1. Introduction
Flos Sophorae Immaturus (FSI) is the dried flower bud of the leguminous plant Sophora japonica L., extensively cultivated in various regions including China, Japan, Great Britain, and Egypt [
1]. This Chinese herbal remedy is known for its liver-clearing, fire-purging, blood-cooling, and hemostatic properties [
2]. FSI is rich in flavonoids such as rutin, quercetin, kaempferol, and sophoricoside, along with polysaccharides, amino acids, and other bioactive compounds [
3,
4,
5,
6]. FSI possesses notable nutritional and pharmacological benefits include antioxidant, anti-aging, antiviral, anti-allergic, antithrombotic, and analgesic effects, and is documented in both the Chinese Pharmacopeia and the European Pharmacopeia [
1,
4]. Despite its potential, FSI remains an underutilized, sustainable plant material with promising applications in high value-added products. Researchers are increasingly directing their efforts towards the development of FSI for use in functional foods, dietary supplements, and cosmetics [
1]. The efficient extraction of total flavonoids from FSI is also a topic of significant interest [
3,
7,
8,
9].
For extracting flavonoids from FSI, green and sustainable technologies should be prioritized. Traditionally, organic solvents such as alcohols and ethyl acetate have been widely used to extract bioactive compounds from natural plant resources. However, these extraction methods require large quantities of flammable and hazardous organic solvents, which conflict with green chemistry principles [
10]. Additionally, they often exhibit low extraction efficiency for the target bioactive substances and high energy consumption. Consequently, there is an urgent need for environmentally friendly solvents to enable efficient green extraction. Deep eutectic solvents (DESs) represent a new type of non-toxic, biodegradable, designable, and reusable solvents that are promising alternatives to traditional solvents [
11,
12,
13]. As an environmentally friendly green solvent, DES is highly suitable for extracting bioactive compounds from natural plant resources. They can be easily synthesized from hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) under mild heating conditions, without further purification. They have many excellent properties, including low volatility, excellent solubility, and high thermal stability. In recent decades, many DESs composed of various components, such as choline chloride, amides, carboxylic acids, and polyols have been developed [
14,
15,
16,
17]. Different combinations of HBA and HBD facilitate the creation of task-specific solvents effective for extracting various natural compounds, including phenolic compounds, flavonoids, carbohydrates, and proteins. Ultrasound-assisted extraction (UAE) is a green and efficient extraction method that offers advantages such as high yield, short extraction time, low solvent consumption, and cost-effectiveness [
18]. It is widely utilized for extracting bioactive compounds from natural sources. UAE improves the dispersibility and mass transfer efficiency of the extraction solvent by inducing cavitation effects, leading to efficient extraction. Numerous studies have reported the synergistic effect of combining DES with UAE for extracting bioactive compounds from plants [
18,
19,
20,
21,
22,
23,
24].
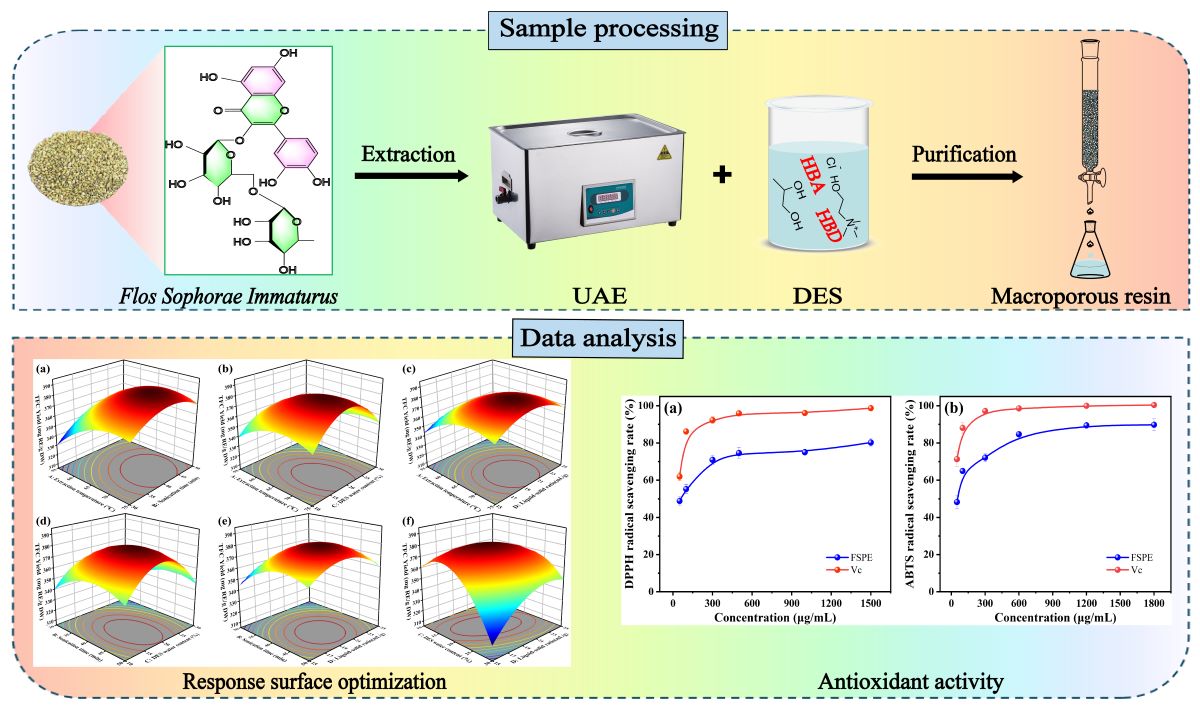
Based on the aforementioned advantages of green extraction, this study proposes a new strategy that combines deep eutectic solvents (DES) and ultrasound-assisted extraction (UAE) for the efficient extraction of total flavonoids from
Flos Sophorae Immaturus (FSI). The optimal extraction solvent for flavonoids from FSI was screened from six freshly prepared DES. Extraction conditions were optimized using the Box-Behnken design (BBD) and response surface methodology (RSM). The effects of process parameters, including extraction temperature, sonication time, DES water content, and liquid-solid ratio, on the total flavonoid extraction rate were analyzed. The target compounds were enriched and isolated from the extract using macroporous resin (D-101), and their antioxidant activity was investigated (
Figure 1). The results of this study aim to provide valuable references for further in-depth research, development, and application of flavonoids from FSI.
2. Materials and Methods
2.1. Plant Materials and Chemicals
Flos sophorae immaturus (FSI, Chinese Huaimi) was collected from Dazhu County, Sichuan Province (China), and authenticated as the dry flower buds from Sophora japonica cv. Jinhuai, an elite cultivar of Sophora japonica L., by Professor Dayi Chen. The FSI samples were oven-dried, ground, and sieved through 60 mesh prior to use.
Choline chloride (ChCl, ≥98%) was purchased from Kelong Chemical Co., Ltd. (Chengdu, China). Betaine, urea (≥98%), glycerol (≥99.5%), 1,2-propanediol (PD, ≥99.5%), 1,3-butanediol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Rutin standard (>99.5%) was sourced from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were purchased from Sigma-Aldrich (Shanghai, China). D-101 macroporous resin was obtained from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). All other chemicals and reagents used in this study were of analytical grade. Deionized water for the experiments was obtained using a Milli-Q water purification system (Millipore, USA).
2.2. Preparing and Screening of DESs
Six kinds of DESs with different molar ratios were prepared using a heating and stirring method as previously described [
25]. Choline chloride and betaine were selected as hydrogen bond acceptors (HBAs), while urea, glycerol, 1,3-butanediol and 1,2-propanediol served as hydrogen bond donors (HBDs). According to the molar ratios given in
Table 1, the components were accurately weighed and mixed in a sealed glass bottle. Additionally, 30% (
v/
v) distilled water was added to reduce the high viscosity. The mixture was then heated in a water bath at 80 °C and stirred until it formed a homogeneous and transparent liquid. The solution was stored at room temperature for later use.
Ultrasonic-assisted extraction (UAE) was performed in an ultrasonic bath (Scientz, SB-5200DTD, Ningbo, China) with an input power of 250 W. An appropriate amount of dry FSI powder was mixed with the previously prepared DESs at a liquid-solid ratio of 18 mL/g, and then subjected to ultrasonic extraction at 65 °C for 39 minutes. Following extraction, the mixture was centrifuged at 6,000 rpm for 10 minutes. The supernatant was collected and stored at 4 °C until further analysis.
2.3. Determination of the Total Flavonoids Content (TFC)
The total flavonoids content (TFC) was measured by a spectrophotometric method using a sodium nitrite-aluminum chloride complex, with slight modifications to the previously described method [
9]. In brief, 1 mL of the extract was placed in a beaker and diluted to 50 mL with 60% ethanol solution. Then, 1.0 mL of this solution was transferred to a 10 mL cuvette and diluted to 5.0 mL with ethanol solution. Subsequently, 0.3 mL of a 5% NaNO
2 solution was added. After standing at room temperature for 8 minutes, 0.3 mL of a 10% AlCl
3 solution was added, followed by a 10-minute incubation. Next, 4.0 mL of 4% NaOH solution was added, and the volume was adjusted to the mark with ethanol solution. The solution was mixed thoroughly and incubated for an additional 10 minutes. The absorbance was measured at 510 nm.
A calibration curve was prepared using different concentrations of rutin (0-40 µg/mL, R2 = 0.999). The results were expressed as milligrams of rutin equivalents (RE) per gram dry weight of the sample (mg RE/g DW).
2.4. Experimental Design
To enhance the yield of total flavonoids from FSI, appropriate experimental factors and their ranges were selected based on the results of preliminary experiments. Single-factor experiments were conducted using the selected deep eutectic solvent (DES-4) to investigate the impact of each factor on the TFC and to establish the optimal ranges for these factors. Each single-factor experiment was replicated three times, and the results were expressed as means ± standard deviation (SD). The selected experimental factors and their ranges in this study were as follows: extraction temperature of 45-85 °C, sonication time of 20-60 min, water content in DES-4 of 0-40% and liquid-solid ratio of 10-30 mL/g.
Based on the results from the single-factor experiments, the extraction of total flavonoids from FSI was optimized using Response Surface Methodology (RSM). The study employed a four-factor, three-level Box-Behnken design (BBD) for experimental design, model construction, and data analysis. A total of 29 experiments were conducted, including five replicates at the central point. The specific experimental design is outlined in
Table 2. The four independent variables were extraction temperature (A, °C), sonication time (B, min), water content in DES-4 (C, %), and liquid-solid ratio (D, mL/g). The corresponding response variable was the yield of total flavonoids (TFC).
2.5. Purification of Flavonoid Compounds Using a Macroporous D-101 Resin
The crude extract from FSI was purified using D-101 macroporous resin, selected through preliminary experiments. The eluent was collected, concentrated, and freeze-dried to obtain the purified extract (FSPE). A precise amount of flavonoid powder was then weighed and dissolved in anhydrous ethanol to prepare a 0.4 mg/mL solution for purity determination.
2.6. Evaluation of in vitro Antioxidant Activity of FSPE
The antioxidant capacity of FSPE was determined using DPPH and ABTS assays. The DPPH free radical scavenging activity was measured with slight modifications to the method described by Xi et al. [
26]. Specifically, the purified extract (FSPE) was dissolved in ethanol to prepare seven concentrations of FSPE (100-1500 μg/mL). Then, 1.5 mL of FSPE solution was mixed with an equal volume of DPPH ethanol solution (0.25 mmol/L), vigorously shaken, and left to stand in the dark at room temperature for 30 minutes. The absorbance was measured at 517 nm spectrophotometrically.
The ABTS radical scavenging activity of FSPE was carried out with slight modifications to the method reported by Liu et al. [
27]. In brief, a certain amount of ABTS was added to 2.6 mmol/L potassium persulfate solution to prepare a 7.4 mmol/L ABTS stock solution, which was stored at 4 °C in the dark for 12-16 hours. The stock solution was then diluted with anhydrous ethanol until its absorbance at 734 nm reached 0.70 ± 0.02, resulting in the ABTS working solution. Next, 0.1 mL of FSPE sample solutions of different concentrations (50-1800 μg/mL) were added to 3.9 mL of ABTS working solution, mixed well, and incubated in the dark at room temperature for 7 minutes. The absorbance of the mixture at 734 nm was recorded.
The DPPH and ABTS radical scavenging assays both used ascorbic acid as a positive control. The antioxidant activity was expressed as the effective concentration of the sample providing 50% inhibition (IC50), and IC50 value was calculated from the linear regression equation.
2.7. Statistical Analysis
All measurements were performed in triplicate, and the data were presented as means ± standard deviation (SD). ANOVA, accompanied by Duncan’s test using SPSS software (version 16.0, SPSS Inc., Chicago, USA), was conducted to identify significant differences between samples (p < 0.05). Design Expert software (Version 13.0.1.0, Stat-Ease Inc., Minneapolis, USA) was used for response surface methodology (RSM) optimization of the extraction conditions.
3. Results and Discussion
3.1. Screening of DESs for the Extraction of Flavonoids
The components of deep eutectic solvents (DESs) significantly affect their physicochemical properties, such as viscosity, polarity, and solubility, which directly influence their extraction efficiency of plant bioactive compounds [
28]. Studies have shown that amide-based DES and polyol-based DES have better extraction efficiency for flavonoids than sugar-based DES [
29]. To effectively extract flavonoids from
Flos Sophorae Immaturus, this study selected choline chloride and betaine as hydrogen bond acceptors (HBAs), amides (urea) and polyols (glycerol, 1,3-butanediol, 1,2-propanediol) as different hydrogen bond donors (HBDs) to synthesize various DESs. For preliminary screening, six DES systems (DES-1 to DES-6) were tested for their efficiency in extracting flavonoids from
Flos Sophorae Immaturus, with extraction efficiency calculated as total flavonoids content (TFC).
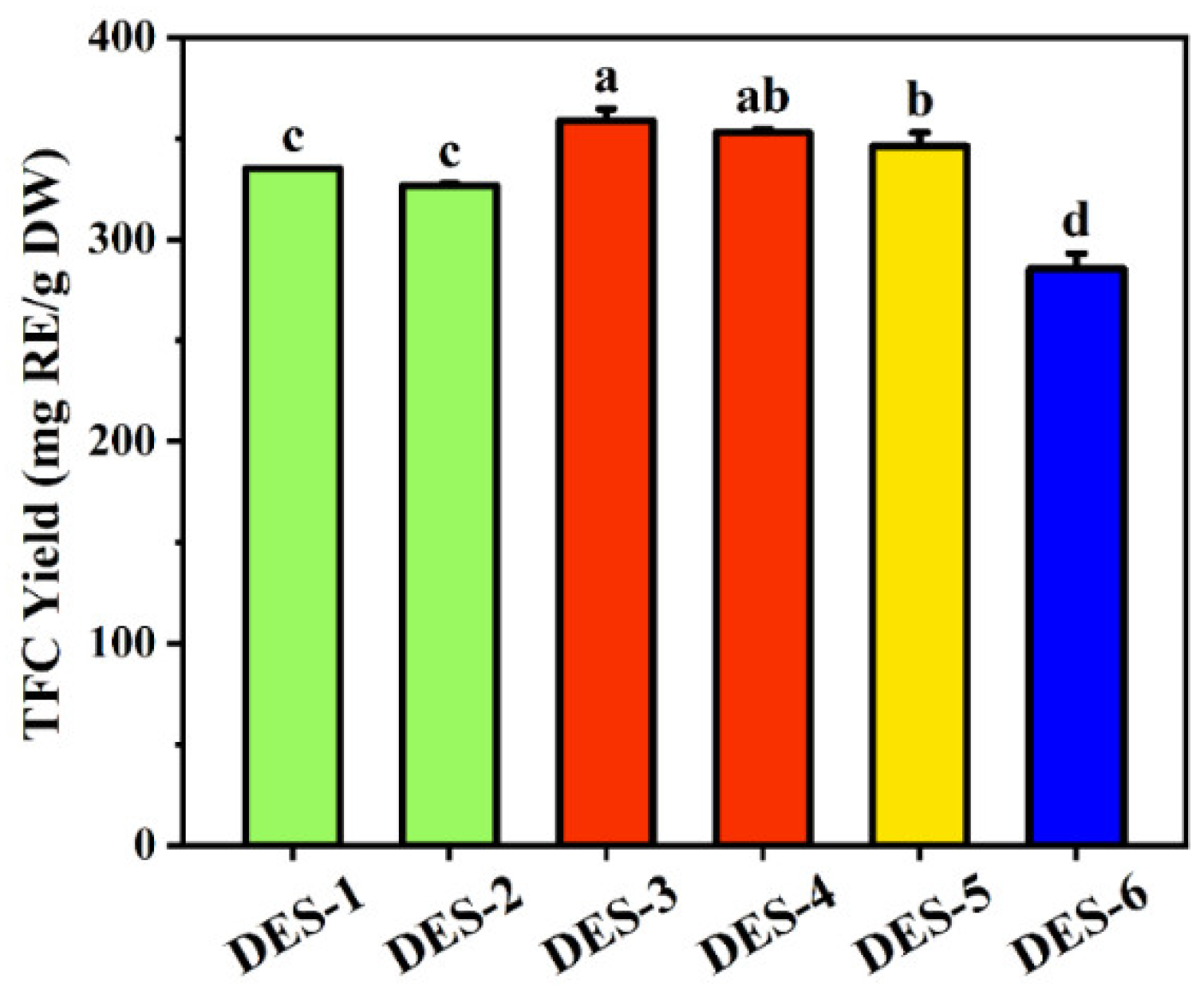
Figure 2 clearly shows that different DESs have varying extraction efficiencies. DES-3 (choline chloride/1,3-butanediol) achieved the highest flavonoid yield from
Flos Sophorae Immaturus, at 358.70 ± 5.85 mg RE/g DW, followed by DES-4 (choline chloride/1,2-propanediol) at 352.91 ± 1.65 mg RE/g DW. DES-6 (betaine-glycerol) had the lowest flavonoid extraction yield at 285.41 ± 7.54 mg RE/g DW. Due to the crystallization tendency of the DES-3 system when stored at room temperature for a week, and its similar extraction yield compared to DES-4, DES-4 (choline chloride/1,2-propanediol, ChCl/PD) was selected as the optimal extractant for subsequent experiments.
3.2. Single-Factor Experiments of UAE-DES Parameters
3.2.1. Effect of Extraction Temperature
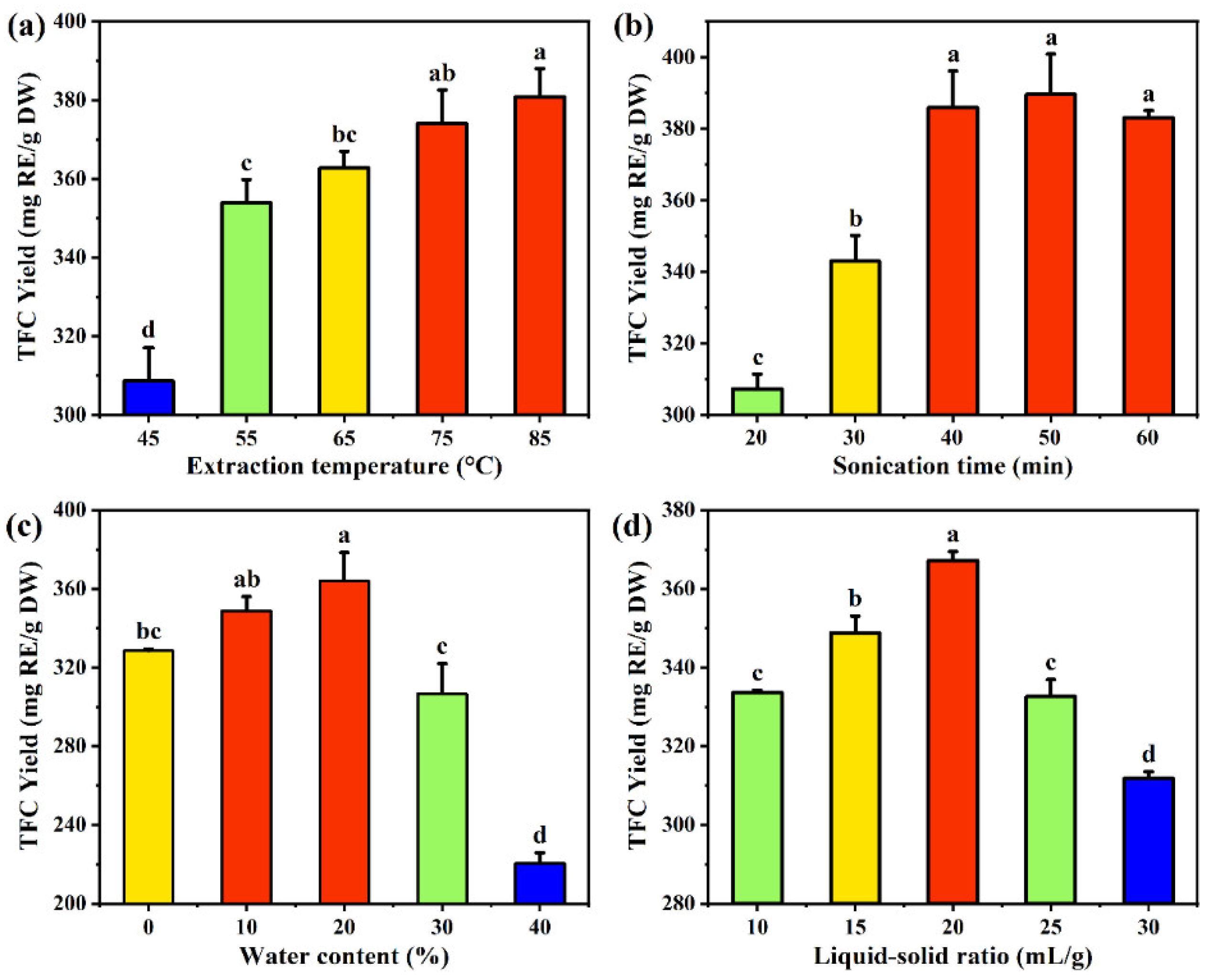
To determine the effect of extraction temperature on flavonoid yield, a range from 45 to 85 °C was studied. The TFC yield continuously increased with rising temperature, significantly from 308.67 mg/g at 45 °C to 374.12 mg/g at 75 °C. However, further increasing the temperature beyond 75 °C had no significant effect on the TFC yield (
Figure 3a). The increase in temperature can reduce the solvent’s viscosity, accelerate the swelling of raw materials, and increase the solubility of extracts. Thus, mass transfer is improved as the temperature gradually rises [
30]. On the other hand, the thermal effect of higher temperature may lead to a higher degradation rate of flavonoids, which could offset the benefits of enhanced mass transfer caused by higher temperature [
31]. Additionally, higher temperatures may promote solvent evaporation, resulting in high energy consumption and the impurity extraction [
32]. In summary, comprehensively considering the principles of green chemistry and minimizing energy consumption, 65 °C, which provides a relatively high TFC yield, was chosen as the optimal extraction temperature to balance yield and energy efficiency, achieving a sustainable and energy-saving extraction process.
3.2.2. Effect of Sonication Time
The effect of sonication time on the TFC yield was investigated with a range of 20-60 minutes. With the increasing sonication time from 20 to 40 min, the TFC yield significantly increased from 307.31 mg/g to 385.96 mg/g, after which a slight declining trend was observed (
Figure 3b). The increase in TFC yield with longer sonication times can be attributed to the ultrasonic cavitation effect. Cavitation causes the rupture of plant cell walls, reducing mass transfer limitations imposed by cell structures, and releases the cell contents into the surrounding medium [
33]. After 40 minutes, the cell walls of FSI are almost completely broken, reducing the concentration gradient, and extended sonication time has no significant effect on TFC yield. Moreover, prolonged sonication may lead to the degradation of flavonoid compounds, resulting in a decrease in TFC yield. Therefore, 40 min was chosen as the optimal sonication time for total flavonoids extraction.
3.2.3. Effect of Water Content in DES
The water content in DES significantly affects extraction efficiency. Adding an appropriate amount of water to hydrophilic DES can reduce its viscosity, thus allow improved mass transfer, resulting in high extraction yield [
34]. In this study, different water contents (0%, 10%, 20%, 30%, 40%,
v/
v) of DES-4 (ChCl/PD) were used to extract flavonoids from
Flos Sophorae Immaturus. As shown in
Figure 3c, within the range of 0% to 20%, the TFC yield continuously increased with the rising of DES water content, reaching a maximum value of 364.11 mg/g at 20%. Increasing the water content further disrupts the hydrogen bonds in the DES system and alters the solvent’s polarity, which is unfavorable for flavonoid dissolution [
35]. Additionally, an excessive water content in DES can disrupt the interactions between DES and the target compounds, leading to a decrease in extraction efficiency [
22]. Therefore, a water content of 20% was selected for subsequent experiments.
3.2.4. Effect of Liquid-Solid Ratio
The liquid-solid ratio is another parameter which affects the mass transfer rate in the extraction process, especially when using high-viscosity and low-volatility DES for ultrasound-assisted extraction. The concentration gradient between the solid raw material and the bulky solvent is the driving force during mass transfer. Within an appropriate range, a high concentration gradient will facilitate the ultrasonic extraction process [
32].
Figure 3d shows the effect of different liquid-solid ratios (10, 15, 20, 25 and 30 mL/g) on the yield of total flavonoids from FSI. The data reveals a statistically significant impact of liquid-solid ratio on TFC yield, as determined by ANOVA (
p < 0.05). The highest yield of 367.20 mg /g was observed at a liquid-solid ratio of 20 mL/g, significantly higher than other ratios tested. This indicates an optimal solvent volume at which flavonoid extraction efficiency is maximized. Ratios below and above 20 mL/g showed lower yields, possibly due to insufficient solvent for mass transfer from the raw material to the solvent at lower ratios, and excessive dilution at higher ratios, reducing the concentration gradient required for effective extraction. This result highlights the crucial role of optimizing the liquid-solid ratio to improve the efficiency of flavonoid extraction, with 20 mL/g identified as the most effective ratio under the given experimental conditions.
3.3. Optimization of Extraction Variables Using Response Surface BBD
Box-Behnken design has been widely employed to determine the optimum extraction parameters from plant material. Based on the single-factor results, RSM-BBD experiments were designed with three levels of independent variables to efficiently optimize the extraction of total flavonoids content (TFC) from FSI. The TFC yield was considered as the response to evaluate the efficiency of the extraction procedure. All experiments were performed in randomized order to avoid systematic errors. The results, including the coded variables and the experimental yields, are shown in
Table 3.
After conducting multiple regression fitting analysis on the experimental data, a second-order polynomial equation was used to represent the proposed model. The final TFC yield as a function of four independent variables is described by the following polynomial equation using actual factors. TFC Yield (mg RE/g DW) = 380.98 + 12.04A + 8.3B − 5.42C + 0.0858D − 4.6AB + 4.77AC + 1.47AD + 2.65BC − 1.7BD + 15.62CD – 13.1A
2 – 13.18B
2 − 27.51C
2 – 14.48D
2. The analysis of variance (ANOVA) was conducted to assess the significance of the model and individual terms (
Table 4). The significance of each coefficient was checked using the F-test and
p-values. As seen in
Table 4, the model was statistically significant with a high F-value of 39.97 and a low
p-value of less than 0.0001, indicating reliable for analyzing the experimental data [
36]. In addition, the non-significant p-value (0.0839) of lack-of-fit implied that the model was fitted with credible prediction. The coefficient of determination (R²) and adjusted determination (adjusted R²) were 0.9756, and 0.9512, respectively, the adequate precision was 20.6445 (> 4), demonstrating excellent predictive power and minimal deviation from the actual data [
37]. Among the factors, extraction temperature (A), sonication time (B), and water content in DES (C) exhibited significant linear and quadratic effects on TFC yield. The liquid-solid ratio (D) was not significant in the linear term but showed significance in the quadratic term (
p < 0.0001). Interaction terms AB and AC were also significant, indicating notable interactions between these factors affecting the response. The above results indicated that the model was statistically ideal, with sufficient accuracy and reliability for this work.
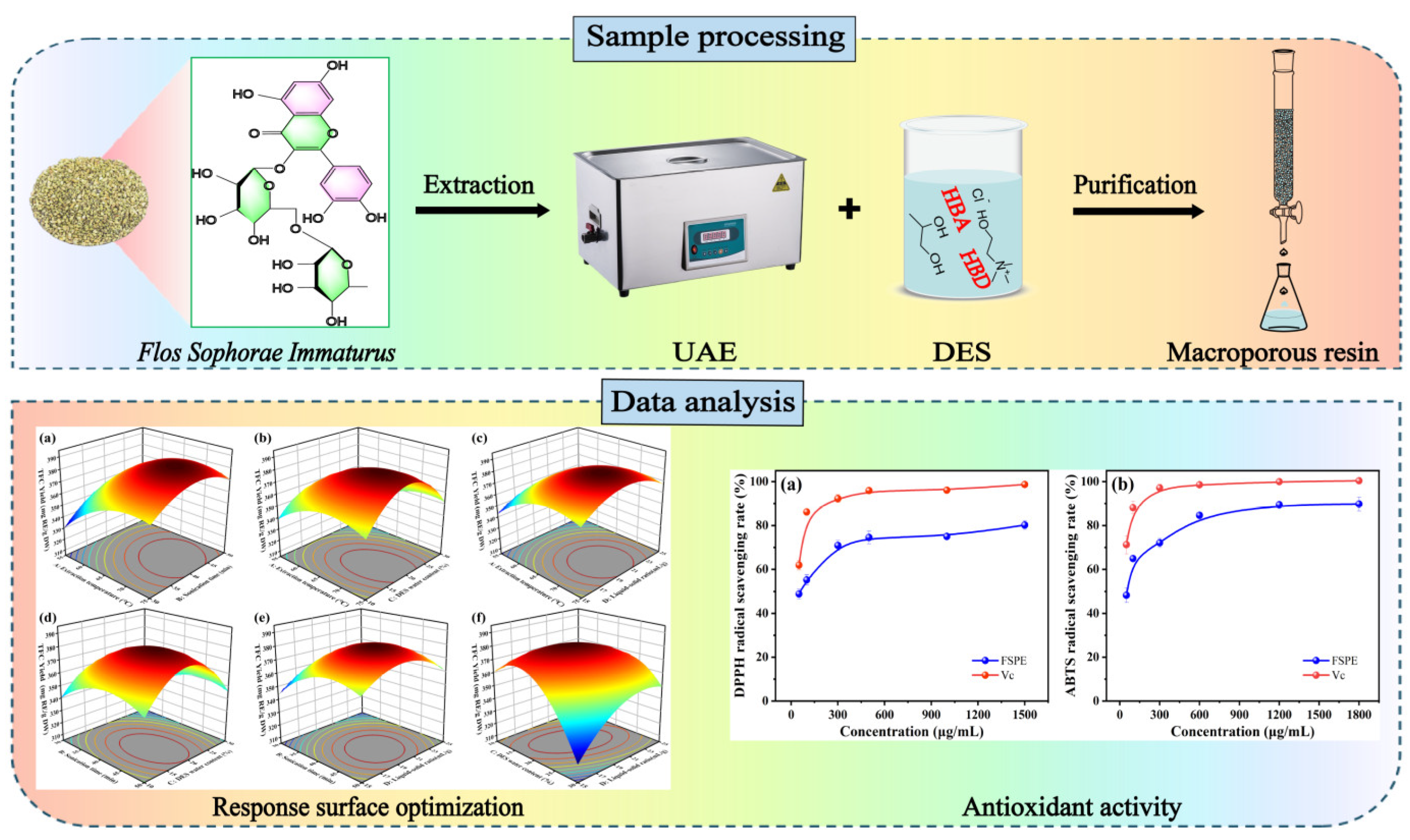
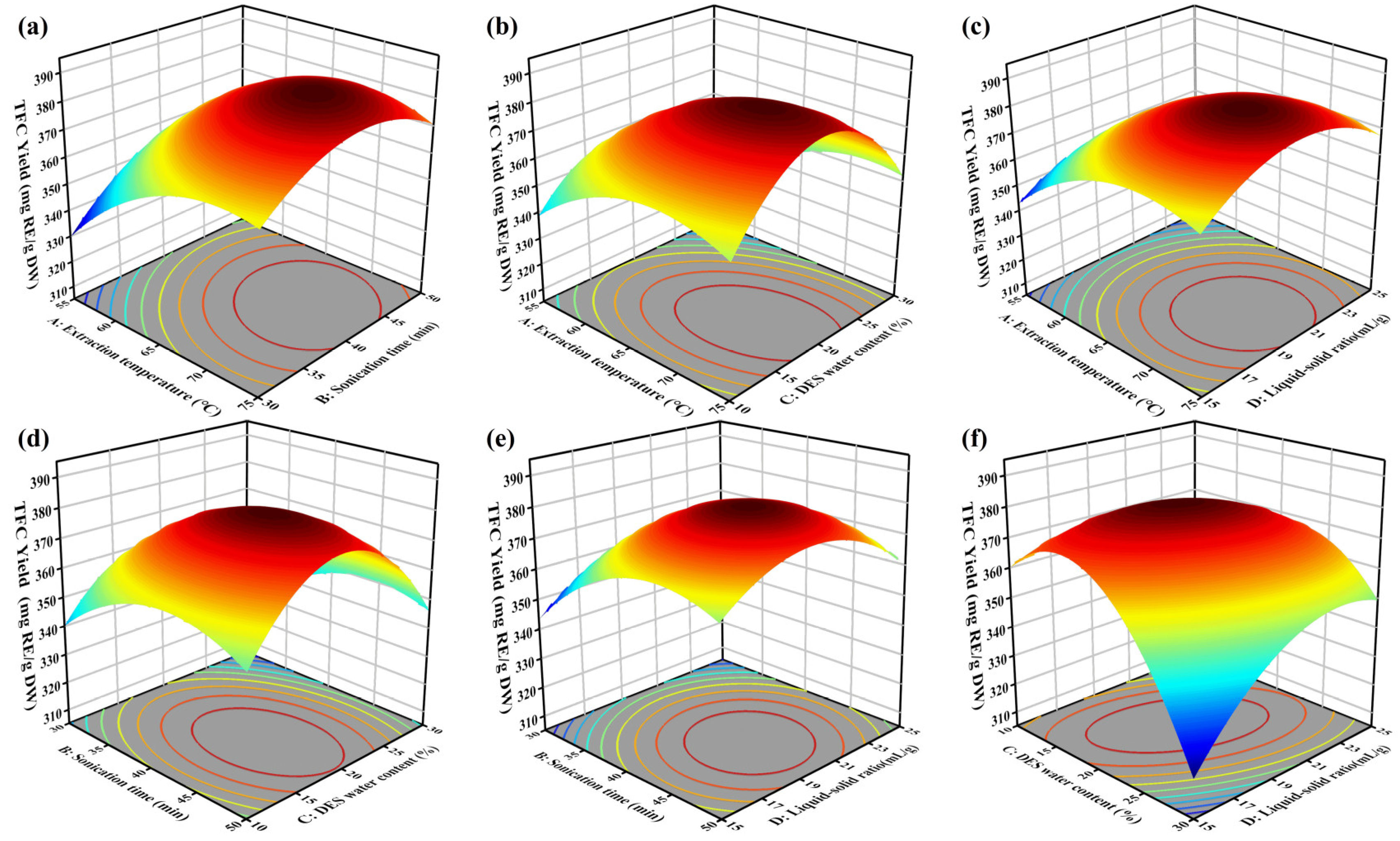
The 3D response surface plots from the multiple quadratic regression model were utilized to evaluate the effects of the different test factors on TFC yield. The interaction effects on TFC extraction were analyzed with other test factors held constant.
Figure 4 showed significant interactions between extraction temperature (A), sonication time (B), water content (C), and liquid-solid ratio (D) on TFC yield, which is consistent with the results of the single-factor experiments. Higher extraction temperature and longer sonication time synergistically increased TFC yield due to enhanced solubility and cell wall disruption. Optimal TFC yield was observed at high temperature with lower water content, as excessive water diluted the DES, reducing extraction efficiency. Similarly, a balanced liquid-solid ratio at elevated temperatures improved TFC yield by optimizing solvent interaction. Extended sonication time with lower water content or optimal liquid-solid ratio further enhanced yield by improving cell disruption and maintaining effective solvent properties. These findings provide a comprehensive understanding of the interactions influencing flavonoid extraction, offering valuable insights for process optimization.
According to the regression model, the highest TFC yield was observed at an extraction temperature of 68.42 °C, a sonication time of 41.42 min, a water content of 21.07%, and a liquid-solid ratio of 20.84 mL/g, with a predicted value of 383.50 mg/g. For convenient operation, the conditions were slightly adjusted (extraction temperature 68°C, sonication time 41 min, water content 21%, liquid-solid ratio 21 mL/g) to verify the predicted yield. Under these optimized conditions, the experimental result (378.77 mg/g) was close to the predicted value, confirming the accuracy and reliability of the fitted model.
The newly developed extraction method was compared with previously published methods. Peng et al. investigated the extraction of rutin from
Sophora japonica bud using a deep eutectic solvent. Under optimal conditions, the maximum yield of rutin was 279.8 mg/g with choline chloride/triethylene glycol (ChCl/TEG) as the extraction medium [
7]. Wang et al. employed deep eutectic solvent-based microwave-assisted extraction (DES-MAE) to obtain flavonoids from
Flos Sophorae Immaturus (FSI), achieving a rutin yield of 116.78 mg/g [
8]. They also invented a negative pressure cavitation-based ultrasound-assisted extraction (NPC-UAE) method, achieving a rutin yield of 125.17 mg/g under optimal conditions [
9]. Compared to the aforementioned methods, ultrasonic-assisted deep eutectic solvent extraction (UAE-DES) was used in this study to significantly increase the yield of flavonoids from FSI. The results showed that UAE-DES is a green and efficient extraction method, that provides a new technical basis for the development and application of
Flos Sophorae Immaturus.
3.4. Enrichment of TFC from Extraction Solution
According to the aforementioned optimal process conditions, a large-scale extraction of total flavonoids was performed. The crude extract was purified using macroporous D-101 resin, selected by preliminary experiments (data not shown). After freeze-drying, a light-yellow powder of flavonoids from FSI was obtained, with a measured purity of 83.11 ± 3.54%. The results proved that the D-101 macroporous resin could effectively enrich the TFC from the UAE-DES crude extract.
3.5. Antioxidant Activity of Purified FSI Flavonoid Extract (FSPE)
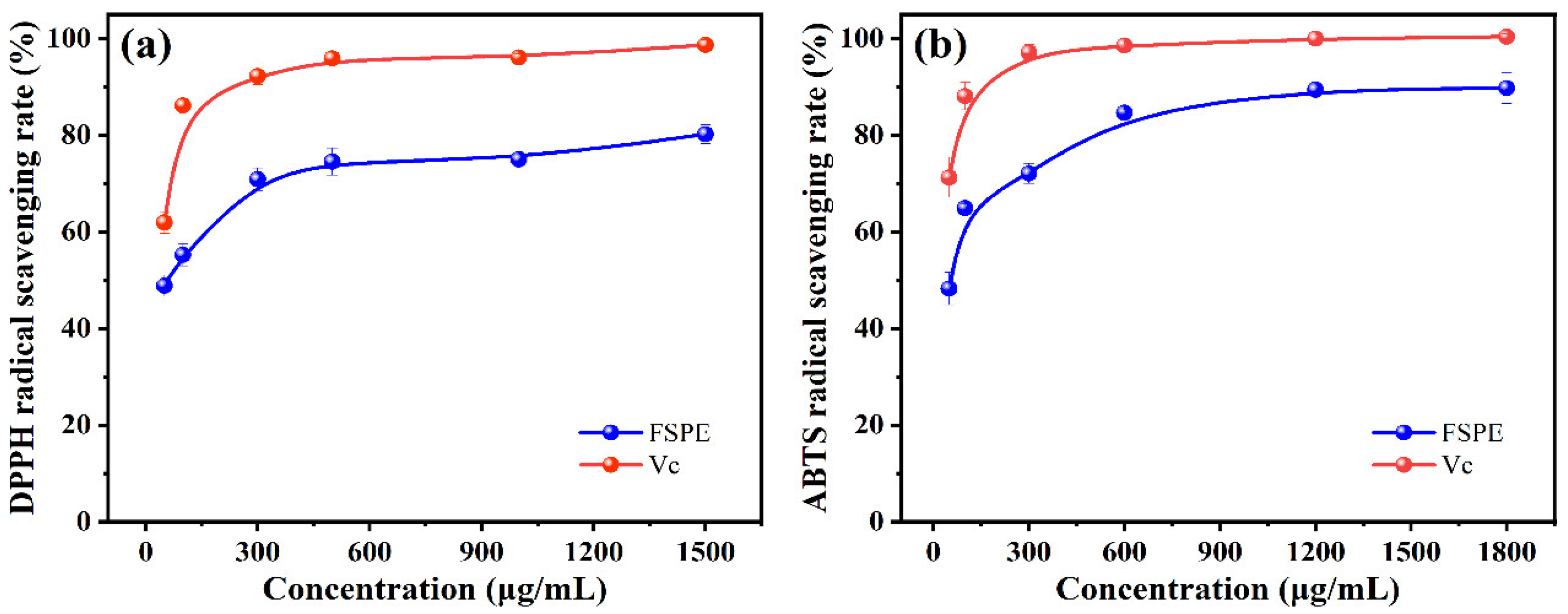
The DPPH radical scavenging assay has been widely used to evaluate antioxidants due to its high stability and excellent reproducibility. This study investigated the DPPH radical scavenging effect of purified FSI flavonoid extract (FSPE) at different concentrations. As shown in
Figure 5a, within the test concentration range, the FSPE scavenged DPPH radicals in a dose-dependent manner, similar to the positive control, ascorbic acid. At lower concentrations, FSPE demonstrated moderate scavenging activity, which substantially increased at higher concentrations, reaching a maximum scavenging rate of 80.22 ± 1.89% at 1500 µg/mL, with an IC
50 value of 52.50 μg/mL. The experimental results indicate that FSPE contains effective antioxidant components, likely due to its high flavonoid content. Flavonoids are well-known for their ability to donate hydrogen atoms or electrons to neutralize free radicals, thereby exhibiting strong antioxidant properties [
38]. This finding aligns with previous studies highlighting the antioxidant potential of flavonoid-rich extracts [
26].
As can be seen in
Figure 5b, the ABTS radical scavenging activity of FSPE also showed a concentration-dependent increase. At the lowest concentration tested, FSPE showed moderate activity, which increased significantly with higher concentrations, reaching a maximum scavenging rate of about 89.75% at 1800 µg/mL. The IC
50 value for FSPE in the ABTS assay was determined to be 49.47 µg/mL. The ABTS assay evaluates the ability of antioxidants to transfer electrons, reflecting their overall antioxidant capacity. The high scavenging rates of FSPE at high concentrations indicate the presence of several active components acting synergistically. The results of the antioxidant assays show that the purified extract of FSI (FSPE) has high antioxidant activity, indicating its potential as a candidate for the development of natural antioxidants in the food, pharmaceutical and dietary supplement industries.
4. Conclusions
The aim of this study was to develop an environmentally friendly and efficient ultrasound-assisted deep eutectic solvent (DES) extraction method for flavonoids from Flos Sophorae Immaturus. DES-4, which consists of choline chloride and 1,2-propanediol in a molar ratio of 1:2, was identified as the optimal extraction solvent. A four-factor, three-level response surface methodology was used to optimize the extraction process. This resulted in optimal conditions with an extraction temperature of 68 °C, a sonication time of 41 minutes, a water content in DES of 21%, and a liquid-solid ratio of 21 mL/g. Under these conditions, the extraction yield of flavonoids from Flos Sophorae Immaturus reached 378.77 mg RE/g DW, significantly higher than the results from previous studies. The crude extract was purified using microporous D-101 resin, achieving a flavonoid purity of 83.11 ± 3.54%. Antioxidant assays indicated that the purified product (FSPE) exhibited high antioxidant activity. The results of this study demonstrate that the UAE-DES (ChCl/PD) technique is an efficient method for extracting flavonoids from Flos Sophorae Immaturus, and the obtained FSPE has potential as a natural antioxidant candidate for applications in food, nutritional supplements, pharmaceuticals, and cosmetics.
Author Contributions
X.Y.: methodology, data curation, formal analysis, writing—original draft preparation; Z.Z.: software, visualization, formal analysis, writing—original draft preparation; Y.Z.: conceptualization, methodology, resources, supervision, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chengdu University of Traditional Chinese Medicine S&T Park College Students’ Innovation and Entrepreneurship Project, grant number KJYYB2315, KJYYB2316
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gong, Y.; Fan, L.; Wang, L.; Li, J. Flos Sophorae Immaturus: Phytochemistry, bioactivities, and its potential applications. Food Rev. Int. 2023, 39, 3185–3203. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume I, p. 370. [Google Scholar]
- Fan, S.; Yang, G.; Zhang, J.; Li, J.; Bai, B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules 2020, 25, 1767. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, C.; Zhang, Y.; Yang, D. Effects of Different Deproteinization Methods on the Antioxidant Activity of Polysaccharides from Flos Sophorae Immaturus Obtained by Ultrasonic Microwave Synergistic Extraction. Agronomy 2022, 12, 2740. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Li, J.; Wang, L.; Fan, L. In Vitro Inhibitory Effects of Polyphenols from Flos sophorae immaturus on α-Glucosidase: Action Mechanism, Isothermal Titration Calorimetry and Molecular Docking Analysis. Foods 2023, 12, 715. [Google Scholar] [CrossRef]
- Peng, F.; Xu, P.; Zhao, B.Y.; Zong, M.H.; Lou, W.A.-O. The application of deep eutectic solvent on the extraction and in vitro antioxidant activity of rutin from Sophora japonica bud. J. Food Sci. Technol. 2018, 55, 2326–2333. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.-J.; Zheng, X.; Gao, M.-Z.; Meng, Y.; Wang, W. Efficient extraction of flavonoids from Flos Sophorae Immaturus by tailored and sustainable deep eutectic solvent as green extraction media. J. Pharm. Biomed. Anal. 2019, 170, 285–294. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.-J.; Li, Y.; Gao, M.-Z.; Meng, Y.; Li, J.; Zhang, S.-D.; Wang, W. Negative pressure cavitation based ultrasound-assisted extraction of main flavonoids from Flos Sophorae Immaturus and evaluation of its extraction kinetics. Sep. Purif. Technol. 2020, 244, 115805. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Mgxadeni, N.; Kabane, B.; Bahadur, I.; Varma, R.S.; Singh, S.K. Deep eutectic solvents as sustainable solvents for industrial separation problems: A recent update. J. Ionic Liquids 2023, 3, 100065. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Tang, W.; Wu, Y.; Wang, M.; Row, K.H.; Qiu, H.; Zhou, J.-L. Emerging application of extraction phase of ionic and non-ionic deep eutectic solvents toward natural herbal medicine. TrAC Trends Anal. Chem. 2023, 165, 117137. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Foroutani, Z.; Afshar Mogaddam, M.R.; Ghasempour, Z.; Ghareaghajlou, N. Application of deep eutectic solvents in the extraction of anthocyanins: Stability, bioavailability, and antioxidant property. Trends Food Sci. Tech. 2024, 144, 104324. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef]
- Kaur, K.; Schmitt-Kopplin, P.; Malik, A.K. Green and efficient extraction of phenolic compounds from Neem leaves using deep eutectic solvents based ultrasonic-assisted extraction. Food Chem. 2024, 451, 139500. [Google Scholar] [CrossRef]
- He, Q.; Tang, G.; Hu, Y.; Liu, H.; Tang, H.; Zhou, Y.; Deng, X.; Peng, D.; Qian, Y.; Guo, W.; et al. Green and highly effective extraction of bioactive flavonoids from Fructus aurantii employing deep eutectic solvents-based ultrasonic-assisted extraction protocol. Ultrason. Sonochem. 2024, 102, 106761. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-assisted extraction of flavonoids from peanut leave and stem using deep eutectic solvents and its molecular mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Wang, R.; Geng, Z.; Jia, J.; Pang, S.; Du, Y.; Jia, S.; Cui, J. Ultrasonic assisted extraction of anthocyanins from rose flower petal in DES system and enzymatic acylation. LWT 2023, 180, 114693. [Google Scholar] [CrossRef]
- Yang, D.; Qiu, W.; Xu, Y.; Hu, Z.; Wang, L. Optimisation and modelling of ultrasonic-assisted extraction of canthaxanthin from Chromochloris zofingiensis using eutectic solvents. J. Clean. Prod. 2023, 202, 117002. [Google Scholar] [CrossRef]
- Trong Le, N.; Huyen Thi Chau, N.; Quynh Dinh Nguyen, P.; Thuy Thi Tran, L.; Thanh Phung, H.; Thi Nguyen, H. Green extraction of berberine from Coscinium fenestratum (Gaertn.) Colebr. using ultrasound-assisted aqueous solutions of organic acids, polyalcohols, and deep eutectic solvents. Sep. Purif. Technol. 2024, 330, 125541. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Xi, J.; Yan, L. Optimization of pressure-enhanced solid-liquid extraction of flavonoids from Flos Sophorae and evaluation of their antioxidant activity. Sep. Purif. Technol. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Wang, X.; Li, C.; Wang, J.; Liu, Z.; Shen, X.; Zhou, D. Response surface methodology-optimized extraction of flavonoids with antioxidant and antimicrobial activities from the exocarp of three genera of coconut and characterization by HPLC-IT-TOF-MS/MS. Food Chem. 2022, 391, 132966. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.-E.; Jang, H.W.; Lim, T.-G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Zhao, L.-D.; Wang, Y. Ultrasonic-assisted extraction of epimedin C from fresh leaves of Epimedium and extraction mechanism. Innovative Food Sci. Emerg. Technol. 2009, 10, 54–60. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Shen, H.; Fan, X.-H.; Shen, Y.; Wang, X.; Song, Y. Changes of gallic acid mediated by ultrasound in a model extraction solution. Ultrason. Sonochem. 2015, 22, 149–154. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of Food Processes by Ultrasound: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef]
- Aslan Türker, D.; Doğan, M. Application of deep eutectic solvents as a green and biodegradable media for extraction of anthocyanin from black carrots. LWT 2021, 138, 110775. [Google Scholar] [CrossRef]
- Liu, Y.-j.; Lou, L.; Huang, Q.; Xu, W.; Li, H. Ultrasonic extraction and purification of scutellarin from Erigerontis Herba using deep eutectic solvent. Ultrason. Sonochem. 2023, 99, 106560. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Hashim, R.; Mohamad Amini, M.H.; Danish, M.; Sulaiman, O. Optimization of activated carbon preparation from cassava stem using response surface methodology on surface area and yield. J. Clean. Prod. 2018, 198, 1422–1430. [Google Scholar] [CrossRef]
- Ismail, B.B.; Guo, M.; Pu, Y.; Wang, W.; Ye, X.; Liu, D. Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrason. Sonochem. 2019, 52, 257–267. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).