Submitted:
05 August 2024
Posted:
06 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
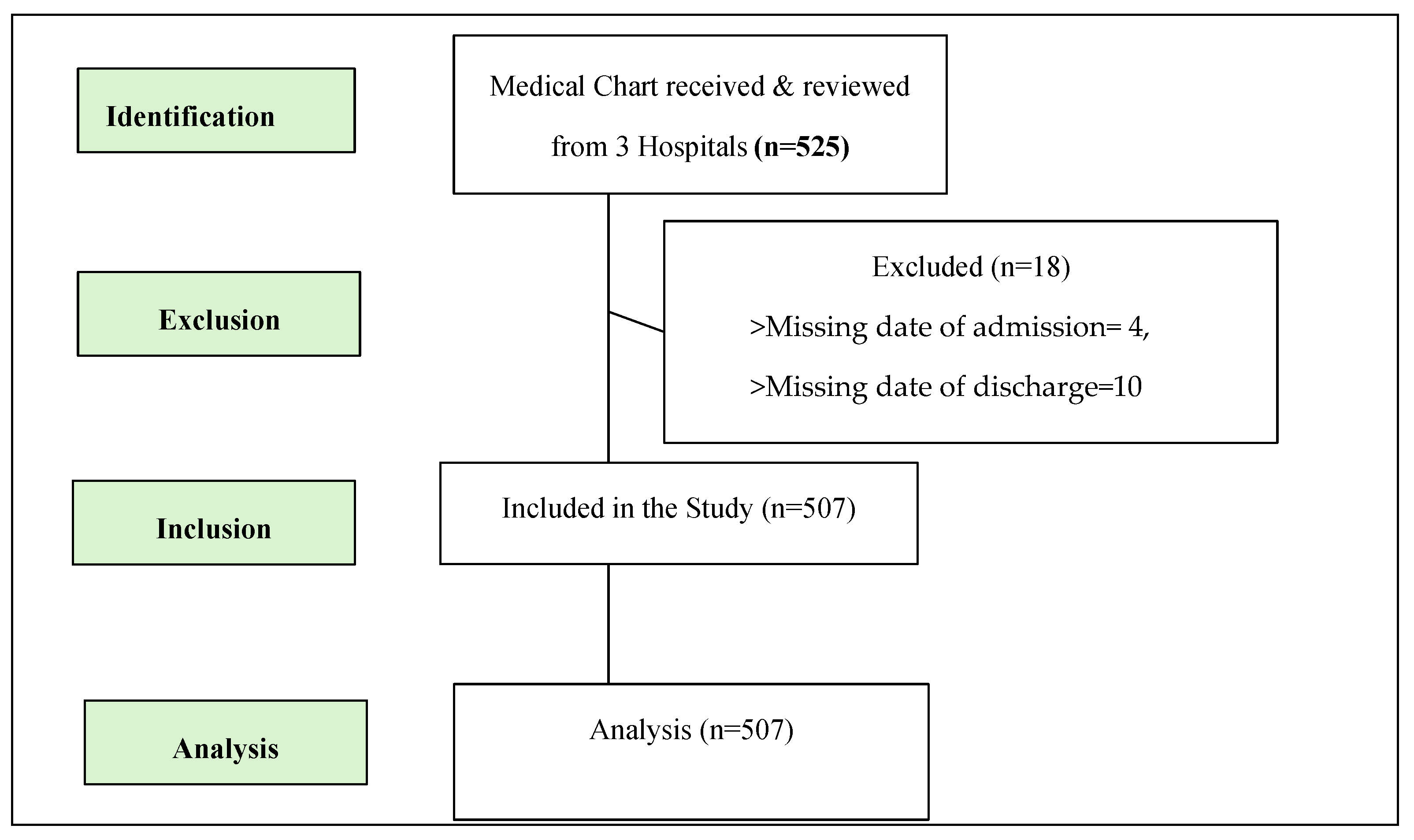
2.1. Study Design and Setting
2.2. Study Population
2.3. Sample Size and Sampling Technique
2.4. Data Extraction and Analysis
2.5. Data Quality Assurance
2.6. Study Variables and Their Measurement
2.7. Statistical Methods
2.8. Ethics Statement
3. Results
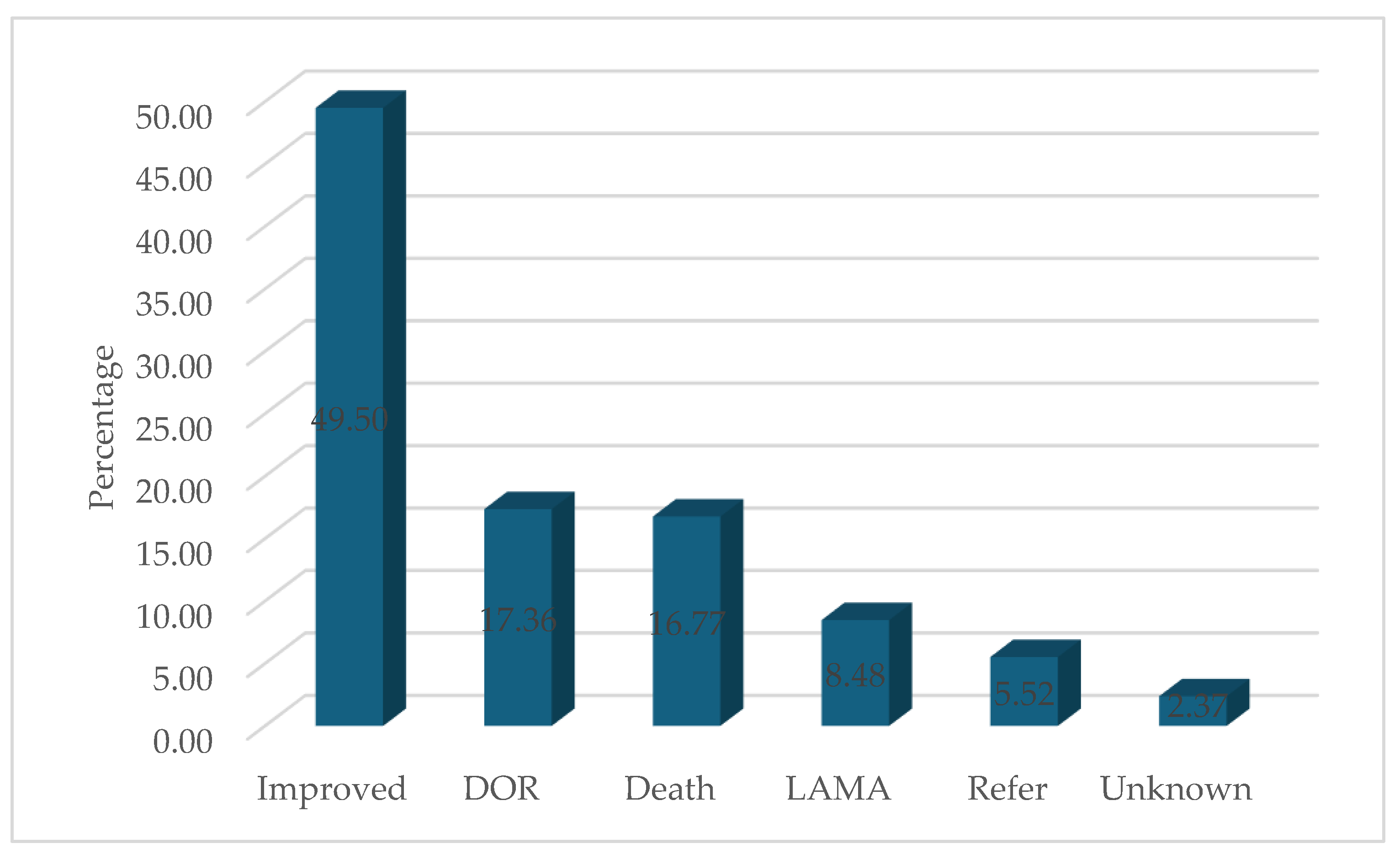
3.1. Treatment Outcomes
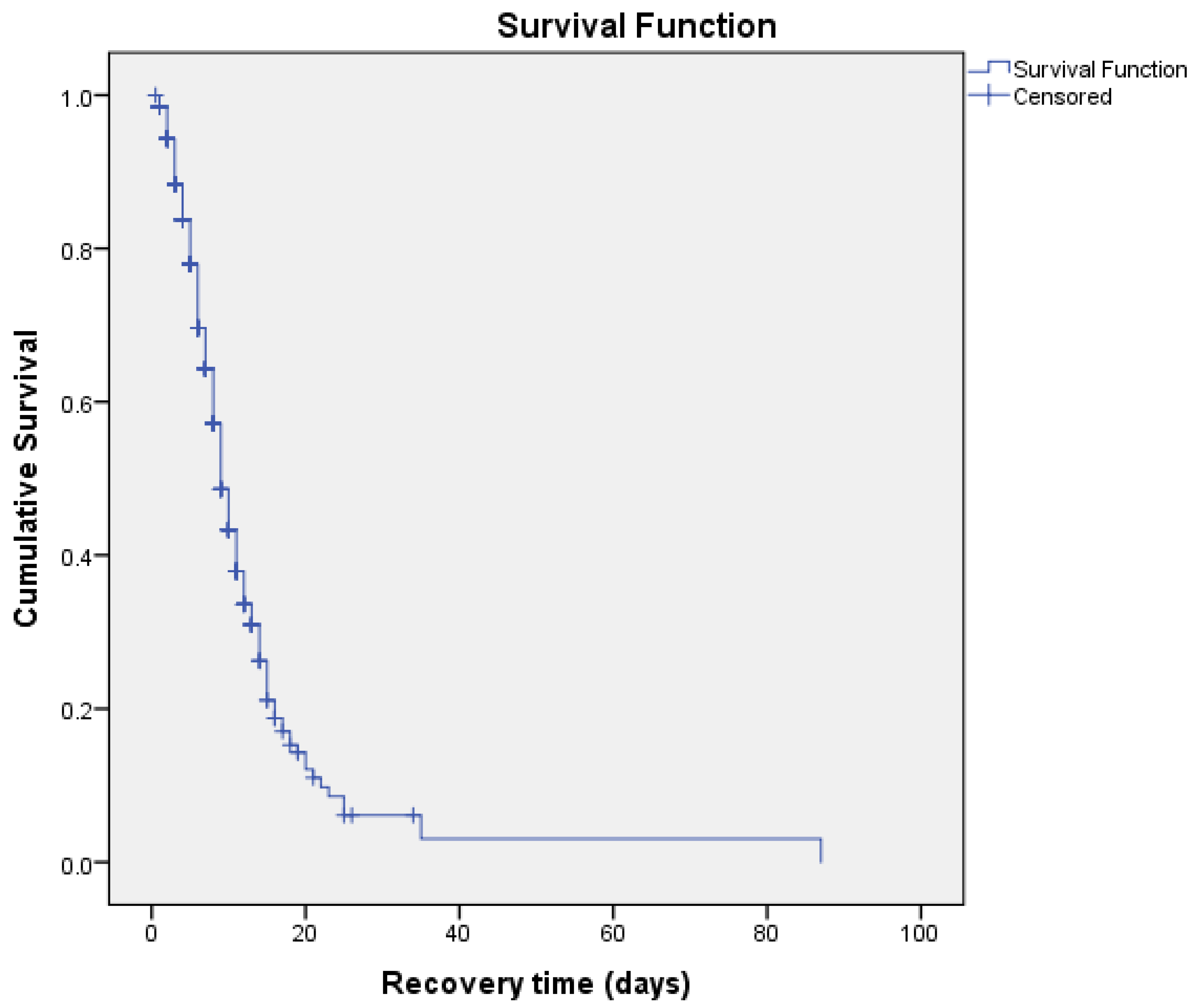
3.2. COVID-19 Recovery Time of Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biancolella, M.; Colona, V.L.; Luzzatto, L.; Watt, J.L.; Mattiuz, G.; Conticello, S.G.; Kaminski, N.; Mehrian-Shai, R.; Ko, A.I.; Gonsalves, G.S.; et al. COVID-19 annual update: a narrative review. Human Genomics 2023, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ito, J.; Uriu, K.; Zahradnik, J.; Kida, I.; Anraku, Y.; Nasser, H.; Shofa, M.; Oda, Y.; Lytras, S.; et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat Commun 2023, 14, 2800. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus disease (COVID-19). Available online: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19) (accessed on 12 November 2023).
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of Autoimmunity 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, 2023-11-12. 2023.
- Bezzio, C.; Saibeni, S.; Variola, A.; Allocca, M.; Massari, A.; Gerardi, V.; Casini, V.; Ricci, C.; Zingone, F.; Amato, A.; et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020, 69, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. American Journal of Physiology-Cell Physiology 2022, 322, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol 2020, 31, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Arab-Zozani, M.; Hashemi, F.; Safari, H.; Yousefi, M.; Ameri, H. Health-Related Quality of Life and its Associated Factors in COVID-19 Patients. Osong Public Health Res Perspect 2020, 11, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Shea, K.M.; Lin, J.; Nguyen, J.L.; Angulo, F.J.; Benigno, M.; Malhotra, D.; Emir, B.; Sung, A.H.; Hammond, J.L.; et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. Journal of Medical Economics 2021, 24, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Practice & Research Clinical Anaesthesiology 2021, 35, 293–306. [Google Scholar] [CrossRef]
- Nizigiyimana, A.; Acharya, D.; Poder, T.G. Impact of COVID-19 pandemic on the health-related quality of life of frontline workers: the case of seven low-income Eastern African countries. Health Qual Life Outcomes 2023, 21, 97. [Google Scholar] [CrossRef]
- Ismaila, H.; Asamani, J.A.; Lokossou, V.K.; Oduro-Mensah, E.; Nabyonga-Orem, J.; Akoriyea, S.K. The cost of clinical management of SARS-COV-2 (COVID-19) infection by level of disease severity in Ghana: a protocol-based cost of illness analysis. BMC Health Serv Res 2021, 21, 1115. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; B. Pearson, C.A.; Group, C.W.; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Medicine 2020, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections. Clinical Infectious Diseases 2021, 74, 1748–1754. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Lemma Tirore, L.; Abose Nadamo, S.; Tamrat Derilo, H.; Erkalo, D.; Sedore, T.; Tadesse, T.; Ermias, D.; Yaekob, T. Time to Recovery from Covid-19 and Its Predictors Among Patients Admitted to Treatment Centers of Southern Nations Nationalities and Peoples Region (SNNPR), ETHIOPIA: Multi-Center Retrospective Cohort Study. Infect Drug Resist 2022, 15, 3047–3062. [Google Scholar] [CrossRef]
- Abrahim, S.A.; Tessema, M.; Defar, A.; Hussen, A.; Ejeta, E.; Demoz, G.; Tereda, A.B.; Dillnessa, E.; Feleke, A.; Amare, M.; et al. Time to recovery and its predictors among adults hospitalized with COVID-19: A prospective cohort study in Ethiopia. PLoS One 2020, 15, e0244269. [Google Scholar] [CrossRef]
- Tolossa, T.; Wakuma, B.; Seyoum Gebre, D.; Merdassa Atomssa, E.; Getachew, M.; Fetensa, G.; Ayala, D.; Turi, E. Time to recovery from COVID-19 and its predictors among patients admitted to treatment center of Wollega University Referral Hospital (WURH), Western Ethiopia: Survival analysis of retrospective cohort study. PLOS ONE 2021, 16, e0252389. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, P.; Lu, W.; Liu, K.; Ma, K.; Huang, L.; Cai, J.; Zhang, H.; Qin, Y.; Sun, H.; et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLOS Pathogens 2020, 16, e1008520. [Google Scholar] [CrossRef]
- Ministry of Health and Population. Health Sector Response to COVID-19; 2021.
- Worldometer, C.-C.P.I. COVID-19 CORONAVIRUS Pandemic [Internet]. Available online: https://www.worldometers.info/coronavirus/ (accessed on 10 November 2023).
- Mininstry of Health and Population Nepal. Coronavirus disease (COVID-19) outbreak updates & resource materials – Health Emergency Operation Center. 2020.
- Diagnostic detection of 2019-nCoV by real-time RT-PCR. Available online: https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf. (accessed on).
- Clinical Spectrum | COVID-19 Treatment Guidelines [Internet]. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 16 July 2022).
- Barman, M.P.; Rahman, T.; Bora, K.; Borgohain, C. COVID-19 pandemic and its recovery time of patients in India: A pilot study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020, 14, 1205–1211. [Google Scholar] [CrossRef]
- Benoni, R.; Campagna, I.; Panunzi, S.; Varalta, M.S.; Salandini, G.; De Mattia, G.; Turrina, G.; Moretti, F.; Lo Cascio, G.; Spiteri, G.; et al. Estimating COVID-19 recovery time in a cohort of Italian healthcare workers who underwent surveillance swab testing. Public Health 2021, 196, 52–58. [Google Scholar] [CrossRef]
- George, N.; Tyagi, N.K.; Prasad, J.B. COVID-19 pandemic and its average recovery time in Indian states. Clinical Epidemiology and Global Health 2021, 11, 100740. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Sitapati, A.M.; Zhang, J.; Zou, J.; Bui, Q.M.; Ren, J.; Longhurst, C.A.; Criqui, M.H.; Messer, K. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. The American Journal of Cardiology 2020, 136, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Faes, C.; Abrams, S.; Van Beckhoven, D.; Meyfroidt, G.; Vlieghe, E.; Hens, N.; Surveillance, B.C.G.o.C.-H. Time between Symptom Onset, Hospitalisation and Recovery or Death: Statistical Analysis of Belgian COVID-19 Patients. International Journal of Environmental Research and Public Health 2020, 17, 7560. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-d.; Ding, M.; Dong, X.; Zhang, J.-j.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.-l.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.H.; Wootten, M.; Moghal, M.; Heron, N.; Martin, R.; Walsted, E.S.; Biswas, A.; Loosemore, M.; Elliott, N.; Ranson, C. Clinical patterns, recovery time and prolonged impact of COVID-19 illness in international athletes: the UK experience. British Journal of Sports Medicine 2022, 56, 4–11. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Abbasian, L.; Solduzian, M.; Ayoobi Yazdi, N.; Jafari, F.; Adibimehr, A.; Farahani, A.; Salami Khaneshan, A.; Ebrahimi Alavijeh, P.; Jahani, Z.; et al. Predictors of the prolonged recovery period in COVID-19 patients: a cross-sectional study. Eur J Med Res 2021, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-existing Comorbidities and Therapeutic Interventions on COVID-19. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Qiu, H.; Wan, L.; Ai, Y.; Xue, Z.; Guo, Q.; Deshpande, R.; Zhang, L.; Meng, J.; Tong, C.; et al. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology 2020, 132, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Gomersall, C.D.; Fowler, R.A. Care for Critically Ill Patients With COVID-19. JAMA 2020, 323, 1499–1500. [Google Scholar] [CrossRef]
- Chivukula, R.R.; Maley, J.H.; Dudzinski, D.M.; Hibbert, K.; Hardin, C.C. Evidence-Based Management of the Critically Ill Adult With SARS-CoV-2 Infection. Journal of Intensive Care Medicine 2021, 36, 18–41. [Google Scholar] [CrossRef]
- Palladino, M. Complete blood count alterations in COVID-19 patients: A narrative review. Biochem Med (Zagreb) 2021, 31, 030501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Qi, G.Q.; Gu, X.; Zhang, X.Y.; Fang, Y.F.; Jiang, H.; Zhao, Y.J. Lymphocyte blood levels that remain low can predict the death of patients with COVID-19. Medicine (Baltimore) 2021, 100, e26503. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Journal of Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef]
- Shenoy, N.; Luchtel, R.; Gulani, P. Considerations for target oxygen saturation in COVID-19 patients: are we under-shooting? BMC Medicine 2020, 18, 260. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City. medRxiv 2004. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, A.; Gálvez-Barrón, C.; Miñarro, A.; Macho, O.; López, G.F.; Robles, M.T.; Dapena, M.D.; Martínez, S.; Milà Ràfols, N.; Monaco, E.E.; et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PLOS ONE 2020, 15, e0239571. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascón, G.A.; Calderón-Tapia, L.E.; García, A.F.; Zarama, V.; Gómez-Álvarez, F.; Álvarez-Saa, T.; Pardo-Otálvaro, S.; Bautista-Rincón, D.F.; Vargas, M.P.; Aldana-Díaz, J.L.; et al. Effect of High-Flow Oxygen Therapy vs Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients With Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. New England Journal of Medicine 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Li, Y.; Ashcroft, T.; Chung, A.; Dighero, I.; Dozier, M.; Horne, M.; McSwiggan, E.; Shamsuddin, A.; Nair, H. Risk factors for poor outcomes in hospitalised COVID-19 patients: A systematic review and meta-analysis. J Glob Health 2021, 11, 10001. [Google Scholar] [CrossRef]
- Liu, B.; Jayasundara, D.; Pye, V.; Dobbins, T.; Dore, G.J.; Matthews, G.; Kaldor, J.; Spokes, P. Whole of population-based cohort study of recovery time from COVID-19 in New South Wales Australia. The Lancet Regional Health - Western Pacific 2021, 12, 100193. [Google Scholar] [CrossRef] [PubMed]

| Variables | All patients (n=507) | Patients who recovered (n=251) | Patients who died/referred* (n=256) | p-value |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 51.09 (14.92) | 48.61 (14.99) | 53.52 (14.48) | <0.001 |
| Gender | ||||
| Male | 345 (68.0) | 173 (50.1) | 172 (49.9) | 0.675 |
| Female | 162 (32.0) | 78 (48.1) | 84 (51.9) | |
| Origin of Residence* | ||||
| Dhanusha | 255 (67.1) | 126 (49.4) | 129 (50.6) | 0.713 |
| Mahottari | 76 (20.0) | 36 (47.4) | 40 (52.6) | |
| Sarlahi | 26 (6.8) | 13 (50.0) | 13 (50.0) | |
| Siraha | 17 (4.5) | 6 (35.3) | 11 (64.7) | |
| Bara/Parsa/Rautahat/Saptari | 6 (1.6) | 4 (66.7) | 2 (33.3) | |
| Area of Residence** | ||||
| Urban | 299 (80.4) | 147 (49.2) | 152 (50.8) | 0.692 |
| Rural | 73 (19.6) | 34 (46.6) | 39 (53.4) | |
| Types of Hospital | ||||
| Public | 285 (56.2) | 149 (52.3) | 136 (47.7) | 0.157 |
| Private | 222 (43.8) | 102 (45.9) | 120 (54.1) | |
| Severity at admission*** | ||||
| Mild | 103 (22.3) | 62 (60.2) | 41 (39.8) | <0.0001 |
| Moderate | 157 (34.0) | 101 (64.3) | 56 (35.7) | |
| Severe | 135 (29.2) | 51 (37.8) | 84 (62.2) | |
| Critical | 67 (14.5) | 13 (19.4) | 54 (80.6) | |
| Respiratory support**** | ||||
| None | 80 (20.0) | 62 (77.5) | 18 (22.5) | <0.0001 |
| Oxygen mask | 260 (64.8) | 136 (52.3) | 124 (47.7) | |
| Mechanical Ventilation | 61 (15.2) | 8 (13.1) | 53 (86.9) | |
| Missing | 106 |
| Variables | All patients (n=507) |
Patients who recovered (n=251) | Patients who died or referred* (n=256) |
p-value |
|---|---|---|---|---|
| Symptoms reported at admission | ||||
| Shortness of breath | 332 (65.5) | 156 (47.0) | 176 (53.0) | 0.233 |
| Fever | 310 (61.1) | 157 (50.6) | 153 (49.4) | 0.680 |
| Cough | 305 (60.2) | 154 (50.5) | 151 (49.5) | 0.603 |
| Fatigue | 56 (11.0) | 27 (48.2) | 29 (51.8) | 0.495 |
| Chest distress | 16 (3.2) | 6 (37.5) | 10 (62.5) | 0.221 |
| Headache | 29 (5.7) | 16 (55.2) | 13 (44.8) | 0.888 |
| Pre-existing conditions | ||||
| Diabetes mellitus | 95 (18.7) | 41 (43.2) | 54 (56.8) | 0.256 |
| Hypertension | 54 (10.7) | 27 (50.0) | 27 (50.0) | 0.686 |
| Chronic obstructive pulmonary disease | 14 (2.8) | 7 (50.0) | 7 (50.0) | 0.785 |
| Asthma | 4 (0.8) | 1 (25.0) | 3 (75.0) | 0.343 |
| Chronic cardiac disease‡ (Excluding hypertension) |
6 (1.2) | 3 (50.0) | 3 (50.0) | 0.839 |
| TB | 4 (0.8) | 2 (50.0) | 2 (50.0) | 0.908 |
| HIV/AIDS | 1 (0.2) | 0 (0.0) | 1 (100.0) | - |
| Thyroid | 16 (3.2) | 9 (56.3) | 7 (43.7) | 0.738 |
| Chronic kidney disease of any stage* | 8 (1.6) | 2 (25.0) | 6 (75.0) | 0.250 |
| Vital signs at hospital presentation | ||||
| Temperature (°F) [n=328] | 98 (97-99) | 98 (97-99) | 98 (97-99) | 0.017 |
| Oxygen saturation (%) [n=475] | 94 (88-97) | 95 (92-97) | 90 (80-95) | <0.0001 |
| Heart rate (beats per min) [n=335] | 88 (80-100) | 86 (80-97) | 89 (80-105) | 0.039 |
| Respiratory rate (breaths per min) [n=173] | 22 (20-28) | 22 (20-24) | 24 (20-32) | 0.018 |
| Systolic blood pressure (mm Hg) [n=303] | 110 (110-120) | 110 (110-120) | 110 (100-120) | 0.066 |
| Diastolic blood pressure (mm Hg) [n=303] | 70 (70-80) | 70 (70-80) | 70 (70-80) | 0.026 |
| Variables | Number | Median recovery time | Log Rank χ2 - value |
p-value |
|---|---|---|---|---|
| Point estimate (95%CI) | ||||
| Age group, years | ||||
| <20 | 10 (2.0) | 9 (6.63-11.36) | 7.11 | 0.212 |
| 20-29 | 30 (5.9) | 9 (6.31-11.68) | ||
| 30-39 | 66 (13.0) | 9 (7.25-10.74) | ||
| 40-49 | 104 (20.5) | 8 (6.88-9.11) | ||
| 50-59 | 127 (25.0) | 9 (7.68-10.31) | ||
| 60-69 | 170 (33.5) | 12 (10.16-13.83) | ||
| Sex | ||||
| Male | 345 (68.0) | 9 (8.11-9.88) | 0.004 | 0.947 |
| Female | 162 (32.0) | 9 (7.07-10.92) | ||
| Origin of Residence | ||||
| Dhanusha | 255 (67.1) | 9 (7.85-10.14) | 2.60 | 0.626 |
| Mahottari | 76 (20.0) | 10 (6.74-13.25) | ||
| Sarlahi | 26 (6.8) | 10 | ||
| Siraha | 17 (4.5) | 18 (10.16-13.83) | ||
| Bara/Parsa/Rautahat/Saptari | 6 (1.6) | 9 (8.03-9.96) | ||
| Missing | 127 | |||
| Area of Residence | ||||
| Urban | 299 (80.4) | 10 (8.89-11.10) | 0.005 | 0.945 |
| Rural | 73 (19.6) | 9 (7.23-10.76) | ||
| Missing | 135 | |||
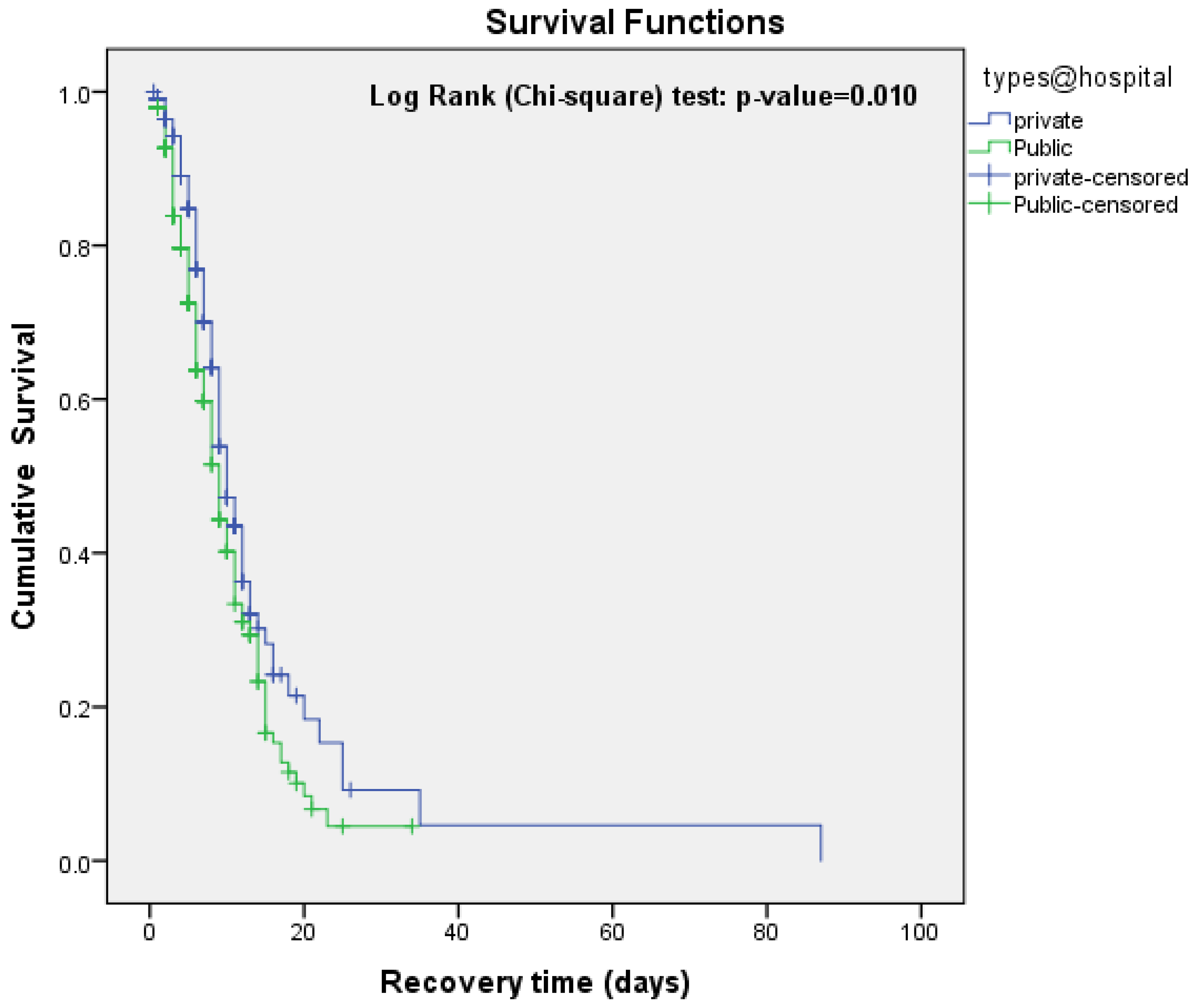
| Types of Hospital | ||||
| Public | 285 (56.2) | 9 (8.05-9.95) | 6.60 | 0.010 |
| Private | 222 (43.8) | 10 (8.78-11.21) | ||
| Severity at admission | ||||
| Mild | 103 (22.3) | 7 (5.18-8.81) | 39.42 | <0.0001 |
| Moderate | 157 (34.0) | 9 (7.78-10.22) | ||
| Severe | 135 (29.2) | 10 (8.59-11.40) | ||
| Critical | 67 (14.5) | 18 (11.96-24.03) | ||
| Missing | 45 | |||
| Respiratory support | ||||
| None | 80 (20.0) | 5 (4.08-5.91) | 90.16 | <0.0001 |
| Oxygen mask | 260 (64.8) | 10 (8.98-11.01) | ||
| Mechanical Ventilation | 61 (15.2) | 22 (9.13-34.86) | ||
| Missing | 106 |
| Variables | Number | Median recovery time | Log Rank χ2 - value |
p-value |
|---|---|---|---|---|
| Point estimate (95%CI) | ||||
| Fever | ||||
| Presence | 310 | 9 (7.93-10.06) | 0.213 | 0.644 |
| Absence | 123 | 9 (7.65-10.34) | ||
| Missing | ||||
| Cough | ||||
| Presence | 305 | 9 (7.84-10.15) | 0.001 | 0.975 |
| Absence | 122 | 9 (7.66-10.33) | ||
| Missing | ||||
| Fatigue | ||||
| Presence | 56 | 10 (7.35-12.64) | 0.700 | 0.403 |
| Absence | 121 | 9 (7.95-10.05) | ||
| Missing | ||||
| Shortness of breath | ||||
| Presence | 332 | 10 (8.66-11.33) | 0.566 | 0.452 |
| Absence | 120 | 9 (7.95-10.04) | ||
| Missing | ||||
| Chest distress | ||||
| Presence | 16 | 10 (7.21-12.78) | 0447 | 0.504 |
| Absence | 119 | 9 (7.95-10.04) | ||
| Missing | ||||
| Headache | ||||
| Presence | 29 | 9 (7.95-10.05) | 0318 | 0.573 |
| Absence | 121 | 8 (5.08-10.91) | ||
| Missing | ||||
| Pre-existing conditions | ||||
| Diabetes mellitus | ||||
| Presence | 95 | 11 (8.07-13.92) | 5.00 | 0.025 |
| Absence | 93 | 9 (7.57-10.42) | ||
| Missing | ||||
| Hypertension | ||||
| Presence | 54 | 11 (8.13-13.86) | 0.137 | 0.712 |
| Absence | 103 | 9 (7.44-10.55) | ||
| Missing | ||||
| Chronic obstructive pulmonary disease | ||||
| Presence | 14 | 12 (10.85-13.14) | 1.81 | 0.178 |
| Absence | 117 | 9 (7.66-10.33) | ||
| Missing | ||||
| Asthma* | ||||
| Presence | 4 | 9 (7.95-10.05) | 0.105 | 0.746 |
| Absence | 212 | 9 | ||
| Missing | ||||
| Chronic cardiac disease‡ (excluding hypertension) * | ||||
| Presence | 6 | 7 (0.01-15.58) | 0.429 | 0.512 |
| Absence | 118 | 9 (7.95-10.04) | ||
| Missing | ||||
| TB * | ||||
| Presence | 4 | 5 (7.69-10.30) | 0.175 | 0.676 |
| Absence | 119 | 9 | ||
| Missing | ||||
| HIV/AIDS | ||||
| Presence | 1 | - | - | - |
| Absence | 121 | - | ||
| Missing | ||||
| Thyroid | ||||
| Presence | 16 | 8 (3.69-12.31) | 1.69 | 0.193 |
| Absence | 112 | 9 (7.61-10.38) | ||
| Missing | ||||
| Chronic kidney disease of any stage* | ||||
| Presence | 8 | 11 | 0.075 | 0.784 |
| Absence | 120 | 9 (7.63-10.06) | ||
| Missing |
| Variables | Univariable HR (95%CI) | Multivariable HR (95%CI) | ||||
|---|---|---|---|---|---|---|
| Model-I | Model-II | |||||
| CHR (95%CI) | p-value | AHR (95%CI) | p-value | AHR (95%CI) | p-value | |
| Age (per 10-year increase) | 0.90 (0.83-0.98) | 0.023 | 0.87 (0.78-0.96) | 0.006 | 0.88 (0.75-1.04) | 0.887 |
| Types of Hospital | ||||||
| Private | Reference | - | Reference | - | Reference | - |
| Public | 1.37 (1.06-1.77) | 0.014 | 1.05 (0.77-1.44) | 0.717 | 3.01 (0.30-29.86) | 0.345 |
| Severity at admission | ||||||
| Mild | Reference | - | Reference | - | Reference | - |
| Moderate | 0.70 (0.51-0.97) | 0.032 | 0.54 (0.37-0.80) | 0.002 | 0.62 (0.23-1.67) | 0.352 |
| Severe/critical | 0.37 (0.26-0.52) | <0.0001 | 0.46 (0.29-0.71) | 0.001 | 0.34 (0.15-0.79) | 0.012 |
| Respiratory support | ||||||
| None | Reference | - | Reference | - | Reference | - |
| Oxygen mask | 0.30 (0.22-0.41) | <0.0001 | 0.34 (0.24-0.48) | <0.0001 | 0.76 (0.35-1.63) | 0.481 |
| Mechanical Ventilation | 0.10 (0.04-0.21) | <0.0001 | 0.11 (0.05-0.25) | <0.0001 | 0.26 (0.05-1.28) | 0.098 |
| Vital signs at hospital presentation | ||||||
| Oxygen saturation (%) | 1.05 (1.03-1.07) | <0.0001 | - | - | 1.09 (1.01-1.17) | 0.018 |
| Temperature (°F) | 0.90 (0.76-1.07) | 0.240 | - | - | 0.96 (0.71-1.29) | 0.810 |
| Heart rate (beats per min) | 0.98 (0.97-0.99) | 0.015 | - | - | 0.99 (0.97-1.01) | 0.547 |
| Respiratory rate (breaths per min) | 0.94 (0.90-0.99) | 0.031 | - | - | 1.02 (0.95-1.09) | 0.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





