1. Introduction
In the complex interplay of factors influencing the dynamics of ungulate populations, factors such as intra-specific competition, environmental fluctuations, and inter-specific interactions (Hixon et al., 2002; Imperio et al., 2012) are of primary importance. Habitat selection is crucial for effective wildlife management and conservation, as it directly influences population dynamics. Further population dynamics is determined by available habitats so that habitat selection is critical for effective wildlife management and conservation (Goldspink et al., 2002; Pearce & Boyce, 2006; Mysterud et al., 2010; Feng et al., 2016). Arctic, alpine, and northern European ungulate populations are notably impacted by stochastic variations in climate (Aanes et al., 2000; Jacobson et al., 2004; Mysterud and Østbye, 2006).
In the cold arid climate of Trans-Himalayan landscapes, climate change is particularly significant, potentially leading to local population declines or extinctions (Rehnus et al., 2018; Lovari et al., 2020). Despite potential negative impacts of large sympatric herbivores on ungulate populations through inter-specific competition for food resources, evidence suggests the potential for non-competitive coexistence facilitated by differential resource use (Bell, 1971; Arsenault and Owen-Smith, 2002). Despite extensive research on ungulate ecology globally (Jarman, 1971; Jarman & Sinclair, 1979; Putman 1996; Arsenault and Owen-Smith 2002; Mysterud et al. 2007; Focardi et al., 2006, 2008; Imperio et al. 2012; Cooke et al. 2016; Rehnus et al., 2018; Lovari et al. 2020; Blum et al., 2023)), our understanding of these dynamics remains limited for Tibetan and Trans-Himalayan mountain ungulates, such as the Tibetan Antelope, locally known as Chiru, and other restricted-range endemic cold-adapted species, particularly under the challenging conditions of harsh cold arid climates.
The Changchenmo Valley in eastern Ladakh, India, harbors a unique assemblage of these ungulates, yet little is known about their population dynamics and habitat interactions in this environment. Previous studies (Schaller 1994, 1998; Fox et al. 1991) have reported significant variations in population structures and grouping sizes among Chiru and other species in the region, with Chiru displaying sexual segregation, with only males occurring in the study area of Changchenmo Valley, while females inhabit the extreme north-western parts of Tibet in India, specifically in Daulet Beg Oldi in Karakoram, and other species are observed in mixed family groups. However, the extent of interspecific competition and potential facilitation among these species remains unclear. The migratory behavior of the Chiru population moving seasonally from the Tibetan province of China to India also remains ambiguous (Fox et al., 1991; Schaller, 1991, 1998). Although, recent studies by Ahmad et al. (2016, 2017) have shed some light on this matter, reporting that approximately 15-20 Chiru individuals have established residency in the Chang Chenmo Valley during winters, unlike other populations in the Qinghai Tibetan Plateau, the extent to which this population engages in migratory patterns similar to those observed elsewhere remains uncertain (Schaller et al., 2000, 2006; Du et al., 2010).
This study aims to address a knowledge gap by investigating the population structure, spatial distribution, and habitat use of Chiru and other sympatric ungulates in the region. Our study offers essential insights and a robust scientific foundation for management and conservation. Additionally, our focus on species interactions in the Trans-Himalayan context enhances the novelty of our research, contributing valuable new perspectives to the broader field of global mountain ungulate ecology and coexistence.
2. Material and Methods
2.1. Study Area
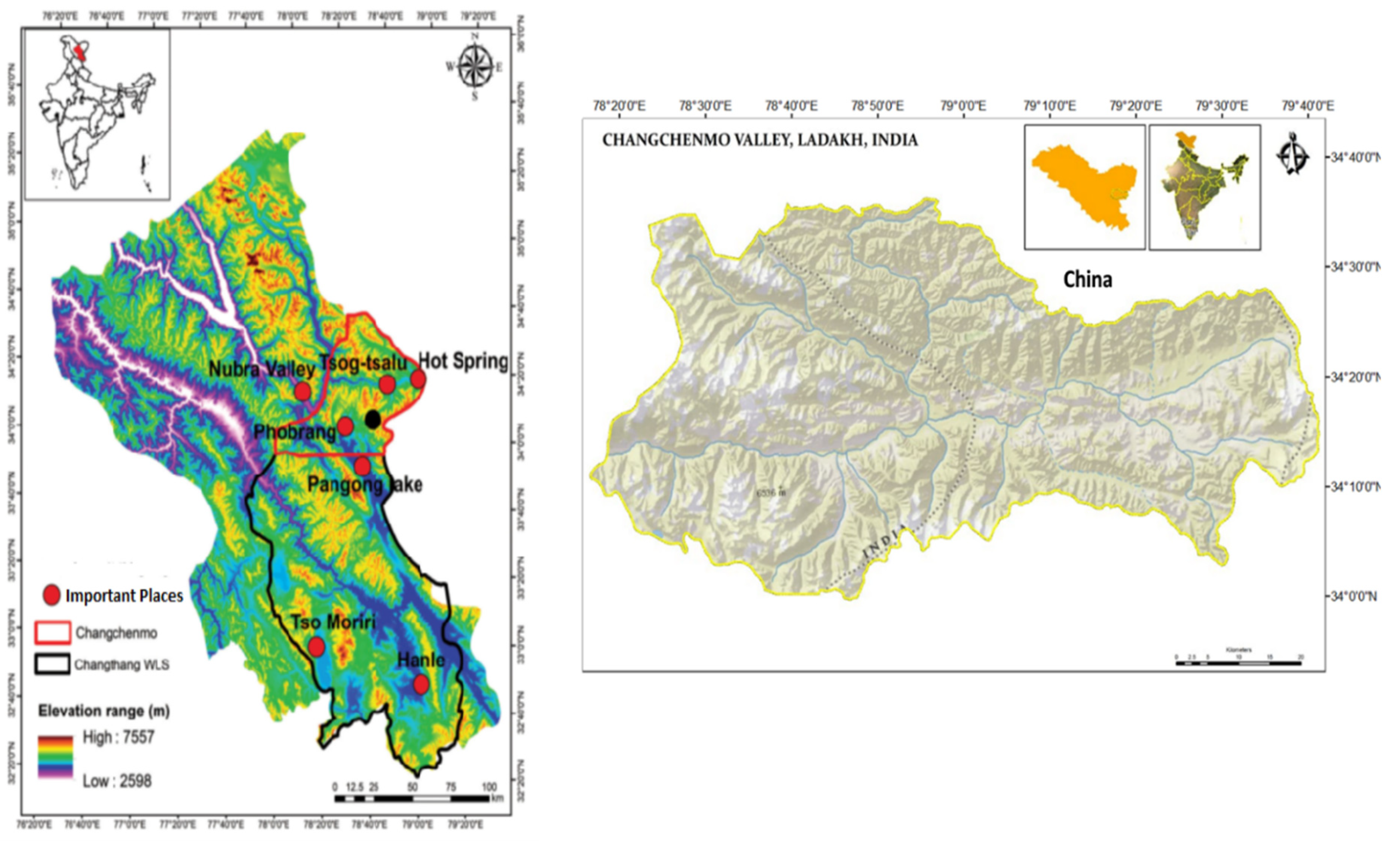
The study was conducted in the Chang Chenmo Valley (34.00° N—34.37° N and 78.10° E—79.00° E), an extension of the Changthang Cold Desert Wildlife Sanctuary located in eastern Ladakh, India, bordering China. Spanning in altitudes from 4450 to 5800 m asl, the area is positioned 150 km north and northeast of Pangong Tso Lake, with Phobrang, the last human-inhabited village, located 84 km away. Focused on the Tibetan Antelope, the study targeted the restricted distribution of this species in the area, conducting intensive field surveys within and around the Chang Chenmo valley (CCV). Efforts were also made to survey the entire landscape from Phobrang to the CCV, covering approximately 500 km
2. Administered by the Durbuk Block of Leh, the CCV lies within the expansive Changthang Wildlife Sanctuary, spanning over 3100 km
2 (Ahmad et al., 2016, 2017) (
Figure 1).
The CCV is drained by the Chang Chenmo and Kurgrang rivers, along with small tributaries like Silug Yogma, Silung Barma, and Silung Kongma. The topography varies from open rolling sandy mountains in the east to undulating terrain in the south (
Figure 1). The area is characterized by a cold arid environment with less than 10% vegetation cover, encompassing high alpine moist and mixed meadows, riverine scrub, desert steppe, and scrub steppe. While perpetual snow and glaciers cover about 12% of the area, moist and marsh meadows account for 1.6%, and over 84% is under sparsely vegetated desert steppe. Notably, the region experiences harsh climatic conditions, low primary productivity, and harbours a unique flora and fauna assemblage. The riverbanks of the Chang Chenmo sustain sedges, herbs, shrubs, and desert communities such as Stipa—
Oxytropis Alyssum and
Christolea crassifolia, reflecting the area’s ecological diversity and significance (Ahmad & Nigam, 2015; Ahmad et al., 2016, 2017).
Figure 1.
Map of the Study Area Chang Chenmo Valley, Ladakh, India.
Figure 1.
Map of the Study Area Chang Chenmo Valley, Ladakh, India.
2.2. Population Abundance and Distribution Monitoring:
In light of the challenging terrain of Tibetan landscapes, distance sampling surveys were deemed impractical (Harris 1993, 1996). We adopted the trail monitoring methodology, according to Rutledge (1982) and Shi et al. (2019), for assessing the population status, distribution and habitat use of Tibetan antelope (Chiru), Tibetan Wild Ass (Kiang), Wild Yak, Blue Sheep, and Tibetan Argali.
The study area was meticulously divided into a standardized network of nine trails, varying in length, altitude, aspect, habitat type, and level of human disturbances (
Table 1). Each trail was designed to encompass representative habitat types and ensure an even coverage of the study area. Observations were conducted systematically on a rotational basis, with each trail surveyed four times a month by foot in morning (5:00–12:00 hrs) and evening (15:00–18:00 hrs). Trails were carefully selected to traverse both open and rugged terrains, maximizing visibility and enabling the observation of ungulates across various habitats. However, due to logistical constraints posed by river swelling, trail no. 4 was surveyed twice a month using a vehicle. In total, we conducted 18 observation sessions on the nine trails (for a total of 162 trail walks), covering a distance of 918.60 km for 1208.85 hours of field effort.
To monitor ungulate populations and quantify habitat use, we employed direct animal sightings and “focal animal plots,” as recommended in the literature (Buckland et al. 2001; Feng et al. 2016). We performed observations in late May and June (spring), July to mid-September (summer), end of September to mid-November (autumn), and mid-November to December (winter). The study area was largely inaccessible during winter and early spring due to heavy snowfall. For each animal sighting, we recorded the number of animals observed, their activity patterns, group size, composition, and age whenever ascertainable. Habitat parameters were also meticulously documented, including altitude (using GPS), aspect (compass/GPS), slope (categorized as flat 0–16°, gentle 16–25°, steep 25–34°, very steep 34–50°), and GPS coordinates for latitude/longitude, following standard methodologies (Rikhari et al. 1989; Wilson et al. 1996). Habitat types were delineated based on predominant landscape features.
Recognizing that vegetation type is a crucial factor in determining habitat suitability for these ruminants (Feng et al. 2016), we recorded detailed vegetation parameters at each sighting location. This included the presence of trees, shrubs, and ground cover, categorized as grass, herb, litter, and exposed soil/rock. This comprehensive data collection approach provides a robust framework for assessing habitat use and the ecological dynamics of ungulate populations in the study area.
A scanning technique following Altman (1952) was also utilized to observe animal behaviour and population biology, including food habits and species interactions, from vantage points using binoculars and spotting scopes. Time was allocated proportionately across all habitats, primarily during the early mornings and, when feasible, in the evening hours (1-3 hrs), to ensure comprehensive coverage.
Spatial Overlap and Habitat Sharing between Species
To assess overlapping occurrences among species, a 36 square-kilometre core zone within the study area was selected, partitioned into one-square-kilometre grids.
Analysis
All statistical analyses were conducted utilizing SPSS® 16.0 software, adhering to the methodologies delineated by Norris (1990) and Carver (2005). Encounter rates were determined by calculating the kilometric abundance index (KIA), obtained by dividing the total number of observed animals by the length of transects/trails. Mean and typical group sizes were computed according to Jarman (1974). To discern differences in habitat utilization patterns based on direct sightings, we employed One-way analysis of variance (ANOVA) and the Chi-square goodness-of-fit test (Zar 1996). ANOVA was applied to all animal use data obtained from direct sightings. The behavioural patterns, food preferences, and feeding habits of Chiru and other ungulates were evaluated based on data collected during 55 (1-hour) scan observations, following the methodology established by Altmann (1951).
3. Results
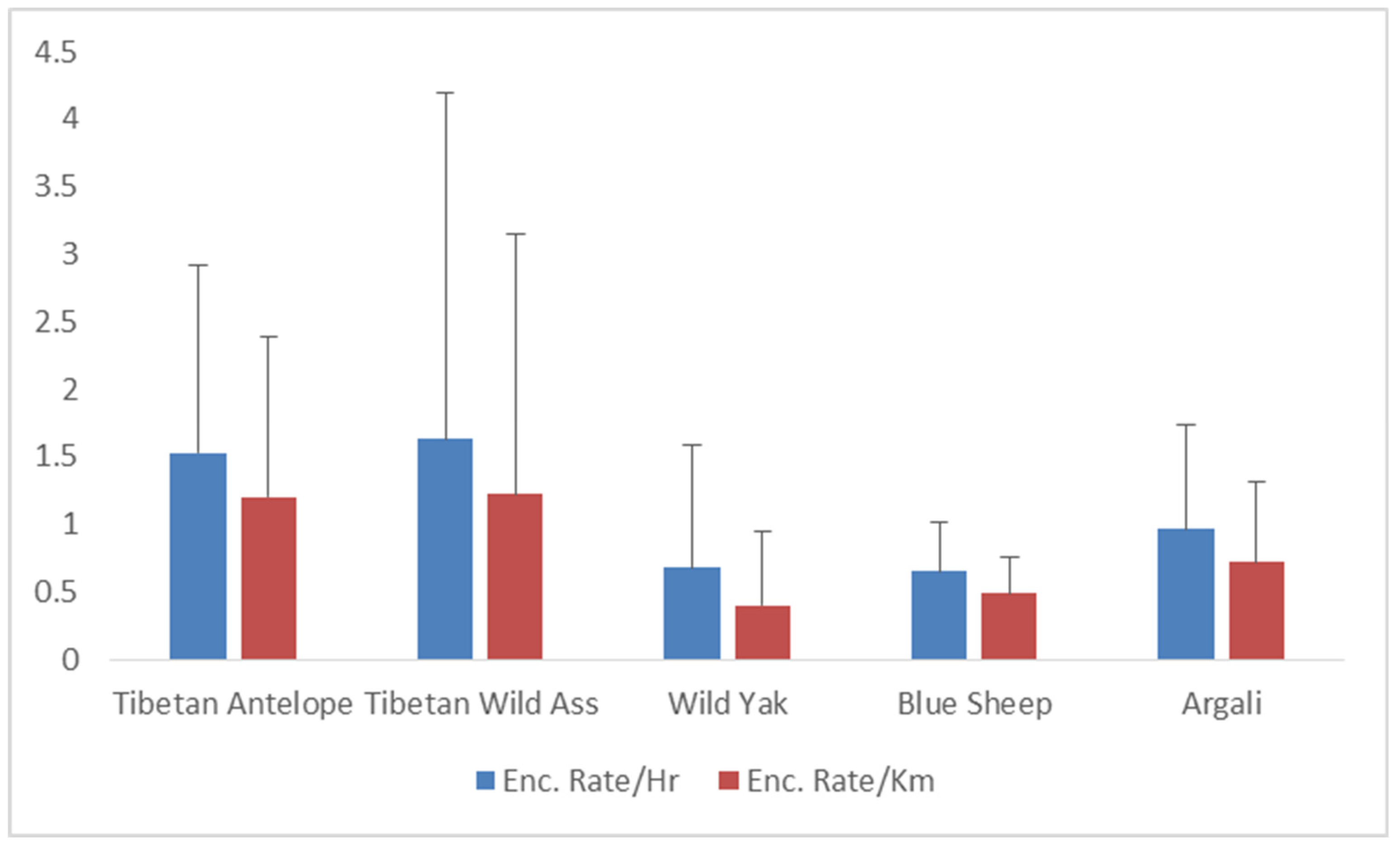
We recorded 113 group sightings for a total of 710 individuals of Tibetan Antelope or Chiru. We had 55 sightings and 561 individuals for the Tibetan Wild Ass, and 21 sightings and 240 individuals for the Tibetan Argali. A smaller number of sightings were recorded for Tibetan Wild Yak (19 sightings with 85 individuals) and Bharal (7 sightings with 74 individuals). Encounter rates varied significantly among the five ungulate species (F= 19.97, P<0.000; F= 17.95, P<0.000, respectively). Kiang exhibited the highest mean encounter rate (1.63 ± 2.57 S.D animals/hr and 1.22 ± 1.93 S.D animals/km), followed by Chiru (1.53 ± 1.37 S.D Chiru/hr and 1.19 ± 1.17 S.D Chiru/km) (
Table 2,
Figure 3 and
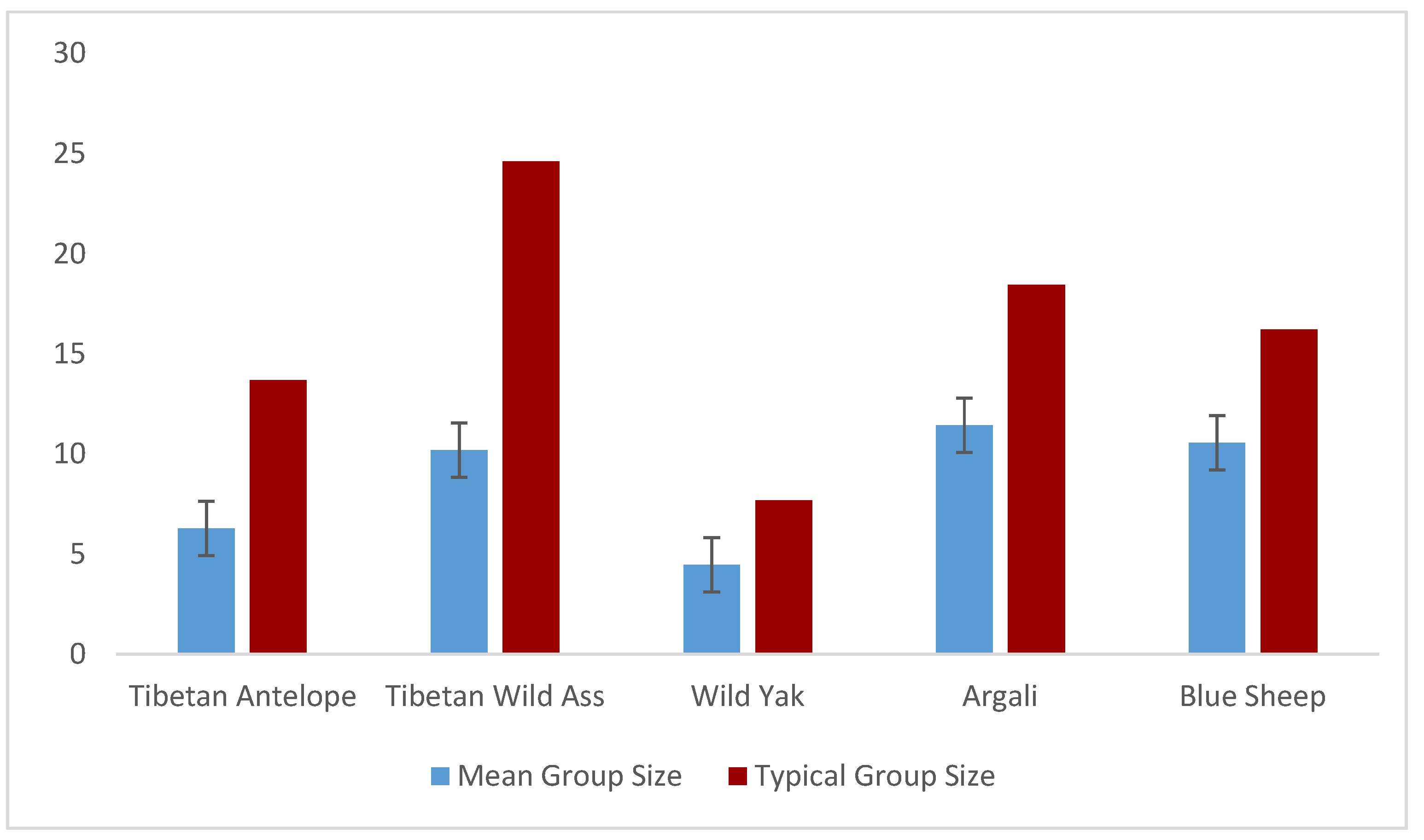
Figure 4). Chiru group sizes varied from solitary animals to 12 animals (1-12), with larger group sizes (23 and 19 individuals) recorded during two sightings. The mean group size of Chiru was 6.28 ± 6.82 S.D, with a typical group size of 13.69 (
Table 2;
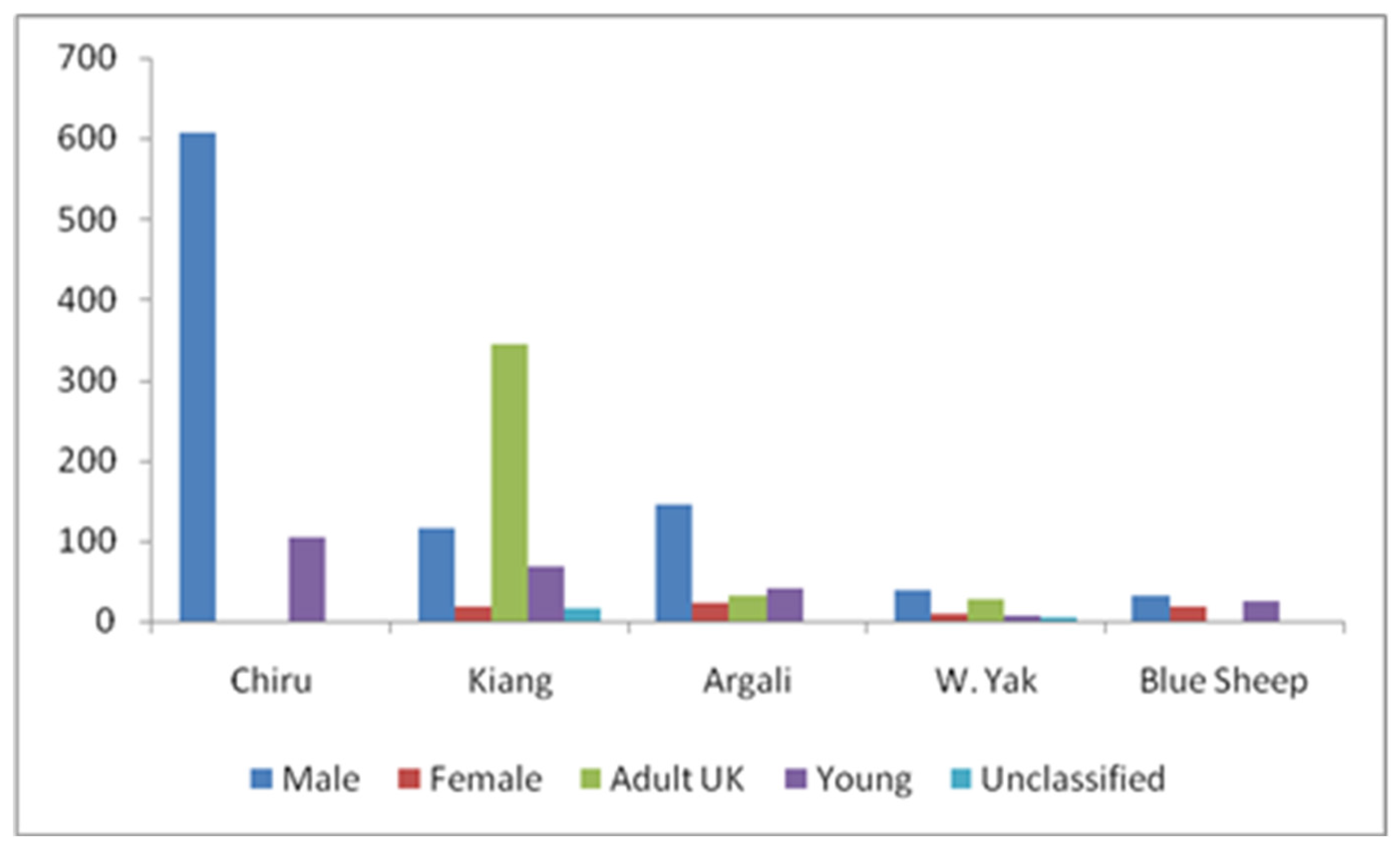
Figure 3). The Chiru population in the Changchenmo Valley comprised solely of males, totalling 710 individuals, including 606 adult males and 104 young males, while other ungulates were observed in mixed groups (
Figure 4).
Table 2.
Estimation of Group Sizes, and Encounter Rates (ER: n/hr. and n/km) of Chiru and other Ungulates in Changchinmo Valley (N= 215 animal sightings).
Table 2.
Estimation of Group Sizes, and Encounter Rates (ER: n/hr. and n/km) of Chiru and other Ungulates in Changchinmo Valley (N= 215 animal sightings).
| Parameters |
Tibetan Antelope |
Argali |
Tibetan Wild Ass |
Wild Yak |
Blue Sheep |
| No of Sightings |
113 |
21 |
55 |
19 |
07 |
| No of Individuals |
710 |
240 |
561 |
85 |
74 |
| Mean Group Size (Mean + S.D) |
6.28 ± 6.82 |
11.43±9.17 |
10.20 ±12.12 |
4.47 ± 3.89 |
10.57± 8.34 |
| Typical Group Size |
13.69 |
18.44 |
24.61 |
7.68 |
16.22 |
| Encounter Rate/Hr. Effort |
1.53 ± 1.37 |
0.96 ±0.78 |
1.63 ± 2.57 |
0.68 ± 0.91 |
0.65 ± 0.36 |
| Encounter Rate/Km. Walked |
1.19 ± 1.17 S.D |
0.72 ± 0.59 |
1.22 ± 1.93 |
0.40 ± 0.54 |
0.49 ± 0.27 |
Figure 2.
Mean Encounter Rates of Tibetan Antelope or Chiru and sympatric ungulates recorded in CCV (N= 1670 Individuals).
Figure 2.
Mean Encounter Rates of Tibetan Antelope or Chiru and sympatric ungulates recorded in CCV (N= 1670 Individuals).
Figure 3.
Mean (Mean ± S.D) & Typical Group Sizes of Chiru and sympatric ungulates in CCV (N= 1670 Individuals sighted).
Figure 3.
Mean (Mean ± S.D) & Typical Group Sizes of Chiru and sympatric ungulates in CCV (N= 1670 Individuals sighted).
Figure 4.
Population Structure of Chiru and sympatric Ungulates in CCV (N= 1670 Individuals).
Figure 4.
Population Structure of Chiru and sympatric Ungulates in CCV (N= 1670 Individuals).
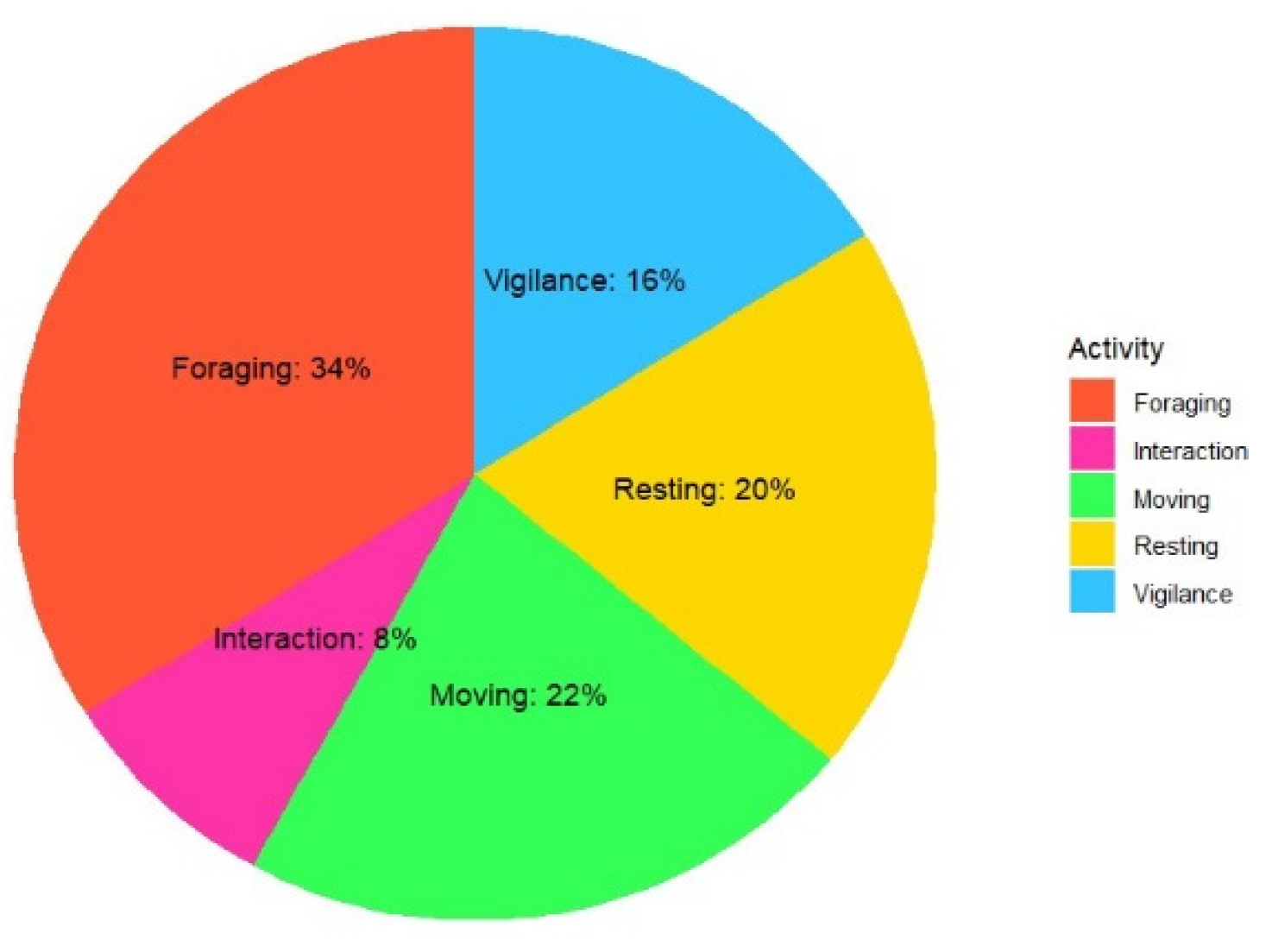
Distinct activity patterns of Chiru groups were revealed through 55 one-hour scan sampling observations exhibiting significant difference (
χ²=18 P=0.0012) among different observed activities. These patterns indicated that the animals primarily engaged in foraging (34% observations), resorting to moving towards cliffs, slopes, and gorges when disturbed, where they resumed feeding after sensing no danger. However, minimal playful or aggressive behaviour was observed (8% of observations) (
Figure 5).
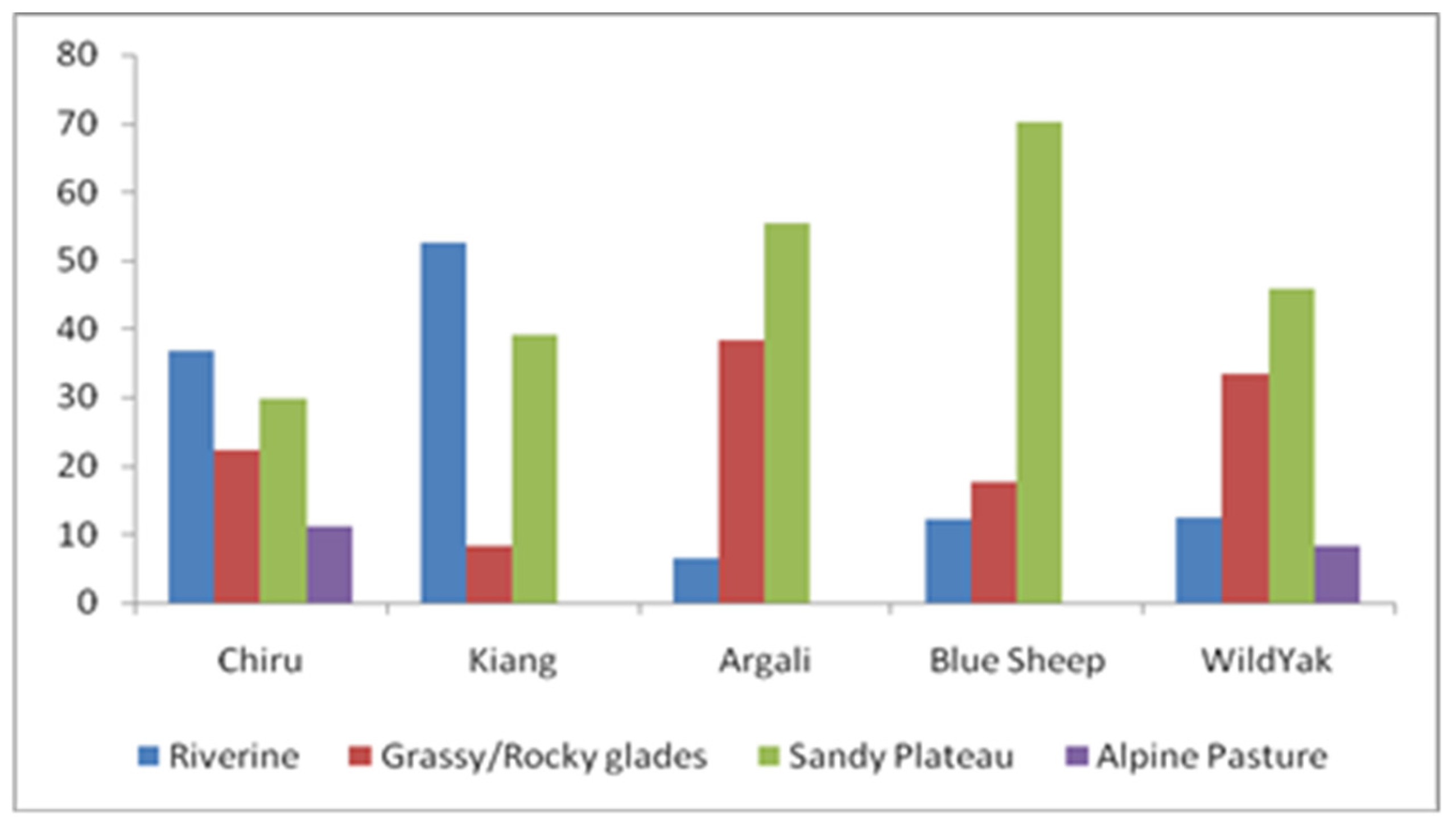
Habitat Use
Chiru sightings were recorded in all habitat types. The highest percentage (36.86%) of Chiru sightings occurred in the Riverine habitat, followed by 22.22% in the Sandy Plateau habitat. The analysis showed no significant differences (F= 0. 695 P ≤ 0. 563) among different habitat types based on direct sightings (
Figure 6). Chiru preferred areas or habitats with 20-40% vegetation cover (grasses/herbs), 10-30% stone cover, and 30-50% barren ground.
Spatial Overlap Between Species
A total of 241 animal sighting locations were recorded, providing robust data on species presence and distribution across the study area. Chiru and kiang were associated (χ² = 12.34, df = 3, p = 0.00123) as the blue sheep and the wild yak (χ² = 11.23, df = 3, p = 0.002) (
Table 3). However, chiru and blue sheep showed no significant association (χ² = 15.67, df = 4, p = 0.456).
Although the six shared grids between wild yak and kiang and the five shared grids between blue sheep and kiang suggested a possible association in specific areas, analysis indicated only moderate levels of co-occurrence (χ² = 9.87, df = 2, p = 0.009) and (χ² = 8.56, df = 2, p = 0.012) respectively.
Table 3.
Spatial Overlap of Chiru and other ungulates in the selected grids of the study area- CCV.
Table 3.
Spatial Overlap of Chiru and other ungulates in the selected grids of the study area- CCV.
| Species |
Summer presence grids |
Summer overlap |
Summer Overlapping grids |
Chi-Square Statistic |
Degrees of Freedom
|
p-value |
| Chiru |
21 |
Chiru-kiang |
12 |
12.34 |
3 |
0.00123 |
| Kiang |
17 |
Blue sheep-Kiang |
6 |
8.56 |
2 |
0.012 |
| Blue sheep |
16 |
Chiru- Blue sheep |
1 |
15.67 |
4 |
0.456 |
| Wild yak |
7 |
Chiru- Yak |
2 |
6.78 |
1 |
0.034 |
| Argali |
4 |
Blue sheep-yak |
4 |
11.23 |
3 |
0.002 |
| Total Grids |
36 |
Yak- Kiang |
5 |
9.87 |
2 |
0.009 |
| Blue sheep- Argali |
3 |
11.23 |
3 |
0.07 |
Discussion
Understanding the population dynamics, habitat requirements, and competitive interactions of large herbivores is crucial for effective wildlife management and conservation planning (Wydeven and Dahlgren, 1985; Gaillard et al., 1998). However, existing research on Tibetan Antelope (Chiru) and other ungulates has predominantly focused on specific regions in China (Schaller & Gu, 1994; Harris and Miller 1995; Miller & Schaller 1996, Schaller 1998), leaving significant knowledge gaps for landscape conservation planning in the Indian Changthang region (Fox et al., 1994, 2009; Schaller, 1998; Mallon, 2008; Feng et al., 2016). To address this, our study conducted an extensive investigation in the Changchenmo Valley (CCV), revealing a noteworthy increase in Chiru, Kiang, and other sympatric ungulate populations, including observed 710 individuals of Chiru in 113 sightings, which is substantially higher compared to previous reports (Anonymous, 2004; Ahmad et al., 2005; Sarkar et al., 2008).
Our findings validate earlier findings (Ahmad et al. 2016, 2017) unveiling significant disparities in population structures and group sizes among Chiru and other species within the Changchenmo Valley. Specifically, we observed sexual segregation in Chiru, where male Chiru predominantly utilized the study area, while females and fawns primarily occupied the adjacent DBO area throughout the majority of the year. In contrast, mixed family groups were noted among the other species present in the study area. The segregation of male and female in Tibetan Antelpe or Chiru is reported in most of the populations but causes for this sexual segregation are not yet well understood (Harris and Miller 1995; Schaller and Gu 1994, Schaller 1998). The group sizes observed in our study for Chiru are comparable to the similar group sizes recorded by other workers in Xinjiang and Kekexili Nature Reserve (KNR) of Qinghai for Chiru and Kiang (Lian et al., 2005; Schaller et al., 2007; J.Shi et al. 2019) although the mean group size of Wild Yak reported by Berger et al. (2014) is twice higher than in our study. The pattern of social organisation and behaviour observed for Chiru aligns with previous research in similar environments (Lian et al. 2005; Schaller et al. 2007; Buuveibaatar et al., 2013; Berger et al. 2014; Ahmad et al. 2015; Ahmad et al. 2017; J.Shi et al. 2019; Luo et al. 2023).
Our study has revealed a previously undocumented presence of a significant Chiru population, comprising 15-20 individuals, in the Chang Chenmo Valley (CCV) during the winter months. This finding challenges earlier reports suggesting that the entire Chiru population migrates to the Tibetan Plateau in China during winters (Fox et al., 1991; Schaller, 1998). Our extensive survey efforts in the Thratsang La area of the Changthang plateau during the late winter months of February 2014 did not result in any direct sightings or indirect evidence of Chiru presence in the area (Ahmad and Nigam 2015; Ahmad et al. 2017). This highlights the variability in Chiru distribution within the region.
Furthermore, our confirmation of Chiru sightings in the ALFA 3 area near CCV during summer, where 80 females with young ones were recorded near Galwan Nullah, underscores the significance of this region as a vital calving ground for Chiru. This highlights the critical role of the ALFA 3 area in supporting the reproductive success and long-term conservation of the Chiru population. The evidence emphasizes the urgent need for focused conservation efforts and habitat protection measures in these key areas to support the Chiru population year-round. These findings offer essential insights into the seasonal distribution of Chiru, informing more effective conservation strategies for this endangered species.
In terms of habitat utilization, similar to other studies (Qi et al. 2015; Feng et al. 2016; J.Shi et al. 2019), Chiru showed a preference for low-elevation areas with abundant vegetation, particularly riverine habitats offering protein-rich diets (Schaller and Gu, 1994; Miller, 1997). Despite sharing similar habitats with Kiang, competitive interactions seemed reduced due to differences in food habits as reported by Harris and Miller (1995). We found that Chiru is a mixed feeder but preferred graminoids compared to forbs. The Chiru fed mostly on sedges (Carex and Kobresia), forbs (Artemesia, Potentilla) and grasses (Poa) whereas Kiang was mainly observed feeding on Stipa.
Our observations of interspecific resource sharing and limited coexistence among multiple species echo findings from other high-altitude environments worldwide (Schaller, 1998; Bonacic et al., 2006). The coexistence of Chiru and Kiang in overlapping habitats within our study area, suggests that facilitation rather than interspecific competition, underscores the complex dynamics of species interactions in high-altitude environments. This observation aligns with findings from various ecosystems worldwide, where sympatric species often exhibit strategies to minimize competition and enhance mutual benefits. For instance, in the Tibetan Plateau, Schaller (1998) documented Wild Yak and Chiru sharing similar habitats during winter, indicating potential facilitative interactions. Similarly, studies in the Andes have revealed instances of sympatric ungulates, such as Vicuña and Guanaco, displaying cooperative behaviors to mitigate competition and promote coexistence (Bonacic et al., 2006).
In our study, the coexistence of Chiru and Kiang in overlapping habitats may be driven by complementary resource use or habitat partitioning, allowing both species to thrive in the same environment. This phenomenon has been observed in various mountainous regions globally, where sympatric ungulates exhibit niche differentiation to reduce competition for resources. For example, in North America, Bighorn Sheep (Ovis canadensis) and Mountain Goats have been documented to utilize different foraging strategies and elevational ranges to minimize resource competition (Bleich et al., 1990; Benavides et al., 2015). In the Himalayas, a study by Chundawat et al. (2001) reported interspecific competition between Blue Sheep and Himalayan Tahr in certain areas.
Our study also unveiled instances of Chiru and other sympatric ungulates sharing resources without significant niche overlap or interspecific competition, except during winter and spring when large mixed groups were observed feeding together. Particularly intriguing is the limited coexistence of all four species, documented in just three grids along the river during winters, suggesting critical ecological factors influencing species distributions and interactions. This overlap underscores the scarcity of habitats where their distributions intersect, highlighting the critical role of specific environmental conditions in either facilitating their coexistence or imperilling the survival of the remaining small Chiru population during harsh winter months. The degree of spatial overlap observed requires further investigation to understand the potential ecological dynamics between these species and to inform effective conservation strategies.
The observed aggregation of Wild Yak, Kiang, and Chiru during winters can also be attributed to various ecological factors, such as births (Byers, 1997; Shi et al., 2016), increased predation pressure (Creel et al., 2014), and anti-predatory strategies to cope with harsh winter conditions (Creel et al., 2014). Additionally, the availability and distribution of food plants in open plains surrounding riverine habitats at lower elevations, combined with severe winter weather, likely drive these aggregations.
As reported by other researchers (Schaller, 1998; Schaller and Liu, 2006; Li et al., 2013; J.Shi et al. 2019), our study suggests that the availability and distribution of food plants in open and flat plains around riverine habitats at lower elevations, together with harsh winter weather, have the potential to lead to the aggregation of wild yak, Kiang, and Chiru. These findings, consistent with patterns observed in other mountainous regions (Benavides et al., 2015), underscore the importance of considering ecological dynamics in landscape conservation planning.
Conclusions
This study significantly advances our understanding of the population dynamics, habitat use, and interspecies interactions of Chiru and other sympatric ungulates in the Changchenmo Valley (CCV) of eastern Ladakh. Our research reveals the presence of a stable Chiru population, including a novel finding of some individuals overwintering in the region—a behavior not previously documented. The confirmed sightings of Chiru in the ALFA 3 area near CCV during summer, where 80 females with young ones were recorded near Galwan Nullah, underscore the significance of this area as a critical calving ground, vital for the reproductive success and long-term conservation of the Chiru population.
Our study reveals a nuanced relationship between Chiru and Kiang, suggesting a complex interplay of ecological factors and adaptive behaviours influencing their coexistence. The limited overlap of ungulate species in winter habitats, with all four species coexisting in only three grids along the river, indicates critical ecological factors at play and highlights the potential for resource competition in these riverine habitats during harsh winter months. This scarcity of intersecting habitats underscores the importance of specific environmental conditions in either facilitating coexistence or threatening the survival of the Chiru population during winter.
Despite these valuable insights, our study faced limitations due to harsh weather conditions and accessibility challenges, particularly during winter months. These constraints hindered continuous monitoring and comprehensive data collection. To address these limitations and enhance year-round monitoring, further research employing advanced technologies such as extensive camera trapping, satellite telemetry, and genomic tools is recommended. These methods can provide consistent, long-term data on Chiru and other Tibetan ungulate populations, deepening our understanding of population dynamics, breeding patterns, and habitat preferences, particularly during winter months. Identifying physical barriers affecting animal movements and gene flow between Chiru populations within India and between India and China is crucial for developing appropriate management strategies.
In conclusion, our findings underscore the critical need for focused conservation efforts, habitat protection measures, and advanced monitoring techniques to support the Chiru and sympatric Tibetan ungulate populations in the Trans-Himalayan region. By understanding the complex ecological dynamics and seasonal distributions, we can develop more effective and informed conservation strategies to ensure the survival and thriving of these endangered species in their challenging habitats. Our findings provide a robust scientific foundation for conservation planning and highlight the need for continuous monitoring, particularly in response to threats from infrastructure development, climate change, and human activities. Special management efforts, including community engagement and policy interventions, are essential to preserve the unique biodiversity of the Tibetan Plateau.
References
- Ahmad, K.; Nigam, P.; Raza, M.; Khan, A.A. Sighting of Black Form of Tibetan Wolf Canis lupus chanco in Changthang Wildlife Sanctuary, Ladakh, India. J. Bombay Nat. Hist. Soc. 2021, 118. [Google Scholar] [CrossRef]
- Ahmad, K R. Ahmed, M.; Nigam, P.; Thapa, J. Analysis of temporal population trend and conservation of Tibetan Antelope in Chang Chenmo Valley and Daulat beg Oldi, Changthang, Ladakh, India. Antelope Specialist Group Newsletter. GNUSLETTER 2017, 34, 16–20. [Google Scholar]
- Ahmad, K.; Kumar, V.P.; Joshi, B.D.; Raza, M.; Nigam, P.; Khan, A.A.; Goyal, S.P. Genetic diversity of the Tibetan antelope (Pant.holops hodgsonii) population of Ladakh, India, its relationship with other populations and conservation implications. BMC Res. Notes 2016, 9, 477. [Google Scholar] [CrossRef]
- Ahmad, K and Nigam, P. (2015). Ecological and Socio-Economic Study on the Tibetan Antelope or Chiru (Pantholops hodgsonii) in Chang Chenmo Wildlife Sanctuary, Leh, Ladakh. Project Completion Report. Submitted to Department of Science and Technology, Govt. of India, Sher-e-Kashmir University of Agricultural Sciences & Technology of Kashmir, Shuhama, Alusteng,Srinagar, J&K. pp 59.
- Ahmad, K. 2006. Aspects of Ecology of Hangul (Cervus elaphus hanglu) in DachigamNational Park, Kashmir, India. Ph.D. thesis. Forest Research Institute (Deemed University), DehraDun, Uttarakhand, India. 220 pp.
- Ahmad, K.; S Sathyakumar and Qamar Qureshi. Conservation status of the last surviving wild population of Hangul or Kashmir Red Deer Cervus elaphus hanglu in Kashmir, India. Journal of the Bombay Natural History Society 2009, 106, 245–255.
- Ahmad, K.Q. Qureshi, G. Agoramoorthy and P. Nigam (2015): Habitat use patterns and food habits of the Kashmir red deer or Hangul (Cervus elaphus hanglu) in Dachigam National Park, Kashmir, India. Ethology Ecology & Evolution. [CrossRef]
- Altmann. Social behaviour of Elk (Cervus Canadensis nelsoni) in the Jackson Hole area of Wyoming. Behaviour. 1952, 4, 116–143. [Google Scholar]
- Arau’ jo, M.B.; PH Williams, and R. J. Fuller. J. Fuller. Dynamics of extinction and the selection of nature reserves. Proceedings of the Royal Society B: Biological Sciences 2002, 269, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.E.; Thomas, C.J.; Houston, D.C.; Thompson, D.B.A. Predicting the spatial distribution of buzzard Buteo buteo nesting areas using a geographical information system and remote sensing. Journal of Applied Ecology 1996, 33, 1541–1550. [Google Scholar] [CrossRef]
- Barja, I.; S, Rosellini. Does habitat type modify group size in roe deer and red deer under predation risk by Iberian wolves? Canadian Journal of Zoology 2008, 86, 170–176. [Google Scholar] [CrossRef]
- Berger, J.; Cheng, E.; Kang, A.; Krebs, M.; Li, L.S.; Lu, Z.X.; Schaller, G.B. Sex differences in ecology of wild yaks at high elevation in the Kekexili Reserve, Tibetan Qinghai Plateau, China. J. Mammal. 2014, 95, 638–645. [Google Scholar] [CrossRef]
- Blank, D.; Yang, W.; Xia, C.; Xu, W. Grouping pattern of the goitered gazelle, Gazella subgutturosa (Cetartiodactyla: bovidae) in Kazakhstan. Mammal 2012, 76, 149–e155. [Google Scholar] [CrossRef]
- Blank, D.; Ruckstuhl, K.; Yang, W.K. Influence of population density on group sizes in goitered gazelle (Gazella subgutturosa Guld 1780). Eur, J. Wildl. Res. 2012, 58, 981–989. [Google Scholar] [CrossRef]
- Bleisch, W.V.; Buzzard, P.J.; Zhang, H.B.; Xü, D.H.; Liu, Z.H.; Li, W.D.; Wong, H.M. Surveys at a Tibetan antelope Pantholops hodgsonii calving ground adjacent to the Arjinshan Nature Reserve, Xinjiang, China: increase and recovery of a population. Oryx 2009, 43, 191–196. [Google Scholar] [CrossRef]
- Bleisch, W.V, Xu, D. & Zhang, H. (2004) A cost-effective approach to monitoring and census of wild Tibetan antelope (Pantholops hodgsonii). In Proceedings of the XIXth International Congress of Zoology (eds China Zoological Society), pp. 263–264. China Zoological Society, Beijing, China.
- Blum, M.E.; Stewart, K.M.; Shoemaker, K.T. ’ et al. Changes in selection of resources with reproductive state in a montane ungulate. Mov. Ecol. 2023, 11, 20. [Google Scholar] [CrossRef]
- Bon, R.; Gonzalez, G.; Im, S.; Badia, J. Seasonal grouping in female Moufflons in relation to food availability. Ethol 1990, 86, 224–236. [Google Scholar] [CrossRef]
- Bowers, M.A.; Matter, S.F. Landscape ecology of mammals: relationships between density and patch size. Journal of Mammalogy 1997, 78, 99–1013. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. 2001. Introduction to Distance Sampling: Estimating Abundance of Biological Populations. Oxford: Oxford University Press.
- Buuveibaatar, B.; Fuller, T.K.; Fine, A.E.; Chimeddorj, B.; Young, J.K.; Berger, J. Changes in grouping patterns of Saiga antelope in relation to intrinsic environmental factors in Mongolia. J. Zool. (London) 2013, 291, 51–58. [Google Scholar] [CrossRef]
- Buzzard, P.J.; Zhang, H.B.; Xü, H.D.; Wong, H.M. . A globally important wild yak Bos mutus population in the Arjinshan Nature Reserve, Xinjiang, China. Oryx 2010, 44, 577–580. [Google Scholar] [CrossRef]
- Buzzard, P.J.; Wong, H.M.; Zhang, H.B. Population increase at a calving ground of the endangered Tibetan antelope Pantholops hodgsonii in Xinjiang, China. Oryx 2012, 46, 266–268. [Google Scholar] [CrossRef]
- Buzzard, P.J.; Xu, D.H.; Li, H. Sexual/aggressive behavior of wild yak (Bos mutus Prejevalsky 1883) during the rut: influence of female choice. Chin. Sci. Bull. 2014, 59, 2756–2763. [Google Scholar] [CrossRef]
- Byers, J.A. American Pronghorn: Social Adaptations and the Ghosts of Predators Past. University of Chicago Press, Chicago, Illinois, 1997.
- Cairns, A.L.; Telfer, E. Habitat use by 4 sympatric ungulates in boreal mixed wood forest. The Journal of Wildlife Management 1980, 44, 849–857. [Google Scholar] [CrossRef]
- Chundawat, R.S. (1992). Ecological studies on Snow leopard and its associated species in Hemis National Park, Ladakh. PhD Thesis, University of Rajasthan, 166pp.
- Chundawat, R.S. Q Qureshi. 1999. Planning Wildlife Conservation in Leh and Kargil Districts of Ladakh. Wildlife Institute of India, Dehradun, India.
- Cooke, Robert S. C., Tim Woodfine, Marie Petretto & Thomas H. G. Ezard Resource partitioning between ungulate populations in arid environments. Ecology and Evolution 2016, 6, 6354–6365. [Google Scholar] [CrossRef]
- Creel, S.; Schuette, P.; Christianson, D. . Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behav. Ecol. 2014, 25, 773–784. [Google Scholar] [CrossRef]
- Dorji, T. (2006) A test of rangeland dynamic theories using grazing gradients in the Aru Basin, north-western Chang Tang, Tibet, China. MSc thesis, University of Tromsø, Tromsø, Norway.
- Dunzhu, G. (2007) Ecological correlates of livestock and antelope winter rangeland use in the Chang Tang Nature Reserve, Tibet Autonomous Region, China. MSc thesis, University of Tromsø, Tromsø, Norway.
- Dorji, T.; Fox, J.L.; Richard, C.; Dhondup, K. An assessment of non equilibrium dynamics in rangelands of the Aru Basin, northwest Tibet, China. Rangeland Ecol. Manage. 2010, 63, 426–434. [Google Scholar] [CrossRef]
- Feng, F.; Z Yang, J.R. OWENS, R. Hou, Z. Zhang and and Dunwu QI An assessment of endangered species habitat at large scale: chiru distribution across the Tibetan region of Chang Tang. Folia Zool.— 2016, 65, 65–71. [Google Scholar] [CrossRef]
- Fox, J.; Dhondup, K.; Dorji, T. Tibetan antelope Pantholops hodgsonii conservation and new rangeland management policies in the western Chang Tang Nature Reserve Tibet: is fencing creating an impasse? Oryx 2009, 43, 183–190. [Google Scholar] [CrossRef]
- Fox, J.L.; Yangzong, C.; Dondhup, K.; Dorji, T.; Richard, C. Biodiversity conservation and pastoralism on the northwest Tibetan Plateau (Byang thang): coexistence or conflict? J. Intl. Tibetan Assoc. Stud. 2008, 4, 1–21. [Google Scholar]
- Fox, J. L.; Bardsen, B.J. Density of Tibetan antelope, Tibetan wild ass and Tibetan gazelle in relation to human presence across the Chang Tang Nature Reserve of Tibet, China. Acta Zool. Sin. 2005, 51, 586–597. (in Chinese). [Google Scholar]
- Fox, J.L.; Yangzom, D. (2005) A Research Program on Wildlife, Nomads and Conservation in the Chang Tang Nature Reserve. Unpublished Report. University of Tromsø, Tromsø, Norway.
- Fox, J.L.; P Mathiesen, D. Yangzom, M. W. Næss, and B. R. Xu. 2004. Modern wildlife conservation initiatives and the pastoralist/hunter nomads of north western Tibet. Rangifer Special 17–27.
- Fox, J.L.; Nurbu, C.; Chundawat, R.S. The mountain ungulates of Ladakh, India. Biological Conservation 1991, 58, 167–190. [Google Scholar] [CrossRef]
- Gaillard, J.M.; Festa-Bianchet, M.; Yoccoz, N.G. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends in Ecology and Evolution 1998, 13, 58–63. [Google Scholar] [CrossRef]
- Goldspink, C.R.; Holland, R.K.; Sweet, G.; Stewart, L. A note on group sizes of oribi (Ourebia ourebi, Zimmermann, 1783) from two contrasting sites in Zambia, with and without predation. African Journal of Ecology 2002, 40, 372–378. [Google Scholar] [CrossRef]
- Green M J B 1985. Aspects of the ecology of the Himalayan musk deer. Ph. D dissertation, Univ. Cambridge, U. K.; 280p,.
- Green M J B 1987. Some ecological aspects of a Himalayan population of musk deer. In Biology and Management of the Cervidae (Wemmer C M, ed)’ 307-319.
- Härkönen, S.; Heikkilä, R. Use of pellet group counts in determining density and habitat use of moose alces alces in finland. Wildlife Biology 1999, 5, 233–239. [Google Scholar] [CrossRef]
- Harris, R.B. ; 1993. Wildlife Management in Yeniugou Qinghai, China. Unpubl. Ph.D. diss., Univ. Montana. 326 pp.
- Harris, R.B.; Miller, D.J. Overlap in summer habitats and diets of Tibetan Plateau ungulates. Mammalia 1995, 59, 197–212. [Google Scholar] [CrossRef]
- Harris, R.B. Wild ungulate surveys in grassland habitats: satisfying methodological assumptions. Chin, J. Zool. 1996, 31, 16–21. [Google Scholar]
- Harris, R.B.; Loggers, C.O. Status of Tibetan plateau mammals in Yeniugou, China. Wildl. Biol. 2004, 10, 91–99. [Google Scholar] [CrossRef]
- Harris, R.B.; Leslie, D. ; 2008. Bos mutus. IUCN Red List of Threatened Species. www.iucnredlist.org. (Accessed 21 March 2015).
- Hilker, T.; Coops, N.C.; Wulder, M.A.; Black, T.A.; Guy, R.D. The use of remote sensing in light use efficiency based models of gross primary production: a review of current status and future requirements. Sci. Total Environ. 2008, 404, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.; Didan, K.; Miura, T.; Rodrigueza, E.P.; Gao, X.; Ferreirab, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- J. Shi, Xiaowen Li, Feiying Lu, Haijin Zhuge, Shikui Dong.Variation in group sizes of sympatric Wild yak, Tibetan wild ass and Tibetan antelope in Arjin Shan National Nature Reserve of Xinjiang Province, China. Global Ecology and Conservation 2019, 20, e00749. [CrossRef]
- Jarman, P.J. The social organisation of antelope in relation to their ecology. Beyond Behav. 1974, 48, 215–267. [Google Scholar] [CrossRef]
- Leslie, D.M.; Schaller, G.B. Pantholops hodgsonii (artiodactyla: bovidae). Mamm. Species 2008, 817, 1–13. [Google Scholar] [CrossRef]
- Li, Z.C.; Yang, Q.S.; Zhang, H.B.; Liu, Z.H.; Yuan, G. Population number and distribution of big mammals in eastern Arjin mountain nature reserve, Xinjiang. Sichuan J. Zool. 2006, 25, 92–95. (in Chinese). [Google Scholar]
- Li, W.D.; Zhang, H.B.; Zhang, X.; Xu, D.H. ; 2013. Comprehensive Exploration of Arjinshan National Nature Reserve. Xinjiang People’s Press House.
- Lian, X.M.; Su, J.P.; Zhang, T.Z.; Cao, Y.F. The characteristics of social groups of the Tibetan antelope (Pantholops hodgsoni) in the Kekexili Region. Acta Ecol. Sin. 2005, 25, 1341–1350. (in Chinese). [Google Scholar]
- Lovari, Sandro, Sara Franceschi, Gianpasquale Chiatante, Lorenzo Fattorini, Niccolò Fattorini & Francesco Ferretti (2020) Climatic changes and the fate of mountain herbivores. Climatic Change. [CrossRef]
- Lu, F.Y.; Shi, J.B.; Zhang, Z.H.; Dong, S.K.; Li, X.W. Surveys of wild yak, Tibetan antelope and Tibetan wild ass populations in arjinshan nature reserve, Xinjiang Province. J. Beijing Normal Univ. (Nat. Sci.) 2015, 54, 1132–1139. (in Chinese). [Google Scholar]
- Luo, Y.; Wang, L.; Yang, L.; Li, X.F.; Anselme, P.; Wang, X.; Tian, X.; Li, Z. . Using a behavior random permutation model to identify displacement grooming in ungulates. Current Zoology 2023, 69, 200–207. [Google Scholar] [CrossRef]
- Mardan, T.; Ma, M.; Zhang, X.; Zhang, T.; Chen, Y. Current population and conservation status of the Tibetan wild ass (Equus kiang) in the Arjin mountain nature reserve, China. Pak. J. Zool. 2013, 45, 1249–1255. [Google Scholar]
- Mallon, D.P. (2008). Pantholops hodgsonii. The IUCN Red List of Threatened Species 2008:e.T15967A5335049. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T15967A5335049.en. [CrossRef]
- Meinertzhagen, R. Ladakh, with Special Reference to Its Natural History. The Geographical Journal 1927, 70, 129–156. [Google Scholar] [CrossRef]
- Miller, D.J.; Schaller, G.B. Conservation threats to the Chang Tang Wildlife Reserve, Tibet. AMBIO 1997, 26, 185–186. [Google Scholar]
- Mueller-Domboise, D.; Ellenberg, H. 1974. Aims and methods of vegetation ecology. John Wiley and Sons. New York. 547 pp.
- Mysterud, A.; Aaserud, R.; Ove, L.; A kra, K.; Olberg, S.; Austrheim, G. Large herbivore grazing and invertebrates in an alpine ecosystem. Basic and Applied Ecology 2010, 11, 320–328. [Google Scholar] [CrossRef]
- Neff, D.J. The pellet-group count technique for big game trend, census, and distribution: a review. The Journal of Wildlife Management 1968, 32, 597–614. [Google Scholar] [CrossRef]
- Pearce, J.L.; Boyce, M.S. Modelling distribution and abundance with presence-only data. J. Appl. Ecol. 2006, 43, 405–412. [Google Scholar] [CrossRef]
- Olson, K.A.; Mueller, T.; Bolortsetseg, S.; Le Imeruber, P.; Fagan, W.F.; Fuller, T.K. A mega-herd of more than 200,000 Mongolian gazelles Procapra gutturosa: a consequence of habitat quality. Oryx 2009, 43, 149–153. [Google Scholar] [CrossRef]
- Pearce, J.L.; Boyce, M.S. Modelling distribution and abundance with presence-only data. J. Appl. Ecol. 2006, 43, 405–412. [Google Scholar] [CrossRef]
- QI Guilan, Y. Hu, R.O. Jacob, Q. Dai, R. Hou, Z. Yang and D. QI. Habitat Suitability for Chiru (Pantholops hodgsonii): Implications for Conservation Management across the Tibetan Region of Chang Tang. The Journal of Wildlife Management 2015, 79, 384–392. [Google Scholar] [CrossRef]
- Rehnus, M.; Bollmann, K.; Schmatz, D.R.; Hackländer, K.; Braunisch, V. Alpine glacial relict species losing out to climate change: the case of the fragmented mountain hare population (Lepus timidus) in the Alps. Glob Chang Biol 2018, 24, 3236–3253. [Google Scholar] [CrossRef] [PubMed]
- Rikhari, H.C.; Chandra, R.; Singh, S.P. Pattern of species distribution and community characters along a moisture gradient within an oak zone of Kumaun Himalaya. Proceedings of Indian National Science Academy 1989, 5, 431–438. [Google Scholar]
- Rodgers, W.A; Panwar, H.S. 1988. Planing a wildlife protected area network in India. Vol.1. Wildlife Institute of India, Dehradun.
- Rodgers, W.A.; Panwar, H.S.; Mathur, V.B. 2000. Wildlife Protected Area Network in India: A Review. Wildlife Institute of India, Dehradun, India.
- Rutledge, R.D. The method of bounded counts: When does it work? Journal of Wildlife Management 1982, 46, 757–761. [Google Scholar] [CrossRef]
- Sankar, K.; Rawat, G.S. and Upadhyay, A.K. (2011). Habitat Ecology and Conservation Status of Wild Ungulates in Northern Parts of Changthang Wildlife Sanctuary, Ladakh. Final Technical Report. Wildlife Institute of India, Dehradun 66 pp.
- Sarkar, P.; Takpa, J. Ahmed R., Tiwari, S.K.; Pendharkar, A.; Milandad, S.J.; Upadhyay, A.; and Kaul, R. (2008). Mountain Migrants: Survey of Tibetan Antelope (Panthalops hodgsonii) and Wild Yak (Bos grunniens) in Ladakh, Jammu and Kashmir, India. Conservation Action Series. Wildlife Trust of India.
- Sathyakumar, S. 1994. Habitat ecology of major ungulates in Kedarnath Musk Deer Sanctuary, Western Himalaya. Ph.D.thesis, Saurashtra University, Rajkhot. 242pp.
- Sathyakumar, S. 2004. Conservation status of mammals and birds in Nanda Devi National Park: An assessment of changes over two decades. In:“Biodiversity monitoring expedition. Nanda Devi 2003. A Report to Ministry of Environment and Forests, Government of India, Uttranchal State Forest Department, Dehra Dun. 1- 14p.
- Schaller, G.B.; JR Ren and, M.J. Qiu 1991. Observations on the Tibetan antelope (Pantholops hodgsonii). App. Anim. Behav. Sci. 29, 361-378.
- Schaller, G.B. 1997. In Litt. To Belinda Wright, Wildlife Protection Society of India (WPSI).
- Schaller G.B. 1998, Wildlife of the Tibetan steppe. University of Chicago Press, Chicago.
- Schaller G.B. 2000: Wildlife conservation in the Chang Tang Reserve, Tibet. In: Lu Z. & Springer J. (eds.), Tibet’s biodiversity, conservation and management. China Forestry Publishing House, Beijing: 21–28. (in Chinese).
- Schaller, G.B. & Binyuan Gu. omparative ecology of ungulates of the Aru Basin of north-west Tibet. National Geographic Research and Exploration 1994, 10, 266–293. [Google Scholar]
- Schaller, G.B.; Liu, W. Distribution, status, and conservation of wild yak Bos grunniens. Biol. Conserv. 1996, 76, 1–8. [Google Scholar] [CrossRef]
- Schaller, G.B.; Kang, A.; Cai, X.; Liu, Y. Migratory and calving behaviour of Tibetan antelope population. Acta Theriol. Sin. 2006, 26, 105–113. [Google Scholar]
- Segelquist C. A., H. L. Short., F. D. Ward and R. G. Leonard 1972. Quality of some winter deer forages in the Arkansas Ozarks. Journal of Wildlife Management 36, 174–177.
- Shang Z.H., Gibb M.J., Leiber F., Ismail M., Ding L.M., Guo X.S. & Long R.J. 2014: The sustainable development of grassland livestock systems on the Tibetan Plateau: problems, strategies and prospects. Rangeland J. 36, 267–296.
- Shawl. T. and Takpa. J. (2009). “Status and distribution of Tibetan antelope (chiru) and associated mammals in Changchenmo Valley and Daulet Beg Oldi Ladakh”, India. Department of Wildlife Protection, Government of Jammu and Kashmir.
- Shi, J.B.; Lu, F.Y.; Li, X.W.; Zhang, Z.H.; Su, X.K.; Dong, S.K.; Xu, H.D.; Zhang, X. 2016. Dietary overlap and co-existence of sympatric wild yak, Tibetan wild ass and Tibetan antelope in Arjin Shan National Nature Reserve, Xinjiang Province, China. Wildl. Res. 43, 323-331.
- Short, H.L. 1975. Nutrition of southern deer in different seasons. J. Wild. Mgmt. 39, 321–329. [CrossRef]
- Vander Wal E, van Beest FM, Brook RK (2013) Density-Dependent Effects on Group Size Are Sex-Specific in a Gregarious Ungulate. PLoS ONE 8, e53777. [CrossRef]
- Wangdwei M. & Fox J.L. 2008: Habitat selection by sympatric chiru and Tibetan gazelle in the Aru Basin, Chang Tang Nature Reserve, Tibet Autonomous Region, China. Acta Theriol. Sin. 28, 225–231.
- Wilson, D.E. , Cole F.R., Nichols J.D., Rudran R. & Foster M.S. 1996. Measuring and monitoring biological diversity: Standard methods for mammals. Washington, DC: Smithsonian Institution Press.
- Wright, B. A Kumar 1997. Fashioned for Extinction: An expose of the Shahtoosh Trade. Wildlife Protection Society of India (WPSI), New Delhi. 48pp.
- Wu Tong, Xinming Lian, Hongqi Li, Dong Wang, Jiaping Chen, Ziyan Miao & Tongzuo Zhang (2021): Adaptation of migratory Tibetan antelope to infrastructure development, Ecosystem Health and Sustainability. [CrossRef]
- Wydeven, A.P. RB DahlGren 1985. Ungulate Habitat Relationship in Wind Cave National Park. J. Wildl. Manage 49 (3): 805-813.
- ZAR, J.H. 1996. Biostatistical analysis (3rd ed.). Upper Saddle River, NJ: Prentice-Hall.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).