Submitted:
02 August 2024
Posted:
06 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
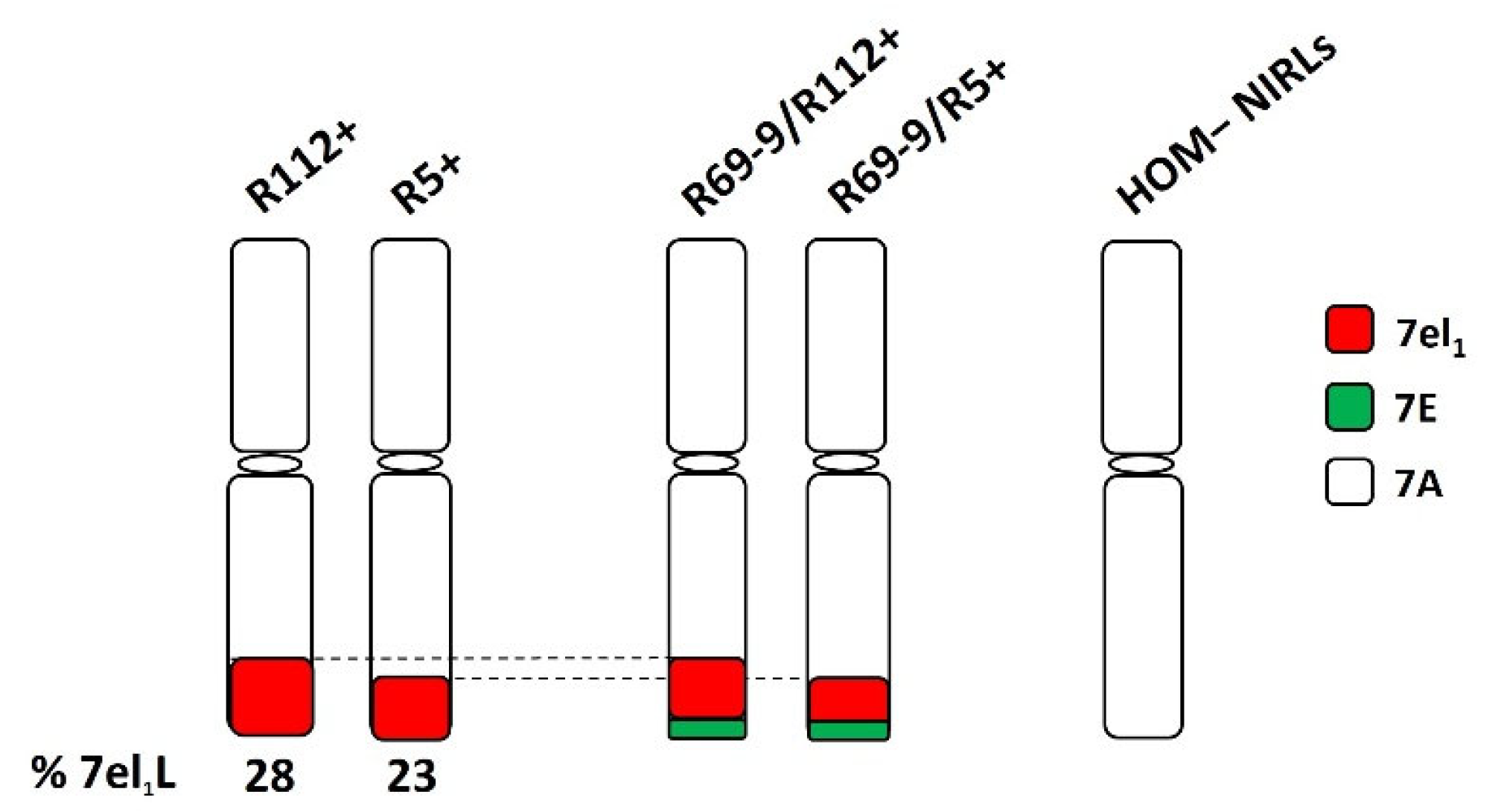
2.1. Plant Materials
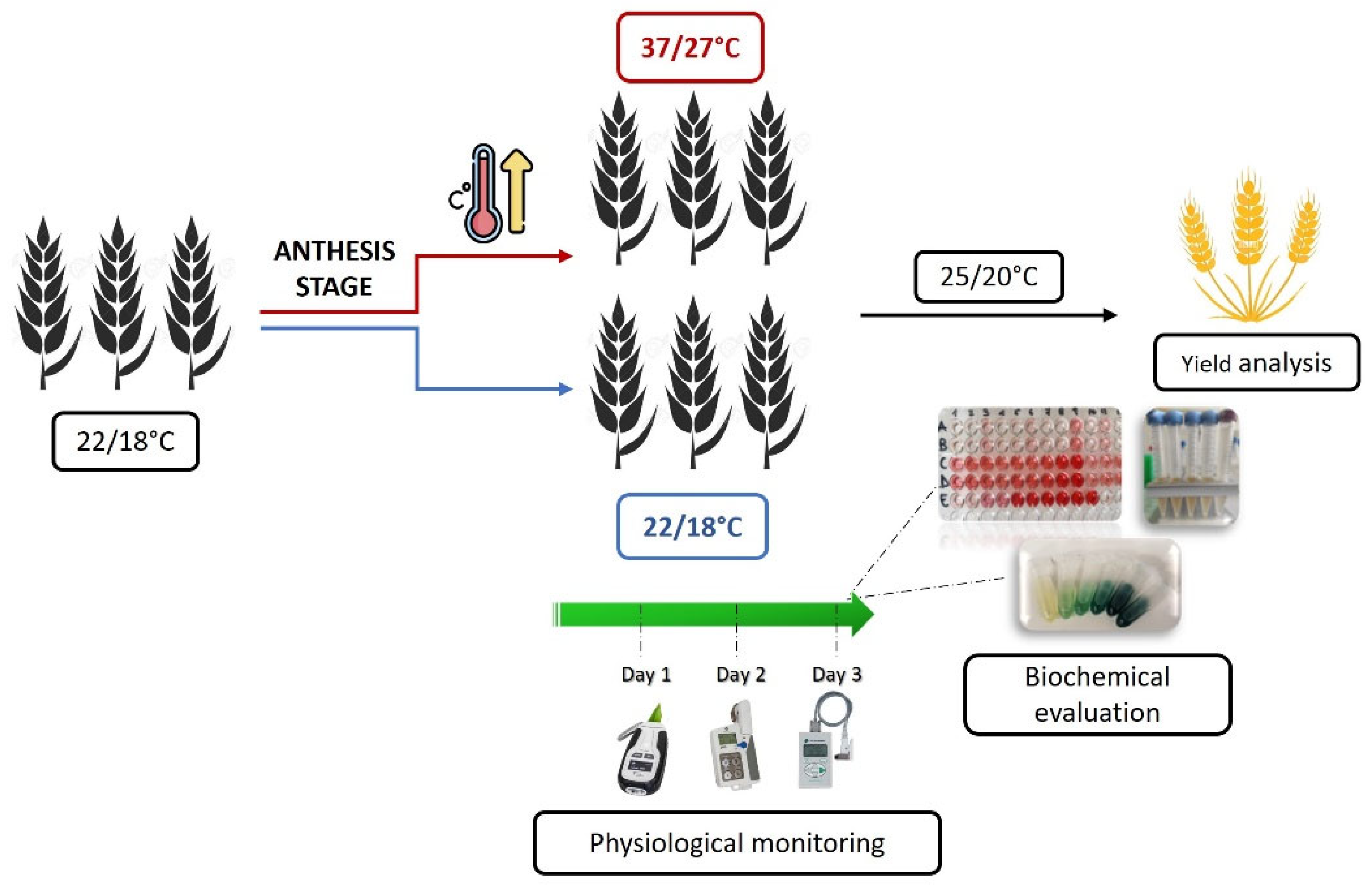
2.2. Plant Growing Conditions and Experimental Design
2.3. Physiological Traits
2.4. Biochemical Assays
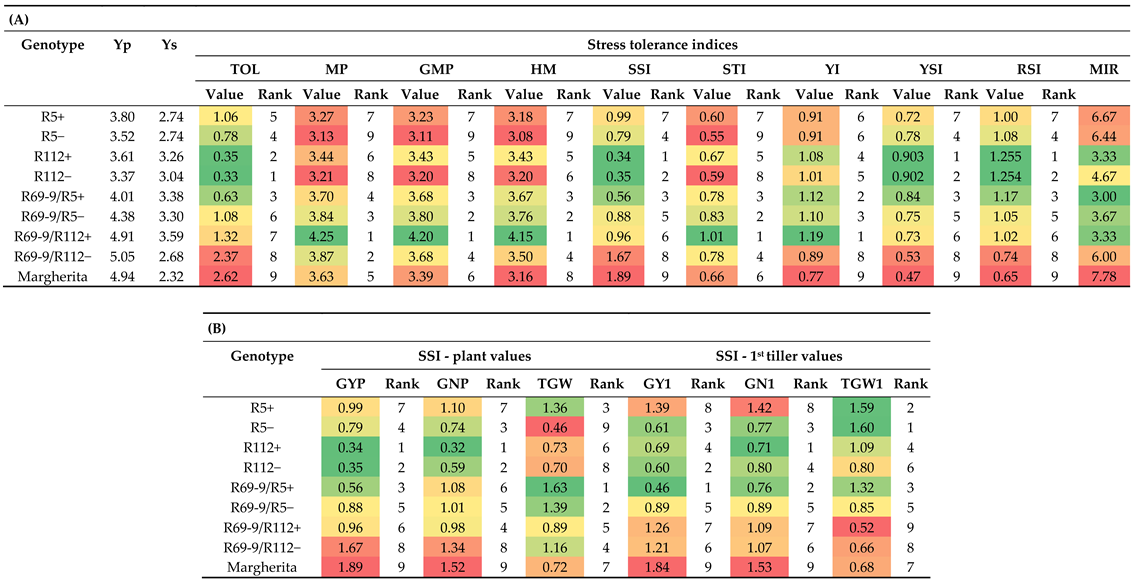
2.5. Yield-related Parameters and Stress Indices
- Tolerance index (TOL) = Yp − Ys
- Stress susceptibility index (SSI) = (Ys ×Yp)/(Ȳp)²
- Stress tolerance index (STI) = (Ys ×Yp)/(Ȳp)²
- Yield index (YI) = Ys/Ȳs
- Yield stability index (YSI) = Ys/Yp
- Relative stress index (RSI) = (Ys/Yp)/(Ȳs/Ȳp)
- Mean productivity (MP) = (Yp + Ys)/2
- Geometric mean productivity (GMP) = √(Ys ×Yp)
- Harmonic mean (HM) = 2(Ys ×Yp)/(Ys + Yp)
2.6. Statistical Analysis
3. Results
3.1. Intense Heat (IH) During Flowering: Main Traits Modulated
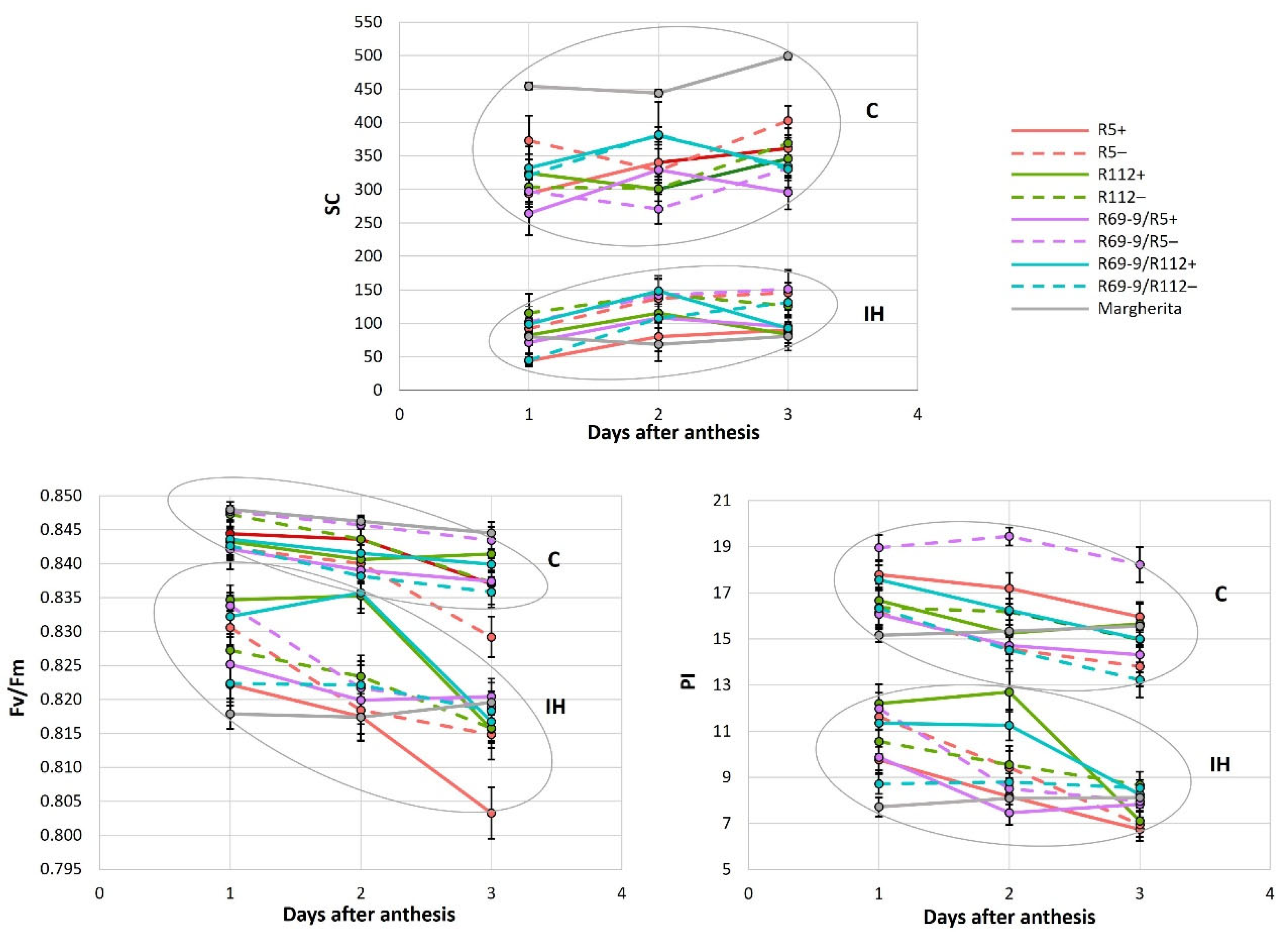
3.2. Physiological perturbations occurring during IH stress application
3.3. Membrane Damage Rate in IH Stressed Leaves
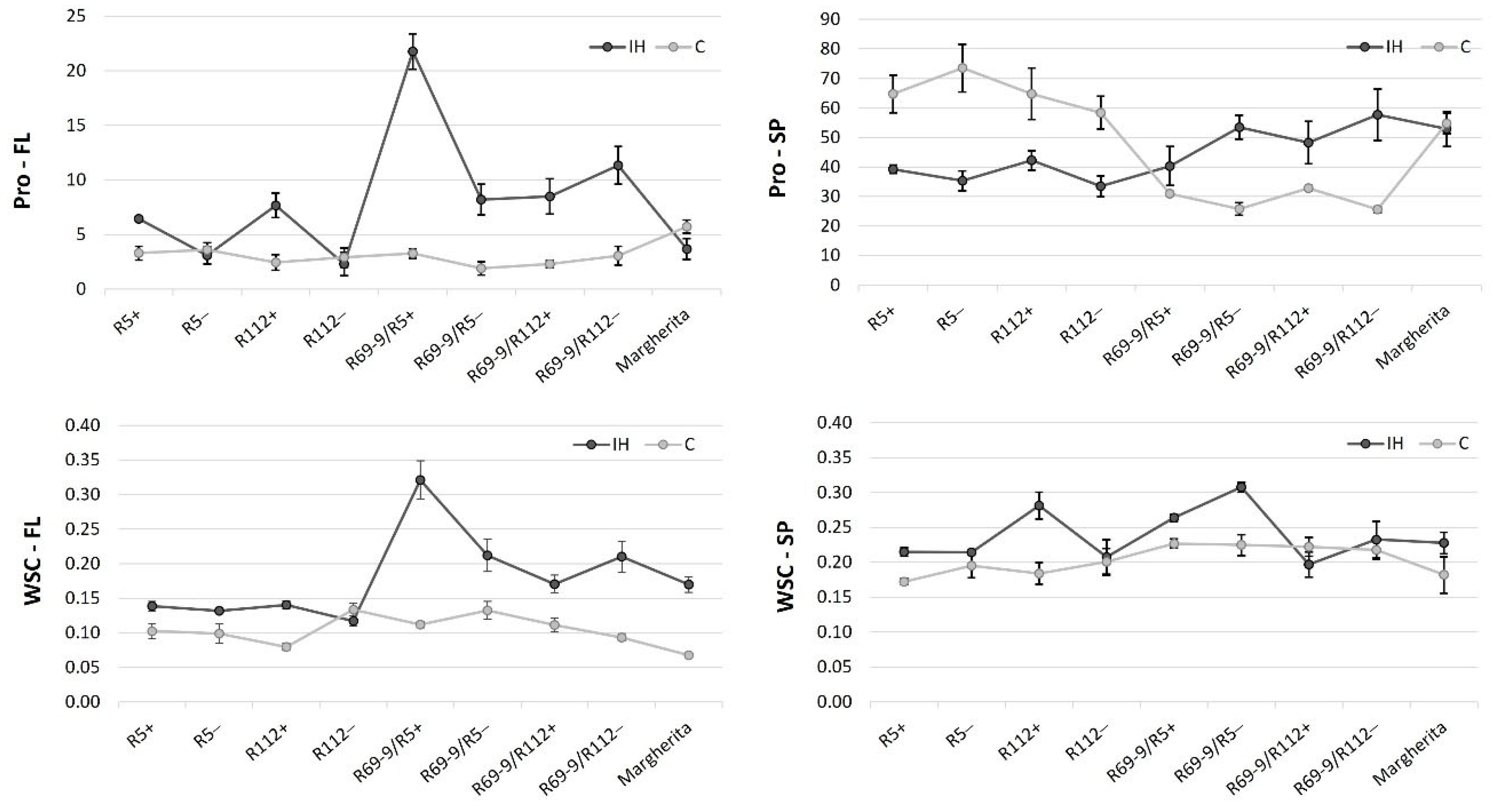
3.4. Osmolytes Accumulation in Flag Leaves and Spikes After Stress
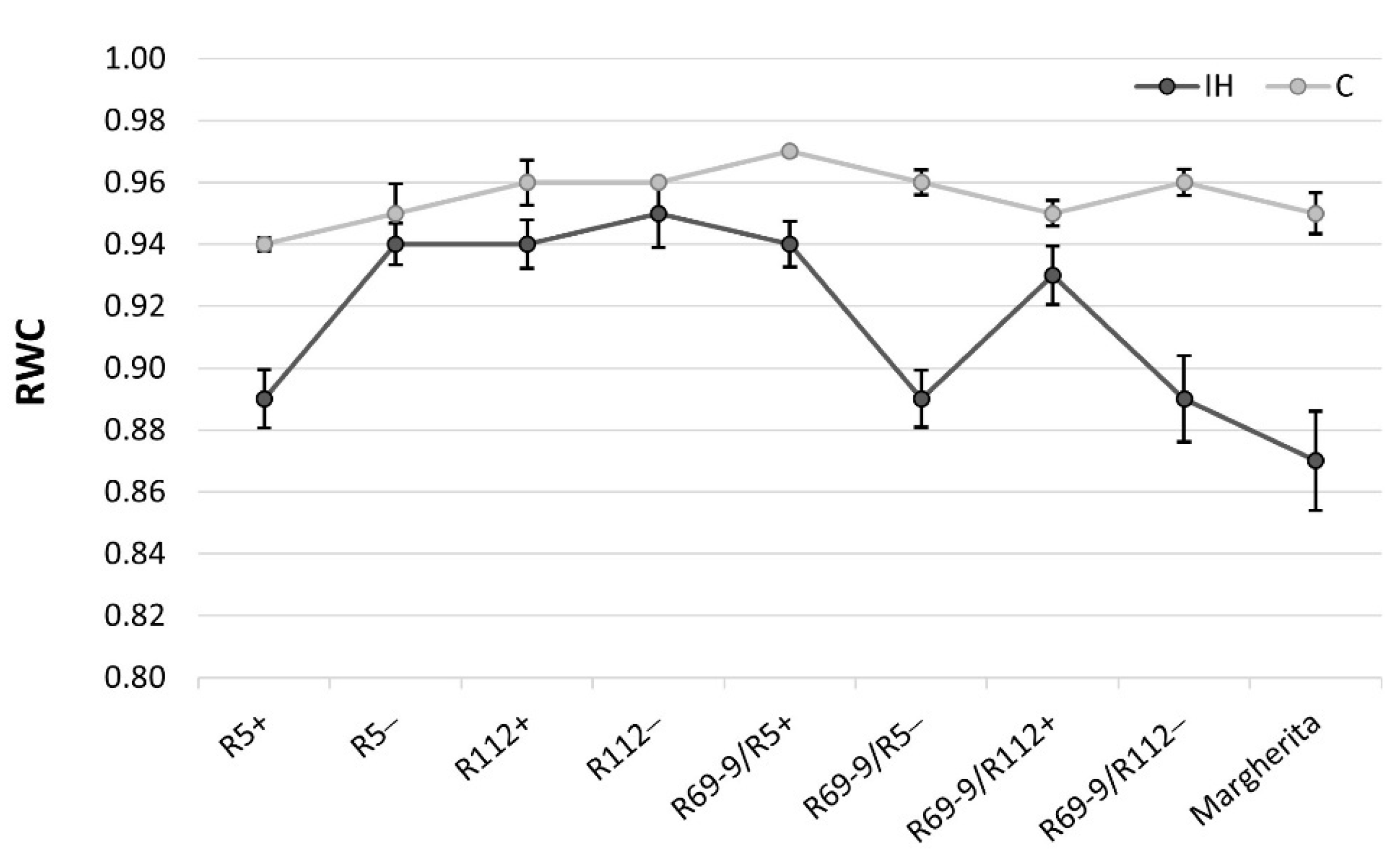
3.5. Relative Water Content Determination
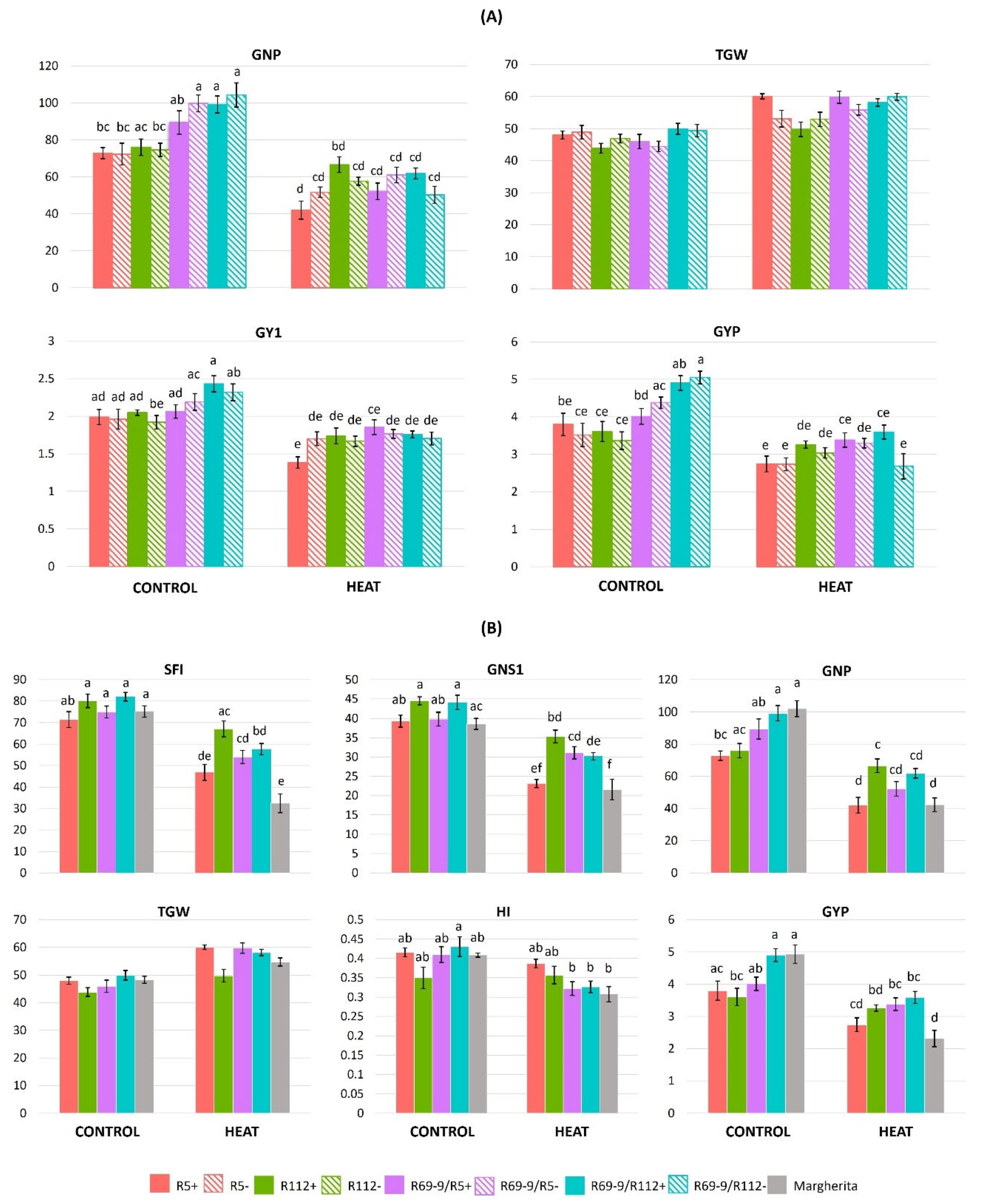
3.6. Effects of Heat Stress on Plant Productivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lidon, F.C.; Almeida, A.S.; Leitão, A.L.; Silva, M.M.; Pinheiro, N.; Maçãs, B.; Costa, R. A synoptic overview of durum wheat production in the Mediterranean region and processing following the European Union requirements. Emir. J. Food Agric. 2014, 26, 693–705. [Google Scholar] [CrossRef]
- Zampieri, M.; Toreti, A.; Ceglar, A.; Naumann, G.; Turco, M.; Tebaldi, C. Climate resilience of the top ten wheat producers in the Mediterranean and the Middle East. Reg. Environ. Change 2020, 20, 41. [Google Scholar] [CrossRef]
- Ali, E.; Cramer, W.; Carnicer, J.; Georgopoulou, E.; Hilmi, N.J.M.; Le Cozannet, G.; Lionello, P. Cross-Chapter Paper 4: Mediterranean Region. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 2233–2272. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum wheat breeding in the Mediterranean region: current status and future prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Ceglar, A.; Toreti, A.; Zampieri, M.; Royo, C. Global loss of climatically suitable areas for durum wheat growth in the future. Environ. Res. Lett. 2021, 16, 104049. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.J. Global trends in wheat production, consumption and trade. In Wheat Improvement; Reynolds, M.P., Braun, H.-J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Dettori, M.; Cesaraccio, C.; Duce, P. Simulation of climate change impacts on production and phenology of durum wheat in Mediterranean environments using CERES-wheat model. Field Crop. Res. 2017, 206, 43–53. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat stress in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Jacott, C.N.; Boden, S.A. Feeling the heat: developmental and molecular responses of wheat and barley to high ambient temperatures. J. Exp. Bot. 2020, 71, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K. Heat stress during flowering in cereals—Effects and adaptation strategies. New Phytol. 2020, 226, 1567–1572. [Google Scholar] [CrossRef]
- Mirosavljević, M.; Mikić, S.; Župunski, V.; Kondić Špika, A.; Trkulja, D.; Ottosen, C.O.; Zhou, R.; Abdelhakim, L. Effects of high temperature during anthesis and grain filling on physiological characteristics of winter wheat cultivars. J. Agron. Crop. Sci. 2021, 207(5), 823–832. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Sun, J.; Mao, F.; Yao, Q.; Li, B.; Wang, Y.; Gao, Y.; Dong, X.; Liao, S.; et al. From the floret to the canopy: High temperature tolerance during flowering. Plant. Commun. 2023, 23, 100629. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sunoj, V.S.J.; Saripalli, G.; Prasad, P.V.V.; Balyan, H.S.; Gupta, P.K.; Grant, N.; Gill, K.S.; Jagadish, S.V.K. Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop. Sci. 2019, 59, 684–696. [Google Scholar] [CrossRef]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant. Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.-S.P. Heat stress effects on source–sink relationships and metabolome dynamics in wheat. J. Exp. Bot. 2020, 71, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustainable Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef] [PubMed]
- Mamrutha, H.M.; Rinki, K.; Venkatesh, K.; Gopalareddy, K.; Khan, H.; Mishra, C.N.; Kumar, S.; Kumar, Y.; Singh, G.; Singh, G.P. Impact of high night temperature stress on different growth stages of wheat. Plant Physiol. Rep. 2020, 25, 707–715. [Google Scholar] [CrossRef]
- Cossani, C.M.; Reynolds, M.P. Physiological traits for improving heat tolerance in wheat. Plant Physiol. 2012, 160, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- García, G.A.; Dreccer, M.F.; Miralles, D.J.; Serrago, R.A. High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Glob. Chang. Biol. 2015, 21, 4153–4164. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.V.; Fritz, A.K.; Boyle, D.L.; Gill, B.S. Impact of high night-time and high daytime temperature stress on winter wheat. J. Agron. Crop Sci. 2015, 201, 206–218. [Google Scholar] [CrossRef]
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell. Environ. 2021, 44, 2049–2065. [Google Scholar] [CrossRef]
- Farhad, M.; Kumar, U.; Tomar, V.; Bhati, P.K.; Krishnan, J.N.; Barek, V.; Brestic, M.; Hossain, A. Heat stress in wheat: A global challenge to feed billions in the current era of the changing climate. Front. Sustain. Food Syst. 2023, 7, 1203721. [Google Scholar] [CrossRef]
- Molero, G.; Coombes, B.; Joynson, R.; Pinto, F.; Piñera-Chávez, F.J.; Rivera-Amado, C.; Hall, A.; Reynolds, M.P. Exotic alleles contribute to heat tolerance in wheat under field conditions. Commun. Biol. 2023, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, M.; Ahmed, M.; Iftikhar Hussain, M. Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Sihag, P.; Kumar, U.; Sagwal, V.; Kapoor, P.; Singh, Y.; Mehla, S.; Balyan, P.; Mir, R.R.; Varshney, R.K.; Singh, K.P.; et al. Effect of terminal heat stress on osmolyte accumulation and gene expression during grain filling in bread wheat (Triticum aestivum L.). Plant Genome 2024, e20307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Liu, G.; Tang, Y.; Zhou, C.; Zhang, L.; Lv, J. The spike plays important roles in the drought tolerance as compared to the flag leaf through the phenylpropanoid pathway in wheat. Plant Physiol. Biochem. 2020, 152, 100–111. [Google Scholar] [CrossRef]
- Vergara-Diaz, O.; Vatter, T.; Vicente, R.; Obata, T.; Nieto-Taladriz, M.T.; Aparicio, N.; Kefauver, S.C.; Fernie, A.; Araus, J.L. Metabolome profiling supports the key role of the spike in wheat yield performance. Cells 2020, 9, 1025. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Shamim, M.; Mishra, U.; Jain, M.; Singh, K.; et al. Biochemical defense response: characterizing the plasticity of source and sink in spring wheat under terminal heat stress. Front. Plant Sci. 2017, 8, 1603. [Google Scholar] [CrossRef]
- Frimpong, F.; Windt, C.W.; van Dusschoten, D.; Naz, A.A.; Frei, M.; Fiorani, F. A wild allele of pyrroline-5-carboxylate synthase1 leads to proline accumulation in spikes and leaves of barley contributing to improved performance under reduced water availability. Front. Plant Sci. 2021, 12, 633448. [Google Scholar] [CrossRef] [PubMed]
- El Habti, A.; Fleury, D.; Jewell, N.; Garnett, T.; Tricker, P.J. Tolerance of combined drought and heat stress is associated with transpiration maintenance and water soluble carbohydrates in wheat grains. Front. Plant Sci. 2020, 11, 568693. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the wild—Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Pour-Aboughadareh, A.; Kianersi, F.; Poczai, P.; Moradkhani, H. Potential of wild relatives of wheat: ideal genetic resources for future breeding programs. Agronomy 2021, 11, 1656. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S. A walk towards wild grasses to unlock the clandestine of gene pools for wheat improvement: A review. Plant Stress 2022, 3, 100048. [Google Scholar] [CrossRef]
- El Haddad, N.; Kabbaj, H.; Zaïm, M.; El Hassouni, K.; Sall, A.T.; Azouz, M.; Ortiz, R.; Baum, M.; Amri, A.; Gamba, F.; Bassi, F.M. Crop wild relatives in durum wheat breeding: Drift or thrift? Crop Sci. 2021, 61, 37–54. [Google Scholar] [CrossRef]
- Monsen, S.B.; Stevens, R.; Shaw, N.L. Grasses. In Restoring Western Ranges and Wildlands; U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; Volume 2, pp. 295–424. [Google Scholar]
- Wang, R.R.C. Agropyron and Psathyrostachys. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–108. [Google Scholar] [CrossRef]
- Ceoloni, C.; Forte, P.; Gennaro, A.; Micali, S.; Carozza, R.; Bitti, A. Recent developments in durum wheat chromosome engineering. Cytogenet. Genome Res. 2005, 109, 328–334. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Forte, P.; Gennaro, A.; Bitti, A. Targeted exploitation of gene pools of alien Triticeae species for sustainable and multi-faceted improvement of the durum wheat crop. Crop Pasture Sci. 2014, 65, 96–111. [Google Scholar] [CrossRef]
- Sears, E.R. Chromosome engineering in wheat. Stadler Genet. Symp. 1972, 4, 23–38. [Google Scholar]
- Ceoloni, C.; Kuzmanović, L.; Forte, P.; Virili, M.E.; Bitti, A. Wheat-perennial Triticeae introgressions: Major achievements and prospects. In Alien Introgression in Wheat—Cytogenetics, Molecular Biology, and Genomics; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 273–313. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Gennaro, A.; Benedettelli, S.; Dodd, I.C.; Quarrie, S.A.; Ceoloni, C. Structural-functional dissection and characterization of yield-contributing traits originating from a group 7 chromosome of the wheatgrass species Thinopyrum ponticum after transfer into durum wheat. J. Exp. Bot. 2014, 65, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, L.; Mandalà, G.; Tundo, S.; Ciorba, R.; Frangella, M.; Ruggeri, R.; Rossini, F.; Gevi, F.; Rinalducci, S.; Ceoloni, C. Equipping durum wheat—Thinopyrum ponticum recombinant lines with a Thinopyrum elongatum major QTL for resistance to Fusarium diseases through a cytogenetic strategy. Front. Plant Sci. 2019, 10, 1324. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Ruggeri, R.; Virili, M.E.; Rossini, F.; Ceoloni, C. Effects of Thinopyrum ponticum chromosome segments transferred into durum wheat on yield components and related morpho-physiological traits in Mediterranean rain-fed conditions. Field Crop. Res. 2016, 186, 86–98. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Ruggeri, R.; Able, J.A.; Bassi, F.M.; Maccaferri, M.; Tuberosa, R.; De Vita, P.; Rossini, F.; Ceoloni, C. Yield of chromosomally engineered durum wheat-Thinopyrum ponticum recombinant lines in a range of contrasting rain-fed environments. Field Crops Res. 2018, 228, 147–157. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Giovenali, G.; Ruggeri, R.; Rossini, F.; Ceoloni, C. Small “nested” introgressions from wild Thinopyrum species, conferring effective resistance to Fusarium diseases, positively impact durum wheat yield potential. Plants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, L.; Menasria, H.; Rouabhi, A.; Giovenali, G.; Capoccioni, A.; Saveriano, M.; Di Romana, M.; Ruggeri, R.; Ceoloni, C. Performance of locally adapted durum wheat germplasm in the Mediterranean basin and recombinant lines with Thinopyrum spp. introgressions across Algerian and Italian environments with different water availability. In Proceedings of the From Seed To Pasta IV International Conference, Bologna, Italy, 26–29 October 2022. Available online: https://www.fromseedtopasta.com/wp-content/uploads/2022/10/45_Ceoloni.pdf.
- Tounsi, S.; Giorgi, D.; Kuzmanović, L.; Jrad, O.; Farina, A.; Capoccioni, A.; Ben Ayed, R.; Brini, F.; Ceoloni, C. Coping with salinity stress: segmental group 7 chromosome introgressions from halophytic Thinopyrum species greatly enhance tolerance of recipient durum wheat. Front. Plant Sci. 2024, 15, 1378186. [Google Scholar] [CrossRef] [PubMed]
- Giovenali, G.; Kuzmanović, L.; Capoccioni, A.; Ceoloni, C. The response of chromosomally engineered durum wheat-Thinopyrum ponticum recombinant lines to the application of heat and water-deficit stresses: effects on physiological, biochemical and yield-related traits. Plants 2023, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Giovenali, G.; Di Romana, M.L., Kuzmanović, L.; Capoccioni, A.; Ceoloni, C. Early selection of stress tolerant durum wheat recombinant lines: targeted introgressions of Thinopyrum spp. chromatin improve seedlings response to heat and water stress. Proceedings of the LXVI SIGA (Italian Society for Agricultural Genetics) Annual Congress, “Climate-smart plants to feed the future”; Bari, Italy, 5-8 September 2023. ISBN: 978-88-944843-4-2. Abstract 2.16. Available online: http://www.geneticagraria.it/congress_abstract.asp?a_pag=4&id=70.
- Sall, A.T.; Bassi, F.M.; Cisse, M.; Gueye, H.; Ndoye, I.; Filali-Maltouf, A.; Ortiz, R. Durum wheat breeding: In the heat of the Senegal river. Agriculture 2018, 8, 99. [Google Scholar] [CrossRef]
- Sall, A.T.; Cisse, M.; Gueye, H.; Kabbaj, H.; Ndoye, I.; Filali-Maltouf, A.; Belkadi, B.; El-Mourid, M.; Ortiz, R.; Bassi, F.M. Heat tolerance of durum wheat (Triticum durum Desf.) elite germplasm tested along the Senegal river. J. Agric. Sci. 2018, 10, 217. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Genotypic performance of Australian durum under single and combined water-deficit and heat stress during reproduction. Sci. Rep. 2019, 9, 14986. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, H.; Wu, Y.; Yan, G. Development and characterization of near-isogenic lines revealing candidate genes for a major 7AL QTL responsible for heat tolerance in wheat. Front. Plant Sci. 2020, 11, 1316. [Google Scholar] [CrossRef] [PubMed]
- Abrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. Methods Mol. Biol. 2010, 639, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 291–297. [Google Scholar] [CrossRef]
- Khan, A.A.; Kabir, M.R. Evaluation of spring wheat genotypes (Triticum aestivum L.) for heat stress tolerance using different stress tolerance indices. Cercet. Agron. Mold. 2015, 47, 49–63. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Moghaddam Vahed, M.; Poczai, P.; Siddique, K.H.M. iPASTIC: An online toolkit to estimate plant abiotic stress indices. Appl. Plant Sci. 2019, 7, e11278. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.B.; Poudel, M.R.; Puri, R.R. Evaluation of heat stress tolerance in spring wheat (Triticum aestivum L.) genotypes using stress tolerance indices in western region of Nepal. J. Agric. Food Res. 2021, 5, 100179. [Google Scholar] [CrossRef]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Sharma, R.; Yashveer, S.; Dalal, M.S. Heat stress tolerance indices for identification of the heat tolerant wheat genotypes. Sci. Rep. 2023, 13, 10842. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Nadeem, F.; Nawaz, A.; Siddique, K.H.; Farooq, M. Heat stress effects on the reproductive physiology and yield of wheat. J. Agron. Crop Sci. 2022, 208, 1–17. [Google Scholar] [CrossRef]
- Kim, J.; Savin, R.; Slafer, G.A. Quantifying pre- and post-anthesis heat waves on grain number and grain weight of contrasting wheat cultivars. Field Crop. Res. 2024, 307, 109264. [Google Scholar] [CrossRef]
- Ni, Z.; Li, H.; Zhao, Y.; Peng, H.; Hu, Z.; Xin, M.; Sun, Q. Genetic improvement of heat tolerance in wheat: recent progress in understanding the underlying molecular mechanisms. Crop J. 2018, 6, 32–41. [Google Scholar] [CrossRef]
- Poudel, P.B.; Poudel, M.R. Heat stress effects and tolerance in wheat: A review. J. Biol. Today’s World 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Dhakal, A.; Adhikari, C.; Manandhar, D.; Bhattarai, S.; Shrestha, S. Effect of abiotic stress in wheat: a review. Rev. Food. Agric. 2021, 2, 69–72. [Google Scholar] [CrossRef]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant. Biol. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bahar, B.; Yildirim, M.; Yucel, C. Heat and drought resistance criteria in spring bread wheat (Triticum aestivum L.): Morpho-physiological parameters for heat tolerance. Sci. Res. Essays 2011, 6, 2212–2220. [Google Scholar] [CrossRef]
- Ramya, K.T.; Jain, N.; Amasiddha, B.; Singh, P.K.; Arora, A.; Singh, G.P.; Prabhu, K.V. Genotypic response for stomatal conductance due to terminal heat stress under late sown condition in wheat (Triticum aestivum L.). Indian. J. Genet. Plant Breed. 2016, 76, 255–265. [Google Scholar] [CrossRef]
- Aparecido, L.M.T.; Woo, S.; Suazo, C.; Hultine, K.R.; Blonder, B. High water use in desert plants exposed to extreme heat. Ecol. Lett. 2020, 23, 1189–1200. [Google Scholar] [CrossRef]
- Alghabari, F.; Shah, Z.H.; Elfeel, A.A.; Alyami, J.H. Biochemical and physiological responses of thermostable wheat genotypes for agronomic yield under heat stress during reproductive stages. Agronomy 2021, 11, 2080. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Kathy Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Virili, M.; Kuzmanović, L.; Bitti, A.; Salvi, S.; Tuberosa, R.; Ceoloni, C. Analysis of seminal root architecture in durum wheat-Thinopyrum ponticum recombinant lines. In Proceedings of the Joint Meeting SIBV-SIGA, Milano, Italy, 8–11 September 2015; Available online: http://www.geneticagraria.it/congress_abstract.asp?a_pag=4&Indice=250&id=59.
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2014, 153, 284–298. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [CrossRef]
- Zhang, J.; Tan, D.K.Y.; Shaghaleh, H.; Chang, T.; Alhaj Hamoud, Y. Response of photosynthesis in wheat (Triticum aestivum L.) cultivars to moderate heat stress at meiosis and anthesis stages. Agronomy 2023, 13, 2251. [Google Scholar] [CrossRef]
- Dias, A.S.; Barreiro, M.G.; Campos, P.S.; Ramalho, J.C.; Lidon, F.C. Wheat cellular membrane thermotolerance under heat stress. J. Agron. Crop Sci. 2010, 196, 100–108. [Google Scholar] [CrossRef]
- Dhyani, K.; Ansari, M.W.; Rao, Y.R.; Verma, R.S.; Shukla, A.; Tuteja, N. Comparative physiological response of wheat genotypes under terminal heat stress. Plant Signal. Behav. 2013, 8, e24564. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dinler, B.S.; Vignjevic, M.; Jacobsen, S.; Wollenweber, B. Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci. 2015, 230, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sinha, K.; Bhunia, R.K. Can wheat survive in heat? Assembling tools towards successful development of heat stress tolerance in Triticum aestivum, L. Mol. Biol. Rep. 2019, 46, 2577–2593. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Basu, S.; Kumar, S.; Kumari, S.; Kumar, A.; Jha, S.; Mishra, J.S.; Bhatt, B.P.; Kumar, G. Enhanced antioxidant enzyme activities in developing anther contributes to heat stress alleviation and sustains grain yield in wheat. Funct. Plant Biol. 2019, 46, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Impa, S.M.; Sunoj, V.S.J.; Krassovskaya, I.; Bheemanahalli, R.; Obata, T.; Jagadish, S.V.K. Carbon balance and source-sink metabolic changes in winter wheat exposed to high night-time temperature. Plant Cell Environ. 2019, 42, 1233–1246. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Jingbo Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, R.; Vergara-Díaz, O.; Schlereth, A.; Höhne, M.; Morcuende, R.; Nieto-Taladriz, M.T.; Araus, J.L.; Aparicio, N.; Vicente, R. Analysis of durum wheat photosynthetic organs during grain filling reveals the ear as a water stress-tolerant organ and the peduncle as the largest pool of primary metabolites. Planta 2023, 257, 81. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bragado, R.; Vicente, R.; Molero, G.; Serret, M.D.; Maydup, M.L.; Araus, J.L. New avenues for increasing yield and stability in C3 cereals: Exploring ear photosynthesis. Curr. Opin. Plant Biol. 2020, 56, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Dreccer, M.F.; Wockner, K.B.; Palta, J.A.; McIntyre, C.L.; Borgognone, M.G.; Bourgault, M.; et al. More fertile florets and grains per spike can be achieved at higher temperature in wheat lines with high spike biomass and sugar content at booting. Funct. Plant Biol. 2014, 41, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Vara Prasad, P.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef]
- Bassi, F.M.; Sanchez-Garcia, M. Adaptation and stability analysis of ICARDA durum wheat elites across 18 countries. Crop Sci. 2017, 57, 2419–2430. [Google Scholar] [CrossRef]
| Measurement/Analysis | Acronym |
|---|---|
| Chlorophyll content (SPAD units) | SPAD |
| Max photochemical efficiency of PSII | Fv/Fm |
| Performance index | PI |
| Stomatal conductance (mmol/[m2s]) | SC |
| Relative water content | RWC |
| Flag leaf proline content (µmol/g FW) | Pro-FL |
| Spike proline content (µmol/g FW) | Pro-SP |
| Flag leaf water soluble carbohydrates content (g/g DW) | WSC-FL |
| Spike water soluble carbohydrates content (g/g DW) | WSC-SP |
| Flag leaf malondialdehyde content (µmol/g FW) | MDA-FL |
| Grain number/spike (main tiller) | GNS1 |
| Spike fertility index (main tiller) | SFI1 |
| Thousand grain weight (main tiller, g) | TGW1 |
| Grain yield (main tiller, g) | GY1 |
| Tiller number/plant | TNP |
| Productive tiller number/plant | PTP |
| Grain number/plant | GNP |
| Spike fertility index | SFI |
| Thousand grain weight (g) | TGW |
| Grain yield/plant | GYP |
| Harvest index | HI |
| A | B | ||||||
| Factors | G | T | G × T | Factors | G | T | G × T |
| df | 7 | 1 | 7 | df | 4 | 1 | 4 |
| TRAITS | p values | TRAITS | p values | ||||
| SPAD | < .001 *** | 0.002 ** | 0.13 | SPAD | < .001 *** | 0.019 * | 0.484 |
| Fv/Fm | < .001 *** | < .001 *** | 0.193 | Fv/Fm | 0.015 * | < .001 *** | 0.013 * |
| PI | < .001 *** | < .001 *** | 0.075 | PI | 0.008 ** | < .001 *** | 0.219 |
| SC | 0.159 | < .001 *** | 0.517 | SC | < .001 *** | < .001 *** | < .001 *** |
| RWC | < .001 *** | < .001 *** | 0.002 ** | RWC | < .001 *** | < .001 *** | 0.016 * |
| Pro-FL | < .001 *** | < .001 *** | < .001 *** | Pro-FL | < .001 *** | < .001 *** | < .001 *** |
| Pro-SP | 0.044 * | 0.33 | < .001 *** | Pro-SP | 0.031 * | 0.248 | 0.014 * |
| WSC-FL | < .001 *** | < .001 *** | < .001 *** | WSC-FL | < .001 *** | < .001 *** | < .001 *** |
| WSC-SP | 0.011 * | < .001 *** | 0.048 * | WSC-SP | 0.018 * | < .001 *** | 0.011 * |
| MDA-FL | 0.003 ** | < .001 *** | 0.181 | MDA-FL | 0.014 * | 0.006 ** | 0.48 |
| GNS1 | < .001 *** | < .001 *** | 0.165 | GNS1 | < .001 *** | < .001 *** | 0.048 * |
| SFI1 | 0.128 | < .001 *** | 0.51 | SFI1 | 0.303 | < .001 *** | 0.589 |
| TGW1 | < .001 *** | < .001 *** | 0.481 | TGW1 | < .001 *** | < .001 *** | 0.32 |
| GY1 | < .001 *** | < .001 *** | 0.044 * | GY1 | < .001 *** | < .001 *** | 0.008 ** |
| GNP | < .001 *** | < .001 *** | < .001 *** | GNP | < .001 *** | < .001 *** | < .001 *** |
| SFI | < .001 *** | < .001 *** | 0.104 | SFI | < .001 *** | < .001 *** | < .001 *** |
| TGW | < .001 *** | < .001 *** | 0.146 | TGW | 0.001 ** | < .001 *** | 0.104 |
| GYP | < .001 *** | < .001 *** | 0.002 ** | GYP | < .001 *** | < .001 *** | < .001 *** |
| HI | 0.106 | < .001 *** | 0.268 | HI | 0.314 | < .001 *** | 0.05 * |
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | Time | Time × G | Time × T | Time × G × T | Factors | Time | Time × G | Time × T | Time × G × T |
| df | 2 | 14 | 2 | 14 | df | 2 | 8 | 2 | 8 |
| TRAITS | p values | TRAITS | p values | ||||||
| SPAD | 0.251 | 0.991 | 0.229 | 0.991 | SPAD | 0.363 | 0.997 | 0.355 | 0.942 |
| Fv/Fm | < .001 *** | 0.037 * | 0.105 | 0.043 * | Fv/Fm | < .001 *** | 0.044 * | 0.077 | 0.047 * |
| PI | < .001 *** | 0.345 | 0.513 | 0.019 * | PI | < .001 *** | 0.013 * | 0.584 | 0.188 |
| SC | 0.002 ** | 0.74 | 0.305 | 0.457 | SC | 0.177 | 0.442 | 0.752 | 0.736 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





