J. Zool. 2019, 43, 516-530.
- González, M.A.; Rodríguez, J.M.; Angón, E.; Martínez, A.; García, A.; Peña, F. Characterization of morphological and meristic traits and their variations between two different populations (wild and cultured) of Cichlasoma festae, a species native to tropical Ecuadorian rivers. Archiv. Tierz. 2016, 59, 435-444. [CrossRef]
- Gonzalez-Martinez, A.; Lopez, M.; Molero, H.M.; Rodriguez, J.; Gonzalez, M.; Barba, C.; García, A. Morphometric and meristic characterization of native chame fish (Dormitator latifrons) in Ecuador using multivariate analysis. Animals, 2020, 10, 1805. [CrossRef] [PubMed]
- Teimori, A.; Schulz-Mirbach, T.; Esmaeli, H.R.; Reichenbacher, B. Geographical differentiation of Aphanius dispar (Teleostei: Cyprinodontae) from Southern Iran. J. Zool. Syst. Res. 2012, 50 (4), 289-304. [CrossRef]
- Aguirre, W.W.; Shervette, V.R.; Navarrete, R.; Calle, P.; Agorastos, S. Morphological and Genetic Divergence of Hoplias microlepis (Characiformes: Erythrinidae) in Rivers and Artificial Impoundments of Western Ecuador. Copeia, 2013, 2, 312-323.
- Gonzalez-Martinez, A.; De-Pablos-Heredero, C.; González, M.; Rodriguez, J.; Barba, C.; García, A. Usefulness of discriminant analysis in the morphometric differentiation of six native freshwater species from Ecuador. Animals, 2021, 11, 111,.
- Siddik, M. A. B.; Hanif, M. A.; Chaklader, M. R.; Nahar, A.; Mahmud, S. Fishery biology of gangetic whiting Sillaginopsis panijus (Hamilton, 1822) endemic to Ganges delta, Bangladesh. Egypt. J. Aquat. Res. 2015, 41(4), 307-313. [CrossRef]
- Dauda, A.; Abbaya, H. Y.; Ebegbulem, V. N. Application of multifactorial discriminant analysis of morphostructural differentiation of sheep. Journal of Genetics and Genetic Engineering, 2018, 2(2), 11-16.
- Mattox, G.M.T.; Bifi, A.G.; Oyakawa, O.T. Taxonomic study of Hoplias microlepis (Günther: 1864): a trans-Andean species of trahiras (Ostariophysi: Characiformes: Erythrinidae). Neotrop. Ichthyol. 2014, 12 (2), 343-352. [CrossRef]
- Rodriguez Vizcaino, N.E. Análisis proximal de pescados continentales de mayor consumo humano en Ecuador. BSc. Pontificia Universidad Católica del Ecuador, Quito-Ecuador. 2017. Available online: https://repositorio.puce.edu.ec/items/6ad10381-fd92-4668-9d18-9b8cc91d5892 (accessed on 25 July 2024).
- Werlinger, C. Biologia Marina y Oceanográfica: Conceptos y procesos. Concepción, Chile. Consejo Nacional del Libro y la Lectura. Universidad de Concepción. 2004.
- Lucas Pozo, A. R. Análisis interanual de cinco especies capturadas en el embalse parque lago Chongón, provincia del Guayas, Ecuador, 2008-2018 (Bachelor’s thesis) La Libertad: Universidad Estatal Península de Santa Elena, Ecuador. 2021. Available online: https://repositorio.upse.edu.ec/handle/46000/6634 (accessed on 25 July 2024).
- Ochoa Ubilla, B.Y.; Mendoza Nieto, K.X.; Vivas Moreira, R.; Zambrano, J.U.; Ferrer-Sánchez, Y. Ecuador Structure of catch sizes and length-weight ratio of native fish in the Abras de Mantequilla wetland, Ecuador (in Spanish). Cien. y Tecnol UTEQ 2016, 9 (2), 19-27.
- Pacheco Bedoya, J.L. Aspectos biológicos y pesqueros de las principales especies capturadas en el río Babahoyo y afluentes en el cantón Samborondón de la provincia del Guayas. Available on line: http://www.institutopesca.gob.ec/wp-content/uploads/2018/01/Aspectos.-Biol%C3%B3gicos-y-Pesqueros-R%C3%ADo-Babahoyo-y-Afluentes-Cant%C3%B3n-Samborond%C3%B3n-2015-2.pdf (accessed on 25 July 2024).
- Ferrito, V.; Mannino, M.C.; Pappalardo, A.M.; Tigano, C. Morphological variation among populations of Aphanius fasciatus Nardo, 1827 (Teleostei, Cyprinodontidae) from the Mediterranean. J. Fish Biol. 2007, 70, 1–20. [CrossRef]
- Mir, F.A.; Mir, J.I.; Chandra, S. Phenotypic variation in the Snowtrout Schizothorax richardsonii (Gray, 1832) (Actinopterygii: Cypriniformes: Cyprinidae) from the Indian Himalayas. Contrib. Zool. 2013, 82, 115-122. [CrossRef]
- Dasgupta, S.; Moqbul Hossain, M.D.; Huq, M.; Wheeler, D. Facing the hungry tide: Climate change, livelihood threats, and household responses in coastal Bangladesh. Clim. Change Econ. 2016, 7, 1650007. [CrossRef]
- Hossain, M.; Nahiduzzaman, M.; Saha, D.; Khanam, M.; Alam, M. Landmark-based morphometric and meristic variations of the endangered carp, Kalibaus labeocalbasu, from stocks of two isolated Rivers, the Jamuna and Halda and a hatchery. Zool. Stud. 2010, 49, 556–563.
- Rodriguez, J. Caracterización de la Cichlasoma festae (Vieja Colorada) en la cuenca hidrográfica del Guayas. Ecuador. Bachelor’s thesis. University of Cordoba, Spain, 2017. Available online: https://helvia.uco.es/xmlui/handle/10396/14921 (accessed on 25 July 2024).
- Prado, M.; Bucheli, R.; Calderón, G. Composición, distribución y abundancia del plancton en sistemas fluviales de la provincia de los ríos-ecuador. Boletín Científico y Técnico 2010, 20 (6), 1-52.
- MAGAP. Aspectos pesqueros de las principales especies capturadas en el embalse parque lago Chongón. Instituto Nacional de Pesca 2008-2013.
- Pazmiño-Rodríguez, J.C.; Zambrano-Ganchozo, G.L.; Coello-Burgos, H.A. Modelización de la calidad del agua del estero Aguas Claras, cantón Quevedo, Ecuador. DYNA 2018, 85, 204-214. [CrossRef]
- Zhang, H.; Kang, M.; Wu, J.; Wang, C.; Li, J.; Du, H.; Yang, H.; Wei, O.V. Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon. Animals 2019, 9, 583. [CrossRef] [PubMed]
- Huayamave, J. Estudio de las Aguas y Sedimentos del Río Daule, en la provincia del Guayas desde el punto de vista Físico químico, orgánico, bacteriológico y toxicológico. Tesis Doctoral. Universidad de las Palmas de Gran Canaria, Departamento de Ingeniería de Procesos. Gran Canaria – España. 2013. Available online: http://hdl.handle.net/10553/11262 (accessed on 25 July, 2024).
- Bermeo, M.; Villa, F.; Toro, P.; Valdiviezo, C. Metales Pesados represa Daule, Peripa. Editorial Grupo Compas S.A. ISBN 978-9942-760-59-3. Guayaquil – Ecuador. 2017.
- Cuero Ordoñez, L.E. (2017). Evaluación de la incidencia de las descargas contaminantes en la calidad del agua del río Quevedo, cantón Quevedo, provincia de los Ríos, año 2016. BSc, Quevedo-Los Rios, Ecuador. Available online: https://repositorio.uteq.edu.ec/items/80926713-fd24-4a8c-9da6-0059335eec0e (accessed on 25 July, 2024).
- Sandal, M. (2019). Efecto de las descargas de aguas residuales de la empresa EMSABA EP sobre la calidad del agua del rio Babahoyo, año 2018. Tesis de maestría. Que-vedo - Ecuador, 2019. Avaiable online: https://repositorio.uteq.edu.ec/server/api/core/bitstreams/8eddc4fa-7955-436c-ac91-d7cfc70f8ea7/content (accessed on 25 July, 2024).
- Less, B.; López, E. Estudios de la Biología de los Peces del Río Vinces. Boletín Científico y Técnico. Instituto Nacional de Pesca 1974, 3 (1), 1- 40.
- Revelo, W. Aspectos biológicos y pesqueros de los principales peces del sistema hídrico de la provincia de los Ríos, durante 2009. Boletín Científico y Técnico 2010, 20 (6), 53-84.
- Canadian Council on Animal Care. Guidelines on the care and use of fish research, teaching and testing. 2005. Available online: https://www.ccac.ca/Documents/Standards/Guidelines/Fish.pdf (accessed on 25 July 2024).
- Norma Española UNE 173300 – “Piscicultura: Guía de prácticas correctas para el sacrificio”. Available online: https://www.mapa.gob.es/es/pesca/temas/calidad-seguridad-alimentaria/pisciculturaguiadepracticascorrectasparaelsacrificio_tcm30-291479.pdf (accessed on 25 July 2024).
- Diodatti, F.C.; Fonseca de Freitas, R.T.; Freato, T.A.; Pérez Ribeiro, P.A.; Solis Murgas, L.D. Parámetros morfométricos en el rendimiento de los componentes corporales de tilapia del Nilo (Oreochromis Niloticus). Anales de Veterinaria (Murcia) 2008, 24, 45–55.
- Nash, R.R.M.; Valencia, A.H.; Geffen, A.J. The Origin of Fulton´s Condition Factor-Setting the Record Straight. Fisheries 2006, 31 (5), 236-238.
- Elliott, N.G.; Haskard, K.; Koslow, J.A. Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. J. Fish Biol. 1995, 46, 202-220. [CrossRef]
- Requiron, E.A.; Torres, M.A.J.; Demayo, C.G. 2012. Applications of relative warp analysis in describing of scale morphology between sexes of the snakehead fish Channa striata. Int. J. Biol.Ecol. Envir. Sci. 2012, 1 (6), 205-209.
- Portillo, JN.; Patulili, R.R.; Lucas, M.M.A.; Alaijos, O.; Demayo, C.G. Body Shape Variation in the Goby.; Glossogobius giuris Collected in Selected Areas in the River of Norzagaray Bulacan Using Landmark-Based Geometric Morphometrics. J. Inform. Math. Sci. 2017, 9 (4), 1109-1116.
- Bussing, W. Peces de las Aguas Continentales de Costa Rica. Editorial de la Universidad de Costa Rica, San Jose, Costa Rica. 1998.
- Laaz Moncayo, E.; Torres Noboa, A. Lista de Peces continentales de la Cuenca del Río Guayas. 2014.
- Gonzáles, A.; Acosta, J.; Andrade, S. (2008). Evaluación de las inundaciones de la cuenca baja del Guayas, datos y manejo. CLIRSEN. In Proceedings of the XI Congreso Ecuatoriano de la Ciencia del Suelo, Quito, Ecuador, 29–31 October.
- Barriga, R. Peces de los Afluentes de la Costa de Ecuador. In Cuencas Pericon-tinentales de Colombia, Ecuador, Perú y Venezuela. Serie Editorial Recursos Hidro-biológicos y Pesqueros Continentales de Colombia; Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt, Bogotá-Colombia. 2015.
- Revelo, W.; Laaz, E. Catálogo de peces de aguas continentales de la provincia de los Ríos – Ecuador. Boletín Especial 2012, 03 (5), 1-56.
- Melvin, G.D.; Dadswell, M.J.; McKenzie, A. Usefulness of meristic and morphometric characters in discriminating populations of American shad (Alosa sapidissima) (Ostreichthys: Clupeidae) inhabiting a marine environment. Can. J. Fish. Aquat. Sci. 1992, 49, 266-280. [CrossRef]
- Ujjania, N.C.; Kohli, M.P.S. Landmark-based morphometric analysis for selected species of indian major carp (catla catla.; ham. 1822). Int. J. Food Agri. Vet. Sci. 2011, 1, 64-74.
- Froese, R. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichth. 2006, 22, 241-253. [CrossRef]
- Trudel, M.; Tucker, S.; Morris, J.; Higgs, D.; Welch, D. Indicators of energetic status in juvenile coho and chinook salmon. N. Am. J. Fish. Manag. 2005, 25, 374-390. [CrossRef]
- Rennie, M.D.; Verdon, R. Evaluation of condition indices for the lake whitefish, Coregonus clupeaformis. N. Am. J. Fish. Manag. 2008, 28, 1270-1293. [CrossRef]
- Cureton, J.C.; Broughton, R.E. Rapid morphological divergence of a stream fish in response to changes in water flow. Biol. Lett. 2014, 10, 20140352. [CrossRef]
- Radojković, N.; Marinoić, Z.; Milosković, A.; Radenković, M.; Duretanović, S.; Luji, J.; Simi, V. Effects of Stream Damming on Morphological Variability of Fish: Case Study on Large Spot Barbell Barbus balcanicus. Turk. J. Fish. & Aquat. Sci. 2018, 19 (3), 231-239.
- Libay, CP.; Ratunil, V.B. Jr.; Ebarsabal, G.A.; Gamboa, G.Z. Jr.; Borja, E.A.; Ga, J.A.A.; Eclipse, M.G.E.; Mahomoc, D.Q.; Cabuga, C.C. Jr. Geometric morphometric analysis in determining phenotypic variability of Bugwan, (Hypseleotris agilis, Herre) in Lake Mainit, Philippines. Int. J. Biosci. 2019, 14 (6), 61-70.
- Caldecutt, WC.; Adams, DC. Morphometrics of trophic osteology in the three spine stickleback, Gasterosteus aculeatus. Copeia 1998, 4, 827-838. [CrossRef]
- Webb, PW. Locomotor patterns in the evolution of actinopterygian fish. Am. Zool. 1982, 22, 329-342. [CrossRef]
- Anders, P.J. Conservation aquaculture: An adaptive approach to prevent extinction of an endangered white sturgeon population. Fisheries 1998, 23 (11), 28-31.

| Habitat | Daule-Peripa dam | Quevedo river | Babahoyo river |
|---|---|---|---|
| Locality description | Reservoir of species. All species | White-water with native species: dama, bocachico, ratón, vieja azul, vieja colorada, among others | Slow-flowing waters which favour benthic species rearing such as guanchiche. |
| Depth | The maximun depth is 85 m with body water of 295 km | The average depth is 5 m in the rainy season, while its depth decreases to 1.92 m in the dry season, with a route of 163 km | It has an average of 5m of depth and 40 km of route |
| Endogeneity and competition | Carnivorous species such as cachama (Colossoma macropomum) and paiche (Arapaima gigas) | Non-native species like tilapia (Oreochromis niloticus) displace native species | Guanchiche coexists with tilapia (Oreochromis niloticus) |
| Plankton-insects | Maximum concentration in October and minimum in August | Rich in phytoplankton | Rich in zooplankton and larvae of different species |
| Fishing vessels | Bongo1, bote1, lancha1 | Balsa, canoe, bongo1, bote1 | Balsa, canoe, bongo1, bote1 |
| Fishing methods | Riverbank manual thrownets, trammel nets, fishing spear | Trawl line, riverbank manual thrownets, trammel nets, thrownets, fishhooks | Trammel nets, thrownets |
| Fishing pressure | Low fishing pressure. The largest reservoir of native freshwater species in Ecuador. | Traditional fishing. Medium fishing pressure of native species | Traditional fishing. Strong fishing pressure of native species |
| Indicators | Daule-Peripa dam | Quevedo river | Babahoyo river |
|---|---|---|---|
| Physical / chemical parameters | |||
| pH | 7.42 | 8.23 | 7.44 |
| Electric conductivity (μS/cm) | 104.9 | 95.3 | 114.3 |
| Temperature (°C) | 19.3 | 19.2 | 27.25 |
| Turbidity (NTU) | 4.9 | 7.4 | 14.1 |
| Colour (UC Pt-Co) | 16 | 5 | 63 |
| Total dissolved solids (mg/l) | 112 | 96 | <150 |
| Alkalinity (mg/l) | 51.80 | 47.73 | 40.2 |
| Total hardness (mg/l) | 38.25 | 41.43 | 29.3 |
| Calcium hardness (mg/l) | 24.22 | 27.97 | 25.9 |
| CO2 (mg/l) | 3.93 | 0.55 | 1.97 |
| SIO2 (mg/l) | 35 | 20.1 | 28 |
| Cations | |||
| Ca2+ (mg/l) | 9.7 | 11.2 | 9.25-12.1 |
| Mg2+ (mg/l) | 3.38 | 3.28 | 3.93 |
| Metals | |||
| Na+(mg/l) | 7.88 | 5.48 | 7.43 |
| Mn (mg/l) | <0.016 | <0.016 | X |
| Cu (mg/l) | <0.024 | <0.024 | <0.03 |
| Cr (mg/l) | < 1 | < 1 | <0.082 |
| Fe (mg/l) | 0.70 | 0.84 | 1.89 |
| K+ (mg/L) | 2.815 | 2.065 | 1.9-1.7 |
| Pb (mg/l) | <1 | <1 | <0.050 |
| Hg (ug/l) | 0 | <0.001 | 0 |
| Cd (mg/l) | <0.010 | <0.001 | <0.010 |
| Anions | |||
| (HCO3)- mg/l | 51.80 | 42.92 | 32.83 |
| (CO3)2- mg/l | 0 | 4.81 | 0 |
| (SO4)2- mg/l | 7.84 | 12.32 | 12 |
| Cl- (mg/l) | <5.30 | <5.3 | <5.0 |
| NH4+ (mg/l9) | 0.33 | 0.03 | <0.0014 |
| NO3- (mg/l) | <0.21 | 0.92 | <0.84 |
| NO2- (mg/l | <0.05 | <0.05 | 0.072 |
| (PO4)3- (mg/l | <0.50 | <0.50 | 2 |
| F-(mg/l) | 0.12 | 0.5 | 0.17 |
| Organic constituents | |||
| Biochemical oxygen demand (BOD5, ml/g) | 5.77 | 3.95 | 2.47 |
| Chemical oxygen demand (DQO, mg/l) | 17.38 | 12.98 | 4.6 |
| Dissolved oxygen (OD, mg/l) | 1.80 | 7.05 | 6.53 |
| OD (% sat) | 48 | 87.6 | 82.7 |
| Measurement | Description | Acronym |
|---|---|---|
| Weight | Total weight including the gut and gonads | BW |
| Total length | Tip of the upper jaw to the top of the caudal superior end of the caudal fin | TL |
| Standard length | Tip of the upper jaw to the tail base | SL |
| Head length | From the front of the upper lip to the posterior end of the opercula membrane | HL |
| Eye diameter | The greatest bony diameter of the orbit | ED |
| Pre-orbital length | Front of the upper lip to the cranial eye edge | Pre-OL |
| Pre-dorsal fin length | Front of the upper lip to the origin of the dorsal fin | Pre-DL |
| Pre-pectoral fin length | Front of the upper lip to the origin of the pectoral fin | Pre-PcL |
| Pre-pelvic fin length | Front of the upper lip to the origin of the pelvic fin | Pre-PvL |
| Pre-anal fin length | Front of the upper lip to the origin of the anal fin | Pre-AL |
| Dorsal fin length | From the base of the first dorsal spine to the base of the last dorsal ray | DFL |
| Dorsal fin ray length | From the base to the tip of the fifth dorsal ray | DFRL |
| Pectoral fin length | From the base to the tip of the pectoral fin | PcFL |
| Pelvic fin length | From the base to the tip of the pelvic fin | PvFL |
| Anal fin length | From the base of the first anal spine to the base of the last anal ray | AFL |
| Anal fin ray length | From the base to the tip of the last anal ray | AFRL |
| Upper jaw length | Straight line measurement between the snout tip and posterior edge of maxilla | UJL |
| Body perimeter 1 | Body perimeter at the level of the first ray of the dorsal fin | P1 |
| Body perimeter 2 | Body perimeter at the level of the first ray of the anal fin | P2 |
| Body perimeter 3 | Body perimeter at the level of the last ray of the dorsal fin | P3 |
| Body perimeter 4 | Body perimeter at the level of the last ray of the anal fin | P4 |
| Body width 1 | Straight line measurement from side to side at the level of the base of the first dorsal spine | LC1 |
| Body width 2 | Straight line measurement from side to side at the level of the base of the first anal spine | LC2 |
| Body width 3 | Straight line measurement from side to side at the level of the base of the last dorsal ray | LC3 |
| Body width 3 | Straight line measurement from side to side at the level of the base of the last anal ray | LC4 |
| Body depth 1 | Body depth at the level of the first ray of the dorsal fin | AC1 |
| Body depth 2 | Body depth at the level of the first ray of the anal fin | AC2 |
| Body depth 3 | Body depth at the level of the first radius of the caudal fin | AC3 |
| Dorsal fin rays | Number of thorns in the dorsal fin | DFR |
| Pectoral fin rays | Number of thorns in the pectoral fin | PcFR |
| Pelvic fin rays | Number of thorns in the pelvic fin | PvFR |
| Anal fin rays | Number of thorns in the anal fin | AFR |
| Caudal fin rays | Number of thorns in the caudal fin | CFR |
| Parameter1 | All | Daule-Peripa Dam (A) |
Quevedo River (B) |
Babahoyo River (C) |
p-Value2 | |
|---|---|---|---|---|---|---|
| A x C | B x C | |||||
| BW | 442.66±29.52 (68.97) | 778.65±74.39 (48.71) | 398.59±31.68 (48.34) | 281.18±20.49 (48.33) | *** | * |
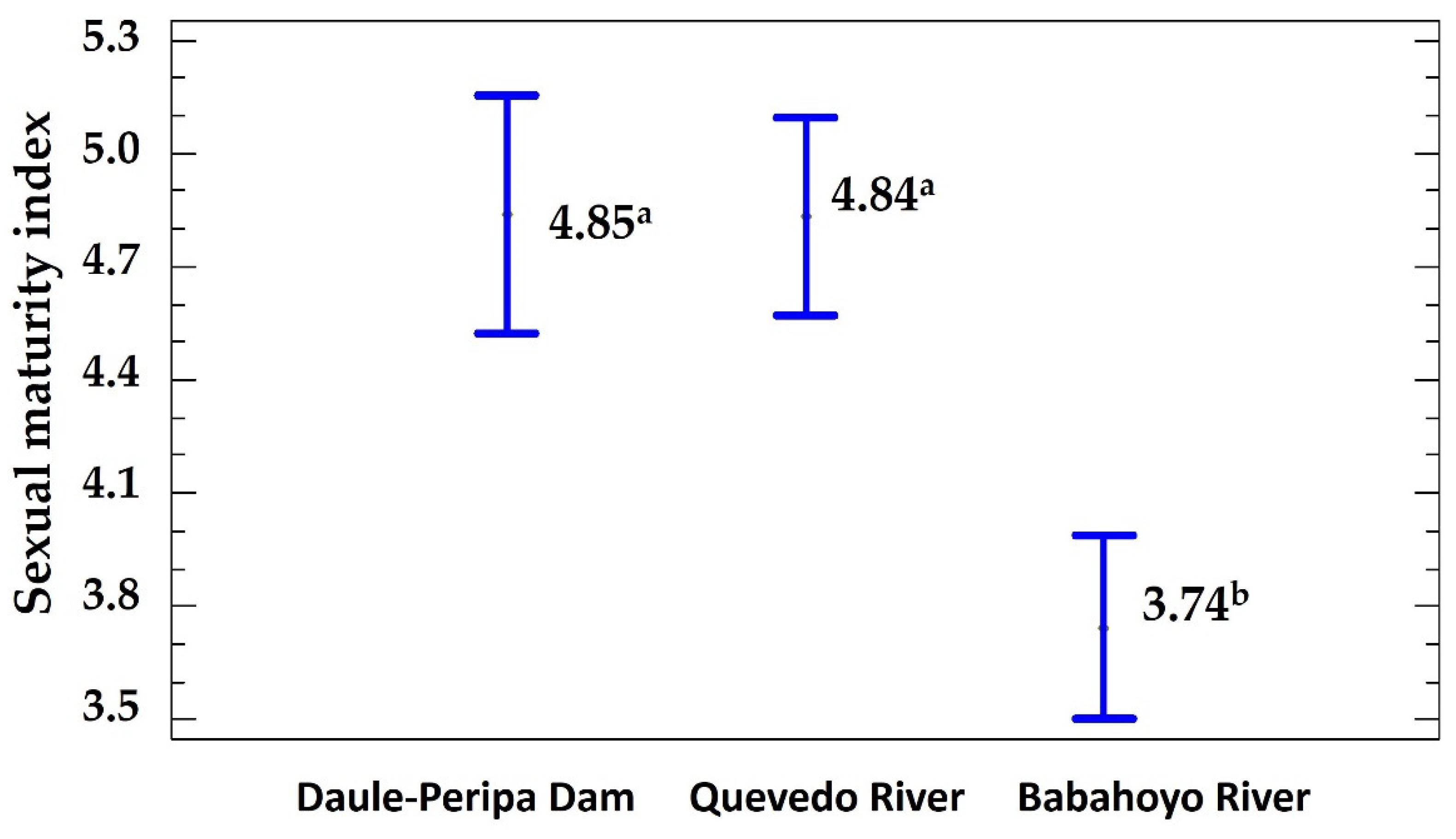
| K | 1.47±0.1 (69.7) | 1.84±0.12 (34.15) a | 1.06±0.1 (60.36) | 1.59±0.2 (83.48) | *** | *** |
| TL | 37.82±0.69 (18.97) | 41.55±1.18 (14.52) | 40.34±0.92 (13.85) | 33.49±1.03 (20.38) | n.s. | n.s. |
| SL | 32.14±0.6 (19.28) | 34.88±1.12 (16.35) | 34.18±0.81 (14.38) | 28.79±0.9 (20.8) | n.s. | n.s. |
| HL | 8.31±0.15 (18.77) | 8.45±0.36 (21.49) | 9.03±0.23 (15.28) | 7.63±0.19 (16.33) | * | n.s. |
| ED | 1.34±0.03 (23.71) | 1.33±0.06 (22.19) | 1.43±0.05 (22.96) | 1.27±0.05 (24.42) | n.s. | n.s. |
| Pre-OL | 1.34±0.04 (31.79) | 1.48±0.06 (22.33) | 1.47±0.08 (32.15) | 1.14±0.05 (30.86) | n.s. | * |
| Pre-DL | 16.16±0.3 (19.25) | 17.59±0.57 (16.41) | 17.31±0.43 (15.11) | 14.35±0.42 (19.2) | n.s. | n.s. |
| Pre-PcL | 8.33±0.17 (20.79) | 8.25±0.4 (24.67) | 8.97±0.22 (14.71) | 7.83±0.26 (21.8) | n.s. | n.s. |
| Pre-PvL | 11.08±0.22 (20.63) | 12.95±0.46 (18.22) | 11.19±0.42 (22.87) | 9.89±0.12 (7.83) | *** | *** |
| Pre-AL | 25.29±0.55 (22.44) | 28.26±1.04 (18.8) | 27.86±0.66 (14.34) | 21.37±0.72 (22.37) | n.s. | n.s. |
| DFL | 3.58±0.08 (22.28) | 3.94±0.19 (24.64) | 3.81±0.13 (21.3) | 3.16±0.06 (12.87) | *** | *** |
| DFRL | 3.99±0.08 (20.37) | 3.59±0.13 (18.92) | 4.14±0.15 (22.43) | 4.10±0.11 (17.44) | n.s. | n.s. |
| PcFL | 2.80±0.08 (28.85) | 2.80±0.18 (33.54) | 3.22±0.13 (23.75) | 2.46±0.09 (23.86) | ** | n.s. |
| PvFL | 3.63±0.09 (25.49) | 4.03±0.16 (20.01) | 4.26±0.12 (17.66) | 2.86±0.07 (16.34) | ** | ** |
| AFL | 2.99±0.08 (28.73) | 3.13±0.18 (29.24) | 3.37±0.15 (27.26) | 2.58±0.08 (21.67) | ** | ** |
| AFRL | 2.95±0.07 (24.3) | 3.04±0.12 (20.54) | 3.56±0.09 (15.25) | 2.39±0.06 (15.95) | ** | * |
| UJL | 2.18±0.05 (24.51) | 2.14±0.11 (26.75) | 1.98±0.07 (22.55) | 2.37±0.08 (21.96) | n.s. | n.s. |
| AC1 | 3.56±0.05 (14.14) | 3.95±0.06 (7.25) | 3.50±0.06 (10.3) | 3.39±0.09 (17.32) | *** | ** |
| AC2 | 4.77±0.06 (12.22) | 5.19±0.08 (8.11) | 4.59±0.07 (9.89) | 4.68±0.1 (13.83) | * | * |
| AC3 | 5.44±0.06 (11.69) | 5.98±0.09 (7.85) | 5.22±0.08 (9.11) | 5.31±0.1 (12.52) | * | * |
| P1 | 13.66±0.19 (14.23) | 13.26±0.49 (18.91) | 14.03±0.17 (7.28) | 13.6±0.32 (15.73) | n.s. | *** |
| P2 | 14.19±0.24 (17.69) | 14.23±0.17 (6.09) | 14.13±0.55 (23.89) | 14.21±0.35 (16.56) | *** | * |
| P3 | 12.36±0.13 (10.91) | 12.00±0.24 (10.35) | 12.59±0.23 (10.93) | 12.38±0.21 (11.06) | n.s. | n.s. |
| P4 | 8.75±0.09 (11.17) | 8.60±0.18 (10.8) | 8.85±0.16 (11.33) | 8.75±0.15 (11.36) | n.s. | n.s. |
| LC1 | 3.11±0.04 (12.55) | 3.10±0.07 (11.39) | 3.13±0.07 (13.07) | 3.11±0.06 (13.01) | n.s. | n.s. |
| LC2 | 3.13±0.04 (13.52) | 3.22±0.06 (9) | 3.06±0.07 (14.86) | 3.15±0.07 (14.6) | * | n.s. |
| LC3 | 2.54±0.04 (17.36) | 2.40±0.07 (14.86) | 2.57±0.08 (18.4) | 2.60±0.07 (17.31) | n.s. | n.s. |
| LC4 | 1.23±0.04 (31.49) | 1.09±0.07 (34.31) | 1.33±0.06 (28.57) | 1.24±0.06 (31.25) | n.s. | n.s. |
| Parameter 1 | All | Daule-Peripa Dam (A) |
Quevedo River (B) |
Babahoyo River (C) |
p-Value2 | |
|---|---|---|---|---|---|---|
| A × C | B × C | |||||
| DFR | 12.96±0.11 (8.73) | 12.85±0.25 (10.02) | 13.00±0.15 (7.25) | 13.00±0.18 (9.24) | n.s. | n.s. |
| PcFR | 10.71±0.20 (19.79) | 10.50±0.47 (22.74) | 10.84±0.34 (19.15) | 10.73±0.31 (18.90) | n.s. | n.s. |
| PvFR | 7.79±0.05 (6.37) | 7.77±0.10 (6.62) | 7.86±0.06 (4.41) | 7.73±0.09 (7.57) | * | n.s. |
| AFR | 9.45±0.12 (13.58) | 9.38±0.25 (13.50) | 9.43±0.20 (12.87) | 9.50±0.21 (14.45) | n.s. | n.s. |
| CFR | 16.96±0.08 (4.99) | 16.92±0.19 (5.77) | 17.00±0.12 (4.38) | 16.95±0.13 (5.08) | * | * |
| G | 3.65±0.05 (14.61) | 3.62±0.11 (15.80) | 3.65±0.09 (14.75) | 3.68±0.08 (14.07) | n.s. | n.s. |
| Parameter1 | Wilks’ Lambda | Partial Lambda | F-Remove | p-Level2 | Toler | 1-Toler |
|---|---|---|---|---|---|---|
| AFRL | 0.22 | 0.85 | 7.60 | ** | 0.69 | 0.31 |
| AC3 | 0.22 | 0.85 | 7.76 | ** | 0.90 | 0.10 |
| Pre-PvL | 0.24 | 0.78 | 12.37 | *** | 0.72 | 0.28 |
| PvFL | 0.22 | 0.85 | 7.65 | ** | 0.68 | 0.32 |
| UJL | 0.20 | 0.92 | 4.09 | * | 0.88 | 0.12 |
| DFRL | 0.21 | 0.90 | 4.79 | * | 0.82 | 0.18 |
| LC4 | 0.20 | 0.94 | 3.05 | n.s. | 0.51 | 0.49 |
| P1 | 0.20 | 0.94 | 2.77 | n.s. | 0.94 | 0.06 |
| HL | 0.19 | 0.96 | 1.85 | n.s. | 0.59 | 0.41 |
| TL | 0.19 | 0.96 | 1.85 | n.s. | 0.68 | 0.32 |
| ED | 0.20 | 0.94 | 2.68 | n.s. | 0.90 | 0.10 |
| LC3 | 0.19 | 0.97 | 1.52 | n.s. | 0.50 | 0.50 |
| Pre-PcL | 0.19 | 0.97 | 1.33 | n.s. | 0.59 | 0.41 |
| Pre-DL | 0.19 | 0.96 | 1.73 | n.s. | 0.64 | 0.36 |
| AFL | 0.19 | 0.97 | 1.21 | n.s. | 0.60 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





