Submitted:
03 August 2024
Posted:
05 August 2024
You are already at the latest version
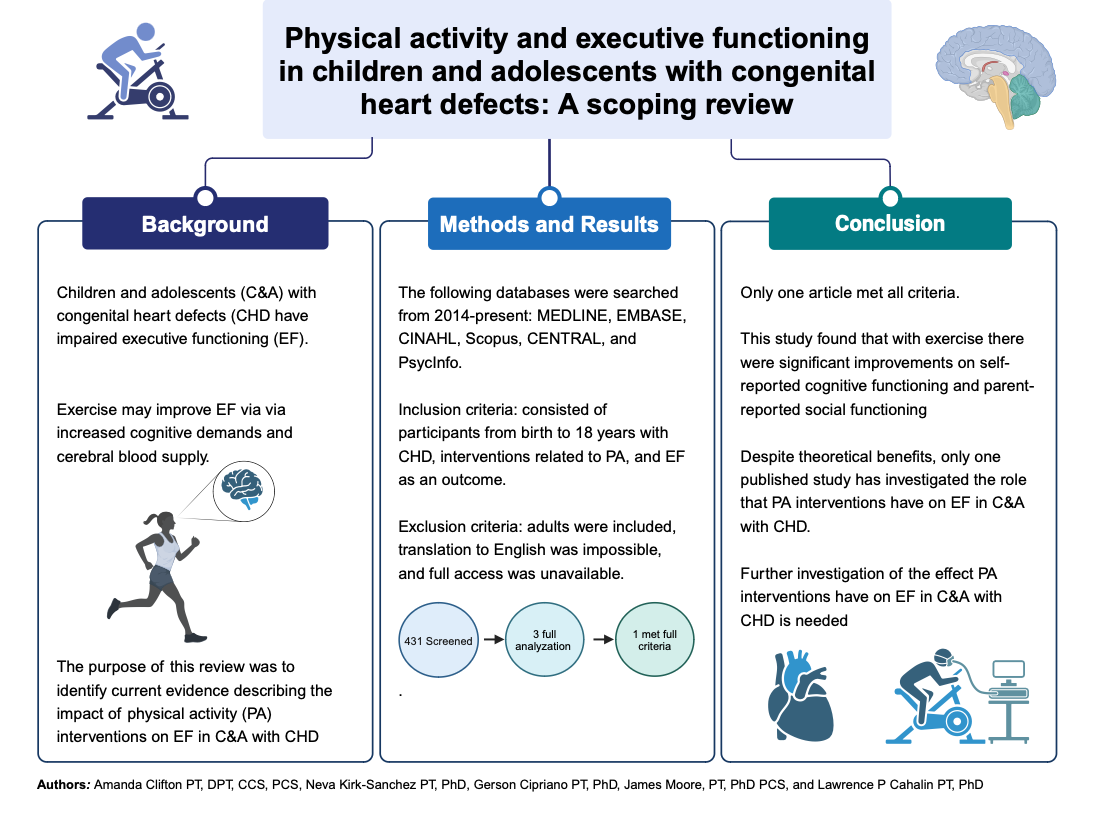
Abstract
Keywords:
1. Introduction
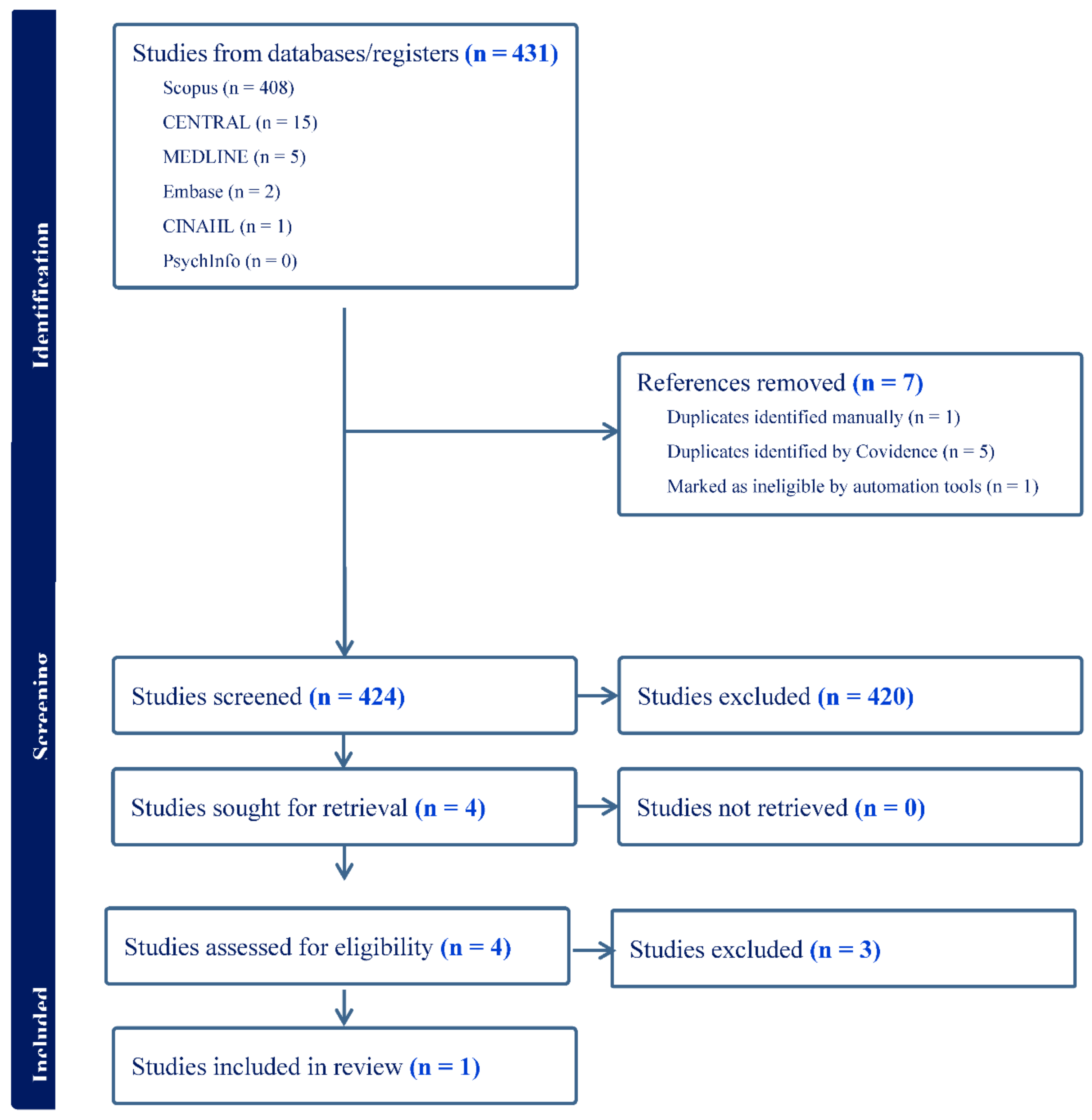
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier N, Curran T, O'Neill JA, Alexander ME, Rhodes J. Establishing a comprehensive pediatric cardiac fitness and rehabilitation program for congenital heart disease. Pediatr Cardiol. 2020;41(8):1569-1579. [CrossRef]
- Best, J.R. Effects of physical activity on children's executive function: Contributions of experimental research on aerobic exercise. Developmental Review. 2010;30(4):331-351. [CrossRef]
- Vassar R, Peyvandi S, Gano D, et al. Critical congenital heart disease beyond HLHS and TGA: neonatal brain injury and early neurodevelopment. Pediatr Res. Published online 2023. [CrossRef]
- Wong R, Al-Omary M, Baker D, et al. Cognitive dysfunction is associated with abnormal responses in cerebral blood flow in patients with single ventricular physiology: Novel insights from transcranial Doppler ultrasound. Congenit Heart Dis. 2019;14(4):638-644. [CrossRef]
- Spillmann R, Polentarutti S, Ehrler M, Kretschmar O, Wehrle FM, Latal B. Congenital heart disease in school-aged children: Cognition, education, and participation in leisure activities. Pediatr Res. Published online 2021. [CrossRef]
- Calderon J, Bellinger DC. Executive function deficits in congenital heart disease: Why is intervention important? Cardiol Young. 2014;25(7):1238-1246. [CrossRef]
- Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive function in children and adolescents with critical cyanotic congenital heart disease. Journal of the International Neuropsychological Society. 2015;21(1):34-49. [CrossRef]
- Bolduc ME, Lambert H, Ganeshamoorthy S, Brossard-Racine M. Structural brain abnormalities in adolescents and young adults with congenital heart defect: a systematic review. Dev Med Child Neurol. 2018;60(12):1209-1224. [CrossRef]
- Sanz JH, Wang J, Berl MM, Armour AC, Cheng YI, Donofrio MT. Executive function and psychosocial quality of life in school age children with congenital heart disease. J Pediatr. 2018;202:63-69. [CrossRef]
- Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity. 2006;14(3):345-356. [CrossRef]
- Byun K, Hyodo K, Suwabe K, et al. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage. 2014;98:336-345. [CrossRef]
- Lambrick D, Stoner L, Grigg R, Faulkner J. Effects of continuous and intermittent exercise on executive function in children aged 8-10 years. Psychophysiology. 2016;53(9):1335-1342. [CrossRef]
- Hsieh SS, Chueh TY, Huang CJ, et al. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J Sports Sci. 2021;39(1):10-22. [CrossRef]
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467-473. [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. [CrossRef]
- Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, eds. JBI manual for evidence synthesis. Published online 202. [CrossRef]
- Dulfer K, Duppen N, Kuipers IM, et al. Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: a randomized controlled trial. J Adolesc Health. 2014;55(1):65-72. [CrossRef]
- Cooney SJ, Campbell K, Wolfe K, DiMaria MV, Rausch CM. Is neurodevelopment related to exercise capacity in single ventricle patients who have undergone Fontan palliation? Pediatr Cardiol. 2021;42(2):408-416. [CrossRef]
- Verrall CE, Tran DL, Yang JYM, et al. Exercise as therapy for neurodevelopmental and cognitive dysfunction in people with a Fontan circulation: A narrative review. Front Pediatr. 2023;11:1111785. [CrossRef]
- Fox KR, Vannatta K, Jackson JL. Difficulties with Executive Function are associated with risky health behaviors among young adult congenital heart defect survivors. J Cardiovasc Nurs. 2023;38(1):60-69. [CrossRef]
- Kobayashi K, Liu C, Jonas RA, Ishibashi N. The current status of neuroprotection in congenital heart disease. Children (Basel). 2021;8(12):1116. [CrossRef]
- van der Mheen M, Meentken MG, van Beynum IM, et al. CHIP-Family intervention to improve the psychosocial well-being of young children with congenital heart disease and their families: results of a randomised controlled trial. Cardiol Young. 2019;29(9):1172-1182. [CrossRef]
- Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143-1172. [CrossRef]
- Bellinger DC, Rappaport LA, Wypij D, Wernovsky G, Newburger JW. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr. 1997;18(2):75-83. [CrossRef]
- Bellinger DC, Wypij D, Kuban KC, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100(5):526-532. [CrossRef]
- Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1385-1396. [CrossRef]
- Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361-1369. [CrossRef]
- Bellinger DC, Watson CG, Rivkin MJ, et al. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc. 2015;4(12). [CrossRef]
- Sananes R, Goldberg CS, Newburger JW, et al. Six-year neurodevelopmental outcomes for children with single-ventricle physiology. Pediatrics. 2021;147(2):e2020014589. [CrossRef]
- Sanz JH, Berl MM, Armour AC, Wang J, Cheng YI, Donofrio MT. Prevalence and pattern of executive dysfunction in school age children with congenital heart disease. Congenit Heart Dis. 2017;12(2):202-209. [CrossRef]
- Rogers S, Dixon B. The new age of cardiac rehab: Do adult protocols meet the needs of children? Arch Phys Med Rehabil. 2021;102(10):e114.
- Kwon SJ, Choi EK, Lee KH, Im YM. Factors influencing physical activity in adolescents with complex congenital heart disease. Child Health Nurs Res. 2019;25(3):262-272. [CrossRef]
- Lui GK, Saidi A, Bhatt AB, et al. Diagnosis and management of noncardiac complications in adults with congenital heart disease: A scientific statement from the American heart association. Circulation. 2017;136(20):e348-e392.
- Soshi T, Andersson M, Kawagoe T, et al. Prefrontal plasticity after a 3-month exercise intervention in older adults relates to enhanced cognitive performance. Cereb Cortex. 2021;31(10):4501-4517. [CrossRef]
- Wilckens KA, Stillman CM, Waiwood AM, et al. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus. 2021;31(3):335-347. [CrossRef]
- Raichlen DA, Klimentidis YC, Bharadwaj PK, Alexander GE. Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle-aged to older adults. Brain Imaging Behav. 2020;14(5):1994-2003. [CrossRef]
- Chaddock L, Erickson KI, Prakash RS, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172-183. [CrossRef]
- Riggs L, Piscione J, Laughlin S, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. Published online 2016:now177. [CrossRef]
- Sanz JH, Berl MM, Armour AC, Wang J, Cheng YI, Donofrio MT. Prevalence and pattern of executive dysfunction in school age children with congenital heart disease. Congenit Heart Dis. Mar 2017;12(2):202-209. [CrossRef]
| Author | Dulfer et al [17] | Cooney et al [18] | Verrall et al [19] |
|---|---|---|---|
| Aims/Purpose | To investigate the effect of an exercise program on HRQoL in children and adolescents with TOF or Fontan circulation. | To characterize the relationship between neurodevelopment and exercise capacity in SVHD post Fontan by evaluating associations between CPET and clinical NPT. |
To discuss current interventions and evidence supporting exercise as a potential intervention for improving cognitive functioning in people with Fontan circulation. |
| Population | 93 participants, ages 10-25 years with surgical repair for ToF or with Fontan circulation. |
23 participants, ages 7- 17 years old with Fontan circulation. |
Discusses impact from fetus to aging adult. |
| Methodology | Stratified, randomized controlled intervention conducted in five pediatric centers in The Netherlands. Random allocation with a ratio of 2:1 to a 12-week period with an exercise program for three times per week or a control group. | Retrospective, cross-sectional pilot study conducted in the United States. One time conduction of CPET with gas analysis and one time conduction of NPT. | Narrative summary and discussion with following categories : established neurodevelopmental and cognitive interventions; exercise, cognition, and Fontan physiology; neural mechanisms underpinning the exercise-cognition relationship; and psychosocial and behavioral mediators of the exercise-cognition relationship |
| Interventions | Exercise program consisted of three one-hour long training sessions a week. Patients already active were encouraged to continue to do activities two times a week. Hour session consisted of 10 minute warm up, 40 minutes aerobic training, and 10-minute cooldown. Participants trained within given heart rate ranges. | Exercise testing was performed with a Medgraphics (Saint Paul, MN) metabolic cart. Breath-by-breath data were collected and averaged over 20 s intervals. Patients performed a symptom-limited test using a ramp protocol on a cycle ergometer. Monitoring of oxygen saturation, electrocardiogram, and blood pressure was also measured. NPT was conducted in a single session on a day separate from the CPET. Standardized scores were assessed from tests of working memory, processing speed, sustained visual attention, executive function, parent ratings of adaptive function, and internalizing problems. Scores were standardized by age. |
Individuals with Fontan physiology have greater risk of neurodevelopmental and cognitive impairments. Exercise is low-risk and has benefits on physical and cognitive functioning. Future research is needed to provide exercise prescription and determine accessible interventions. |
| Outcomes | At baseline and follow up after 12 weeks, participants and parents as appropriate completed the HRQoL measures: for the 10-15 group, TACQOL CF and TACQOL PF; for the 16-25 group, SF-36 and CONHD-TAAQOL; and for the total group 10-25, LAS. |
CPET measures: VO2max indexed to body weight, anaerobic threshold, peak heart rate, ventilatory efficiency, pulmonary vasodilator use, and RER. Results were compared to percent predicted values for VO2max and peak heart rate based on gender, height, and weight. NPT measures: executive function (WAIS-IV or WISC-V and TOL), attention (CPT-2,3), adaptive function (ABAS-2,3), and emotional function (BASC-2,3) |
|
| Key Findings | Compared with the control-group, children, aged 10-15 years in the exercise-group improved significantly on self-reported cognitive functioning and parent-reported social functioning. Increase noted in this group with lower baseline HRQoL. Participants aged 16-25 years did not change their HRQoL. | Higher VO2max and anaerobic threshold were related to better adaptive functioning scores and higher peak heart rate was related to better scores on a measure of sustained visual attention. The relationship appeared strongest in relation to adaptive function, as both higher VO2 max and anaerobic threshold were significantly associated with a higher global adaptive composite score. CPET variables related to working memory, processing speed, executive functioning, or internalizing symptoms were not significant. Ventilatory efficiency was not significantly related to any of the NPT variables | Interventions for impaired neurodevelopment and cognitive dysfunction in people with Fontan circulation are lacking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





