Submitted:
02 August 2024
Posted:
06 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
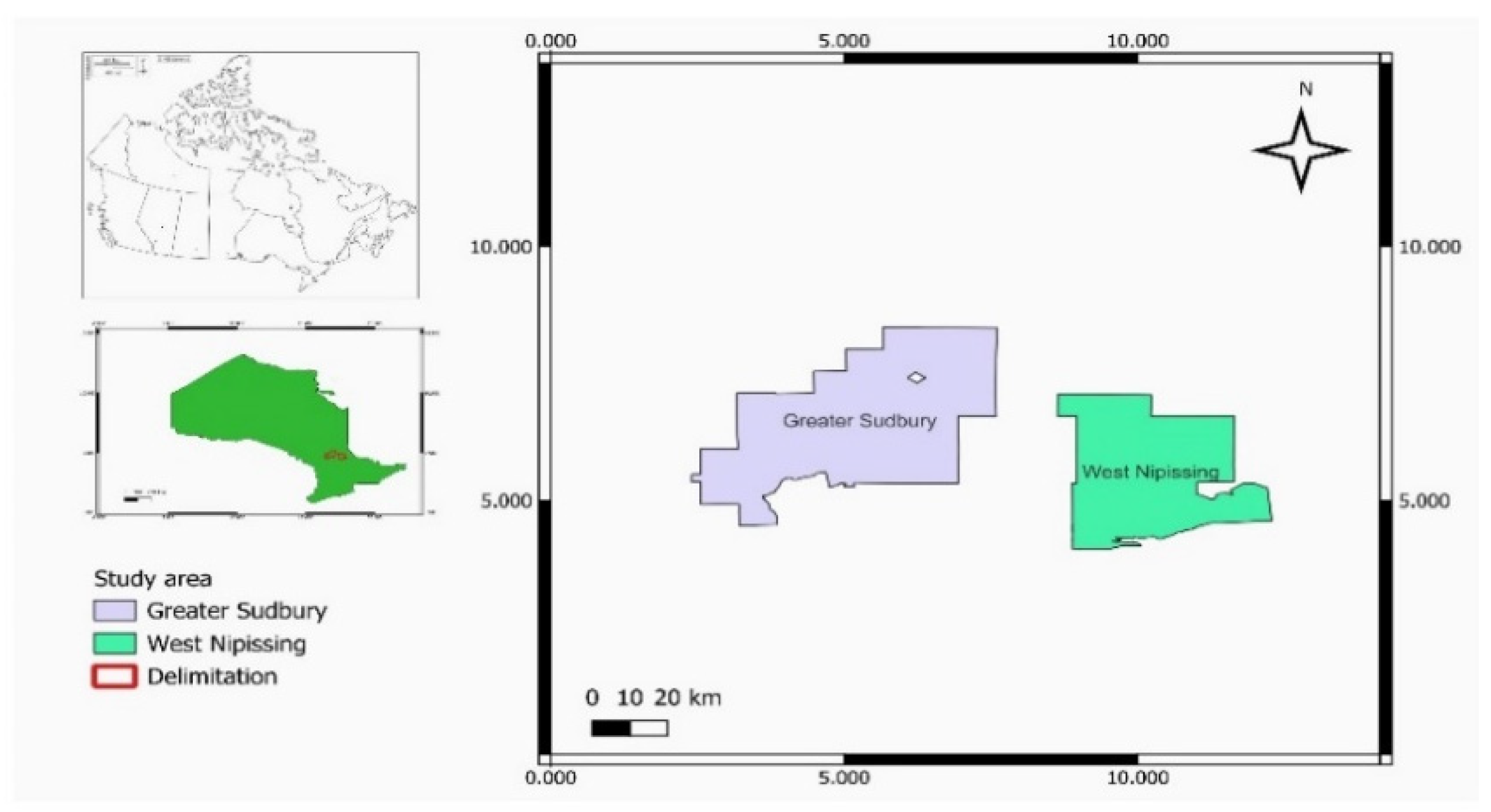
2.1. Experimental Site
2.2. Inoculum, Vectors and Distributors
2.3. Strawberry Varieties Used
2.4. Experimental Methodology
2.5. Statistical Analysis
3. Results
3.1. Distribution of B. bassiana by Bumblebees
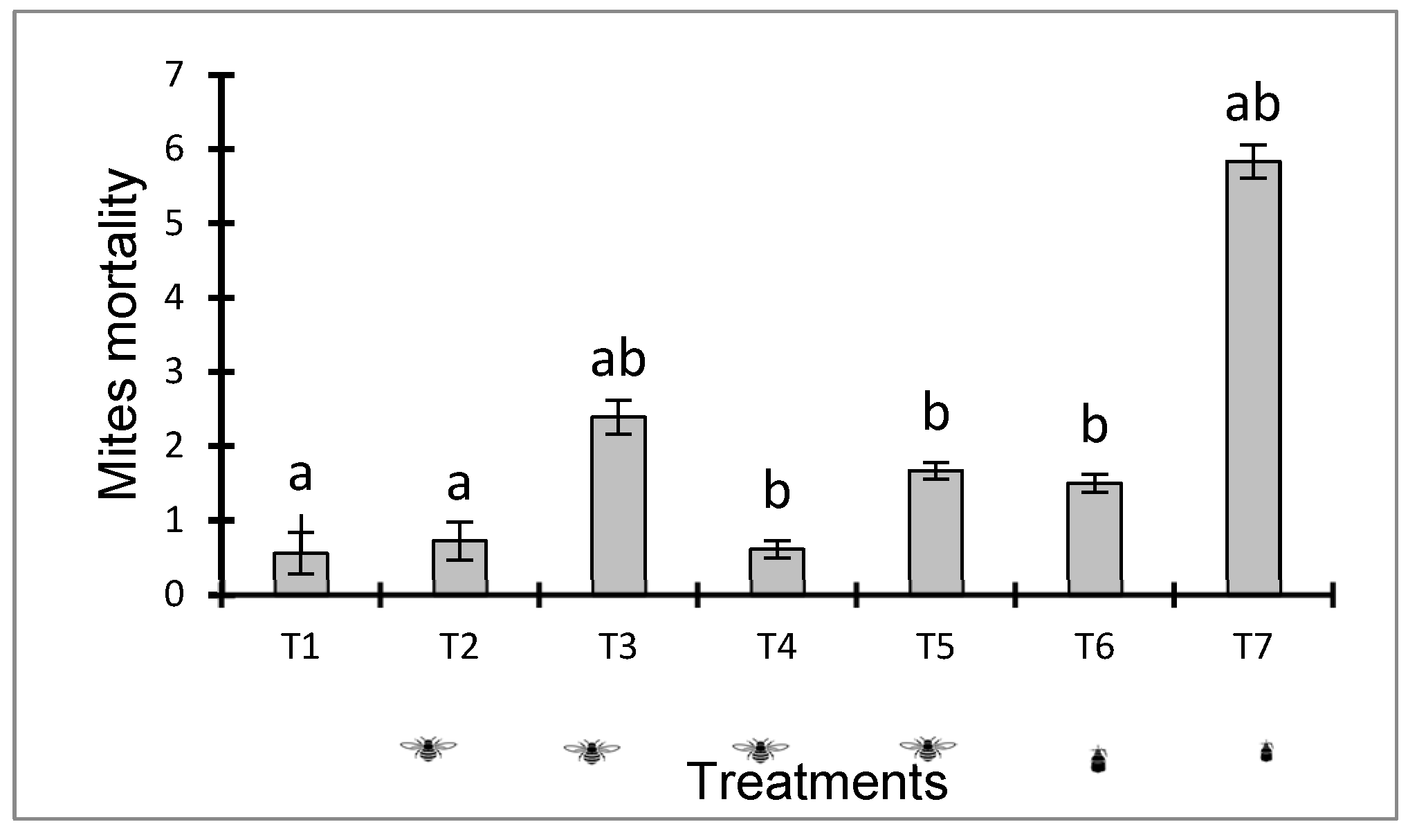
3.2. Effect of Treatments on the Cyclamen Mite and Damage Recorded
3.3. Efficacy of the Spraying Mode of B. bassiana and Neem oil on P. pallidus
3.4. Effectiveness of Bumblebee Biocontrol of P. pallidus and B. bassiana
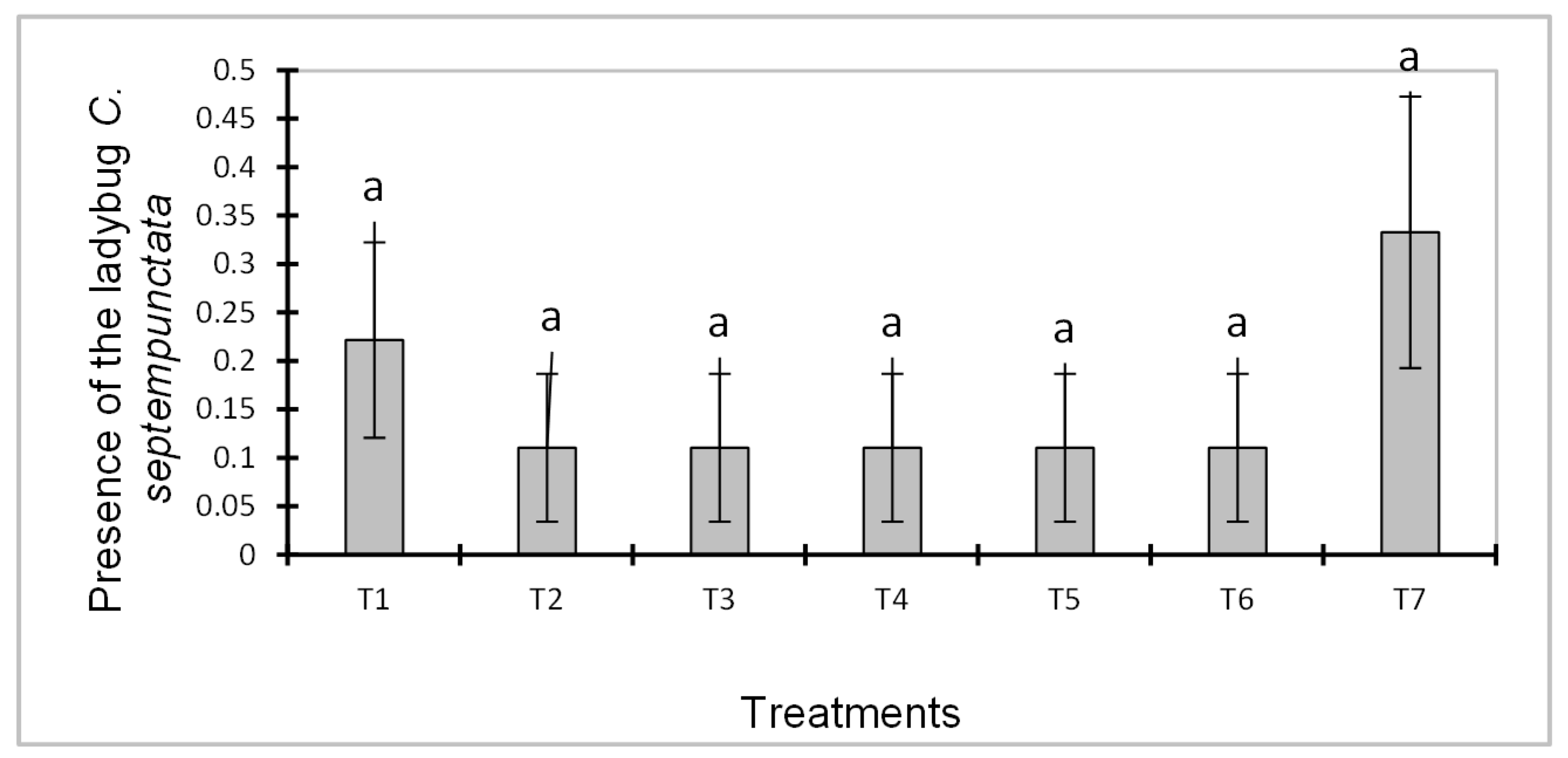
3.5. Effect of B. bassiana Transmitted by Bumblebees and Spray Treatments on Beneficial Organisms
4. Discussion
5. Conclusion
Acknowledgments
References
- Agriculture et Agroalimentaire Canada, A. et A. Produire des fraises tout aussi savoureuses, mais plus résistantes aux maladies grâce aux rayons ultraviolets. http://agriculture.canada.ca/fr/science/science-racontee/realisations-scientifiques-agriculture/produire-fraises-savoureuses-plus-resistantes-aux-maladies-grace-aux-rayons-ultraviolets (accessed 2024-02-11).
- Gouvernement du Canada, S. C. Envie de vous bourrer la fraise? https://www.statcan.gc.ca/o1/fr/plus/1300-envie-de-vous-bourrer-la-fraise (accessed 2024-02-11).
- Patenaude, S.; Tellier, S.; Fournier, V. Cyclamen Mite (Acari: Tarsonemidae) Monitoring in Eastern Canada Strawberry (Rosaceae) Fields and Its Potential Control by the Predatory Mite Neoseiulus Cucumeris (Acari: Phytoseiidae). Can. Entomol. 2020, 152 (2), 249–260. [CrossRef]
- Renkema, J. M.; Pate, E.; Olivier, C. The Temporal Distribution of Cyclamen Mite, Phytonemus Pallidus (Acari: Tarsonemidae), in Strawberry and Comparison of Sampling Methods. Can. Entomol. 2022, 154 (1), e33. [CrossRef]
- Wiesmann, R.; Wiesmann, R. Investigations on the Biology and Control of the Strawberry Mite, T. Pallidus. Landw Jb Schweiz Bern 1941, 55, pts. 3, 259–329.
- Johansen, N. S.; Trandem, N.; Le, V. H.; Stensvand, A. The Potential for Using Aerated Steam to Eradicate Strawberry Mite and Two-Spotted Spider Mite on Strawberry Transplants. Exp. Appl. Acarol. 2022, 88 (3–4), 243–262. [CrossRef]
- Tamm, L.; Speiser, B.; Niggli, U. Réduction des produits phytosanitaires en Suisse: la contribution de l’agriculture biologique. 2018, 9 (2), 8.
- Réseau d’avertissement phytosanitaire. Bulletin d’informations Général; Message important de Santé Canada aux utilisateurs d’insecticides à base d’endosulfan. 3; 2017; p 2. https://www.agrireseau.net/documents/Document_95140.pdf.
- Temmermans, J.; Smagghe, G. Different Bees as Vectors for Entomovectoring with Enhanced Pollination and Crop Protection Control: Current Practices, Use Cases and Critical View on Transport. Rev. Sci. Tech. Int. Off. Epizoot. 2022, 41, 107–116. [CrossRef]
- Hazlegreaves, S. How is apivectoring innovating agricultural systems?. Open Access Government. https://www.openaccessgovernment.org/how-is-apivectoring-innovating-agricultural-systems/107527/ (accessed 2024-02-11).
- Smagghe, G., Boecking, O., Maccagnani, B., Mänd, M., Kevan, P. G. Entomovectoring for Precision Biocontrol and Enhanced Pollination of Crops; Eds. Springer International Publishing: Cham, 2020. [CrossRef]
- Kapongo, J. P.; Shipp, L.; Kevan, P.; Sutton, J. C. Co-Vectoring of Beauveria Bassiana and Clonostachys Rosea by Bumble Bees (Bombus Impatiens) for Control of Insect Pests and Suppression of Grey Mould in Greenhouse Tomato and Sweet Pepper. Biol. Control 2008, 46 (3), 508–514. [CrossRef]
- Karise, R.; Dreyersdorff, G.; Jahani, M.; Veromann, E.; Runno-Paurson, E.; Kaart, T.; Smagghe, G.; Mänd, M. Reliability of the Entomovector Technology Using Prestop-Mix and Bombus Terrestris L. as a Fungal Disease Biocontrol Method in Open Field. Sci. Rep. 2016, 6 (1), 31650. [CrossRef]
- Crepet, W. L. Advanced (Constant) Insect Pollination Mechanisms: Pattern of Evolution and Implications Vis-a-Vis Angiosperm Diversity. Ann. Mo. Bot. Gard. 1984, 71 (2), 607. [CrossRef]
- Michener, C. D. The Bees of the World, second edition edition.; The Johns Hopkins University Press, 2007.
- Wahengbam, J.; Raut, A.; Pal, S.; Banu, A. N. Role of Bumble Bee in Pollination. Ann. Biol. 2019, 35, 290–295.
- Dara, S. K. Implementation of IPDM in Strawberries and Other Berries. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M. L., Albajes, R., Nicot, P. C., Eds.; Plant Pathology in the 21st Century; Springer International Publishing: Cham, 2020; pp 597–624. [CrossRef]
- Michereff-Filho, M.; Navia, D.; Alexopoulos Quevedo, I.; de Almeida Magalhães, M.; Wagner da Silva Melo, J.; Biaggioni Lopes, R. The Effect of Spider Mite-Pathogenic Strains of Beauveria Bassiana and Humidity on the Survival and Feeding Behavior of Neoseiulus Predatory Mite Species. Biol. Control 2022, 176, 105083. [CrossRef]
- Agence de réglementation de la lutte antiparasitaire du Santé Canada. Projet de décision d’homologation PRD2020-01. 2009, No. H113-9/2009-3F (H113-9/2009-3F-PDF), 89.
- Macharia Kanyi, J.; Gikungu, M.; Karanja, R.; Okoth, S. African Journal of Agricultural Research Managed Bees as Pollinators and Vectors of Bio Control Agent against Grey Mold Disease in Strawberry Plantations. Afr. J. Agric. Res. 2020, Vol. 16(12), 1674–1680. [CrossRef]
- Sabbahi, R. Utilisation du champignon entomopathogène Beauveria bassiana dans une stratégie de gestion phytosanitaire des principaux insectes ravageurs en fraiseraies. phd, Université du Québec, Institut National de la Recherche Scientifique, Québec, 2008. https://espace.inrs.ca/id/eprint/285/ (accessed 2024-02-11).
- Shipp, L.; Kapongo, J. P.; Park, H.-H.; Kevan, P. Effect of Bee-Vectored Beauveria Bassiana on Greenhouse Beneficials under Greenhouse Cage Conditions. Biol. Control 2012, 63 (2), 135–142. [CrossRef]
- Sayed, S.; El Arnaouty, S.; Alotaibi, S.; Salah, M. Pathogenicity and Side Effect of Indigenous Beauveria Bassiana on Coccinella Undecimpunctata and Hippodamia Variegata (Coleoptera: Coccinellidae). Insects 2021, 12, 12. [CrossRef]

| Time after application of treatments | Leaves and flowers | |
| **% P Damage. pallidus | *Number of dead mites of P. pallidus | |
| 7JHA | 56,571 ± 0.041ab | 1.119 ± 0.38ab |
| 12JHA | 22,571 ± 0.054a | 2.524 ± 0.81a |
| 17JHA | 56,571 ± 0.041ab | 2,048 ± 0.57ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





