1. Introduction
According to The Global Health Observatory, almost all countries all over the world applied an excise duty to production and consumption of alcohol and alcoholic beverages. Excise duty exemption is applicable through a denaturing process, which consists in the addition of certain substances which make the alcohol unsuitable for human consumption.
In the European Union excise duties are regulated by Directive 92/83/EEC [
1] on the harmonization of the structures of excise duties on alcohol and alcoholic beverages, and by Regulation (EC) 3199/93 [
2] (and its amendments), which establishes mutual recognition of procedures for the complete denaturing of alcohol.
Ethanol content determination has a relevant role in the process of complete denaturation of alcohols (CDA) in the European Union. As the majority of the denaturing formulas are expressed in litres or grams of substance per hectolitre of absolute ethanol, it is necessary to have an exact quantification of the ethanol content in alcoholic products which are subjected to denaturing processes. In particular, European Customs Agencies’ task is to check the ethanol content in alcoholic products both before and after the denaturation process, respectively to find the correct denaturation formula or possible frauds. For these reasons, a good method of quantification must be able to determine the correct amount of alcohol in different kind of matrixes: denatured, crude alcohols and generic ethyl alcohols of agricultural or synthetic origin. Generally, this determination is carried out by a gaschromatographic technique with a flame ionization detector (GC-FID), necessary to separate and quantificate ethanol from other substances (i.e. methanol, or other interfering volatile substances).
Customs Laboratories European Network (CLEN) has developed an analytical method for this purpose: CLEN/ILIADe 143:2023. It is a GC-FID method that allows to determine ethanol content by a calibration curve using an internal standard, 15 mL of methanol to dilute 1 ml of the sample and about 20 min chromatographyc course. A quality control sample must be prepared every day to check the calibration curve validity for each analytical sequence and calibration procedure should be performed frequently to achieve sufficient accuracy in analytical results. All these aspects can be considered disadvantages of GC technique compared to the use of 1H LF-NMR spectroscopy for the same determination, how discuss below.
Nuclear magnetic resonance (NMR) is a non-destructive technique that allows to recognize chemical structures of molecules selectively, because each molecule has its own spectrum. Among different nuclei, the proton is the most studied for this purpose. The capability of NMR for quantitation (qNMR) to simultaneously detect several compounds in complex mixtures without separation proves to be advantageous as a method to use for analyses of natural products [
3,
4] including food, fruit juices, or alcoholic beverages [
5,
6,
7]. In particular,
1HNMR methodologies have been applied to determine chemical constituents (phenolics, sugars, and organic acids) and to measure the amount of alcohol for authenticating the quality of wine [
8,
9,
10,
11]. The LF-NMR is a technique characterized by the use of a low intesity field (80 MHz in this study), which involves the reduction of costs and mainteinance procedures, as well as the felling of samples analysis time. As a disadvantage, the low field could be reflected on a lower signal sensitivity, but this is offset by the high ethyl alcohol content in some alcoholic products (> 80 %vol). The purpose of this article was to investigate the use of a low-field NMR spectrometer to determine the ethanol content on different alcoholic products, especially in crude and denatured alcohols, verifying validation parameters and comparing the results with those obtained by the reference GC-FID method, in according with the
ILIADe 143:2021| CLEN Method “Determination of Ethanol in Alcoholic Products by GC-FID” Version 2 February 2021. [
12]
2. Materials and Methods
2.1. Samples
For this study were used 25 real alcohol samples, analysed by Customs Laboratory of Bologna, to perform repeatability test, a reference material of the “Proficiency test on completely denatured alcohol (burning alcohol)” organized by CLEN to verify trueness, and some alcoholic solution containing different common denaturants of ethyl alcohol to study specificity.
2.2. Chemical Analysis
All samples were analysed using cromatographic, densimetric and spectroscopic (LF-NMR) methods, according to the ILIADe 143:2021| CLEN Method “Determination of Ethanol in Alcoholic Products by GC-FID” Version 2 February 2021 and Reg CE 2870/2000 19/12/2000 GU CE L333 29/12/2000 All I App II Met B + Reg UE 383/2023 16/02/2023 GU UE L53 21/02/2023 All, for the first two methods respectively. The NMR spectroscopic analysis is described below.
2.2.1. Cromatographic Method
Cromatographic analyses, in according to the CLEN method mentioned above, accredited by the Italian Customs and Monopolies Agency (ADM) - Laboratory of Bologna, were carried out by a GC-FID Shimadzu GC 2030 with autosampler (Shimadzu Corporation, Kyoto, Japan) equipped with a GC column DB-624, fused silica capillary 60 m x 0,25 mm i.d., and 1,40 mm film thickness (Agilent, Santa Clara, CA, USA). The carrier gas was hydrogen, at a flow rate of 50 ml min-1, with a flow of nitrogen and air both respectively 30 ml min-1 and 400 ml min-1. The injection volume was 0.8 mL with a split ratio of 1:150; the injection temperature Tinj and the FID temperature Tdet was, respectively, 200 °C and 230 °C. The column temperature was as follows: 37 °C held for 3 min, then to 90 °C (rate of 4.50 °C min-1) and finally to 220 °C (rate of 40 °C min -1). The total analysis time was around 20 min. The ethanol content in sample is expressed in %v/v. For sample preparation 15 mL of methanol were mixed with 0.5 ml of internal standard (4-methyl-2-pentanol) and 1.0 mL of sample. Quantification of ethanol content is based on a calibration curve, performed every 4 months (verifying an R2 >0,99). A quality control (QC) by a 99.9 %vol ethanol CRM (certified reference material) is carrued out in duplicate before each sequence analysis to verify the reliability if analysis.
2.2.2. Densimetric Method
Alcohol content (%v/v) in anhydrous ethyl alcohol samples and hydroalcoholic mixture solutions was determined by an Anton Paar DMA 5000M electronic densimeter with Xsample 520 autosampler.
2.2.3. Spectroscopic (LF-NMR) Method
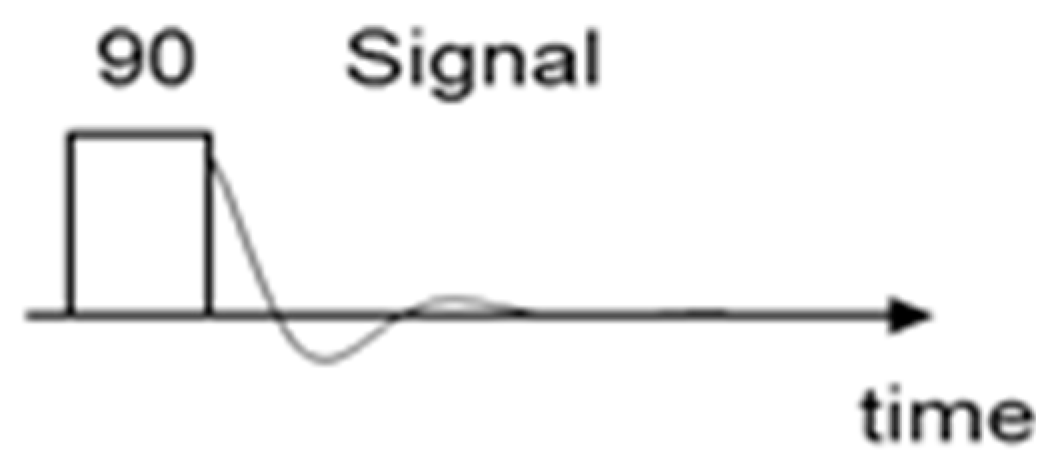
1H LF-NMR spectroscopy analyses were carried out by a Spinsolve 80 Multi-X Ultra benchtop NMR spectrometer, characterized by a magnetic field strength of 80 MHz. The sample was introduced into a classic NMR glass tube and for the analysis the operators used the pre-installed sequence PROTON 1D (1 minute). The parameters of the sequence were setted directly by the instrument. The general scheme of this sequence was reported below in
Figure 1.
In this sequence the sample is radiated by a 90 degrees impulse for 1 minute. The experiment was repeated four times. The data report was created using the Spinsolve 2.2.4 version software and the chemical shift values were extrapolated using Mestrenova software.
A mixture of about 450 ml of internal standard (salicylaldehyde 99.0 % w/w) and 150 ml of alcoholic sample weighed in a GC-vial using an analytical balance with a sensitivity of 0.1 mg was transferred to a NMR glass tube and analysed. The ethanol content was expressed in term of %w/w, calculated as reported in
Equation 1:
Equation 1. Formula used to calculate the analyte concentration
Where Cx is the ethanol content in w/w%, and Ix, Istd, NSTD, Nx, Mx, Mstd, mstd, mx and pstd are, respectively, the integral area, proton’s number generating peaks, molecular mass, sample and standard weights (in grams) and purity related to ethanol (x) and IS (std). Integrated signals are referred to of methylene (or methyl) group of ethanol peak and aldehydic group of salicylaldehyde (IS) peaks.
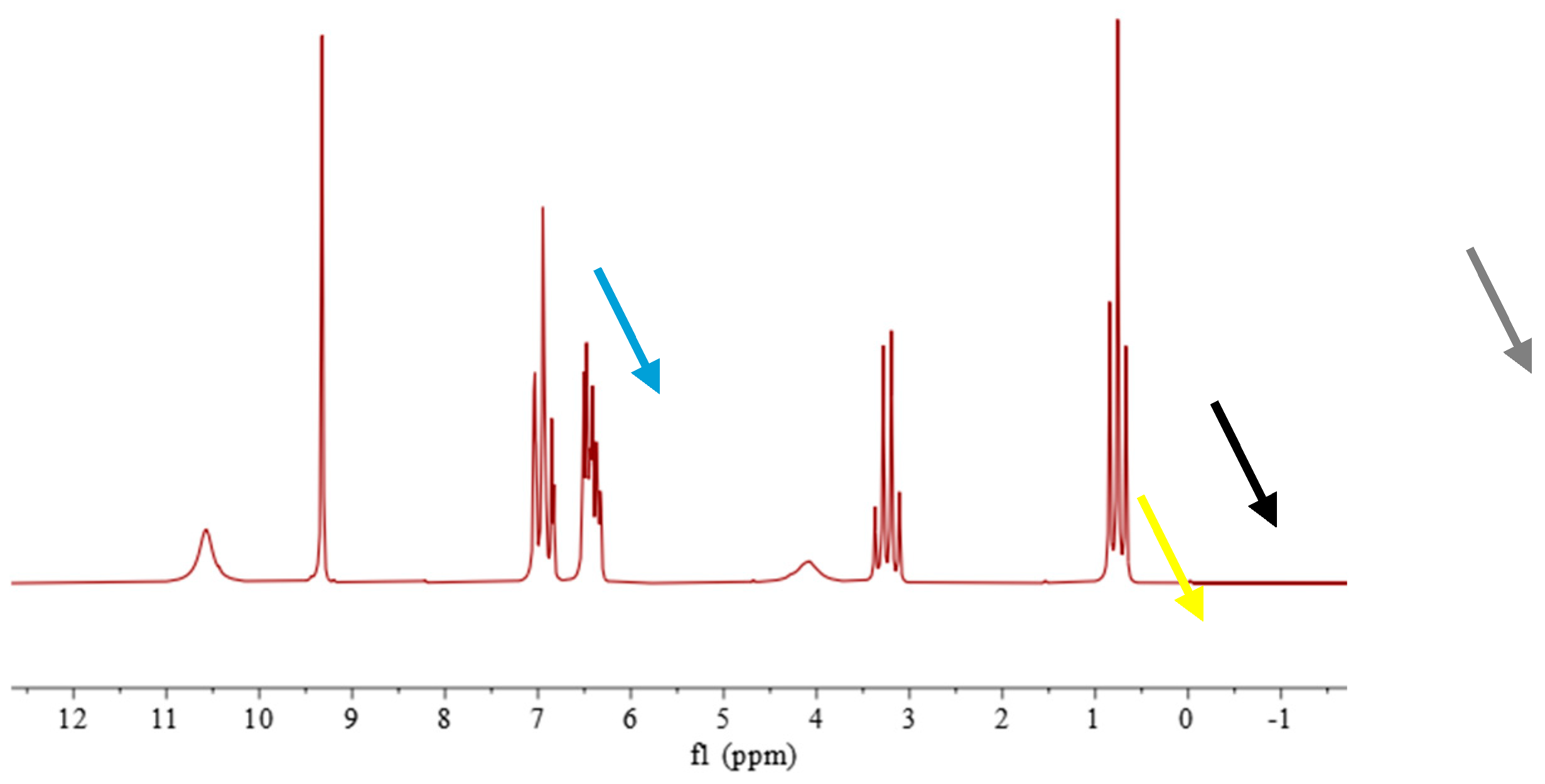
A typical spectrum of the mixture consisting of the alcohol sample and salicylaldehyde is shown in
Figure 2.
The quantification was done using the aldehyde’s group integral at 9.4 ppm and both ethanolic peaks at 3.9 ppm for CH2 and 0.8 ppm for CH3 groups. The expression of ethanol content in %v/v is performed by multiplying the result obtained in %w/w by the ratio of sample’s and ethanol’s densities.
3. Results and Discussion
The validation parameters studied in this article for ethanol content determination by 1H LF-NMR content were the following:
Precision data;
Trueness;
Specificity;
Application field;
Linearity;
Robustness.
3.1. Precision Data (Repeatabilty and Uncertainty of Measurement)
The method’s precision was estimated by eleven indipendent repeatability determination on four different kind of alcoholic matrices, as explained in
Table 1.
The matrices 1, 2 and 3 represent the majority of samples analyzed by the ADM Laboratory of Bologna. The last matrix was studied to evaluate the solubility of the internal standard in samples with a higher water content than typical alcohol samples. The repeatability of laboratory r
LAB was calculated as reported in
Equation 2.
Equation 2. Formula used to calculate the repeatability of laboratory rLAB
Where t is the t of Student and sr is the repeatability standard deviation. Considering that there is not precision data regarding this method, the Horwitz’s equation was used. [
13] Using this approach, reproducibility standard deviation was calculated from ethanol content
(1), then multiplied by two to obtain the expanded uncertainty, as reported in the
Equations 3A-3B.
Equation 3. Equations used to calculate the expanded uncertainty UH
In this equations, s
R is the reproducibility standard deviation calculated with Horwitz’s approach, C is the average of the concentrations of ethyl alcohol expressed in w/w and U
H is the expanded uncertainty of measurement . Refer to the
Supporting Information for further details. The summary of the results obtained for repeatability and measurement uncertainty (expressed in %v/v) are shown in
Table 2.
3.2. Trueness
The trueness of the method was evaluated through the following approaches:
Comparison between results obtained by NMR spectroscopic and reference cromatographic methods, using both standards and real samples.
3.2.1. Comparison with a PT Sample
The method is considered reliable if the analysis on a PT sample residual (Burning Alcohol, organized by CLEN) yields a result such that the z-score is between ±2. The sample of the “Proficiency test on completely denatured alcohol 27 June 2019- Burning Alcohol” of CLEN was used as reference material. The analyisis was performed in triplicate. The z-score was defined as reported in the
Equation 4.:
Equation 4. Equation for the calculation of z-score
In the equation (4), x is the result obtained by the laboratory with spectroscopic method; xpt is the reference value of the Proficeinecy test and spt is the reproducibility standard deviation of the circuit.
The results are reported in
Table 3.
As highlighted, it can be inferred that the method used provides accurate results using the methylene group (-CH2) signal, unlike the underestimated outcome obtained by the methyl group (-CH3) signal. It is noteworthy as results were reliable on a denaturated alcohol sample notwithstanding the presence of denaturants that could interfere, based on their concentration (see Specificity below).
3.2.2. Comparison between Results Obtained by NMR Spectroscopic and Reference Cromatographic Methods
Another check on trueness was performed by comparing the results of 1H-NMR method with those obtained by gas chromatographic quantification.
For the purpose, the same four matrices (anhydrous ethyl alcohol, denatured ethyl alcohol: euro DG, crude and Hydroalcoholic mixture ethyl alcohol-water 80 %v/v) were analysed also by the reference GC-FID method, carrying out six determination for each kind of sample.
As reported in literature [
14], this approach is used when certified reference material are not available, as in our case for crude alcohol.
For both methods normal distribution of data was verified, standard deviations calculated and homogeneity of variances tests by the F-Test. Therefore, the two methods were compared and considered statistically equal if the following
Equation 5 was satified:
Equation 5. Equation used to evaluate the deviation between the spectroscopic method (A) and the GC reference method (N)
Where
and
are respectively the average ethanol content values determined by NMR (alternatively using -CH
2 and -CH
3 signals) and GC-FID methods,
t is the Student’s t and
is a combination of standard deviations, as defined in full in
Supporting Information. The results were reported in
Table 4.
Results highlighted that NMR and GC methods provide statistically comparable results using the methylene group (-CH2) signal, unlike using methyl group (-CH3) signal which tend to underestimated the outcomes, as reported in the previous paragraph.
As an exception, it also emerged that the comparison on absolute ethyl alcohol sample was negative. However, this was not due to a NON-accuracy of the NMR method determination, but rather to the overestimation obtained by the gas chromatographic method (100.88 %v/v). Indeed, NMR provided an average value of 100.03 %v/v, certainly more accurate considering the type of sample (moreover stated 99.9% v/v by the supplier). Anyway, to further support the trueness on anhydrous ethyl alcohol, the sample was characterized by densimeter and considered as a reference. Therefore, to value the NMR result the following
Equation 6 (t-test) was used:
Equation 6. t-test applied on NMR and densimeter reference value on anhydrous ethyl alcohol sample
Where and are the average value obtained with NMR and densimeter and; uand urif are the uncertainty of the laboratory associated to the mean values obtained by the two techniques, respectively.
Moreover, the comparison between NMR and GC methods was carried out on 25 real crude heads-tails alcohol samples, with a 85-96 %vol range alcohol content, analysed in duplicate by each technique.
Firstly, for all samples was verified the repeatability condition for each duplicate determination, both for NMR and GC analysis, according to the
Equation 7:
where Dm is the difference of duplicate results obtained by NMR (both with -CH
3 and -CH
2 group signals) or GC tecnique.
Hence, the comparison between NMR and GC analysis was evaluated by the
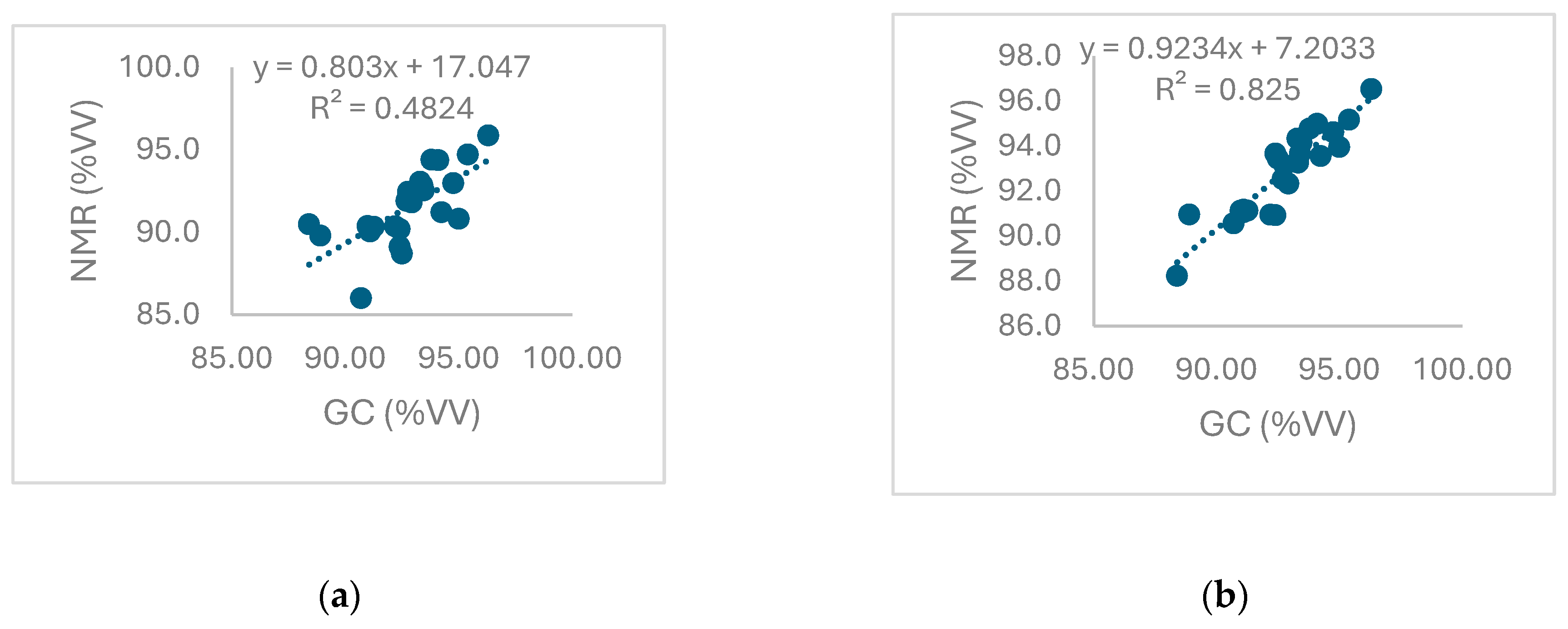
Equation 6 for each analysed sample and by the correlation graph reported in
Figure 3.
Once more, it was showed that NMR analyses based on -CH2 group signal provides results more accurate (precise and true) and definetely comparable with GC outcomes.
Indeed, even the graphs highlighted that by NMR -CH
2 group signal, besides an higher degree of correlation (as indicate R
2 value), the slope and the intercept values are not significantly different from one and zero respectively, excluding constant or proportional systematic errors and confirming the perfect comparability of the two methods, as reported in
Table 6.
3.3. Specificity
LF-NMR spectroscopy tecnique has several advantages, such as short time analysis as well as an easy sample preparation. Despite many advantages, this technique may suffer specificity problems, since it does not provide, unlike the GC technique, a preventive separation of the components of a sample. The main and potential interferents for alcohol matrices object of this study are the following substances, sometimes naturally contained or typically added as denaturants in ethyl alcohol samples: methanol, acetone, 2-propanol tert-butanol, n-hexane, n-propanol, ethyl-methyl ketone (MEK), ethyl acetate, ciclohexane, isopropyl acetate, toluene, ethylen glycol, eucalyptol and diethylphtalate. The addition of denaturants listed above are regulated both at National and European level, the latter by Reg. UE 2017/2236 [
17]. In crude heads-tail alcohols samples, this problem may be even more relevant, due to the possible presence of different volatile substances. To verify possible interferences in ethanol determination by LF-NMR, specificity tests were carry on following solutions:
ethanol standard solution containing all the 14 denaturants mentioned above;
ethanol standard solution containing the two most common volatile substances generally found in crude alcohol: methanol and ethyl acetate;
ethanol standard solutions containing, singularly, common or critical denaturants as received by our laboratory.
3.3.1. Analysis of Ethanol Standard Solution Containing 14 Denaturants
A 55% ethanol standard solution containing 5 l/hl a.a. of each of the 14 denaturants listed above, prepared from a 99.9 % v/v absolute ethyl alcohol as reference material, was analised in triplicate. The interference assessment was based on the recovery calculation of ethanol content compared to the nominal value, defined as reported in the
Equation 8:
Equation 8. Equation for ethanol recovery calculation
A recovery within 3% (equal toU) is to be considered accettable.
The LF-NMR spectra are reported below in
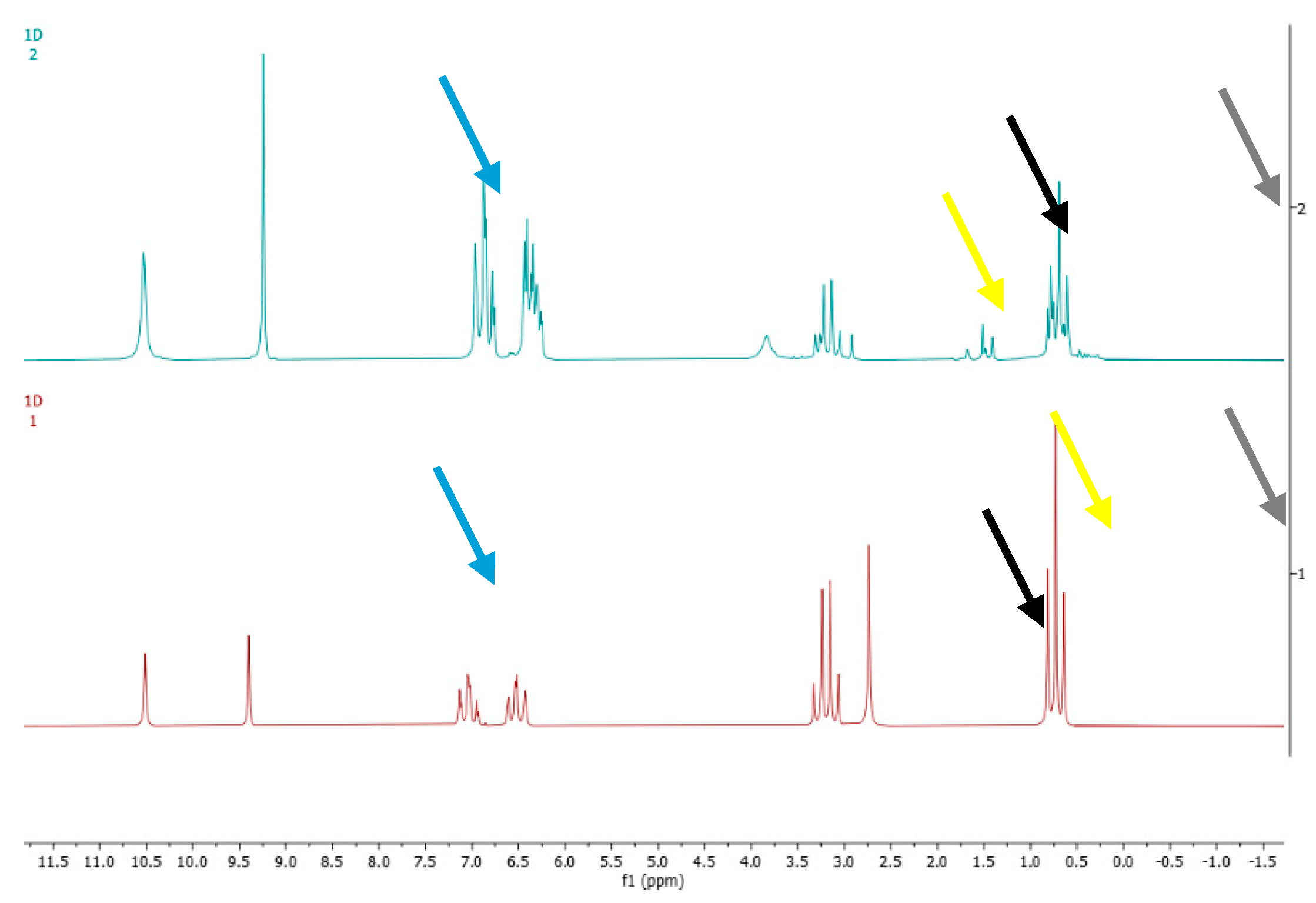
Figure 4:
The colored arrows represent the different peaks examined for analysis: the orange arrow indicates the singlet (at 9.4 ppm) of the proton of salicylic aldehyde, used as the internal standard; the yellow arrow indicates the signal (at 3.9 ppm) of the proton of the hydroxyl group of ethyl alcohol, the black arrow indicates the quadruplet (at 3.2 ppm) related to the two methylene group’s protons of ethyl alcohol (-CH2); the grey arrow indicates the triplet (at 0.8 ppm) related to the three methyl group’s protons of ethyl alcohol (-CH3)
Comparing the two spectra, interferences due to denaturants are already showed by shape of methylene or methyl group of ethanol, as confirmed by recovery calculation reported in
Table 7.
Therefore, established that, in general, denaturants may interfere with ethanol determination, further tests were carried out on most common denaturants to determine the critical amount beyond which ethanol content determination is not reliable.
3.3.2. Analysis of Ethanol Standard Solution Containing Methanol and Ethyl Acetate
An 80% ethanol standard solution containing 10% of methanol and 10% of ethyl acetate was prepared and analysed in triplicate, since they represent the two most common volatile substances generally found, even at high concentrations, in crude alcohol.
As in the previous paragraphs, interference assessment was based on the recovery calculation of ethanol content compared to the nominal value, as reported in
Table 8.
It was highlighted that quantification of ethanol content in the mixture met acceptability criterion for specificity if based on -CH2 group signal.
3.3.3. Analysis of Ethanol Standard Solutions Containing, Singularly, Common or Critical Denaturants
Further five standard solutions containing 85-90% of ethanol and, singularly, 10-15% of other common or critical denaturants were prepared and analysed, since these com-pounds in particular produce signals that may overlap with those of ethanol. They are: acetone, ethyl-methyl ketone (MEK), 2-propanol, ethylen glycol, and n-propanol. The results were reported in
Table 9.
Considering the acceptability criterion of 3%, only ethylen glycol and n-propanol gave interferences respectively at a concentration of 15% and 10%, when using -CH2 group signal. Anyway, although representing critical substances, n-propanol is generally present in trace in crude alcohol and ethylene glycol is a specific denaturant, not used in a such high concentration. Quantification based on -CH3 group signal failed recovery test only using 2-propanol, but generally, as highlighted so far, results are not accurate.
Since, generally, denaturants added not exceed 3l/hl a.a. for completely denatured alcohol (see Reg. UE 2017/2236), LF-NMR method may be considered definitely reliable.
Anyway, in case of doubt, in particular whenever alterated peaks’ shape is observed, qualitative or semiquantitative GC screeening is suggested, to evaluate if ethanol content determination may be affected by interferences.
3.4. Application Field
Matrices analysed in this study allow to set an application field ranged between 80 and 100 %v/v of ethanol in hydroalcoholic, even denaturated, samples.
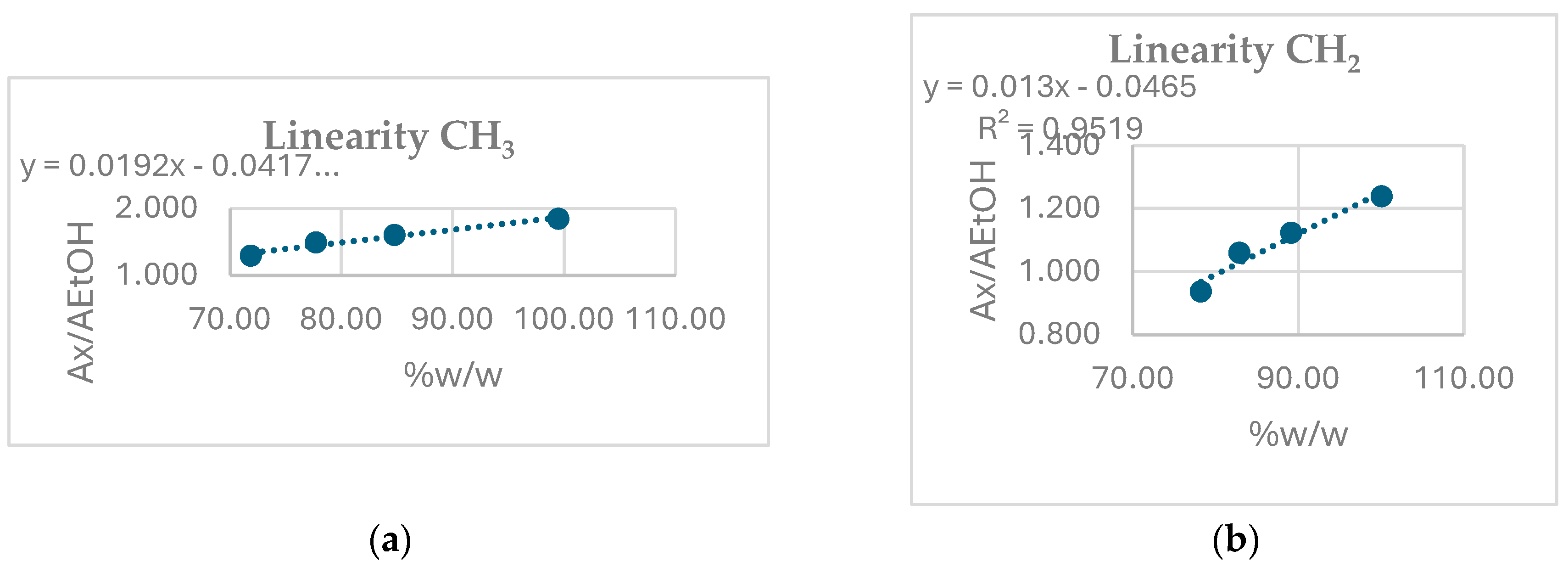
3.5. Linearity
Calibration is not necessary since quantification is made by the Equation (1).
Anyway, linearity was investigated in the concentration range of application field (80.0-100.0 %w/w of ethanol) by four ethanol standard solution, both using -CH
3 and CH
2 groups signal. As expected, a linearity with R
2>0,95 was obtained, as reported in
Figure 5.
3.6. Robustness
Robustness represents capacity of an analytical procedure to produce unbiased results when small changes in the experimental conditions are made voluntarily. Considering the proposed method, some procedural changes may occur only during sample preparation, therefore different ratios weighing between sample an internal standard were tested for robistness, using the OFAT (one-factor-at-time) approach.
Sample preparation consists of weighing a mixture of internal standard (salicylaldehyde) and alcohol sample in a 3:1 ratio. It was tested a 2:1 and a 4:1 ratios.
As reported in Supporting Informations the method resulted robust for the 2:1 ratio, unlike the 4:1 one.
4. Conclusions
In this paper, a quantitative, reliable, rapid and cost-effective determination of ethyl alcohol in alcoholic matrices by 1H LF-NMR was proposed.
Indeed, ethanol content determination in alcohol products has a relevant role in the process of complete denaturation of alcohol (CDA) in the European Union as denaturing formulas are expressed in litres or grams of denaturing agents (denaturants) per hectolitre of absolute ethanol.
The method herein discussed was evaluated for accuracy (precision and trueness), specificity, application field, linearity and robustness. Expanded measurement uncertainty was estimated by Horwitz approach, proving to be approximately 2%.
Results of experiments show that the 1H LF-NMR technique, if based on ethanol CH2 group signal for quantification, is reliably and comparable with the gascromatographic method, with the advantage of being faster and even more precise in the set application field (80-100 %vol). Instead, quantification based on ethanol CH3 group signal results lacking of accuracy.
About specificity the study showed that the ethanol NMR determination may suffer the presence of some interfering substances, naturally occurred in crude alcohol or added as denaturants, if their content is above 10 %vol. However, typically, only methanol and ethyl acetate are found in crude heads-tails alcohol that may interfere with the NMR signal if exceed such concentration. In case of doubt, also deduced from the shape of the reference signals of NMR spectra, a qualitative chromatography is recommended to evaluate the presence of any interferents.
Finally, robustness tests suggest maintaining the weight ratio of internal standard and sample between 2:1 and 3:1 during sample preparation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1. 1H-NMR spectrum of a mixture consisting of the alcohol sample and salicylaldehyde in 1:3 volume ratio; Table S1. Raw data, analysed by 1H-NMR spectroscopy; Equation S1.: Fischer’s test formula; Equation S2.: Bartlett’s test formula; Equation S3. Repeatibilities equations for CH2 and CH3 peaks datas; Table S2. Repeatability of the method for both CH2 and CH3 peaks; Table S3. Raw data collected with CLEN/ILIADe 143:2023 version 25 July 2023 method; Equation S4. Formulas used to statistically compare the two datasets obtained by the two methods analysed (LF-NMR and GC-FID); Table S4. Comparison between two methods acceptability criteria; Table S5. Repeatability tests for real alcoholic samples received in the laboratory in the last two years; Equation S5 t-test applied on NMR and densimeter reference value on anhydrous ethyl alcohol sample; Table S6. Resulsts obtained from the comparison between two methods (GC-FID and LF-NMR); Equation S5. OFAT approach; Table S7. Results obtained with OFAT approach.
Author Contributions
Conceptualization: G.F and S.S.; methodology: G.F. and M.S.; software: G.F. and M.S.; validation: G.F. and S.S.; formal analysis: G.F., M.S. and C.C.; investigation: G.F.; resources: S.R.; data curation: G.F.; writing–original draft preparation: G.F, C.C, S.S and M.P.; writing–review and editing: G.F., S.S., C.C. and M.P. ; visualization: S.S. and C.C.; supervision: S.S., G.F. and M.P.; project administration, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Available data are reported in Supporing Information.
Acknowledgments
The paper is published with the contribution of all the staff of the Italian Customs Chemical Laboratory of Bologna.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Council Directive 92/83/EEC of 19 October 1992 on the harmonization of the structures of excise duties on alcohol and alcoholic beverages, OJ L 316, 31.10.1992, p. 21–27 (ES, DA, DE, EL, EN, FR, IT, NL, PT).
- COMMISSION REGULATION (EC) No 3199/93 of 22 November 1993 on the mutual recognition of procedures for the complete denaturing of alcohol for the purposes of exemption from excise duty.
- I.W. Burton, M. A. Quilliam, and J. A. Walter, “Quantitative 1H NMRwith external standards: use in preparation of calibrationsolutions for algal toxins and other natural products,” Analytical Chemistry, vol. 77, no. 10, pp. 3123–3131, 2005. [CrossRef]
- S. K. Bharti and R. Roy, “Quantitative 1H NMR spectroscopy,” TrAC Trends in Analytical Chemistry, vol. 35, pp. 5–26, 2012.
- H.-S. Son, M. K. Kim, F. Van den Berg et al., “ 1H nuclearmagnetic resonance-based metabolomic characterization of wines by grape varieties and production areas,” Journal of Agricultural and Food Chemistry, vol. 56, no. 17, pp. 8007–8016, 2008. [CrossRef]
- G. F. Pauli, T. G¨odecke, B. U. Jaki, and D. C. Lankin, “Quantitative 1H NMR. Development and potential of an analytical method: an update,” Journal of Natural Products, vol. 75, no. 4, pp. 834–851, 2012. [CrossRef]
- J. C. Edwards, J. M. Hunter, and B. V. Nemzer, “Multinuclear NMR of calcium fructoborate complex—structure, stability, and quantitation in the presence of other ingredients, excipients or adulterants,” Journal of Food Research, vol. 3, no. 3, pp. 115–131, 2014. [CrossRef]
- G.Ramtahal, I. C. Yen, I. Bekele et al., “Cost-effective method of analysis for the determination of cadmium, copper, nickel and zinc in cocoa beans and chocolates,” Journal of Food Research, vol. 4, no. 1, pp. 193–199, 2014. [CrossRef]
- P. K. Bowyer, “The measurement of alcohol levels in wine,” in The AustralianGrapegrower andWinemaker, pp. 90–96,AnnualTechnical Issue, 2006.
- P. Maes, Y. B. Monakhova, T. Kuballa, H. Reusch, and D. W. Lachenmeier, “Qualitative and quantitative control of carbonated cola beverages using 1H NMR spectroscopy,” Journal of Agricultural and Food Chemistry, vol. 60, no. 11, pp. 2778–2784,2012. [CrossRef]
- B. Lorrain, I. Ky, L. Pechamat, and P.-L. Teissedre, “Evolution of analysis of polyphenols from grapes, wines, and extracts,”Molecules, vol. 18, no. 1, pp. 1076–1100, 2013. [CrossRef]
- ILIADe 143:2023| CLEN Method “Determination of Ethanol in Alcoholic Products by GC-FID” Version 25 July 2023.
- EA guidelines on the expression of uncertainty in quantitative testing, European co-operation for Accreditation, EA-4/16.
- UNICHIM Manuals n. 179/0, 179/1, 179/2 2011 Edition “Guidelines for the validation of analytical methods in chemical laboratories”.
- Decreto legge del 30/08/1993 n.331 “Armonizzazione delle disposizioni in materia di imposte sugli oli minerali, sull’alcole, sulle bevande alcoliche, sui tabacchi lavorati e in materia di IVA con quelle recate da direttive CEE e modificazioni conseguenti a detta armonizzazione, nonche’ disposizioni concernenti la disciplina dei centri autorizzati di assistenza fiscale, le procedure dei rimborsi di imposta, l’esclusione dall’ILOR dei redditi di impresa fino all’ammontare corrispondente al contributo diretto lavorativo, l’istituzione per il 1993 di un’imposta erariale straordinaria su taluni beni ed altre disposizioni tributarie”; Gazzetta Ufficiale n. 203 del 30/08/1993 null n. 427 del 29/10/1993.
- Decreto ministeriale 9/07/1996, n. 524 , “Regolamento recante norme per disciplinare l’impiego dell’alcole etilico e delle bevande alcoliche in usi esenti da accisa”, (GU Serie Generale n.237 del 09-10-1996).
- COMMISSION IMPLEMENTING REGULATION (EU) 2017/2236 of 5 December 2017 amending Regulation (EC) No 3199/93 on the mutual recognition of procedures for the complete denaturing of alcohol for the purposes of exemption from excise duty.
Figure 1.
Proton 1D pulse sequence.
Figure 1.
Proton 1D pulse sequence.
Figure 2.
1H-NMR spectrum of a mixture consisting of the alcohol sample and salicylaldehyde in 1:3 volume ratio. The colored arrows represent the different peaks examined for analysis: the orange arrow indicates the singlet (at 9.4 ppm) of the proton of salicylic aldehyde, used as the internal standard; the yellow arrow indicates the signal (at 3.9 ppm) of the proton of the hydroxyl group of ethyl alcohol, the black arrow indicates the quadruplet (at 3.2 ppm) related to the two methylene group’s protons of ethyl alcohol (-CH2); the grey arrow indicates the triplet (at 0.8 ppm) related to the three methyl group’s protons of ethyl alcohol (-CH3).
Figure 2.
1H-NMR spectrum of a mixture consisting of the alcohol sample and salicylaldehyde in 1:3 volume ratio. The colored arrows represent the different peaks examined for analysis: the orange arrow indicates the singlet (at 9.4 ppm) of the proton of salicylic aldehyde, used as the internal standard; the yellow arrow indicates the signal (at 3.9 ppm) of the proton of the hydroxyl group of ethyl alcohol, the black arrow indicates the quadruplet (at 3.2 ppm) related to the two methylene group’s protons of ethyl alcohol (-CH2); the grey arrow indicates the triplet (at 0.8 ppm) related to the three methyl group’s protons of ethyl alcohol (-CH3).
Figure 3.
Correlation between results obtained by NMR, based on-CH3 (a) and -CH2 (b) group signals respectively, and GC-FID methods
Figure 3.
Correlation between results obtained by NMR, based on-CH3 (a) and -CH2 (b) group signals respectively, and GC-FID methods
Figure 4.
The spectra 1 and 2 referee, respectively, to the reference absolute ethyl alcohol and ethanol standard solution containing 14 denaturants.
Figure 4.
The spectra 1 and 2 referee, respectively, to the reference absolute ethyl alcohol and ethanol standard solution containing 14 denaturants.
Figure 5.
Linearity using CH3 (A) and CH2 (B) group signals of ethanol.
Figure 5.
Linearity using CH3 (A) and CH2 (B) group signals of ethanol.
Table 1.
Matrices studied for repeabiltility tests.
Table 1.
Matrices studied for repeabiltility tests.
| Matrix |
EtOH content
determined by GC-FID |
| Anhydrous ethyl alcohol |
EtOH: 99.9 % v/v |
| Denatured ethyl alcohol (euro DG) |
EtOH: 88.5 % v/v |
| Crude alcohol |
EtOH: 92.7 % v/v |
| Hydroalcoholic mixture ethyl alcohol/water 80/20 %v/v |
EtOH: 82.7 % v/v |
Table 2.
Summary of results obtained for the evaluation of method accuracy data.
Table 2.
Summary of results obtained for the evaluation of method accuracy data.
| Matrix |
rLAB (%v/v)
-CH3 signal |
rLAB (%v/v)
-CH2 signal |
UH (%v/v)
|
| Anhydrous ethyl alcohol |
1.64 |
0.74 |
2.00 |
| Denatured ethyl alcohol (euro DG) |
1.84 |
| Crude alcohol |
1.90 |
| Hydroalcoholic mixture ethyl alcohol/water 80/20 %v/v |
1.76 |
Table 3.
Comparison between laboratory and proficiency test results and acceptability criteria.
Table 3.
Comparison between laboratory and proficiency test results and acceptability criteria.
| Matrix |
X
(%v/v) |
Xpt
(%v/v) |
spt |
Z-score |
Acceptability
(-2 ≤ z ≤ +2) |
| PT CLEN 2019-Burning Alcohol (-CH3) – Repetition 1 |
86.76 |
89.62
|
0.65 |
-4.40 |
NO |
| PT CLEN 2019-Burning Alcohol (-CH3) – Repetition 2 |
87.61 |
-3.09 |
NO |
| PT CLEN 2019-Burning Alcohol (-CH3) – Repetition 3 |
86.31 |
-5.09 |
NO |
| PT CLEN 2019-Burning Alcohol (-CH2) – Repetition 1 |
89.25 |
-0.57 |
YES |
| PT CLEN 2019-Burning Alcohol (-CH2) – Repetition 2 |
90.04 |
0.65 |
YES |
| PT CLEN 2019-Burning Alcohol (-CH2) – Repetition 3 |
88.78 |
-1.30 |
YES |
Table 4.
Comparison between NMR and GC methods.
Table 4.
Comparison between NMR and GC methods.
| |
|
-CH3 signal |
-CH3 signal |
-CH3 signal |
Acceptability |
-CH2 signal |
-CH2 signal |
-CH2 signal |
Acceptability |
| Anhydrous ethyl alcohol |
100.88 |
99.49 |
1.39 |
0.16 |
NO |
100.03 |
0.85 |
0.14 |
NO |
| Denatured ethyl alcohol (euro DG) |
88.08 |
89.07 |
1.00 |
0.26 |
NO |
88.28 |
0.20 |
0.40 |
YES |
| Crude alcohol |
92.73 |
92.76 |
0.03 |
0.56 |
YES |
92.92 |
0.19 |
0.65 |
YES |
| Hydroalcoholic mixture ethyl alcohol/water 80/20 %v/v |
82.72 |
81.32 |
1.40 |
0.25 |
NO |
82.99 |
0.26 |
0.33 |
YES |
Table 5.
Trueness test for anhydrous ethyl alcohol.
Table 5.
Trueness test for anhydrous ethyl alcohol.
| |
CH3
|
Acceptability |
CH2
|
Acceptability |
| Anhydrous ethyl alcohol - CRM |
0.47 |
YES |
0.11 |
YES |
Table 6.
Correlation equations of NMR and GC methods. The intercept and slope values based on -CH2 signal showed no significant differences between the two methods, as both are respectively different from zero and one.
Table 6.
Correlation equations of NMR and GC methods. The intercept and slope values based on -CH2 signal showed no significant differences between the two methods, as both are respectively different from zero and one.
Equation
-CH3 signal |
a (intercept) ± s(a)
-CH3 signal |
b (slope) ± s(b)
-CH3 signal |
Equation
-CH2 signal |
a (intercept) ± s(a)
-CH2 signal |
b (slope) ± s(b)
-CH2 signal |
| y = 0.803x + 17.047 |
17.0±16.1 |
0.80±0.17 |
y = 0.9234x + 7.2033 |
7.20±8.22 |
0.92±0.09 |
Table 7.
Specificity on ethanol standard solution containing 14 denaturants .
Table 7.
Specificity on ethanol standard solution containing 14 denaturants .
| |
Cexperimental
-CH3 signal |
Cexperimental
-CH2 signal |
Ctheoretical |
Recovery
-CH3 signal |
Recovery
-CH2 signal |
| Ethanol standard solution containing 14 denaturants |
98.65w/w % |
103.41w/w % |
54.99 w/w % |
179.39 % |
188.06% |
Table 8.
Specificity on ethanol standard solution containing methanol and ethyl acetate .
Table 8.
Specificity on ethanol standard solution containing methanol and ethyl acetate .
| |
Cexperimental
-CH3 signal |
Cexperimental
-CH2 signal |
Ctheoretical |
Recovery
-CH3 signal |
Recovery
-CH2 signal |
Ethanol std +
methanol & ethyl acetate
|
83.13 %w/w |
81.31%w/w |
80.26 %w/w |
103.58% |
101.31% |
Table 9.
Specificity on ethanol standard solution containing, singularly, different denaturants.
Table 9.
Specificity on ethanol standard solution containing, singularly, different denaturants.
| |
Cexperimental
-CH3 signal |
Cexperimental
-CH2 signal |
Ctheoretical |
Recovery
-CH3 signal |
Recovery
-CH2 signal |
| Ethanol std + acetone |
88.52 |
91.43 |
90.10 |
98.25 |
101.47 |
| Ethanol std + MEK |
89.99 |
90.53 |
89.99 |
100.60 |
101.75 |
| Ethanol std + 2-propanol |
103.69 |
92.69 |
90.25 |
114.89 |
102.70 |
| Ethanol std + ethylen glycol |
85.67 |
83.41 |
85.67 |
97.37 |
124.80 |
| Ethanol std + n-propanol |
89.54 |
90.95 |
89.54 |
101.58 |
109.98 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).