Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
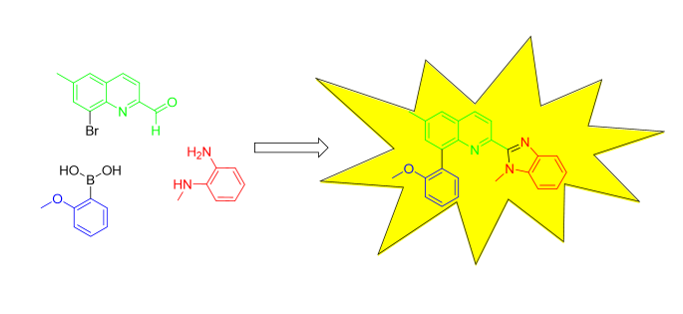
Keywords:
1. Introduction
2. Results and Discussion
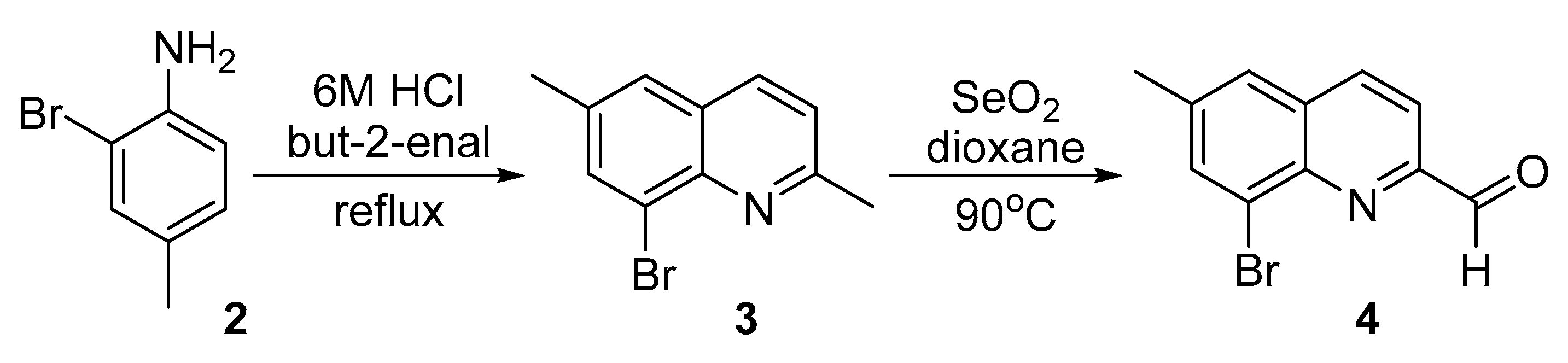
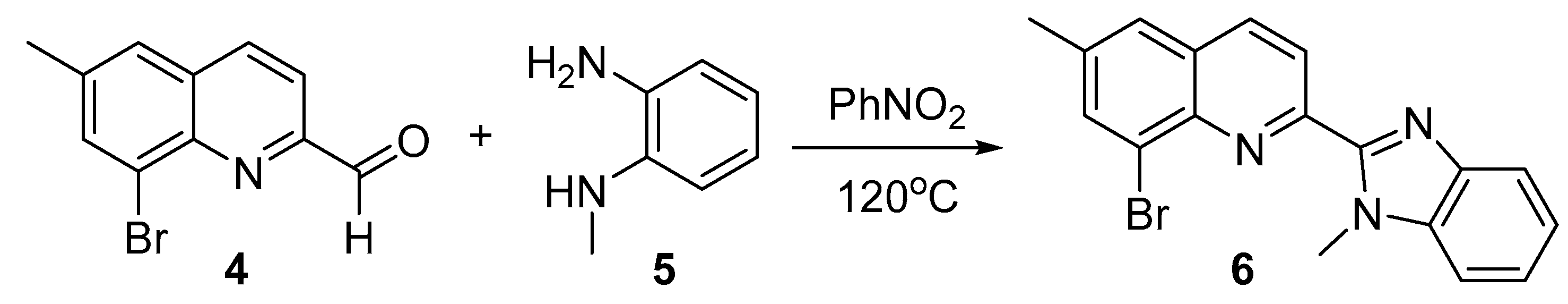
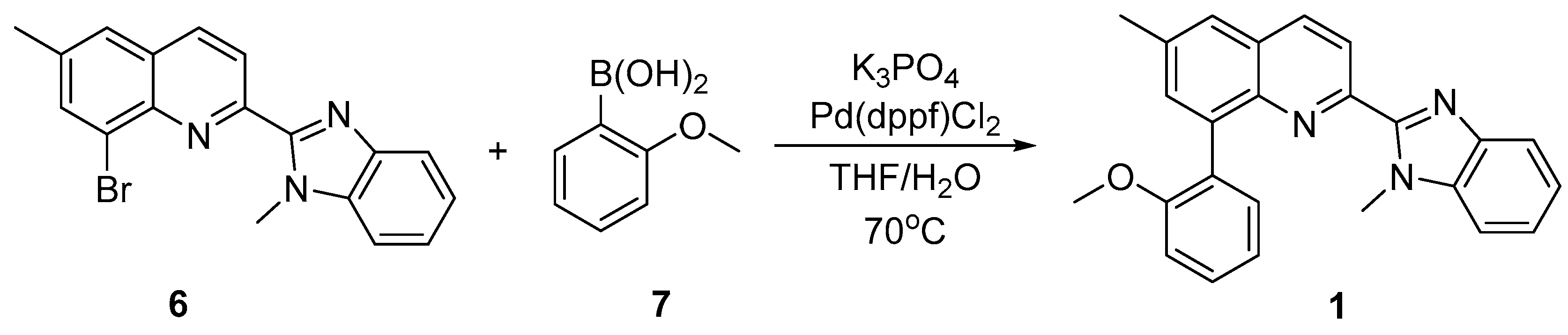
2.1. Synthesis
2.2. NMR Studies
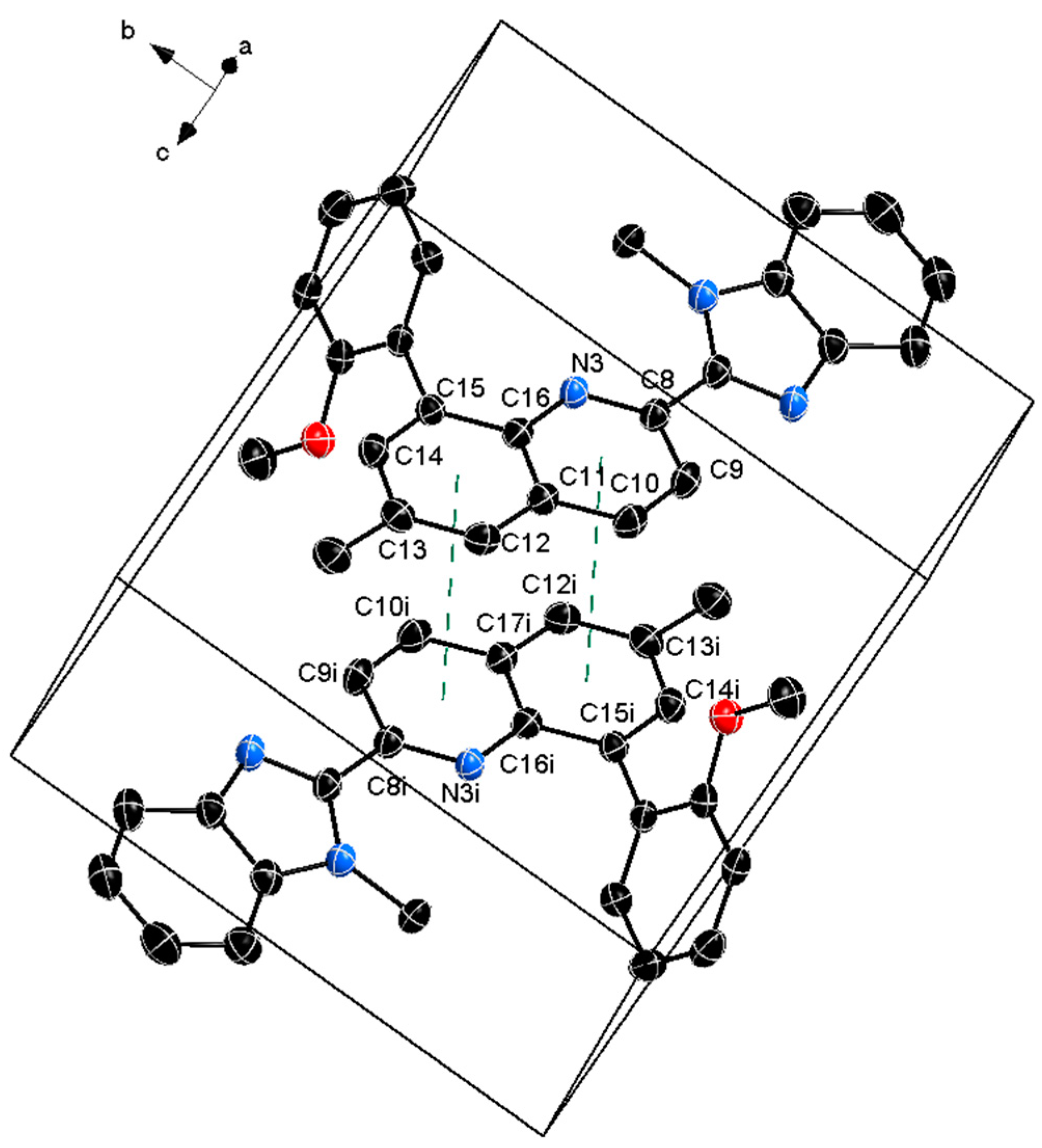
2.3. Single Crystal Diffraction Studies
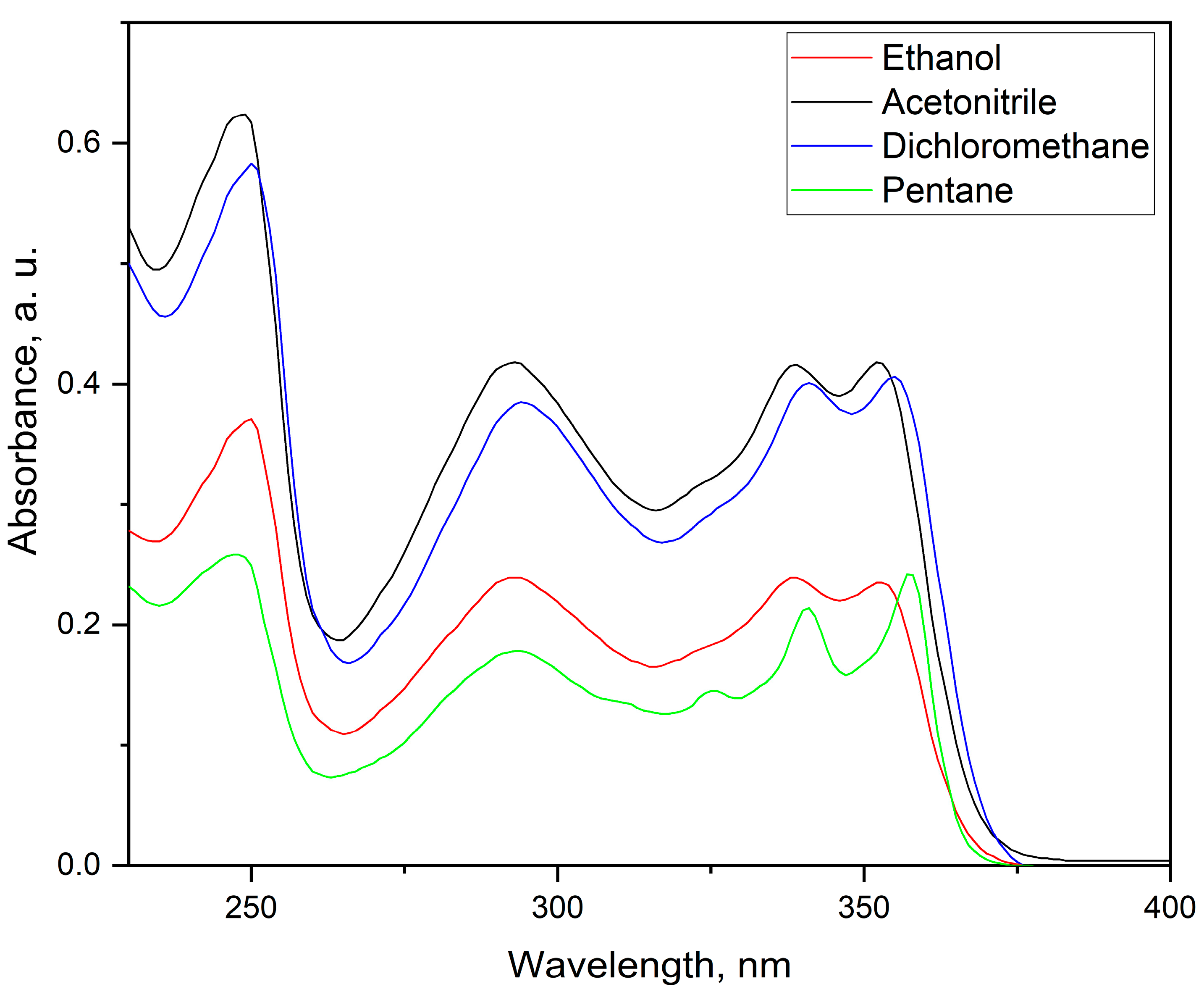
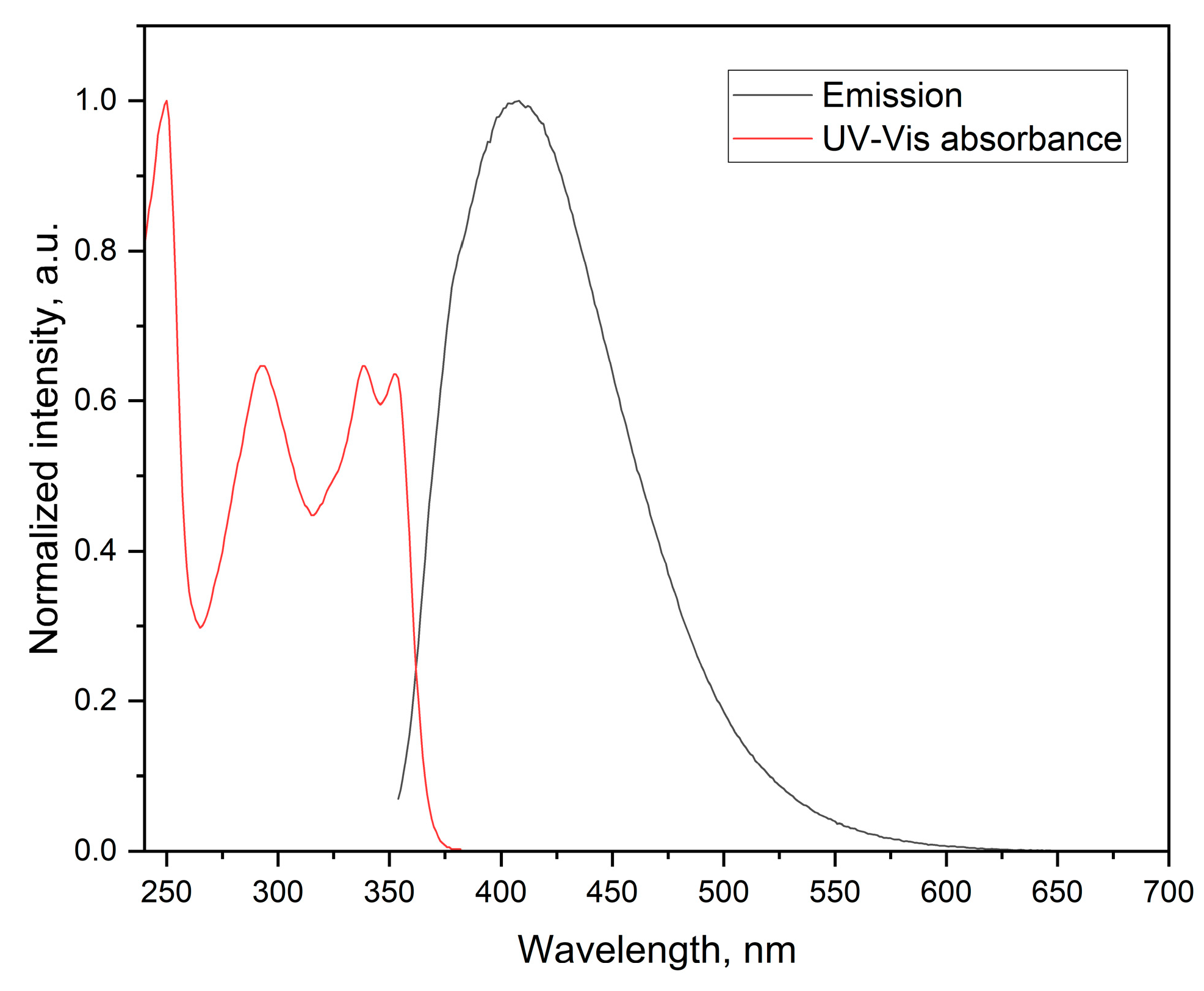
2.4. Optical Properties
4. Materials and Methods
Synthesis of 8-Bromo-2,6-dimethylquinoline 3
Synthesis of 8-Bromo-6-methylquinoline-2-carbaldehyde 4
Synthesis of 8-Bromo-6-methyl-2-(1-methyl-1H-benzo[d]imidazol-2-yl)quinoline 6
Synthesis of 8-(2-Methoxyphenyl)-6-methyl-2-(1-methyl-1H-benzo[d]imidazol-2-yl)quinoline 1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973–115998. [Google Scholar] [CrossRef]
- Mohammed, L.A.; Farhan, M.A.; Dadoosh, S.A.; Alheety, M.A.; Majeed, A.H.; Mahmood, A.S.; Mahmoud, Z.H. A Review on Benzimidazole Heterocyclic Compounds: Synthesis and Their Medicinal Activity Applications. SynOpen 2023, 7, 652–673. [Google Scholar]
- Ebenezer, O.; Oyetunde-Joshua, F.; Omotoso, O.D.; Shapi, M. Benzimidazole and its derivatives: Recent Advances (2020–2022). Results Chem. 2023, 3, 100925–100955. [Google Scholar] [CrossRef]
- Hisano, T.; Ichikawa, M.; Tsumoto, K.; Tasaki, M. Synthesis of Benzoxazoles, Benzothiazoles and Benzimidazoles and Evaluation of Their Antifungal, Insecticidal and Herbicidal Activities. Chem. Pharm. Bull. 1982, 30, 2996–3004. [Google Scholar] [CrossRef]
- Baig, M.F.; Shaik, S.P.; Nayak, V.L.; Alarifi, A.; Kamal, A. Iodine-catalyzed Csp3-H functionalization of methylhetarenes: One-pot synthesis and cytotoxic evaluation of heteroarenyl-benzimidazoles and benzothiazole. BMCL, 2017, 27, 4039–4043. [Google Scholar] [CrossRef]
- Dayan, O.; Tercan, M.; Özdemir, N. Syntheses and molecular structures of novel Ru(II) complexes with bidentate benzimidazole based ligands and their catalytic efficiency for oxidation of benzyl alcohol. J. Mol. Struct. 2016, 1123, 35–43. [Google Scholar] [CrossRef]
- Sahki, F.A.; Messaaadia, L.; Merazig, H.; Chibani, A.; Bouraiou, A.; Bouacida, S. Synthesis, X-ray structure and theoretical investigation of 2-(2’-quinolyl)benzimidazole metal complexes. J. Chem. Sci. 2017, 129, 21–29. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, Y.; Cui, X.; Li, Y.; Wang, H.; Qi, X. 2-Benzoimidazol-8-alkylquinolinylnickel(II) complexes as efficient catalysts for ethylene oligomerization and vinyl polymerization of norbornene. J. Coord. Chem., 2015, 68, 3825–3838. [Google Scholar] [CrossRef]
- Hisano, T.; Hisano, K. Studies of Organosulphur Compounds. I. Reaction between Active Methyl Groups and o-Substituted Bifunctional Molecule in the Presence of Sulfur. Yakugaku Zasshi 1971, 91, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yaragorla, S.; Babu, P.V. Oxidative Csp3-H functionalization of 2-methylazaarenes: A practical synthesis of 2-azaarenyl-benzimidazoles and benzothiazoles. Tetrahedron Lett. 2017, 58, 1879–1882. [Google Scholar] [CrossRef]
- Lee, J.S.; Song, I.; Warkad, S.D.; Seong, Y.G.; Shinde, P.B.; Song, K.; Nimse, S.B. Synthesis and evaluation of 2-Aryl-1H-benzo[d]imidazole derivatives as potential microtubule targeting agents. Drug Dev. Res. 2022, 83, 769–782. [Google Scholar] [PubMed]
- Tynebor, R.; Millings, E. Novel Method of Synthesizing Various Five Membered Heterocycles from an Aryl Tribromomethyl Group. Synth. Commun. 2013, 43, 1902–1908. [Google Scholar] [CrossRef]
- Johnson, J.R.; Sandborn, L.T. 3-Bromo-4-aminotoluene. Org. Synth. 1926, 6, 8–9. [Google Scholar]
- Leir, C.M. An Improvement in the Doebner-Miller Synthesis of Quinaldines. J. Org. Chem., 1977, 42, 911–913. [Google Scholar] [CrossRef]
- Garrod, R.E.; Jones, H.O.; Evans, P.E. Some quinoline and tetrahydroquinoline derivatives obtained from aldol bases. J. Chem. Soc., Trans., 1912, 101, 1389–1394. [Google Scholar] [CrossRef]
- Barsoum, D.N.; Kirinda, V.C.; Kang, B.J.; Kalow, A. Remote-Controlled Exchange Rates by Photoswitchable Internal Catalysis of Dynamic Covalent Bonds. J. Am. Chem. Soc. 2022, 144, 10168–10173. [Google Scholar] [CrossRef] [PubMed]
- Seynah, M.; Fernelius, W.C. Formazyl Complexes of the Quinoline Series. J. Org. Chem. 1957, 22, 217–219. [Google Scholar]
- Martínez-González, S.; Rodríguez-Arístegui, S.; Oliva, C.A.G.; Hernández, A.I.; Cantalapiedra, E.G.; Varela, C.; García, A.B.; Rabal, O.; Oyarzabal, J.; Bischoff, J.R.; Klett, J.; Albarrán, M.I.; Cebriá, A.; Ajenjo, N.; García-Serelde, B.; Gómez-Casero, E.; Cuadrado-Urbano, M.; Cebrián, D.; Blanco-Aparicio, C.; Pastor, J. Discovery of novel triazolo[4,3-b]pyridazin-3-yl-quinoline derivatives as PIM inhibitors. Eur. J. Med. Chem. 2019, 168, 87–109. [Google Scholar] [CrossRef]
- Kumar, G.; Paul, K.; Luxami, V. Deciphering the excited state intramolecular charge-coupled double proton transfer in an asymmetric quinoline–benzimidazole system. New J. Chem. 2020, 44, 12866–12874. [Google Scholar] [CrossRef]
- Saha, A.; Ghosh, A.; Guin, S.; Panda, S.; Mal, D.K.; Majumdar, A.; Akita, M.; Maiti, D. Substrate-Rhodium Cooperativity in Photoinduced ortho-Alkynylation of Arenes, Angew. Chem., Int. Ed. Engl. 2022, 61, e202210492. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr C Struct Chem 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J Appl Crystallogr 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
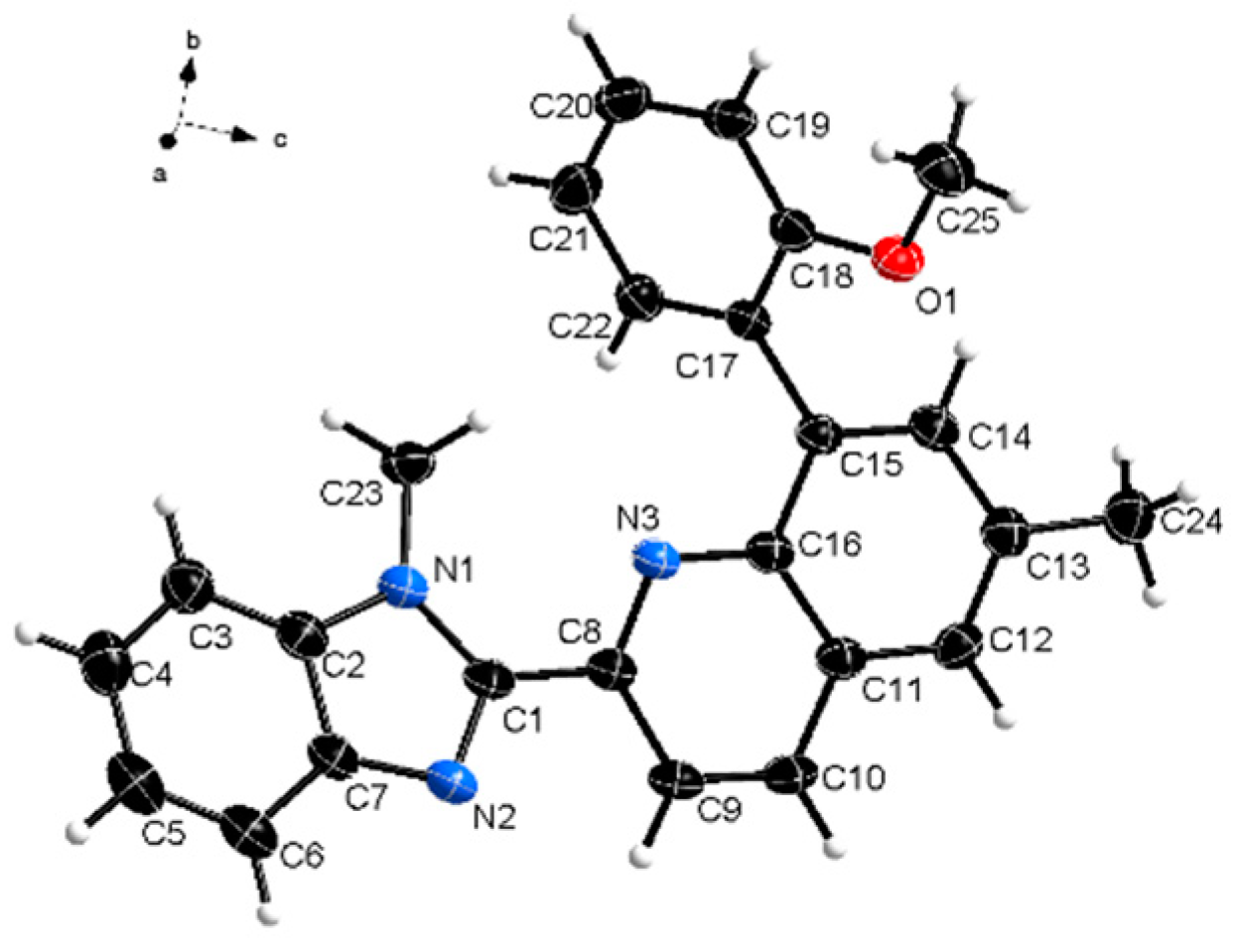
| Crystal data | |
|---|---|
| CCDC numbers | 2374257 |
| Empirical formula | C25H21N3O |
| Molecular weight, (g/mol) | 379.45 |
| Crystal system, space group | Triclinic, P |
| Temperature (K) | 133(2) |
| a, b, c(Å) | 7.0467(5), 10.8556(7), 13.5823(9) |
| α, β, γ(°) | 79.469(6), 79.307(6), 71.684(6) |
| V, (Å3) | 960.56(12) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ, (mm−1) | 0.082 |
| Crystal size (mm) | 0.200 x 0.080 x 0.060 |
| Data collection | |
| Diffractometer | Rigaku XtaLAB Synergy |
| Absorption correction | Spherical Harmonics implemented in SCALE3 ABSPACK scaling algorithm |
| Tmin, Tmax | 0.8728, 1.0000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4365, 4365, 3704 |
| (sin θ/λ)max, (Å−1) | 0.625 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0501, 0.1409, 1.066 |
| No. of reflections | 4365 |
| No. of restrains | 0 |
| No. of parameters | 266 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.169, -0.240 |
| π – π interaction | Cg – Cg, Å | α, o | Slippage, Å |
|---|---|---|---|
| Cg2 – Cg4i | 3.8224(14) | 0.58(11) | 1.639 |
| Cg2i – Cg4 | 3.8224(14) | 0.58(11) | 1.606 |
| Interaction | H∙∙∙π, Å | C∙∙∙ π, Å | C – H∙∙∙ π, o |
|---|---|---|---|
| C23 – H23B∙∙∙Cg3ii | 2.62 | 3.513(3) | 152 |
| C24 – H24A∙∙∙Cg5iii | 2.94 | 3.723(3) | 138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





