Submitted:
04 August 2024
Posted:
05 August 2024
You are already at the latest version
Abstract
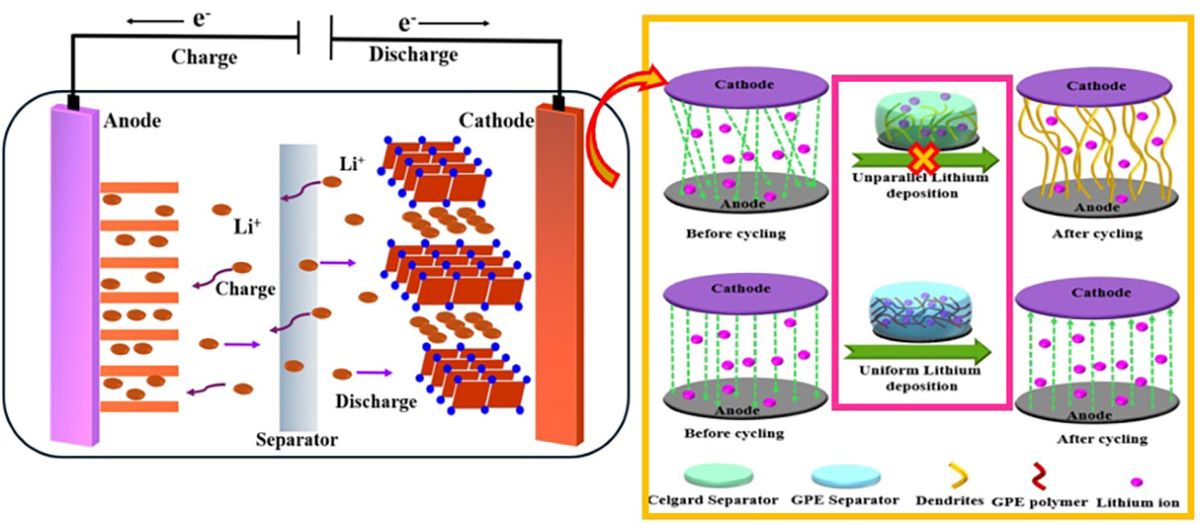
Keywords:
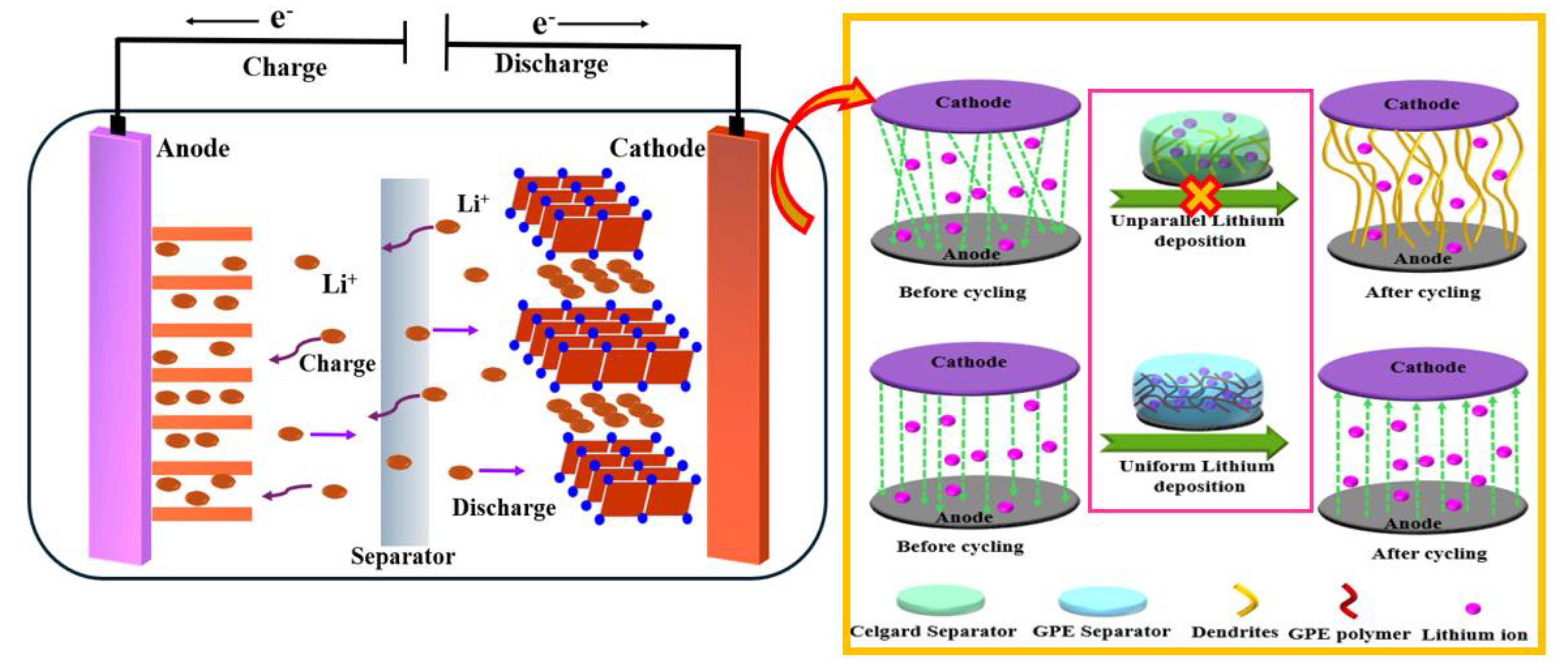
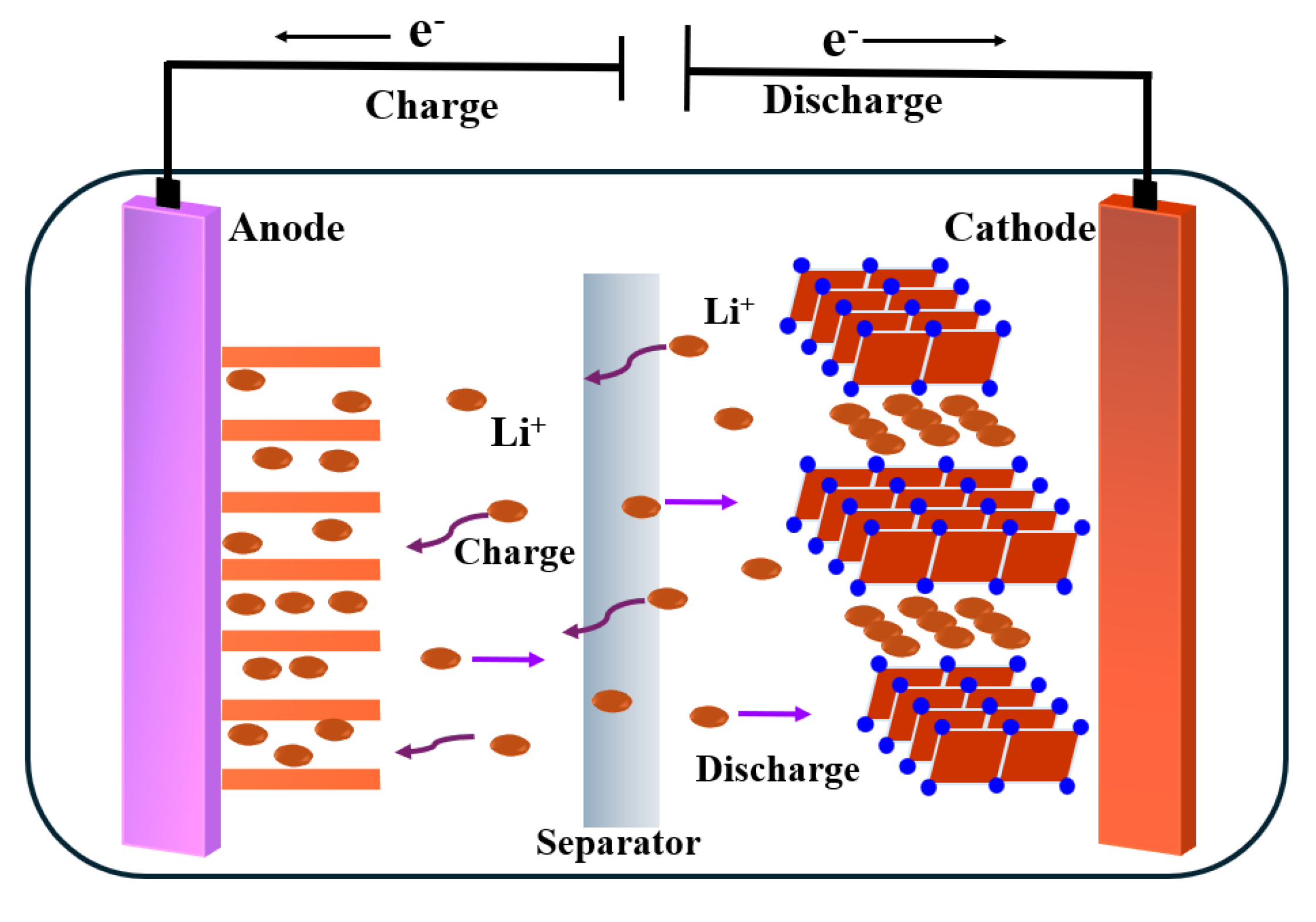
1. Introduction
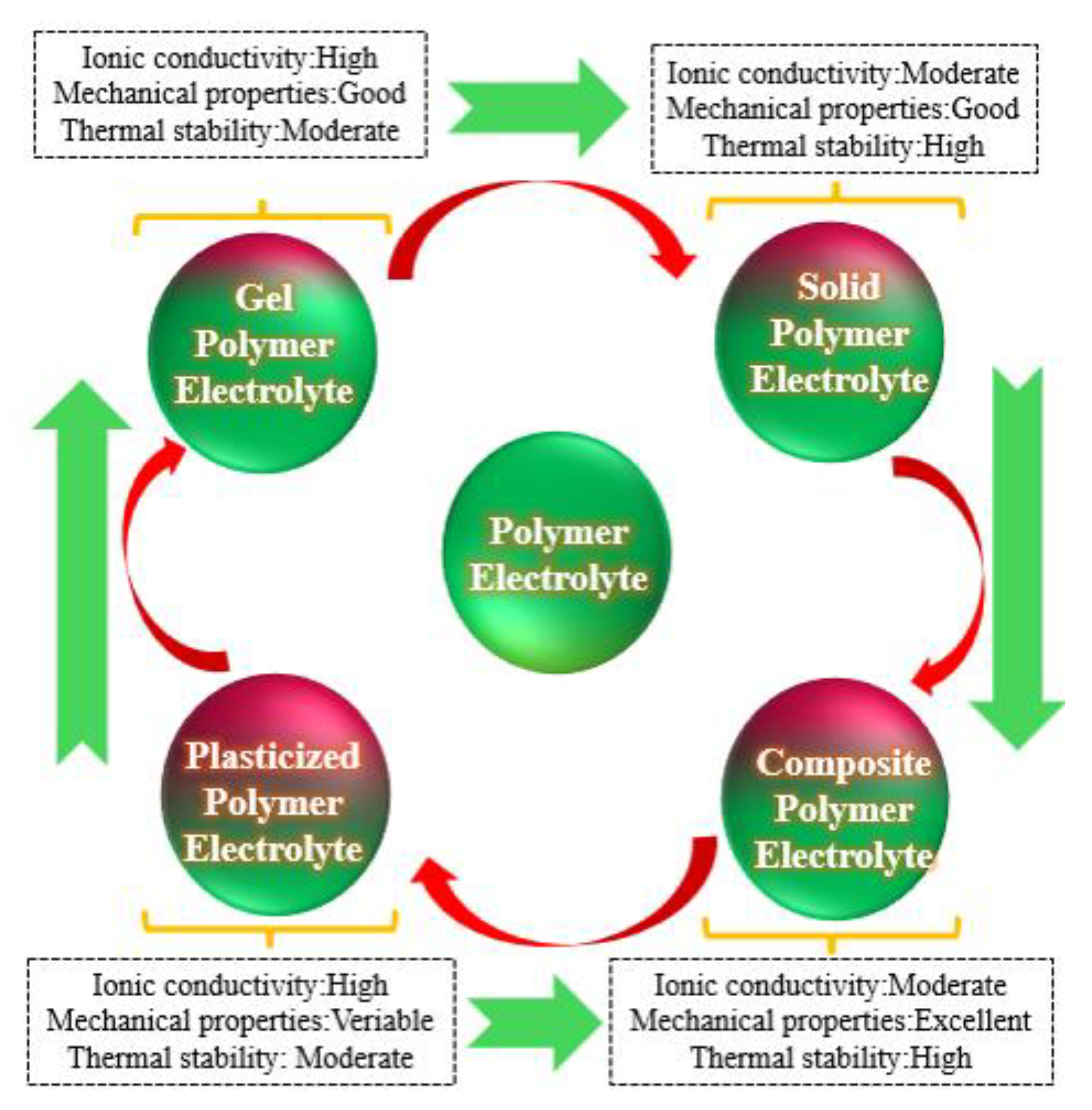
1.1. Solid Polymer Electrolytes
1.2. Gel Polymer Electrolytes
2. The Creation of the Polymer Framework in Gel Polymer Electrolytes (GPEs)
3. Polymer Backbone Based GEPs in Lithium-Ion Battery
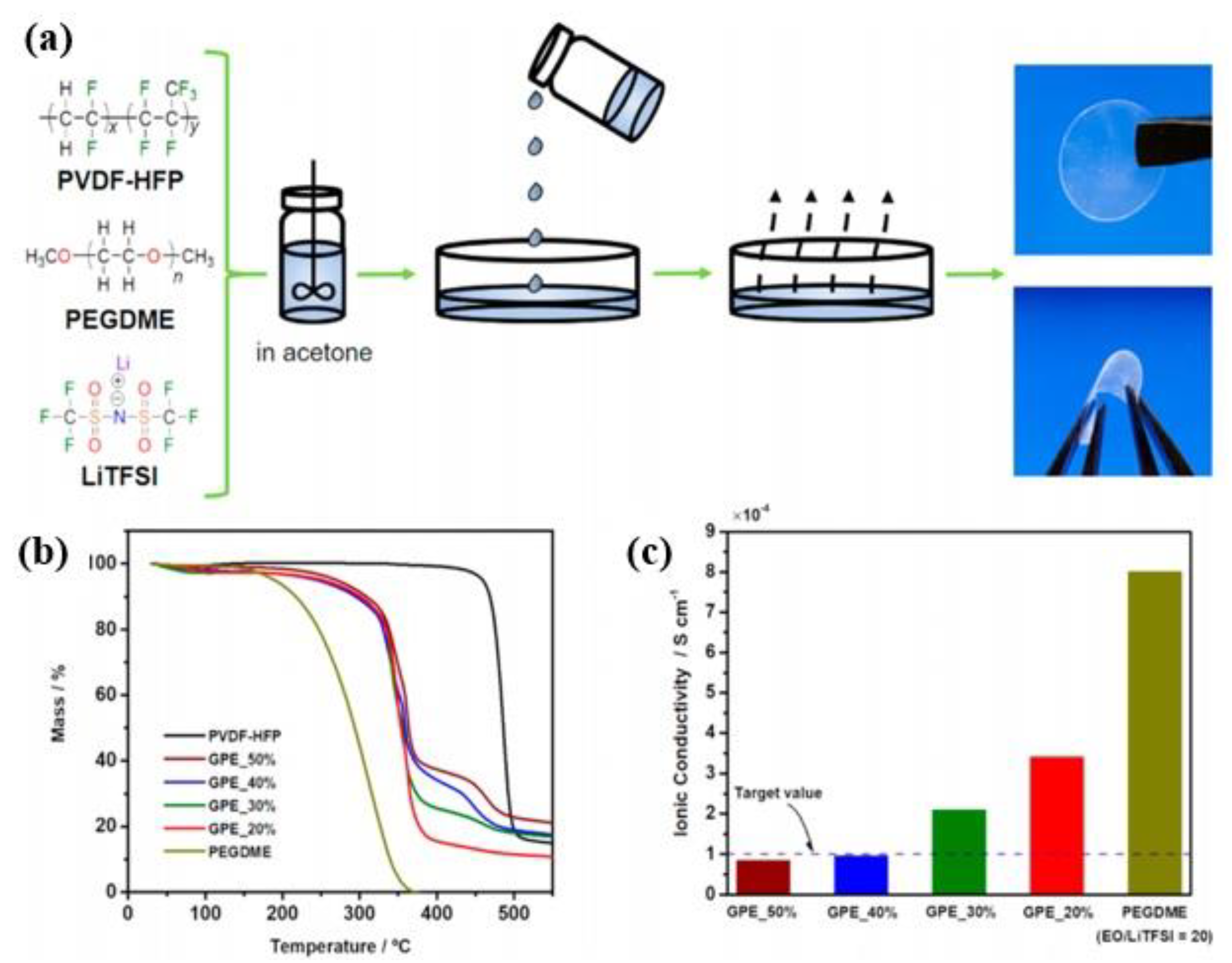
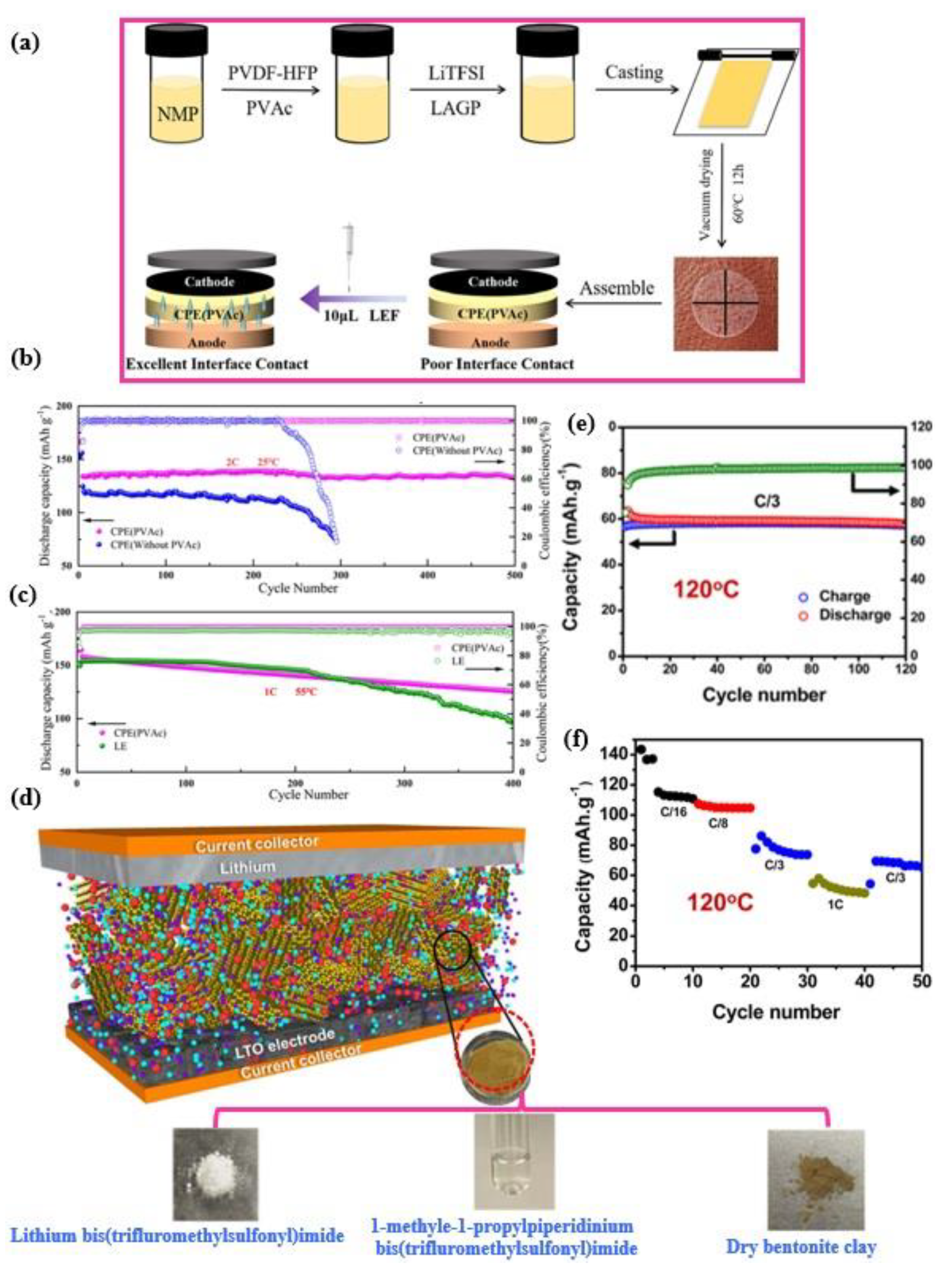
3.1. PVDF Based GPEs
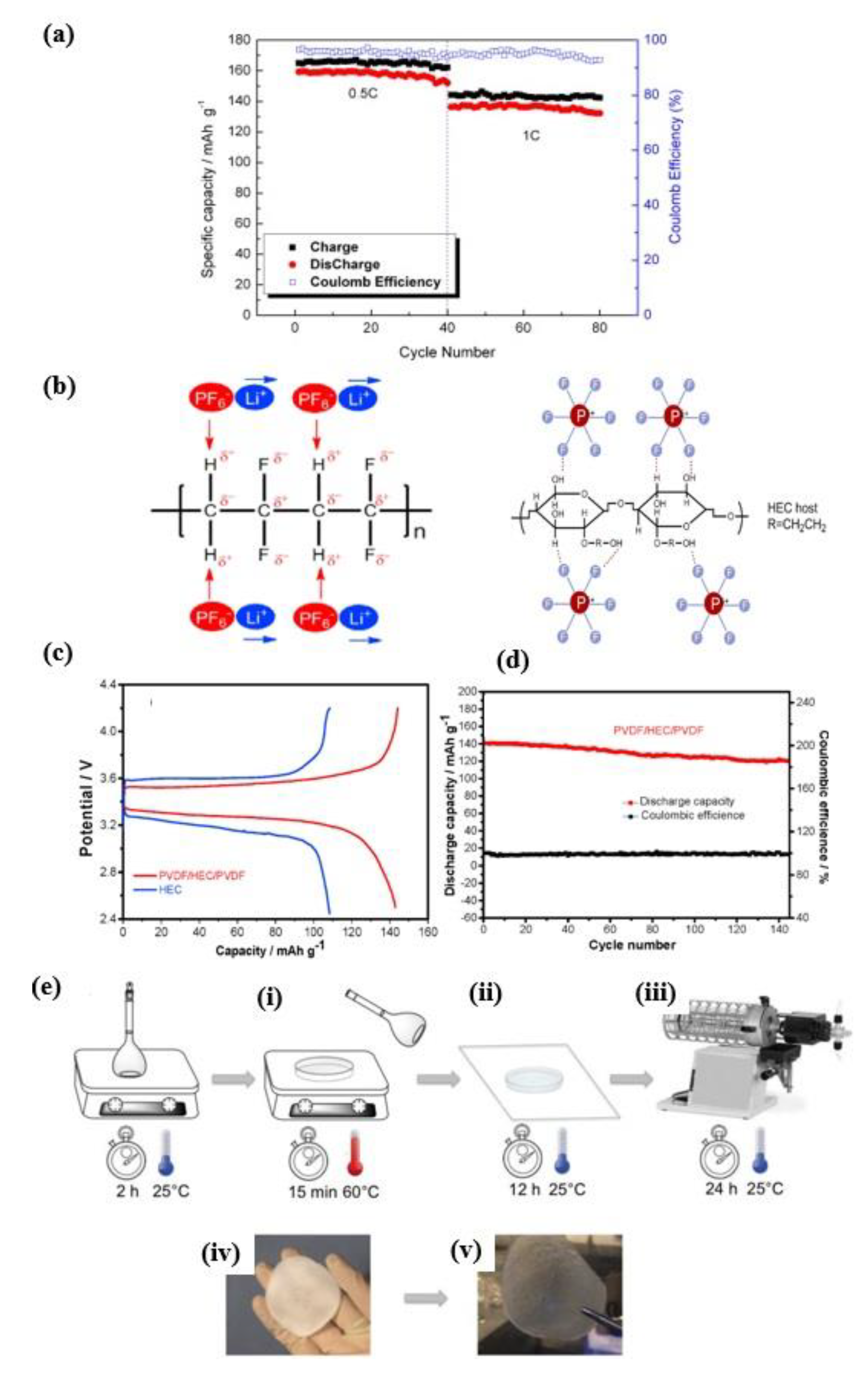
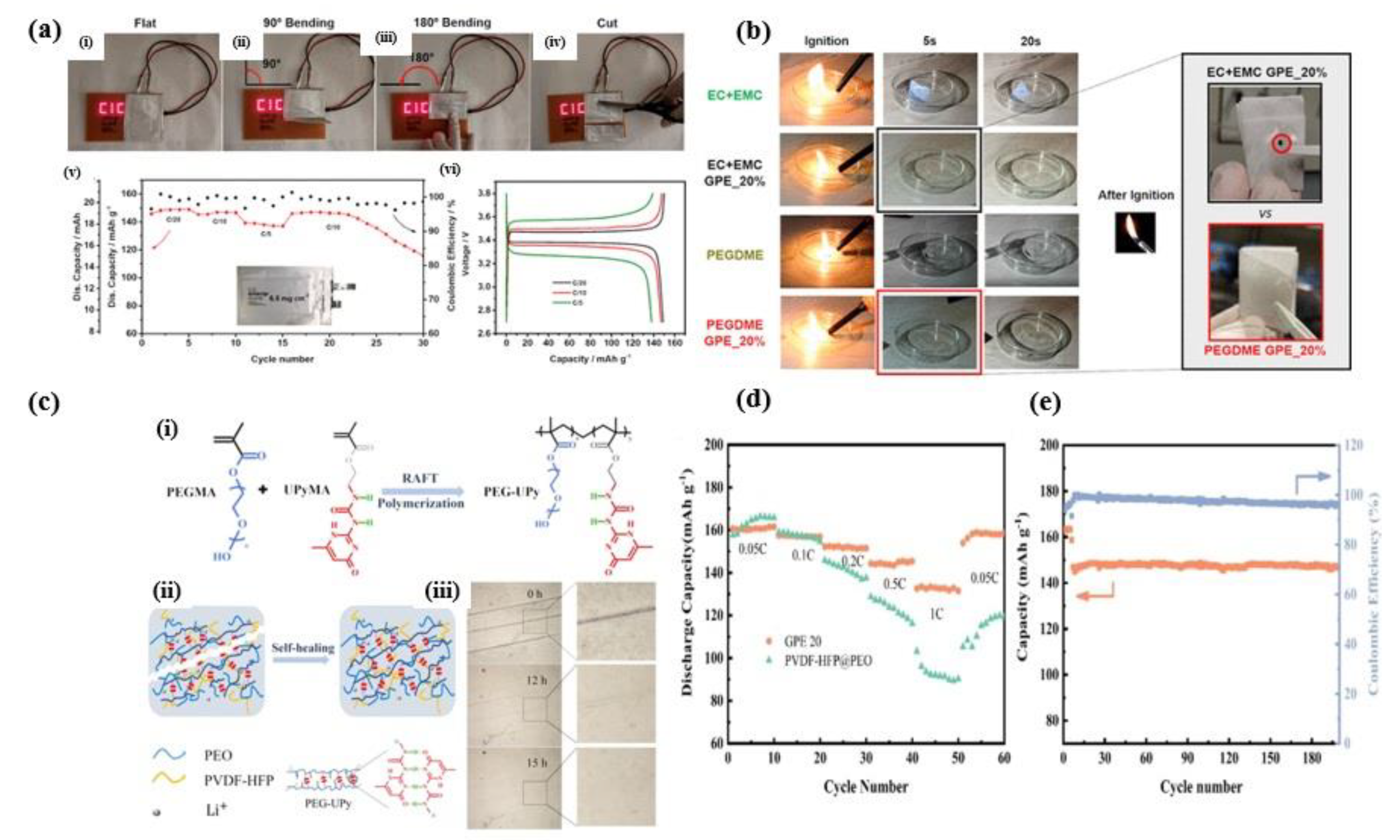
3.2. Cellulose Gel Membrane Gel Polymer Electrolyte
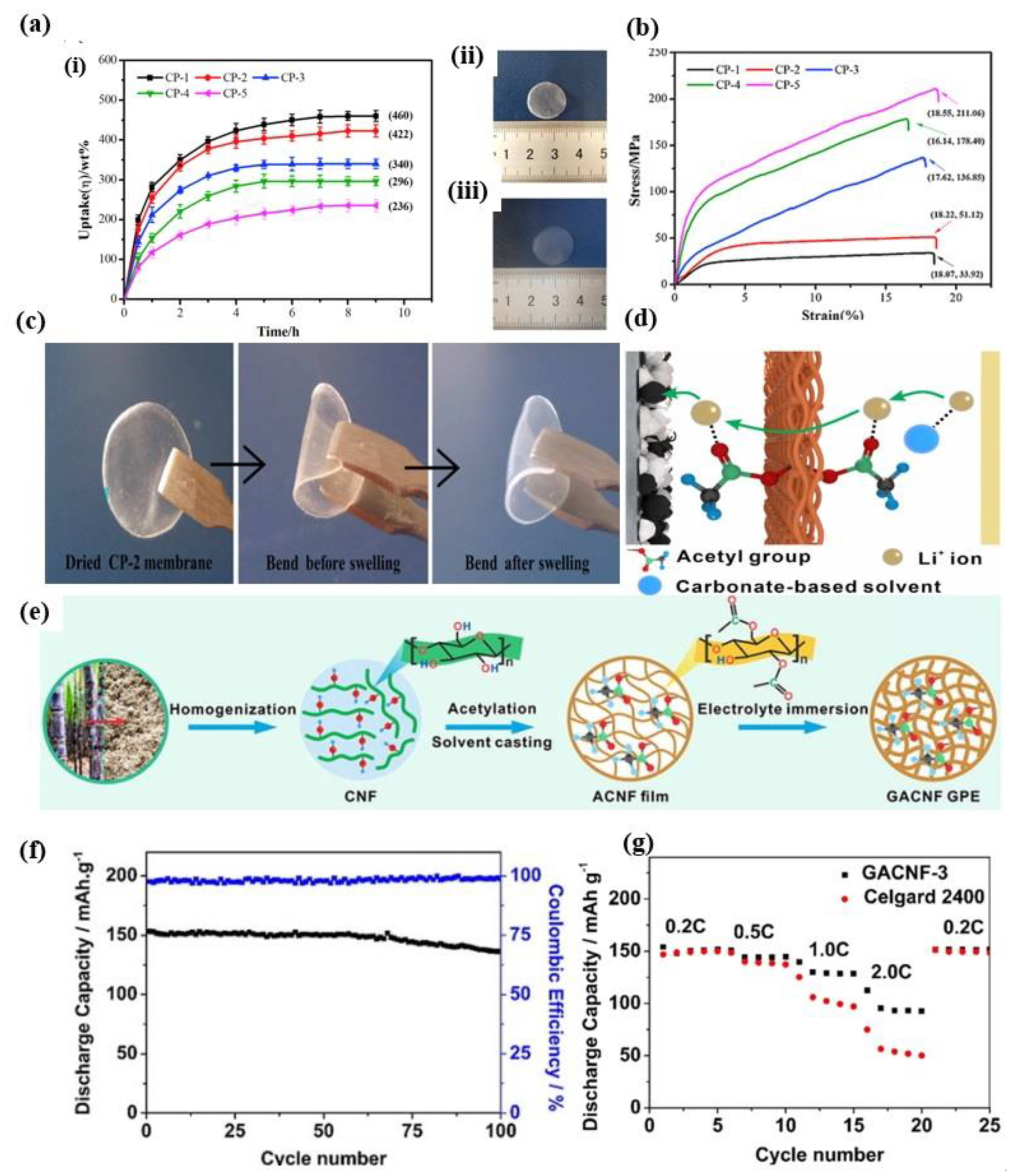
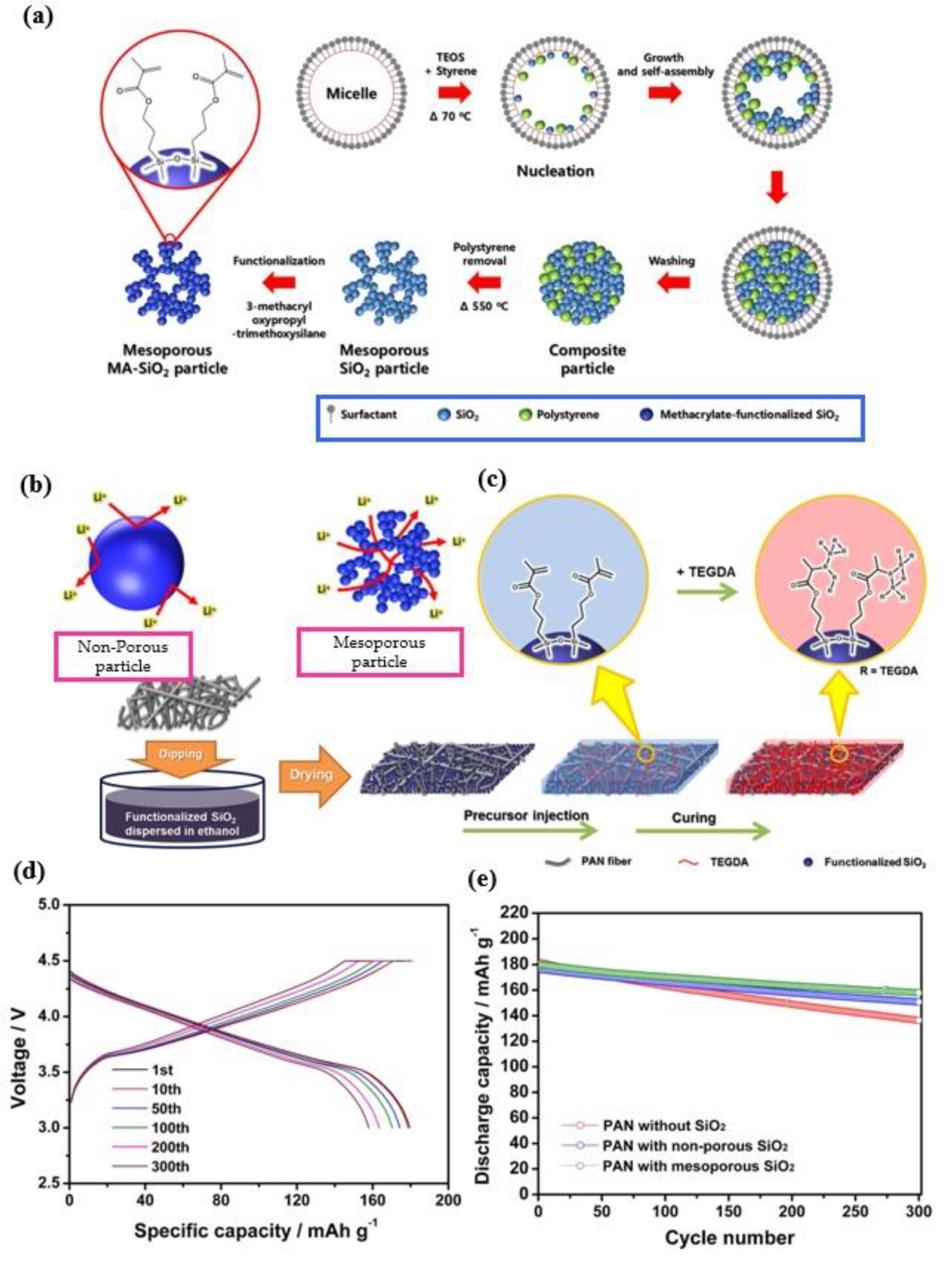
3.3. Methacrylamide Based GPEs
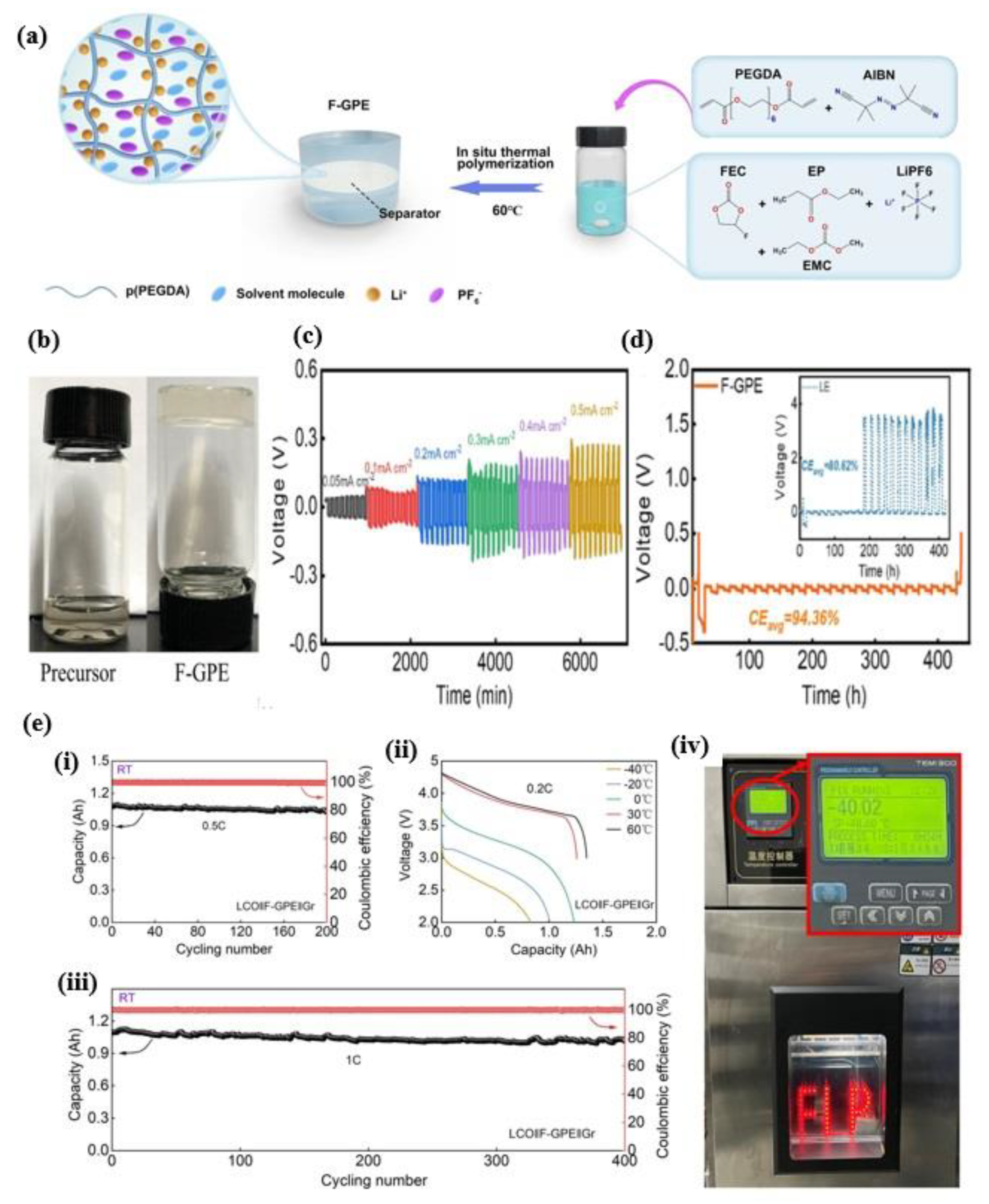
3.4. Fluorinated Gel Polymer Electrolyte Derived from PEGDA Facilitated
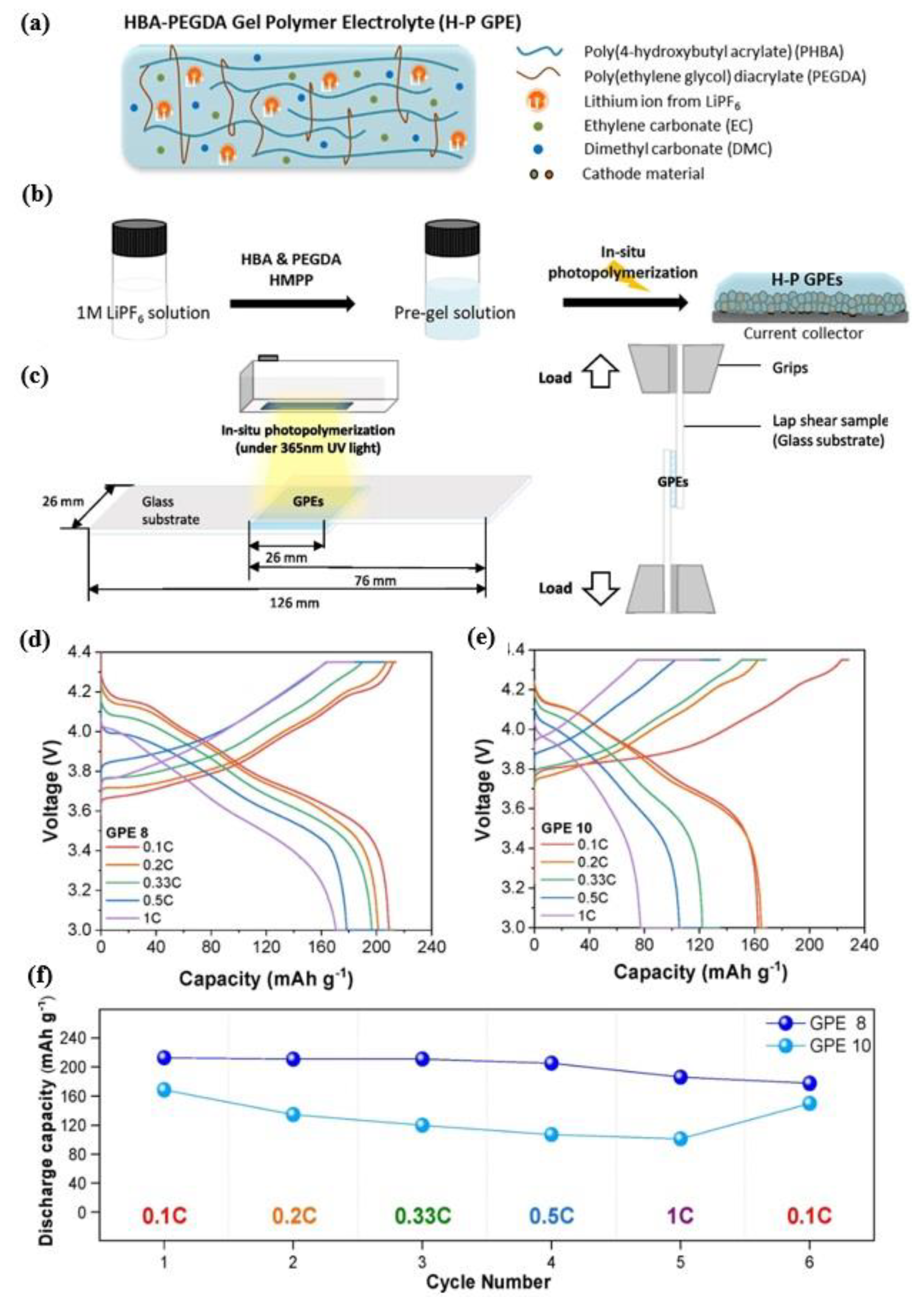
3.5. Poly (4-Hydroxybutyl Acrylate) for Applications in Lithium-Ion Batteries
3.6. PEO Based GEPs
3.7. Succinonitrile-Based Gel Polymer Electrolyte
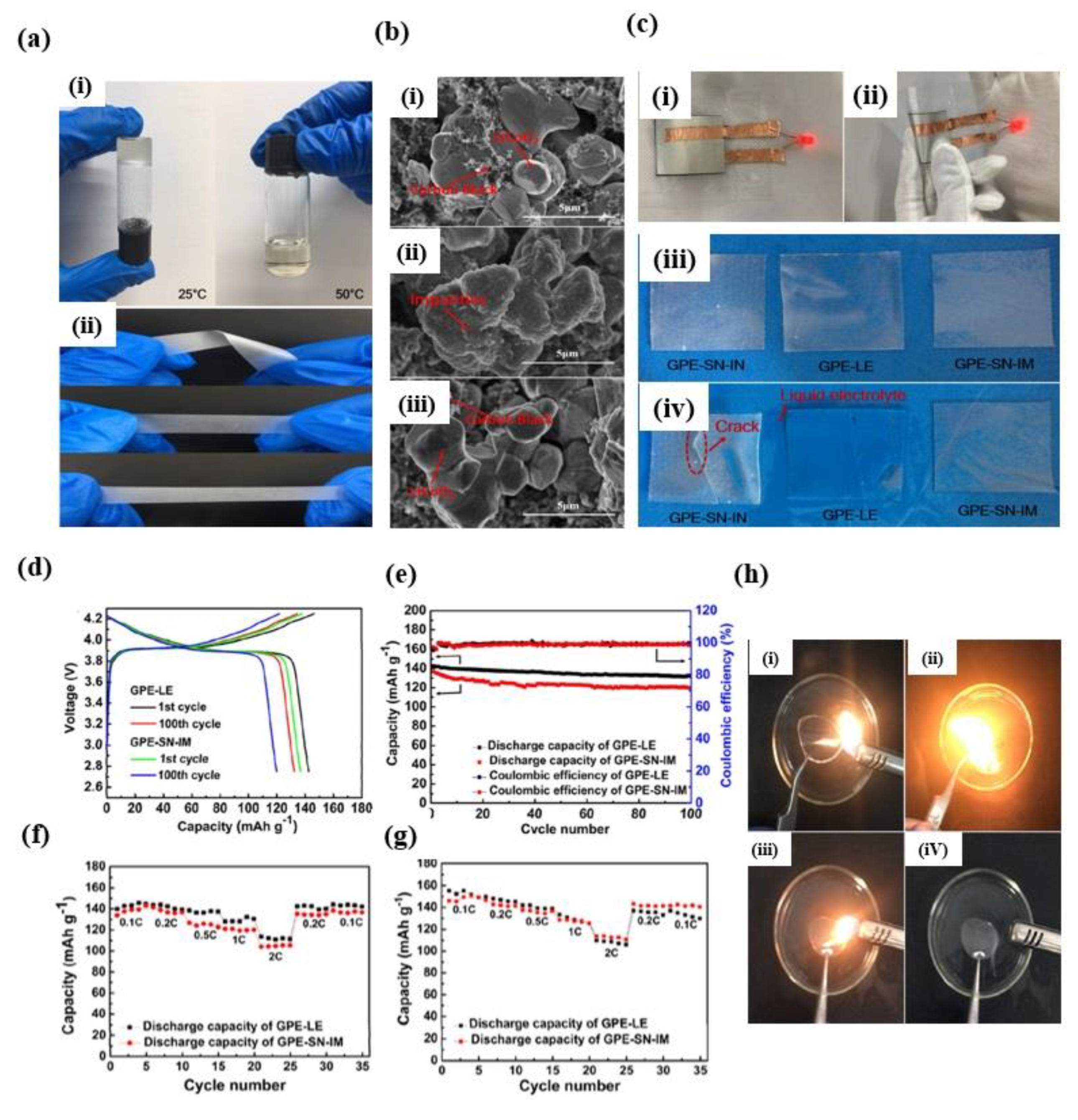
4. Application of Lithium-Ion Battery in Long Cycle Life Span
5. Application Gel Polymer Electrolytes for Several Beneficial Properties
5.1. Thermal and Chemical Stability
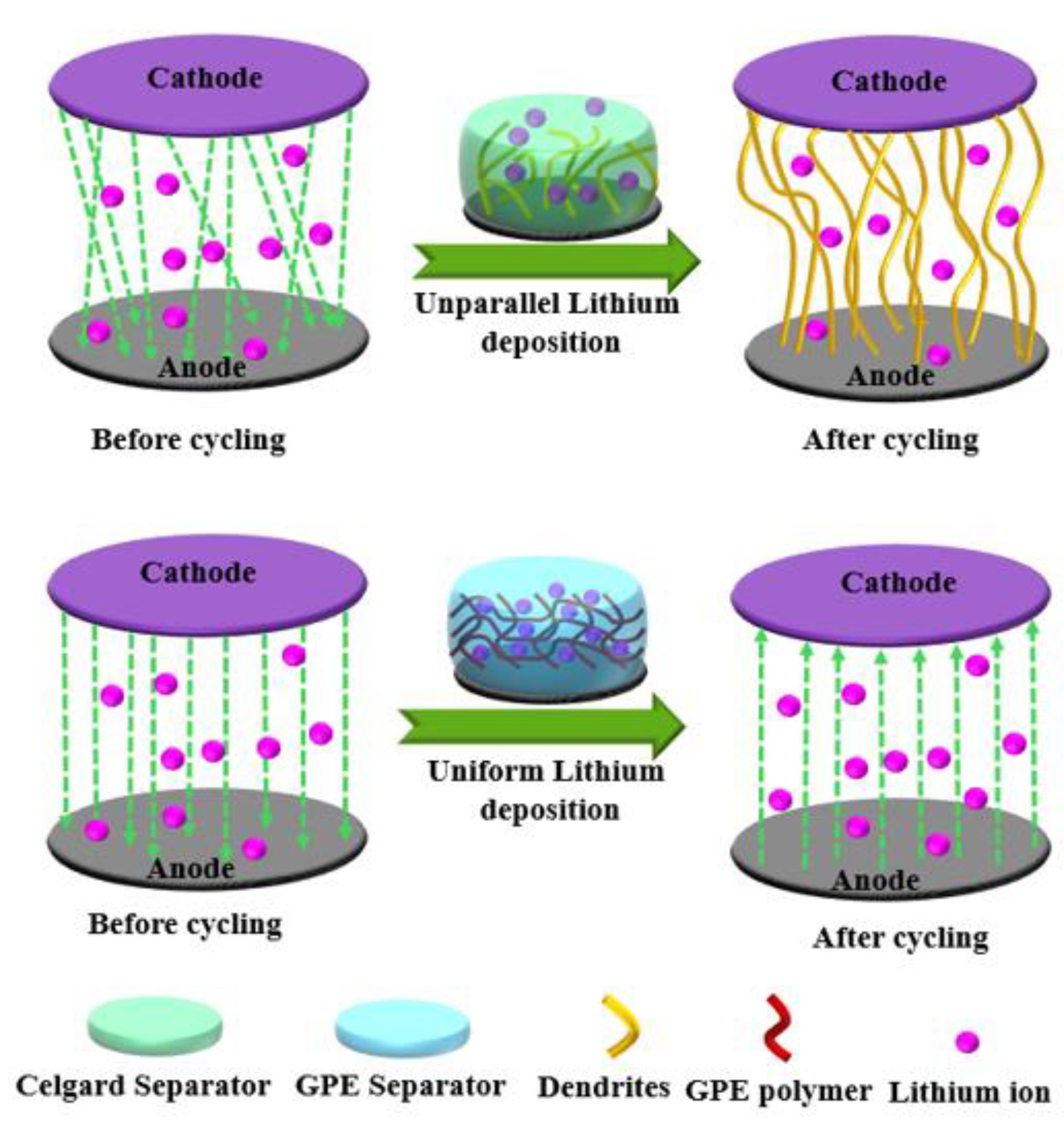
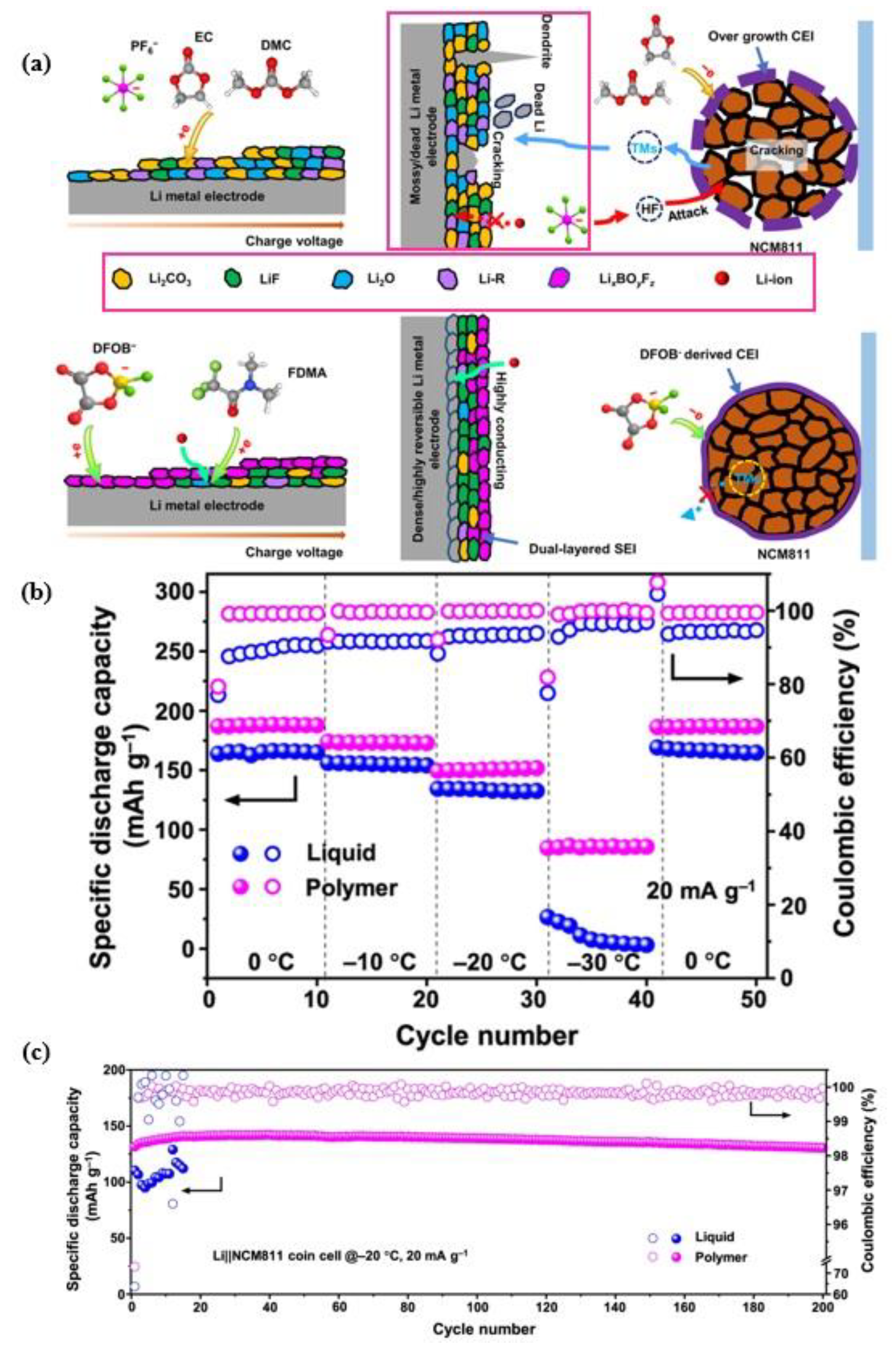
5.2. Improved Interfacial Stability
5.3. Wide Operating Temperature Range
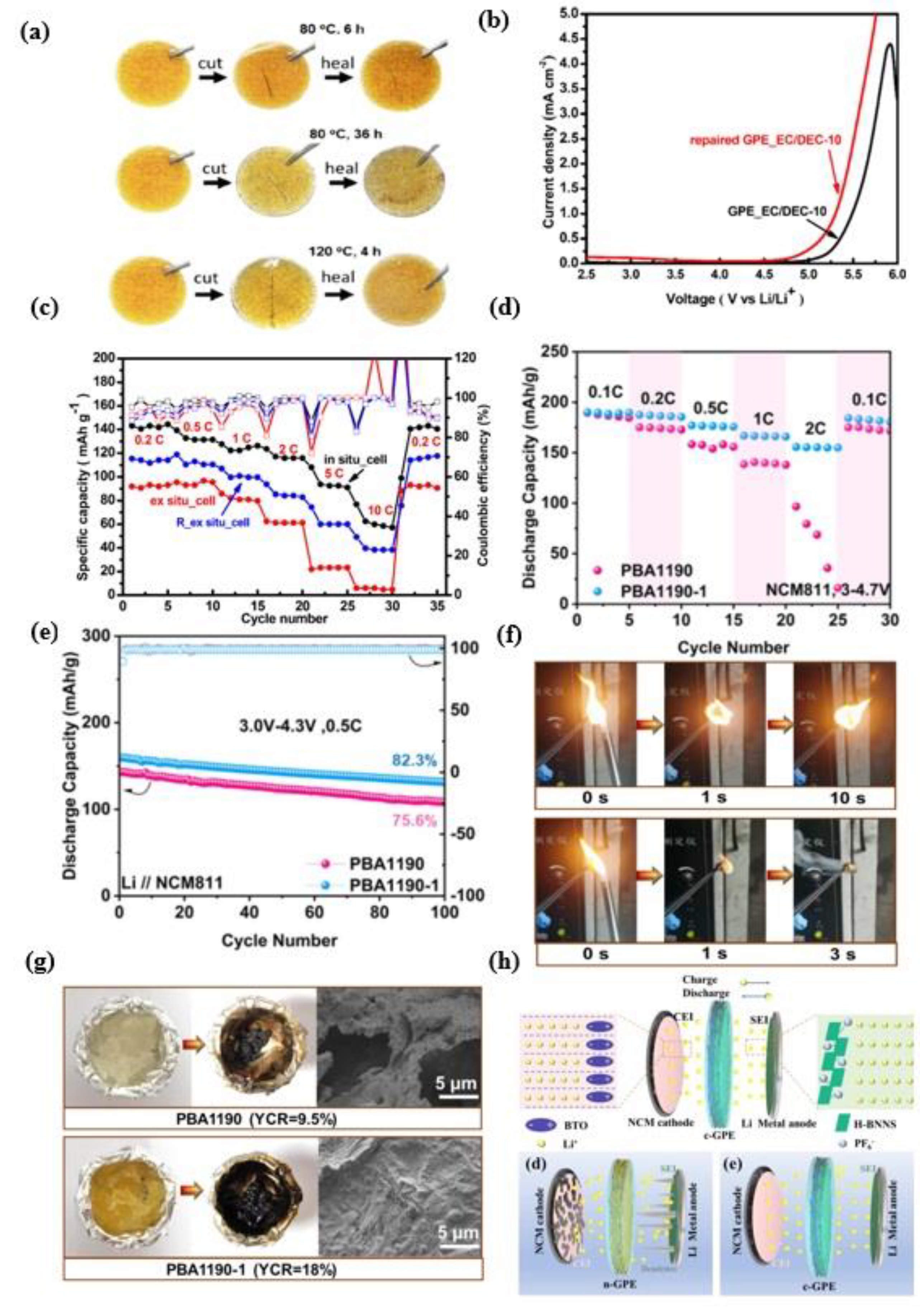
5.4. Mechanical Stability
5.5. Electrochemical Stability
6. Application of Gel Polymer Electrolyte in High Nickel Cathode
6.1. Application in High Performance Cathode
6.2. NCA and NCM Cathode
| Polymer marix | Liquide electrolyte | Cathode name | Voltage range | Cycle life | Refference |
|---|---|---|---|---|---|
| GPE PAN_LAGP_PEGDA | LiTFSI/DMF | NCM-811 | 2.8-4.3 | 270 @ 0.5C | [76] |
| GPE PVDF-HFP_BaTiO3 | LiPF6/EC/DMC | NCM-532 | 2.5-4.5 | 200 @ 5C | [77] |
| GPEMMA_TEGDMA_ETA | LiDFOB/FEC | NCA | 3-4.5 | 200 @ 0.2C | [78] |
| GPE UPyMA_TEGDA | LiFSI/FEC/EMC/EGA | NCM-811 | 2.8-4.7 | 500 @ 0.5C | [79] |
| GPE LiNO3_DMAA | LiPF6 /EC/DEC/FEC | NCM-622 | 3-4.3 | 400 @ 1C | [80] |
| GPE DOL-PEE | LiTFSI/LiDFOB/FEC | NCM-622 | - | 300 @ 0.5C | [81] |
| GPE TFPO-PEE | LiDFOB/LiTFSI | NCM-622 | 2.7-4.5 | 300 @ 0.5C | [82] |
| GPE PEGDA_PETA_PPO | LiPF6/EMC/FEC | NCM-811 | 2.8-4.3 | 400 @ 1C | [83] |
| GPE PMBA | LiTFSILiBOB/EC/EMC | NCM-622 | 3.0-4.3 | 700 @ 1C | [84] |
| SPE PVC-TF3 | LiTFSI/NMP | NCM-811 | 2.5-4.3 | 150 @ 0.1C | [85] |
| SPE B-PEGMA_VC_AN | LiTFSI | NCM-811 | 2.8-4.3 | 100 @ 0.5C | [86] |
| CPE LATP_PEO_PAN | LiTFSI/DMF | NCM-622 | 2.8-4.3 | 120 @ 0.5C | [87] |
6.3. Application of GPEs in High Nickel Cathode to Enhance Safety
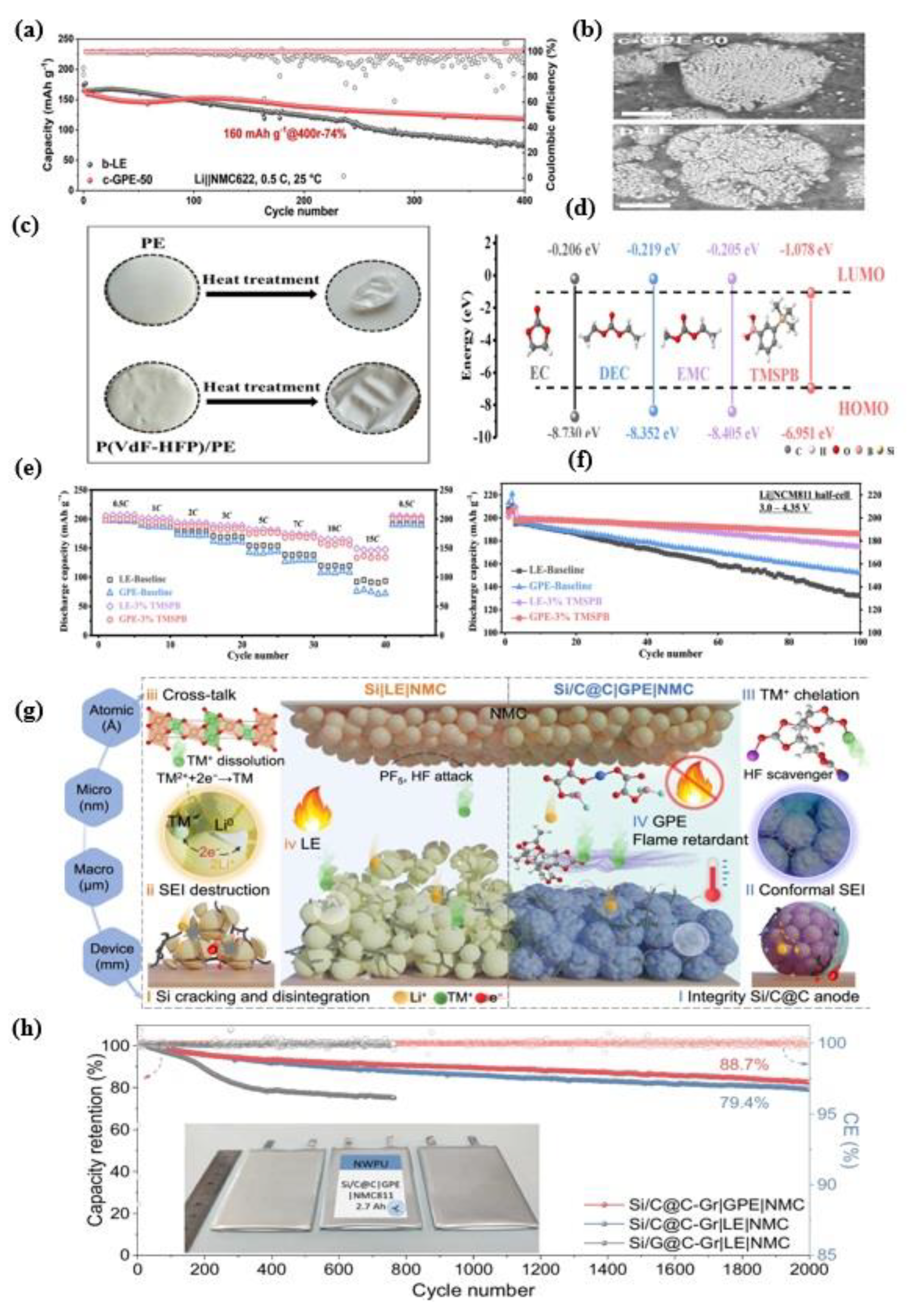
6.4. Application of LiFePO4 Cathode for Flame Safety
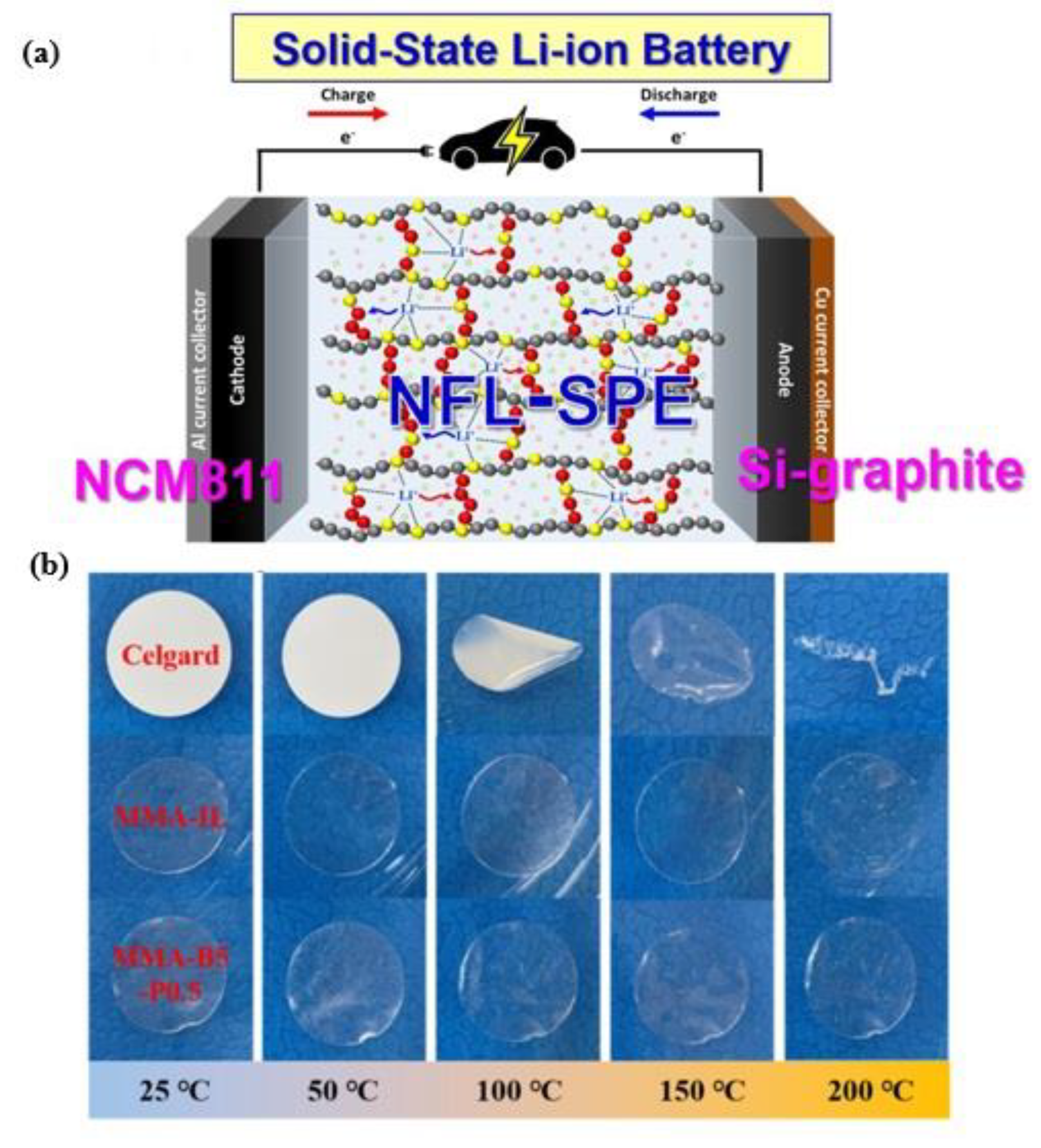
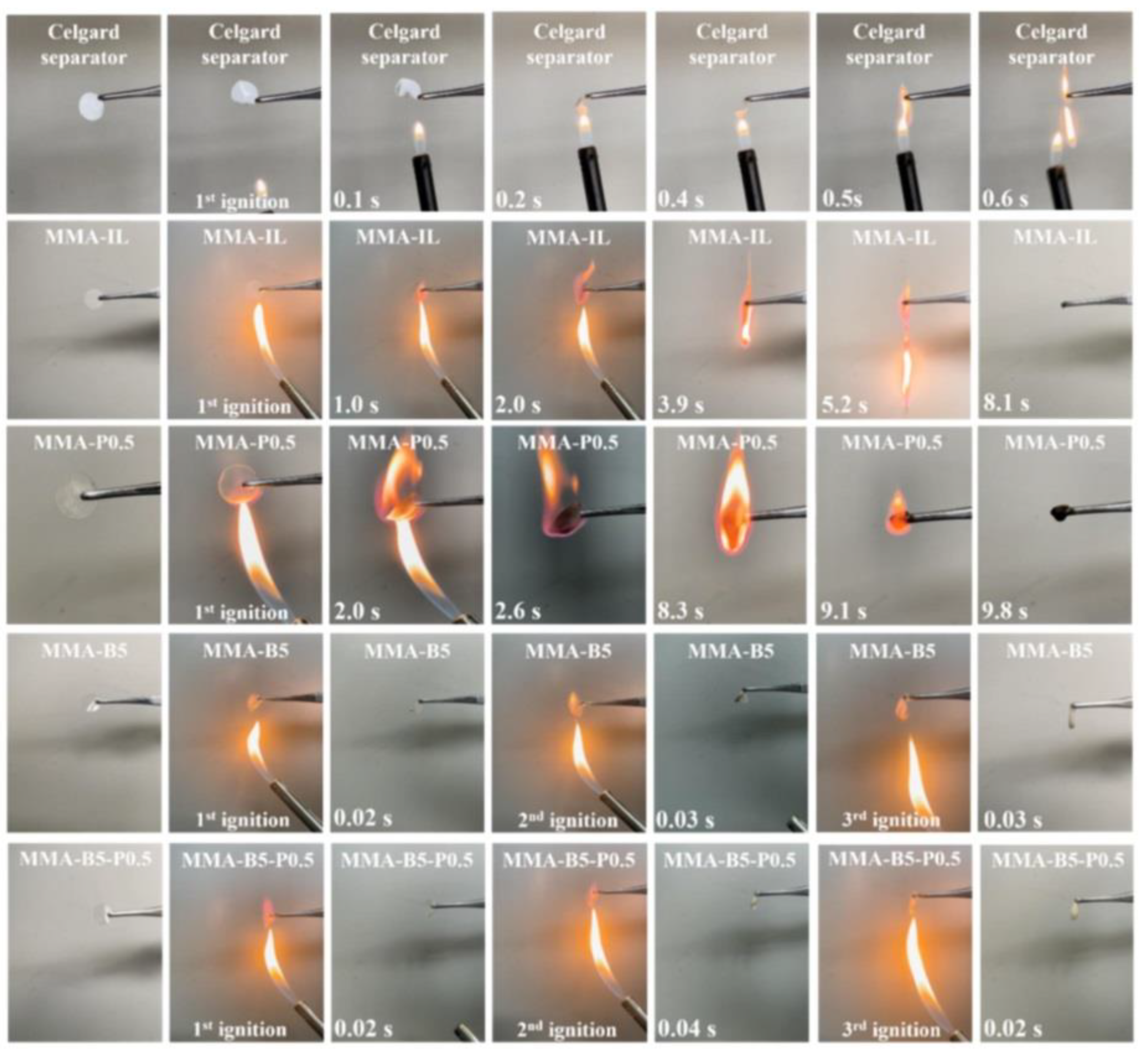
7. Conclusion and Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A. 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid. A Battery of Choices. Science. 2011, 334, 928. [Google Scholar]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001, 414, 359. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries A review. J. Power Sources. 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium-ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium-ion battery. J.Power Sources. 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium-ion battery for electric vehicles A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zhang, P.; Li, R.; Huang, J.; et al. Flexible poly(vinylidene fluoride-co hexafluoropropylene)-based gel polymer electrolyte for high-performance lithium-ion batteries. RSC Adv. 2021, 11, 11943–11951. [Google Scholar] [CrossRef] [PubMed]
- Tleukenov, Y.T.; Kalimuldina, G.; Arinova, A.; Issatayev, N.; Bakenov, Z.; Nurpeissova, A. Polyacrylonitrile-Polyvinyl Alcohol-Based Composite Gel-Polymer Electrolyte for All-Solid-State Lithium-Ion Batteries. Polymers (Basel). 2022, 14, 2357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, X.; Chi, Z.; et al. Designing a new-type PMMA based gel polymer electrolyte incorporating ionic liquid for lithium oxygen batteries with Ru-based Binder-free cathode. Appl. Surf. Sci, 2021; 565, 150612. [Google Scholar]
- Deng, X.; Huang, Y.; Song., A.; et al. Gel polymer electrolyte with high performances based on biodegradable polymer polyvinyl alcohol composite lignocellulose. Mater. Chem. Phys. 2019, 229, 232–241. [Google Scholar] [CrossRef]
- Li, H.; Ma, X.T.; Shi, J.L.; Yao, Z.K.; Zhu., B.K.; Zhu, L.P. Preparation and properties of poly (ethylene oxide) gel filled polypropylene separators and their corresponding gel polymer electrolytes for Li-ion batteries. Electrochim Acta. 2011, 56, 2641–2647. [Google Scholar] [CrossRef]
- Hassoun, J.; Scrosati, B. Review Advances in Anode and Electrolyte Materials for the Progress of Lithium-Ion and beyond Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2582–A2588. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer electrolytes for lithium-ion batteries a critical study. Ionics. 2017, 23, 497–540. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A. 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; Kasprzyk, M.; Łęzak, P.; Zukowska, Z.; Zalewska, A.; Wieczorek, W. Electrolytes for Li-ion transport − Review. Solid State Ionics. 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Dias, F.B.; Plomp, L.; Veldhuis, J.B.J. Trends in polymer electrolytes for secondary lithium batteries. J. Power Sources. 2000, 88, 169–191. [Google Scholar] [CrossRef]
- Jeong, D.; Yook, J.; Hong, D.G.; Lee, J.C. Lithium dendrite suppression by single ion conducting gel polymer electrolyte cross-linked with graphene oxide. J.Power Sources. 2022, 534, 231424. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Yen, H.J. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Zhu M, Wu J, Wang Y, et al. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Yang, P.; Liu, L.; Li, L.; et al. Gel polymer electrolyte based on polyvinylidenefluoride-co- hexafluoropropylene and ionic liquid for lithium ion battery. Electrochim Acta. 2014, 115, 454–460. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, M.X.; Chang, Z.; et al. A Sandwich PVDF/HEC/PVDF Gel Polymer Electrolyte for Lithium Ion Battery. Electrochim Acta. 2017, 245, 752–759. [Google Scholar] [CrossRef]
- Fasciani, C.; Panero, S.; Hassoun, J.; Scrosati, B. Novel configuration of poly(vinylidenedifluoride)-based gel polymer electrolyte for application in lithium-ion batteries. J. Power Sources. 2015, 294, 180–186. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Shi, Y.; Yang, Y.; Hou, Y.; Wu, Y. A composite gel polymer electrolyte with high performance based on poly(vinylidene fluoride) and polyborate for lithium ion batteries. Adv Energy Mater. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Castillo, J.; Santiago, A.; Judez, X.; et al. Safe, Flexible, and High-Performing Gel-Polymer Electrolyte for Rechargeable Lithium Metal Batteries. Chem Mater. 2021, 33, 8812–8821. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.; Zou, C.; et al. High-Performance Gel Polymer Electrolyte with Self-Healing Capability for Lithium-Ion Batteries. ACS Appl Energy Mater. 2022, 5, 5267–5276. [Google Scholar] [CrossRef]
- Zhang, P.; Li, R.; Huang, J.; et al. Flexible poly(vinylidene fluoride-co-hexafluoropropylene)-based gel polymer electrolyte for high-performance lithium-ion batteries. RSC Adv. 2021, 11, 11943–11951. [Google Scholar] [CrossRef]
- Du, Z.; Su, Y.; Qu, Y.; et al. A mechanically robust, biodegradable and high performance cellulose gel membrane as gel polymer electrolyte of lithium-ion battery. Electrochim Acta. 2019, 299, 19–26. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, J.; Du, Z.; et al. High-strength and flexible cellulose/PEG based gel polymer electrolyte with high performance for lithium ion batteries. J Memb Sci. 2020, 593, 117428. [Google Scholar] [CrossRef]
- Wangn, W.; Li, Z.; Huang, H.; Li, W.; Wang, J. Facile design of novel nanocellulose-based gel polymer electrolyte for lithium-ion batteries application. Chem Eng J. 2022, 445, 136568. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, F.; Yang, Y.; Chang, Z.; Wu, Y. An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. RSC Adv. 2014, 4, 76. [Google Scholar] [CrossRef]
- Nakano, Y.; Shinke, K.; Ueno, K.; Tsutsumi, H. Gel polymer electrolytes based on poly(methacrylamide) derivative having branched pendant with terminal nitrile groups. Solid State Ionics. 2016, 293, 13–17. [Google Scholar] [CrossRef]
- Rao, M.M.; Liu, J.S.; Li, W.S.; Liang, Y.; Zhou, D.Y. Preparation and performance analysis of PE-supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery application. J Memb Sci. 2008, 322, 314–319. [Google Scholar] [CrossRef]
- Isken, P.; Winter, M.; Passerini, S.; Lex-Balducci, A. Methacrylate based gel polymer electrolyte for lithium-ion batteries. J. Power Sources. 2013, 225, 157–162. [Google Scholar] [CrossRef]
- Tillmann, S.D.; Isken, P.; Lex-Balducci, A. Gel polymer electrolyte for lithium-ion batteries comprising cyclic carbonate moieties. J Power Sources. 2014, 271, 239–244. [Google Scholar] [CrossRef]
- Shin, W.K.; Cho, J.; Kannan, A.G.; Lee, Y.S.; Kim, D.W. Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Li, M.; Qiu, J.; Wen, Y.; Chen, J. In situ cross-linked fluorinated gel polymer electrolyte based on PEGDA-enabled lithium-ion batteries with a wide temperature operating range. Chem. Eng. J. 2023, 467, 143311. [Google Scholar] [CrossRef]
- Choi, H.J.; Jeong, Y.J.; Choi, H.S.; et al. Gel polymer electrolyte with improved adhesion property based on poly(4-hydroxybutyl acrylate) for lithium-ion batteries. Chem. Eng. J. 2023, 474, 145673. [Google Scholar] [CrossRef]
- Li, W.; Pang, Y.; Liu, J.; Liu, G.; Wang, Y.; Xia, Y. A PEO-based gel polymer electrolyte for lithium ion batteries. RSC. Adv. 2017, 7, 23494–23501. [Google Scholar] [CrossRef]
- Li, X.; Qian, K.; He, Y.B.; et al. A dual-functional gel-polymer electrolyte for lithium ion batteries with superior rate and safety performances. J. Mater. Chem. A. 2017, 5, 18888–18895. [Google Scholar] [CrossRef]
- Lv, P.; Li, Y.; Wu, Y.; et al. Robust Succinonitrile-Based Gel Polymer Electrolyte for Lithium-Ion Batteries Withstanding Mechanical Folding and High Temperature. ACS Appl. Mater. Interfaces. 2018, 10, 25384–25392. [Google Scholar] [CrossRef]
- Li, Z.; Yu, R.; Weng, S.; Zhang, Q.; Wang, X.; Guo, X. Tailoring polymer electrolyte ionic conductivity for production of low- temperature operating quasi-all-solid-state lithium metal batteries. Nat Commun. 2023, 14, 1–12. [Google Scholar]
- Zhu, G.R.; Zhang, Q.; Liu, Q.S.; et al. Non-flammable solvent-free liquid polymer electrolyte for lithium metal batteries. Nat. Commun. 2023, 14, 4617. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources. 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Dias, F.B.; Plomp, L.; Veldhuis, J.B.J. Trends in polymer electrolytes for secondary lithium batteries. J. Power Sources. 2000, 88, 169–191. [Google Scholar] [CrossRef]
- Liang, S.; Yan, W.; Wu, X.; Zhang, Y.; Zhu, Y.; Wang, H.; Wu, Y. Gel polymer electrolytes for lithium-ion batteries: Fabrication, characterization and performance. Solid State Ionics. 2018, 318, 2–18. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; Kasprzyk, M.; Łęzak, P.; Zukowska, Z.; Zalewska, A.; Wieczorek, W. Electrolytes for Li-ion transport − Review. Solid State Ionics. 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources. 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Yang, Y.Q.; Li, M.X.; Wang, F.X.; Chang, Z.; Wu, Y.P.; Liu, X. A composite membrane based on a biocompatible cellulose as a host of gel polymer electrolyte for lithium-ion batteries. J. Power Sources. 2014, 270, 53–58. [Google Scholar] [CrossRef]
- Yuan, J.J.; Sun, C.C.; Fang, L.F.; et al. A lithiated gel polymer electrolyte with superior interfacial performance for safe and long-life lithium metal battery. J. Energy Chem. 2021, 55, 313–322. [Google Scholar] [CrossRef]
- Yang, P.; Gao, X.; Tian, X.; et al. Upgrading Traditional Organic Electrolytes toward Future Lithium Metal Batteries: A Hierarchical Nano-SiO2-Supported Gel Polymer Electrolyte. ACS Energy Lett. 2020, 5, 1681–1688. [Google Scholar] [CrossRef]
- Chen, L.; Wu, H.; Ai, X.; Cao, Y.; Chen, Z. Toward wide-temperature electrolyte for lithium–ion batteries. Batter Energy. 2022, 1, 20210006. [Google Scholar] [CrossRef]
- Yong, H.; Park, H.; Jung, C. Quasi-solid-state gel polymer electrolyte for a wide temperature range application of acetonitrile-based supercapacitors. J. Power Sources. 2020, 447, 227390. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A. 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Ni, W.; Yang, D.; Cheng, J.; Li, X.; Guan, Q.; Wang, B. Gel-type polymer separator with higher thermal stability and effective overcharge protection of 4.2 v for secondary lithium-ion batteries. RSC Adv. 2016, 6, 52966–52973. [Google Scholar] [CrossRef]
- Song, D.; Liao, J.; Huang, S.; Yuan, W.; Li, C.; He, J. A rigid-flexible gel polymer electrolytes with long cycle life and dendrite-free in lithium metal batteries. J. Energy Storage. 2024, 75, 109591. [Google Scholar] [CrossRef]
- Kalaga, K.; Rodrigues, M.T.F.; Gullapalli, H.; Babu, G.; Arava, L.M. .;, Ajayan P.M. Quasi-Solid Electrolytes for High Temperature Lithium Ion Batteries. ACS Appl.Mater.Interfaces. 2015, 7, 25777–25783. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Lu, Y.H.; Liu, YL. In Situ Self-Healing of Gel Polymer Electrolytes Enhancing the Cycling Stability of Lithium Ion Batteries. ACS Sustain Chem. Eng. 2024, 12, 7894–7902. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Lu, Y. Designing safer lithium-based batteries with nonflammable electrolytes: A review. Science. 2021, 1, 163–177. [Google Scholar] [CrossRef]
- Rinkel, B.L.D.; Vivek, J.P.; Garcia-Araez, N.; Grey, C.P. Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries. Energy Environ. Sci. 2022, 15, 3416–3438. [Google Scholar] [CrossRef] [PubMed]
- Cabañero, M.M.A.; Boaretto, N.; Naylor, A.J.; et al. Are Polymer-Based Electrolytes Ready for High-Voltage Lithium Battery Applications? An Overview of Degradation Mechanisms and Battery Performance. Adv. Energy Mater. 2022, 12, 2201264. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, SK.; Gupta, H.; et al. Electrochemical performance of Li-rich NMC cathode material using ionic liquid-based blend polymer electrolyte for rechargeable Li-ion batteries. J. Alloys Compd. 2020, 843, 155615. [Google Scholar] [CrossRef]
- Mi, J.; Ma, J.; Chen, L.; et al. Topology crafting of polyvinylidene difluoride electrolyte creates ultra-long cycling high-voltage lithium metal solid-state batteries. Energy Storage Mater. 2022, 48, 375–383. [Google Scholar] [CrossRef]
- Long, M.C.; Wu, G.; Wang, X.L.; Wang, Y.Z. Self-adaptable gel polymer electrolytes enable high-performance and all-round safety lithium-ion batteries. Energy Storage Mater. 2022, 53, 62–71. [Google Scholar] [CrossRef]
- An, H.; Liu, Q.; Deng, B.; et al. Eliminating local electrolyte failure induced by asynchronous reaction for high-loading and long-lifespan all-solid-state batteries. Adv Funct Mater. 2023, 33, 2305186. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Wang, T.; et al. Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers. Adv. Energy Mater. 2018, 8, 1801528. [Google Scholar] [CrossRef]
- Duan, H.; Fan, M.; Chen, W.P.; et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv. Mater. 2019, 31, 1807789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wu, J.; Liu, B.; et al. Multifunctional polymer electrolyte improving stability of electrode-electrolyte interface in lithium metal battery under high voltage. J Membr. Sci. 2019, 588, 117194. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Ma, Y.; et al. Li-ion transfer mechanism of gel polymer electrolyte with sole fluoroethylene carbonate solvent. Adv. Mater. 2023, 35, 2300998. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Zheng, S.; Li, D.; Guo, X. Self-healing polymer electrolyte for dendrite-free Li metal batteries with ultra-high-voltage Ni-rich layered cathodes. Small 2022, 18, 2200891. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Dai, K.; Liu, D.; et al. Crosslinked solubilizer enables nitrate-enriched carbonate polymer electrolytes for stable, high-voltage lithium metal batteries. Sci. Bull. 2024, 69, 209–17. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Zhao, R.; et al. In situ 3D crosslinked gel polymer electrolyte for ultra-long cycling, high-voltage, and high-safety lithium metal batteries. Energy Stor. Mater. 2023, 57, 92–101. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, R.; Zhang, J.; et al. Long-cycling and high-voltage solid state lithium metal batteries enabled by fluorinated and crosslinked polyether electrolytes. Angew. Chem. Int. Ed. 2024, 63, 202400303. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Guo, Y.; et al. In situ high-performance gel polymer electrolyte with dual-reactive cross-linking for lithium metal batteries. Energy Environ. Mater. 2024, 7, 12497. [Google Scholar] [CrossRef]
- Chen, M.; Ma, C.; Ding, Z.; et al. Upgrading electrode/electrolyte interphases via polyamide-based quasi-solid electrolyte for long-life nickel-rich lithium metal batteries. ACS Energy Lett. 2021, 6, 1280–1289. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Liang, Y.; et al. Molecular-level designed polymer electrolyte for high-voltage lithium-metal solid-state batteries. Adv. Funct. Mater. 2023, 33, 2209828. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Z.; Lin, Z.; Zhang, C.; Dai, K.; Wei, W. Organoboron- and cyano-grafted solid polymer electrolytes boost the cyclability and safety of high-voltage lithium metal batteries. ACS Appl. Mater. Interfaces. 2023, 15, 21112–21122. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Zeng, X.X.; Zhang, X.D.; et al. Engineering janus interfaces of ceramic electrolyte via distinct functional polymers for stable high-voltage Li-metal batteries. J. Am. Chem. Soc. 2019, 141, 9165–9169. [Google Scholar] [CrossRef]
- Kalhammer, F.R. Polymer electrolytes and the electric vehicle. Solid State Ionics. 2000, 135(1-4), 315-323.
- Zhang, J.; Sun, B.; Huang, X.; Chen, S.; Wang, G. Honeycomb-like porous gel polymer electrolyte membrane for lithium-ion batteries with enhanced safety. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem. 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Wu, M.; Han, S.; Liu, S.; Zhao, J.; Xie, W. Fire-safe polymer electrolyte strategies for lithium batteries. Energy Storage Mater. 2024, 66, 103174. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Wang, X.; Kan, Y.; Gui, Z.; Hu, Y. Noncombustible gel polymer electrolyte inspired by bio-radical chemistry for high voltage and high safety Ni-rich lithium batteries. J. Colloid Interface Sci. 2024, 670, 114–123. [Google Scholar] [CrossRef]
- Choudhury, S.; Tu, Z.; Nijamudheen, A.; et al. Stabilizing polymer electrolytes in high-voltage lithium batteries. Nat. Commun. 2019, 10, 1–11. [Google Scholar]
- Zhu. Y.; Chen, D.; Su, Y.; et al. Multifunctional gel polymer electrolyte suppressing lithium dendrites and stabling cathodes by asymmetric structural design. J. Electroanal.Chem. 2022, 912, 116263. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Zhao, R.; et al. In situ 3D crosslinked gel polymer electrolyte for ultra-long cycling, high-voltage, and high-safety lithium metal batteries. Energy Storage Mater. 2023, 57, 92–101. [Google Scholar] [CrossRef]
- Cho, YG.; Jung, S.H.; Jeong, J.; et al. Metal-Ion Chelating Gel Polymer Electrolyte for Ni-Rich Layered Cathode Materials at a High Voltage and an Elevated Temperature. ACS Appl. Mater. Interfaces. 2021, 13, 9965–9974. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Singh, S.K.; Meghnani, D.; Singh, R.K. High-Voltage-driven Li/Mn-rich Li1.2Mn0.6Ni0.1Co0.1O2 Cathode with nanocomposite blend gel polymer electrolyte for long cyclic stability of rechargeable Li-battery. Energy Storage. 2022, 4, 1–16. [Google Scholar] [CrossRef]
- Xu, N.; Zhao, Y.; Ni, M.; et al. In-Situ Cross-linked F- and P-Containing Solid Polymer Electrolyte for Long-Cycling and High-Safety Lithium Metal Batteries with Various Cathode Materials. Angew. Chemie. – Int. Ed. 2024, 63, 202404400. [Google Scholar] [CrossRef]
- Li, G.; Feng, Y.; Zhu, J.; et al. Achieving a Highly Stable Electrode/Electrolyte Interface for a Nickel-Rich Cathode via an Additive-Containing Gel Polymer Electrolyte. ACS Appl. Mater. Interfaces. 2022, 14, 36656–36667. [Google Scholar] [CrossRef]
- Bai, M.; Tang, X.; Zhang, M.; et al. An in-situ polymerization strategy for gel polymer electrolyte Si||Ni-rich lithium-ion batteries. Nat. Commun. 2024, 15, 1–13. [Google Scholar] [CrossRef]
- Yee, M.; An, K.; Nguyen, D.T.; et al. Confining nonflammable liquid in solid polymer electrolyte to enable nickel-rich cathode-based 4.2 V high-energy solid-state lithium-metal and lithium-ion batteries. Mater. Today Energy. 2022, 24, 100950. [Google Scholar] [CrossRef]
- Du, Y.; Xie, Y.; Chen, L.; et al. High-performance nonflammable gel polymer electrolyte with 3D interpenetrating network for advanced lithium-ion batteries. Chem. Eng. J. 2024, 493, 152810. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Xie, Y.; et al. A gel polymer electrolyte film based on chitosan derivative and ionic liquid for the LiFePO4 cathode solid Li metal battery. Mater. Today Commun. 2022, 31, 103597. [Google Scholar] [CrossRef]
- Yun, Y.S.; Song, S.W.; Lee, S.Y.; Kim, S.H.; Kim, D.W. Lithium metal polymer cells assembled with gel polymer electrolytes containing ionic liquid. Curr. App.l Phys. 2010, 10, 97–100. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chen, Y.M.; Hou, S.S.; Jan, J.S.; Lee, Y.L.; Teng, H. Diode-like gel polymer electrolytes for full-cell lithium ion batteries. J Mater. Chem. A. 2017, 5, 17476–17481. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Wang, J.; et al. Asymmetric gel polymer electrolyte with high lithium ion conductivity for dendrite-free lithium metal batteries. J. Mater. Chem A. 2020, 8, 8033–8040. [Google Scholar] [CrossRef]
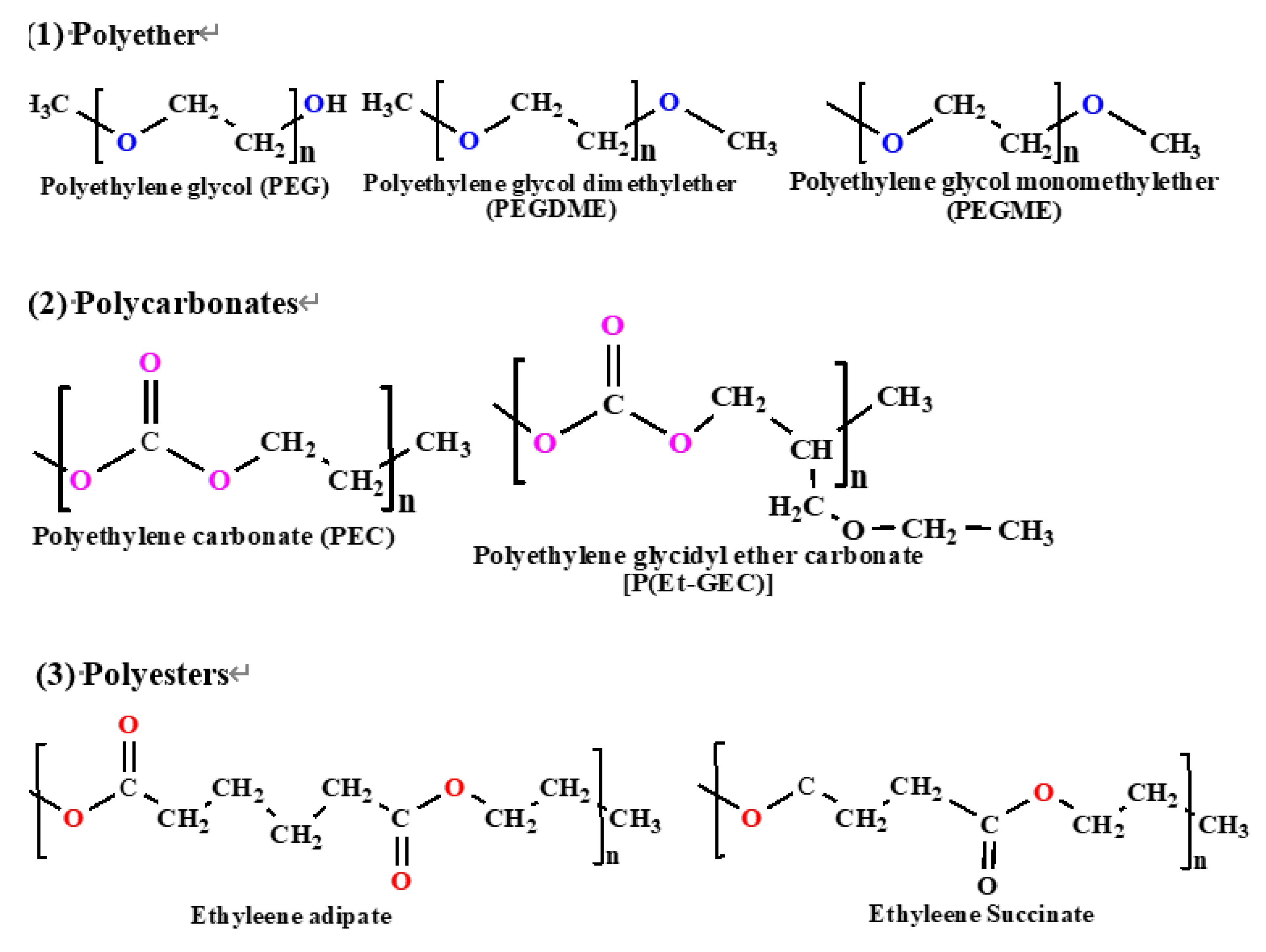
| Polymer Backbone | Chemical Structure | Key Properties | References |
|---|---|---|---|
| Polyvinylidene Fluoride (PVDF) | -(CH2-CF2)n- | High chemical and thermal stability, good mechanical properties, and electrochemical stability | [15] |
| Polyacrylonitrile (PAN) | -(CH2-CH(CN))n- | Good thermal stability, high ionic conductivity with plasticizers and salts | [16] |
| Polymethyl Methacrylate (PMMA) | -(CH2-C(CH3)(COOCH3))n- | Good mechanical strength, transparency, and stable matrix | [17] |
| Polyvinyl Alcohol (PVA) | -(CH2-CHOH)n- | Good film-forming properties, highly flexible, can form hydrogels | [18] |
| Polypropylene Oxide (PPO) | -(CH2-CH(CH3)-O)n- | Good flexibility, high ionic conductivity, good solvating power for salts | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





