Introduction
The gastrointestinal tract (GIT), an integral component of the human digestive system, plays a pivotal role in digestion, immunity, and absorption. The human gut microbiota constitutes the largest immunological organ in the body, harboring 60-80% of immune cells (1). The human gut microbiota is renowned for its significant influence on various host functions, especially immune modulation. Recent advancements in next-generation sequencing methodologies have enriched our understanding of the gut microbiota’s essential role. It sustains the equilibrium between human health and microorganisms, including bacteria, fungi, and archaea (2,3). Althougmicrobiota icrobiome maintains a stable composition throughout an individual’s lifetime, the proportions of different bacteria can be influenced by alterations in the intestinal micro-ecological system (4–6).
The gut microbiota is a key component in maintaining health and can contribute to disease, including cancer. Certain bacteria and viruses have been linked to cellular dysplasia and carcinogenesis (7). For instance, Salmonella typhi and Helicobacter spp. are associated with biliary cancer (8,9), while Helicobacter pylori are linked to gastric cancer (10). H. pylori can exert genotoxic effects and alter intracellular signaling pathways, contributing to gastric adenocarcinoma and MALT lymphoma, classified as a class I carcinogen by the WHO (11). Thus, general gut microbiota dysbiosis is connected with carcinogenesis (12).
Gut Microbiota Composition, Function, and Its Role in Immunotherapy
Overview of the Gut Microbiota and Its Microbial Diversity
The gut microbiota incorporates genetic material from bacteria, fungi, protozoa, and viruses inhabiting the digestive systems of humans and animals (13). The microbiota’s composition is unique to each mammalian gut, harboring a diversity of microorganisms, especially Firmicutes and Bacteroidetes. Additionally, environmental and ethnic backgrounds play a key role in shaping the distinct gut microbe composition. According to Qin J et al. (2012), distinctions in the European and Chinese gut microbiotas are due to a confluence of environmental and genetic factors, although the underlying mechanisms are still ambiguous (14).
Furthermore, the literature reports a strong relationship between diet and the gut microbiota. Evidence suggests that the host has a considerable influence on the human gut microbiota makeup through dietary factors, with age, gender, race, and body weight playing comparatively subordinate roles (13). Moreover, gut microbiota can be classified into essential, autochthonous (resident), and nonessential, allochthonous (traveler) microbes specific to everyone. For instance, Ruminococcus is a substantial ecotype, and in Taiwanese individuals, Enterobacteriaceae seems to be a crucial ecotype (13). Autochthonous microbes reside permanently in the colonic mucosa, while allochthonous appear during digestion and serve various roles in the ecosystem (15). Hence, it could be hypothesized that several environmental and genetic factors play a role in the unique gut microbiota of everyone, including essential and nonessential bacteria, which can be dichotomized into resident and transient categories.
Roles of Gut Microbes on the Host Immune System
Gut microbiota actively contribute to the improvement of the Immune system and are integral to disease progression and therapy. The single layer of epithelial cells, or mucosa, comprises intestinal epithelial cells (IECs) which are categorized as Paneth cells, goblet cells, and intraepithelial lymphocytes (9,13). The Paneth cells secrete antimicrobial peptides, while goblet cells produce mucus (9). Bacterial byproducts, such as short-chain fatty acids, and bacteria cause local dendritic cells to migrate to mesenteric lymph nodes (9). There, adult dendritic cells turn on naive T cells, accelerating their differentiation into effector T cells, regulatory T cells (Tregs), or Th17 cells, which then either return to the gut or disseminate consistently (9). Tregs contribute by secreting interleukin-10 (IL-10)), establishing a localized anti-inflammatory cytokine environment (9,13). Meanwhile, the secretion of cytokines, including IL-17, by Th17 cells prompts IECs to fortify tight junctions and release antimicrobial proteins. IL-17 can also trigger the release of other inflammatory cytokines. This mechanism contributes to the local immune responses (9).
Systemic immune reactions are affected by microbiota-mediated briefing of immune cells. Antigens from gut bacteria in mesenteric lymph nodes mLNs induce B cells and T cell subsets, facilitating immune reactions against identical remote antigens via cross-reactivity (9). Thus, the symbiotic relationship between gut microbiota and the intestinal epithelial cells not only fosters immunity through the secretion of antimicrobial peptides and mucus but also plays a pivotal role in disease progression and therapeutic interventions, as bacterial byproducts influence the activation and differentiation of immune cells, shaping the overall immune landscape.
Factors Influencing Gut Microbiota Composition
Diet and host genetics are crucial elements framing the gut microbiota (9,16). Alteration in diet can significantly influence major gut bacteria, including Bacteroides thetaiotaomicron , Eubacterium rectale, and Methanobrevibacter smithii (17). Studies have emphasized the importance of dietary elements, specifically alcohol, in altered gut microbiota in both animals and humans, as studied in the literature (13). Conversely, modification in the composition of these key gut bacteria can, in turn, influence host metabolism and contribute to the development of associated diseases.
In the gut environment, microbial products, such as lipopolysaccharides (LPS) generated by Gram-negative bacteria and delivered by chylomicrons (18). Lipopolysaccharides prompt innate immunity in various organisms. The survival of peritoneal dialysis patients is intricately linked with plasma LPS and C-reactive protein levels, demonstrating their role as markers for inflammation and prognosis (13). LPS, a component of the outer membrane of Gram-negative bacteria, can trigger systemic inflammation. Elevated LPS levels in peritoneal dialysis patients may indicate bacterial translocation or endotoxin exposure, contributing to an inflammatory state (19). LPS can promote cancer progression by stimulating chronic inflammation. It activates immune cells and inflammatory pathways, leading to an environment supporting tumor growth and metastasis.
CRP is a protein produced by the liver in response to inflammation. Elevated CRP levels are associated with a higher risk of cancer progression and poor prognosis. CRP reflects systemic inflammation, which can contribute to tumor development and resistance to treatment (20). Moreover, Trimethylamine (TMA), metabolized into trimethylamine N-oxide (TMAO), a dietary metabolite of gut flora, influences patient morbidity, highlighting the vast impact of microbiota on host health and disease (21). The combined influence of diet, genetics, and the gut microbiota leads to significant health consequences, offering potential efficacy for disease prevention and treatment. In addition, microbial products like LPS and TMAO reveal systemic microbiota imputations (13). The gut microbiota of horses is susceptible to several factors such as stresses, drugs, and diet, as revealed by advanced sequencing and bioinformatics. (22).
Gut Microbiota; A Critical Factor in Cancer Immunotherapy
The term ‘microbiota’ refers to individual microorganisms, while their collective genomes are called ‘microbiotas’. The human body contains billions of microorganisms that constantly interact with the host (7,8). The gut microbiota can modulate the host immune system at both local and systemic levels (7).
The gut microbiota has emerged as a remarkable factor in cancer immunotherapy; however, maintaining its delicate equilibrium is essential for therapeutic efficacy. Gut microbes influence the peripheral immune system (23) and serve as crucial contributors to the effectiveness of immune checkpoint inhibitors (ICIs) for cancer treatment (12). However, the disruption of the gut microbiota, referred to as ‘intestinal dysbiosis’, is epidemiologically linked to several chronic inflammatory diseases. (24). Thus, the effectiveness of ICIs should be properly balanced against the potential risks for long-term inflammatory disorders.
The microbiota significantly influences tumor development and the response to cancer treatments, particularly immunotherapy (25,26). Immunotherapy, notably using immune checkpoint inhibitors (ICIs) such as antibodies against cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death/ligand 1 (PD-1/PD-L1), has markedly improved survival outcomes for advanced metastatic cancers, including melanoma and lung carcinoma (27). Immunotherapy is an alternative therapeutic approach for cancer treatment but unfortunately, not all patients respond effectively. Furthermore, patients who exhibit positive responses experience adverse side effects, such as diarrhea, colitis, hepatitis, skin problems, and hormonal problems (28). Therefore, in certain individuals, immunotherapy accelerates the growth of the tumor raising concerns regarding its clinical use. Proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells and activation of oncogenic signaling pathways (29). Risk factors for hyperprogression during immunotherapy have not been validated. However, some associations have been reported between hyperprogression and MDM2 or MDM4 amplifications and EGFR alterations in tumor cells as well as older age, female sex, and the presence of more than 2 pretreatment sites of metastases (29). Identifying biomarkers and predictive factors that can anticipate hyperprogression is an active area of research to improve the safety and efficacy of immunotherapy treatments.
The Impact of Gut Microbiota on Immunotherapy Outcomes
Increasing evidence indicates the significance of the human gut microbiota in cancer immunotherapy. The human gastrointestinal tract is colonized by 3 × 1013 microorganisms (30), and the body’s immune system serves as the main force controlling tumor development (11). Hence, the microbiota of the gastrointestinal (GI) tract plays a significant role in the immune system response toward immunotherapy, which has substantial implications for tumor development and progression.
The normal human gut microbiota has a positive influence on immune responses, exerting an anticancer effect. According to previous studies, the gut microbiota influences tumor vulnerability to therapies like immunotherapy (31), targeting co-inhibitory molecules such as PD-1/PD-L1, immune checkpoint inhibitors (ICIs), and enhancing host immunity (32). However, an abnormal composition can lead to adverse effects such as immune-related colitis, respiratory infections, and myocarditis (31). Certain gut microorganisms have been identified to safeguard against these complications (33), enhancing treatment sensitivity while minimizing drug side effects (34). For further clarification, the tendency of the microbiota to affect the outcome of cancer treatment is summarized in Table 1
Diet has a profound impact on intestinal bacterial diversity, which influences the metabolism of anticancer and immunotherapeutic drugs. Recent studies have shown that a combination of butyrate with irinotecan enhances drug effectiveness (35,36). Moreover, certain bacterial taxa, including Lactobacillus fermentum BR11, act as a probiotic and minimize 5-FU-induced mucositis (37). Likewise, prebiotics, inulin, and oligofructose have shown promising results in reducing tumor growth and increasing survival in rat models (37) (refer to Table 1).
Antibiotics can alter gut microbiota diversity and enhance treatment efficacy. Co-administration of ciprofloxacin and gemcitabine has demonstrated improved treatment efficacy, despite potential side effects, making antibiotics an intensively used strategy for microbiota modification (38). However, antibiotics can lower microbiota diversity and enhance the proliferation of harmful bacteria in colorectal cancer.(39)
Immunomodulatory treatment (IMT) has shown benefits in murine models, with enhanced efficacy observed in mice that responded positively to anti-PD-L1, while contrasting outcomes were observed in patients who did not respond to the treatment (40). Another successful combination involves Saccharomyces cerevisiae UFMG A-905 and selenium, which significantly improves therapeutic results in 5-FU treatment, maintaining intestinal integrity and suppressing inflammation (41)(Table 1).
Mechanism Underlying Microbiota-Immune System Interaction
The host-microbiota connection is a complex interplay, influenced by food, microbiota, host metabolites, and antibiotics, with significant consequences on metabolism, the immune system, and health. The host actively contributes to this symbiotic interaction by producing antimicrobial peptides such as defensing (42,43), IgA (44), and miRNAs, which shape microbiota development and anatomical settings, thereby influencing bacterial transcription and growth (45). Furthermore, commensal gut microbes, in turn, adapt to host immunity and activate metabolic pathways for coexistence (46). The host recognition of microbial compounds triggers immune responses. This interaction establishes tolerance to commensals and susceptibility to pathogens (47).
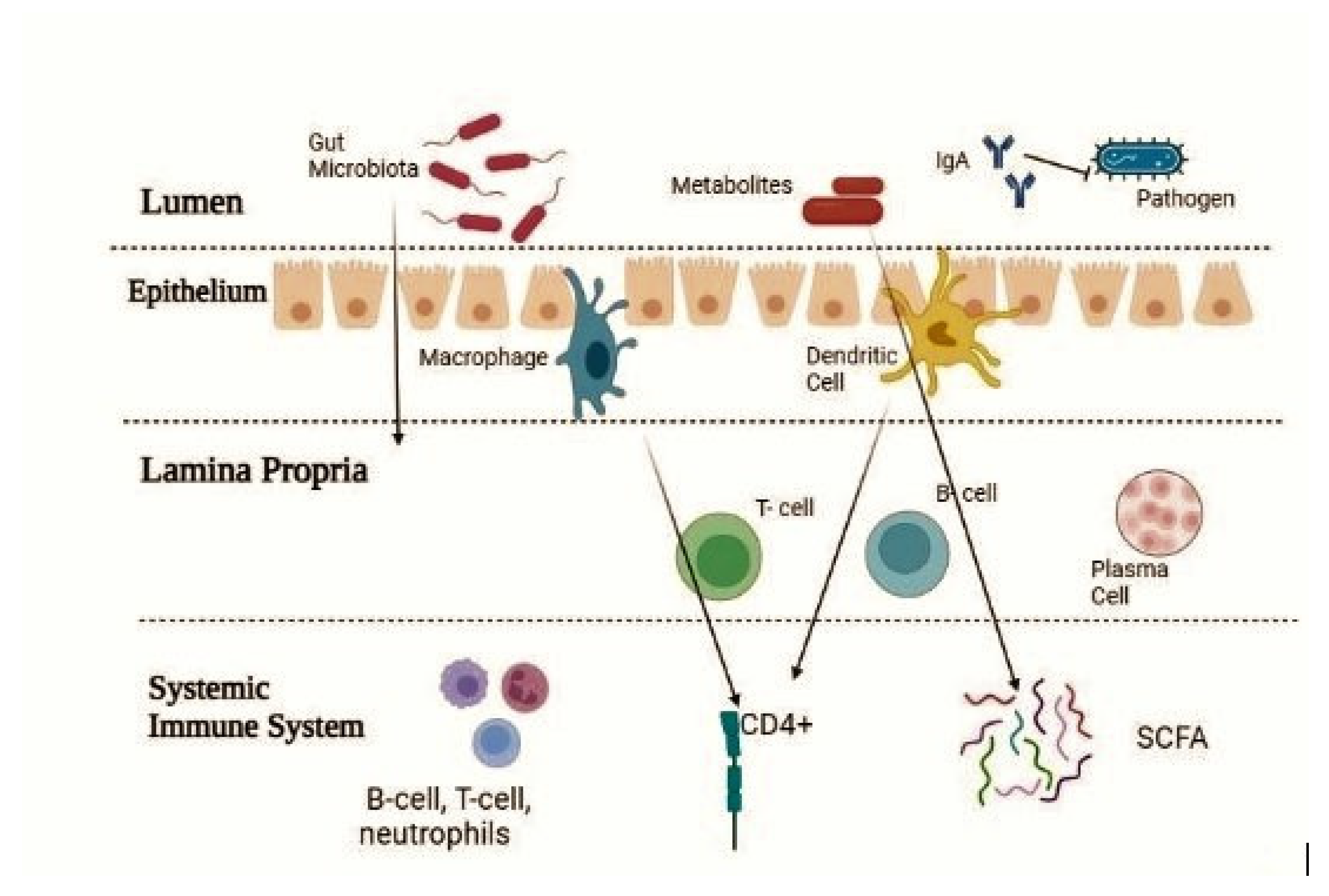
The disorganization of the gut microbiota can initiate an immunological imbalance, contributing to immune dysfunction and vulnerability to diseases. Pattern recognition receptors (PRRs) detect microbiota, reinforce host-microbiota connections, and defend against pathogens. Microbial signals generate pro-inflammatory cytokines, such as IL-23 and IL-1b, triggering T-cell manufacturing of IL-17 and IL-22, which foster the production of antimicrobial peptide (AMP). Dendritic cells pass on microbial antigens to gut lymphoid follicles, promoting Th17 and regulatory T cell development, and increasing IgA-producing plasma B cells. The gut microbiota sustains the host’s innate immunity by monitoring the gut environment (48). PRRs, including TLRs and NLRs, secrete microbial metabolites to control epithelial barriers, phagocyte durability, AMPs, and IgA production. Beneficial gut bacteria, through the fermentation of dietary fibers, generate short-chain fatty acids, which boost anti-inflammatory cytokines and Treg production (49) (
Figure 1).
The gut microbiota sustains intestinal immunological stability, triggering T-cell responses and inflammasome signaling, and producing cytokines such as TNF-α, IL-6, pro-IL-1b, and pro-IL-18. It promotes Th17 and Treg cell growth and tolerance via pattern recognition (48). Host immunity measures microbial metabolism through metabolites recognized by pattern recognition receptors (PRRs) (49). The microbiota, in turn, digests compounds like non-digestible fibers, tryptophan, arginine, and hepatic bile acids (50). Therefore, these transformations affect the gut immune system, increase antimicrobial activity, and facilitate sustained colonization. Moreover, the activity of inflammasomes enables microbial-mediated metabolic processes, controlling immune signaling pathways (51).
Immune Checkpoint Modulation by Gut Microbes
The immune system employs a network of inhibitory pathways known as Immune checkpoint inhibitors (ICIs), which enhance immune responses for eliminating tumor cells. Three categories of Immune checkpoint inhibitors, including monoclonal antibodies targeting PD-1, PD-L1, and CTLA-4, have received clinical approval. Notably, the gut microbiota produces essential compounds that affect metabolism, endocrine function, and immunity, which can influence the effectiveness of Immune Checkpoint Inhibitors. This leads to significant implications for drug development aimed at improving ICI efficacy (52).
The gut microbiota has the potential to enhance the efficacy of immunotherapy by modulating innate and adaptive immunity, as well as immunogenicity of tumor antigens, thereby restructuring the tumor microenvironment (53). Hence, the role of the gut microbiota in the regulation of intestinal immunity and modulation of the host immune system via mechanisms including systemic metabolic processes and immunological regulation. The role of the gut microbiota in immune regulation is continuously evolving, encompassing the inhibition of pro-inflammatory cytokines, overcoming regulatory T-cell (Treg) density, and promoting anti-tumor dendritic cell maturation and T-cell accumulation in the tumor microenvironment (12,54). Additionally, cytokines and metabolites play a crucial role in regulating gut microbiota, preserving the mucosal barrier, maintaining endocrine homeostasis, and modulating intestinal immunity (55). Moreover, the gut microbiota’s influence on immunotherapy effectiveness can be triggered by altering MHC class I/II genes (55). Disruption of microbial metabolism plays a significant role in regulating gut mucosa immunological balance. Some bacterial species contribute to anti-tumor tolerogenesis owing to lower short-chain fatty acids and increased primary bile acid conversion to secondary bile acids by Clostridiales, positively related to immune checkpoint inhibitor responses (56).
Cancer Immunotherapy: A Paradigm Shift in Cancer Treatment
The human body acquires all host-related microorganisms through vertical transmission after birth. These microorganisms undergo evolutionary changes through environmental exposure throughout life (57,58). Microbiota inhabits the human gut and tumor microenvironmentsas depicted in Table (2) and can influence both tumor development and treatment response (25). Consequently, cancer immunotherapy harnesses the body’s immune system to combat cancer, making a major scientific breakthrough.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) have emerged as one of the most innovative and promising cancer treatments in the past decade (57). Numerous reports indicated that these inhibitors function by blocking immune checkpoints, which are membrane-bound molecules used by cancer cells to evade the immune system (58,59). The most prominent immune checkpoints include the programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). These checkpoints suppress T cell activation, thereby weakening immune responses against cancer. However, it’s noteworthy that less than 30% of patients derive benefit from Immune checkpoint blockade (ICB) (60) which is a type of immunotherapy that has demonstrated efficacy against metastatic tumors (61).
The role of PD-1 as an immune checkpoint was confirmed with the discovery of one of its ligands, PD-L1 (26). Researchers proposed that inhibiting PD-1 would result in sustained T-cell responses. This concept gained support from several preclinical researchers, which finally led to the development of ipilimumab, a monoclonal antibody that targets human CTLA-4 and is used in clinical trials (59,62). Immune checkpoint inhibitors (ICIs) have achieved multiple approvals from both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), leading to a transformation in the treatment approach for certain metastatic cancers (35). Various immune checkpoint inhibitors (ICIs) have now become the standard treatment for several advanced cancer types, including lung cancer, renal cell carcinoma (RCC), urothelial carcinoma, and head and neck cancer (63).
Chimeric Antigen Receptor T Cell (CAR-T) Therapy
Chimeric antigen receptor (CAR)-T cell therapy is a revolutionary treatment, demonstrating highly effective and long-lasting clinical outcomes (64). CARs, synthetic receptors engineered to redirect immune cells, primarily T cells, are helpful in the identification and elimination of cells bearing a particular target antigen (65). These receptors interact directly with target antigens on the cell surface instead of utilizing the conventional major histocompatibility complex (MHC) receptor. It utilizes chimeric antigen receptors to empower T cells for tumor antigen recognition (66), leading to abundant T-cell activation and anti-tumor responses (67). Therefore, due to its exceptional success in combating B-cell malignancies, the US Food and Drug Administration (FDA) approved anti-CD19 CAR-T cell therapy in 2017 (66,67). FDA-approved treatments involve Tisagenlecleucel and Axicabtagene ciloleucel, with ongoing development targeting solid tumors (66).
CARs are considered modular synthetic receptors, comprising four primary elements:
An antigen-binding domain (64).
A hinge domain (64,65).
A transmembrane domain (64).
One or more intracellular signaling domains (65).
Gut Microbiota and Cancer Development:
The gut microbiota is also a major factor in the development of cancer, according to recent studies, which have prompted an investigation into the complex mechanisms underlying this correlation. To create focused interventions to reduce cancer risk and progression, it is crucial to comprehend these pathways.
Inflammation and Immune Modulation:
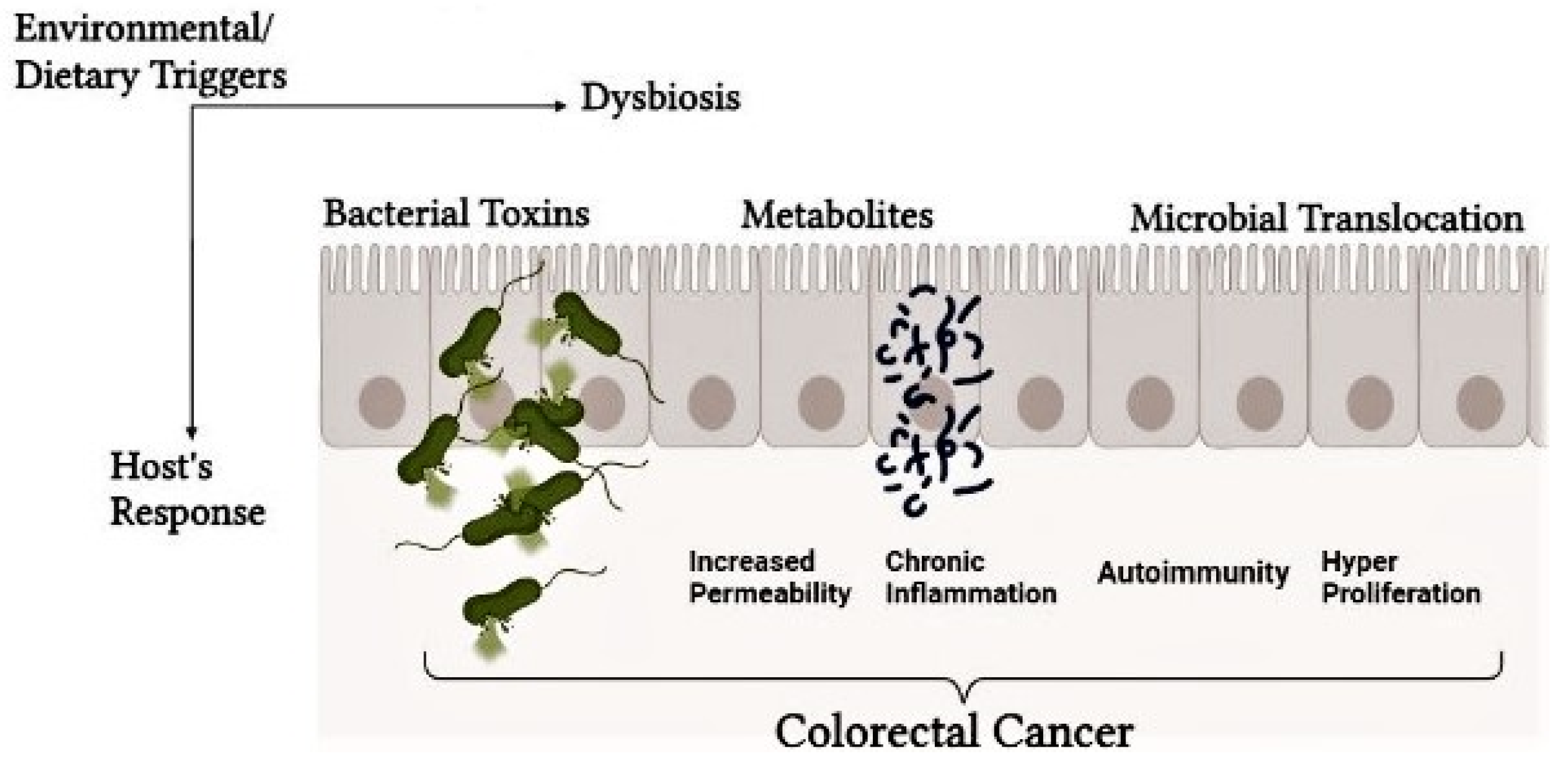
Chronic inflammation is an indication of cancer, with gut microbiota controlling host immune reactions. However, Intestinal carcinogenesis is also influenced by the gut microbiota. In addition to known particular carcinogens like Salmonella typhi and Helicobacter pylori, a general change in the gut microbiota may possibly play a role in the development of cancer(27). Dysbiosis can also induce persistent inflammation and cancer progression (68). Microbial components like LPS regulate pro-inflammatory pathways via Toll-like receptors (TLRs) (69). The gut microbiota influences the immune cell community, affecting antitumor immunity and fostering a pro-tumorigenic environment (
Figure 2).
Genotoxicity and DNA Damage:
Some gut microbial species can directly cause DNA damage in host cells by producing genotoxins such as cytolethal distending toxin (CDT) and colibactin. Type I deoxyribonuclease (DNase I) in mammals is functionally analogous to bacteria’s genotoxins, which cause single-strand breaks (SSBs) and double-strand breaks (DSBs) on genetic material in targeted cell populations, thereby inducing an initiation of the DNA damage response (DDR)(73). This promotes the development and spread of tumors by causing cell death, apoptosis, or mutations in genes as a result of these reactions (74).
Modulation of Host Signaling Pathways:
Mood, digestion, cognitive function, and motor skills are all significantly impacted by the relationship between the gut bacteria and the central nervous system. A multitude of illnesses, including cancer, can be attributed to disturbances in the microbiota-gut-brain pathway. The gut microbiota can affect cell division, death, and differentiation by interacting with host signaling pathways (75). Microbial metabolites may act as mediator for the activation of oncogenic pathways such as Wnt/-catenin and the epidermal growth factor receptor (EGFR) pathway, accelerating the development of cancer (76).
Microbial Dysbiosis and Cancer:
Microbial dysbiosis, a disruption of the gut microbiota’s delicate equilibrium, is linked to the emergence of cancer by various mechanisms (77). First, inflammation, a precipitating factor for tumors, can damage DNA promoting cell proliferation and creating a preferable environment for cancer growth (78). Second, microbiota alteration produces metabolites affecting host cells, leading to host immune system dysfunction and potentially impairing the body’s ability to recognize and combat cancer cells. Third, certain bacteria can activate procarcinogens, which become carcinogenic when metabolized. Thus, the connection between microbial dysbiosis and cancer emphasizes the importance of microbiota health in oncology.
Microbiota and Immunotherapy Response:
The gut microbiota influences immunotherapy efficiency by regulating anti-tumor immune responses, enhancing T-cell activation, and improving immune checkpoint inhibitor efficacy (79,80). Therefore, understanding and manipulating it holds promise for improving the efficacy of immunotherapy in cancer patients.
Mechanisms of Influence:
Researchers are still investigating the processes through which the microbiota affects the efficacy of cancer immunotherapy. The generation of chemicals by gut bacteria that can have an impact on the immune system is one mechanism. These metabolites can either stimulate or decrease the immune response, affecting how well immunotherapies work.
Microbiota and Immunotherapy Toxicity:
The toxicity connected with immunotherapy may potentially be influenced by the microbiota (81). The gut microbiota’s makeup may have an impact on how quickly and severely adverse effects from immunotherapy manifest. For instance, specific gut flora may affect the immunological reactions and inflammation that lead to adverse outcomes associated with immunotherapy (82).
Specific Cancer Types Influenced by the Gut Microbiota:
Gut microbiota such as Fusobacterium nucleatum are related to colorectal cancer risk (83). Particular gut microbes boost anti-tumor immune responses and immunotherapy efficacy in melanoma (84). Specific bacteria interlink with enhanced response and persistent survival in lung cancer individuals on immunotherapy (85). Gut microbiota alterations impact liver inflammation and liver cancer risk (86). Recent research explores a significant interaction between the gut microbiota and breast cancer (87). A changed gut microbiota may influence immune response and inflammation, impacting pancreatic cancer development and treatment response (88). The gut microbiota’s role in gastroesophageal cancers, including esophageal and gastric cancers, is under analysis for its influence on inflammation, immunity, and cancer development (89).
Several studies have highlighted the tumor specificity of certain bacterial taxa and their potential role in cancer treatment. Some of these bacteria are manipulated due to their characteristics such as localied cytotoxicity, tumor specific germination, spore formation under specific conditions. Neospora caninum and Clostridium species spores, offer a promising approach to cancer therapy due to their ability to germinate specifically in the hypoxic and necrotic environments of tumors, minimizing toxicity to healthy tissues (90). Once activated, these spores secrete enzymes that degrade tumor cells while simultaneously triggering an immune response that enhances the infiltration of immune cells, such as neutrophils and monocytes, into the tumor microenvironment. Engineered strains like Clostridium novyi-NT, which have reduced toxicity, can effectively induce tumor regression and promote long-term immune activation(90). Additionally, these spores can be designed to release prodrug-converting enzymes at the tumor site, transforming non-toxic prodrugs into potent cytotoxic agents. This multifaceted mechanism highlights the potential of bacterial spores as a targeted and effective cancer treatment strategy.
Tumor Micro-Environnement Immune Response Modulation:
The gut microbiota affects the tumor microenvironment and induce immune cells against tumors, having an anti-tumor response. In the tumor microenvironment, angiogenesis and tissue remodeling caused by gut bacteria influence tumor growth and interference (91). Considering these relationships is necessary for personalized cancer treatment. Modifying the gut microbiota via diet, probiotics, or fecal microbiota transplantation (FMT) can change the tumor microenvironment and potentially enhance responses to immunotherapies and cancer treatments.
Strategies to Manipulate the Gut Microbiota to Improve Cancer Immunotherapy:
The composition and activity of gut bacteria can be changed to improve the efficacy of immunotherapeutic methods as part of strategies to regulate the gut microbiota to improve cancer immunotherapy. The objective is to alter the gut microbiota in a way that promotes an immune response that fights cancer and enhances the effectiveness of cancer immunotherapies such as immune checkpoint inhibitors. The following are some of the approaches under consideration and investigation:
Probiotics and Prebiotics:
Probiotics, live microorganisms administered in sufficient dosages, offer positive effects on health (92). Certain probiotic strains have been investigated for their potential to modulate the gut microbiota and enhance the response to immunotherapy (93). On the other hand, prebiotics, or indigestible fibers, provide nourishment for good microorganisms in the gut. These symbiotic microbial species encourage the growth of helpful bacteria, thereby improving the immune system’s capacity to fight tumors.
Administration of Probiotics to Improve the Efficacy of Immunotherapy and Chemotherapy:
Fighting cancer is a challenging task, as cancerous cells possess the ability to surpass the host’s immune system despite advanced therapies. However, probiotics hold the promise to overcome such barriers and act as anti-cancerous agents by promoting the development of anti-oxidant, anti-cancerous, and anti-inflammatory products (94). Furthermore, Probiotics combat cancer through a variety of methods, including enhancing the intestinal barrier, translocation of bacteria, and preserving the homeostasis of the gut microbiota(95).
Probiotics play a key role in improving immune checkpoint inhibitor therapy for cancer patients. Probiotics such as Lactobacillus and Bifidobacterium, are essential parts of the healthy gut flora that aid in halting the growth and spread of tumors. By inducing phagocytes to eliminate cancer cells in the initial stages and controlling anti-inflammatory cytokines, they improve the body’s reaction to immunological checkpoint inhibitors (ICIs). Probiotics’ control over the host’s immunological history is directly related to their capacity to affect the effectiveness of ICIs(96). Among probiotics, Lactobacillus rhamnosus GG (LGG) constitutes one of the most researched and thoroughly characterized archetypes. Lactobacilli is one of the probiotics being researched as a supplementary therapy for intestinal damage linked to chemotherapy(97). Moreover,several onging clinical studies focus on using probiotics to prevent and manage chemotherapy-induced side effects (Table 3). Notably, probiotics may help alleviate symptoms like diarrhea and mucositis, enhancing patients’ quality of life during cancer treatment.
Dietary Interventions:
Dietary modifications have a significant effect on the gut flora. A diet high in plant-based foods and fiber can encourage the development of good gut flora, which provides compounds that support an anti-inflammatory and anti-tumor environment (98).
Antibiotics and Microbiota Modulation:
Antibiotics have a short-term impact on the gut microbiota and studies suggest that their usage can influence the efficacy of cancer immunotherapy treatments (99). Therefore, Antibiotic use should be careful, though, as it can potentially eliminate helpful bacteria.
Synbiotics:
Synbiotics are a combination of probiotics and prebiotics. They aim to enhance the survival and activity of beneficial bacteria in the gut by providing the necessary nutrients for their growth and maintenance. Hence, improving the therapeutic effect of gut microbiota (100).
Fecal Microbiota Transplantation (FMT):
Fecal Microbiota Transplantation restores a healthy gut microbiota by transplanting feces from a healthy donor into the recipient’s digestive system. FMT has been investigated as a potential method to modify the gut microbiota and enhance cancer immunotherapy response rates (101). Fecal microbiota transplantatio is a promising treatment option. However, research studies have shown both positive and negative outcomes in relation to immunotherapy and cancer treatment. Clinical trials have also identified both beneficial and adverse effects of FMT on cancer (Table 4).
Conclusion
microbiotas emerge as powerful tools, both in the clinical and pre-clinical context, revealing their potential for treating cancer through immunotherapy. Since the discovery of immune checkpoint inhibitors, the modulation of the gut microbiota for optimal health has evolved significantly in cancer therapy. However, it presents certain challenges that necessitate comprehensive research to implement this immunotherapeutic strategy for optimal results. A thorough analysis of the microbiota’s influence on the immune system, along with the identification of unique beneficial bacterial taxa, for different individual ethnic and environmental backgrounds, and their pathways linked to immunity, is necessary. This can be beneficial in overcoming challenges in novel therapy strategies, such as the association of fecal microbiota transplantation (FMT) with infections. A systematic classification of individuals into groups, discerning the shared or disparate factors (including diet, environment, and genetics) influencing their microbiota composition. The adoption of distinctive methodologies, such as profiling techniques to delineate a group of individuals’ microbiotas, holds significant promise for attaining favorable therapeutic outcomes
Challenges and Future Directions
Unanswered Questions and Areas for Further Research
The gut microbiota plays a pivotal role in eradicating cancer via modulation therapies, especially immunotherapy; however, certain unanswered questions need to be highlighted. A novel microbiota modulation therapy, Fecal Microbiota Transplantation is an efficient way of treating diseases caused by bacterial species possessing antibiotic resistance, such as Clostridium difficile (102), and enhances insulin resistance in diabetic patients. Despite these achievements, various complications in FMT therapy need to be addressed, specifically the selection of the best donor which should have a broad diversity of microbial species having beneficial bacteria. To date, Akkermansia muciniphili, Bifidobacteria spp, Bacteroides spp, and E. hirae have been considered advantageous bacteria that increase anti-cancer immunity. Further, it elevates concerns about the potential transmission of infectious pathogens, necessitating careful examination, and disregarding viruses and bacteria (103).
Harmful bacteria can weaken immunotherapy efficiency but are indiscriminately eliminated by antibiotics, causing dysbiosis. Prebiotics, whether dietary or chemical, can increase the growth of beneficial bacteria, enhancing the role of immunotherapy. The complete digestion of dietary fiber elements produces fatty acids with anti-tumor characteristics (104). The effectiveness of prebiotics relies on the host’s specific bacteria. Merging specific bacteria with prebiotics as symbiotics can be helpful (105). Moreover, bacteriophages selective for specific bacterial species may assist in eliminating harmful intestinal bacteria (106). Challenges from the early 20th century regarding perniciousness and impurity still affect microbiota-related tumor diagnosis and therapies (107). Precise studies with selected samples are needed to mitigate toxicities and understand microbial effects on oncogenesis and therapeutic responses.
References
- McDermott AJ, Huffnagle GB. The microbiota and regulation of mucosal immunity. Immunology. 2014 May;142(1):24–31. [CrossRef]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The Application of Ecological Theory Toward an Understanding of the Human microbiota. Science. 2012 Jun 8;336(6086):1255–62. [CrossRef]
- Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, et al. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia. 2017 Oct;19(10):848–55. [CrossRef]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al.. Host-gut microbiota metabolic interactions. Science (2012) 336:1262–7. [CrossRef]
- Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015 Jan;37(1):47–55. [CrossRef]
- Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr Cardiol Rep. 2015 Dec;17(12):120. [CrossRef]
- Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiota on cancer, immunity, and cancer immunotherapy. Cancer cell. 2018 Apr 4;33(4):570. [CrossRef]
- Morgan XC, Huttenhower C. Chapter 12: Human microbiota Analysis. Lewitter F, Kann M, editors. PLoS Comput Biol. 2012 Dec 27;8(12):e1002808. [CrossRef]
- Li W, Deng Y, Chu Q, Zhang P. Gut microbiota and cancer immunotherapy. Cancer Letters. 2019 Apr;447:41–7.
- Kroemer G, Zitvogel L. The breakthrough of the microbiota. Nat Rev Immunol. 2018 Feb;18(2):87–8. [CrossRef]
- Mager L.F, Burkhard R, Pett N, Cooke N.C.A, Brown K, Ramay H, Paik S, Stagg J, Groves R.A, Gallo M.et al.Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy.Science. 2020; 369: 1481-1489. [CrossRef]
- Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Annals of Oncology. 2019 Dec;30(12):2012. [CrossRef]
- Liang D, Leung RKK, Guan W, Au WW. Involvement of gut microbiota in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018;10:3. [CrossRef]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012 Oct 4;490(7418):55–60. [CrossRef]
- Tilg H, Cani PD, Mayer EA Gut microbiome and liver diseasesGut 2016;65:2035-2044. [CrossRef]
- Igartua C, Davenport ER, Gilad Y, Nicolae DL, Pinto J, Ober C. Host genetic variation in mucosal immunity pathways influences the upper airway microbiome. Microbiome. 2017 Feb 1;5(1):16. [CrossRef]
- Knights D, Silverberg MS, Weersma RK, Gevers D, Dijkstra G, Huang H, et al. Complex host genetics influence the microbiota in inflammatory bowel disease. Genome Med. 2014;6(12):107. [CrossRef]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–71. [CrossRef]
- Garcia-Vello P, Di Lorenzo F, Zucchetta D, Zamyatina A, De Castro C, Molinaro A. Lipopolysaccharide lipid A: A promising molecule for new immunity-based therapies and antibiotics. Pharmacology & Therapeutics. 2022;230:107970. [CrossRef]
- Kim ES, Kim SY, Moon A. C-Reactive Protein Signaling Pathways in Tumor Progression. Biomol Ther (Seoul). 2023 Sep 1;31(5):473–83. [CrossRef]
- Sharma A, Buschmann MM, Gilbert JA. Pharmacomicrobiomics: The Holy Grail to Variability in Drug Response? Clin Pharmacol Ther. 2019 Aug;106(2):317–28. [CrossRef]
- Garber A, Hastie P, Murray JA. Factors Influencing Equine Gut Microbiota: Current Knowledge. J Equine Vet Sci. 2020 May;88:102943. [CrossRef]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017 Jan;541(7637):321–30. [CrossRef]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiota influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018 Jan 5;359(6371):91–7. [CrossRef]
- Elinav E, Garrett WS, Trinchieri G, Wargo J. The cancer microbiota. Nat Rev Cancer. 2019 Jul;19(7):371–6.
- Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016 Jun 21;44(6):1255–69. [CrossRef]
- Roviello G, Iannone LF, Bersanelli M, Mini E, Catalano M. The gut microbiota and efficacy of cancer immunotherapy. Pharmacol Ther. 2022 Mar;231:107973. [CrossRef]
- Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10:1758835918764628. [CrossRef]
- Sehgal, K. Hyperprogression in Patients With Cancer Receiving Immune Checkpoint Inhibitors. JAMA Network Open [Internet]. 2021 Mar 24 [cited 2024 Jun 30];4(3):e211839. [CrossRef]
- Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016 Aug;14(8):e1002533. [CrossRef]
- Velikova T, Krastev B, Lozenov S, Gencheva R, Peshevska-Sekulovska M, Nikolaev G, et al. Antibiotic-Related Changes in microbiota: The Hidden Villain behind Colorectal Carcinoma Immunotherapy Failure. Int J Mol Sci. 2021 Feb 10;22(4):1754. [CrossRef]
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018 Sep;8(9):1069–86. [CrossRef]
- Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022 Apr 29;15(1):47. [CrossRef]
- Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer. 2019 Jun 10;19(1):559. [CrossRef]
- Immuno-oncology drug development goes global - PubMed [Internet]. [cited 2024 Jun 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/31780841/.
- Encarnação JC, Pires AS, Amaral RA, Gonçalves TJ, Laranjo M, Casalta-Lopes JE, et al. Butyrate, a dietary fiber derivative that improves irinotecan effect in colon cancer cells. J Nutr Biochem. 2018 Jun;56:183–92. [CrossRef]
- Smith CL, Geier MS, Yazbeck R, Torres DM, Butler RN, Howarth GS. Lactobacillus fermentum BR11 and fructo-oligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr Cancer. 2008;60(6):757–67. [CrossRef]
- Taper HS, Roberfroid MB. Nontoxic potentiation of cancer chemotherapy by dietary oligofructose or inulin. Nutr Cancer. 2000;38(1):1–5. [CrossRef]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiota influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018 Jan 5;359(6371):91–7. [CrossRef]
- Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017 Sep 15;357(6356):1156–60. [CrossRef]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiota influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018 Jan 5;359(6371):91–7. [CrossRef]
- Treatment with selenium-enriched Saccharomyces cerevisiae UFMG A-905 partially ameliorates mucositis induced by 5-fluorouracil in mice - PubMed [Internet]. [cited 2024 Jun 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/31079219/.
- Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006 Feb 3;281(5):2858–67. [CrossRef]
- Wieland WH, Orzáez D, Lammers A, Parmentier HK, Verstegen MWA, Schots A. A functional polymeric immunoglobulin receptor in chicken (Gallus gallus) indicates ancient role of secretory IgA in mucosal immunity. Biochem J. 2004 Jun 15;380(Pt 3):669–76. [CrossRef]
- Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, et al. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe. 2016 Jan 13;19(1):32–43. [CrossRef]
- Kogut M, XiaoNan Y, JianMin Y, Broom L. Gut health in poultry. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2017 Oct 25;12.
- Bene K, Varga Z, Petrov VO, Boyko N, Rajnavolgyi E. Gut Microbiota Species Can Provoke both Inflammatory and Tolerogenic Immune Responses in Human Dendritic Cells Mediated by Retinoic Acid Receptor Alpha Ligation. Front Immunol. 2017;8:427. [CrossRef]
- Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016 Jul 15;30(14):1589–97. [CrossRef]
- Blacher E, Levy M, Tatirovsky E, Elinav E. microbiota-Modulated Metabolites at the Interface of Host Immunity. J Immunol. 2017 Jan 15;198(2):572–80. [CrossRef]
- Göbel TW, Schneider K, Schaerer B, Mejri I, Puehler F, Weigend S, et al. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: conservation of a Th1-like system in a nonmammalian species. J Immunol. 2003 Aug 15;171(4):1809–15. [CrossRef]
- Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, et al. Nlrp6 regulates intestinal antiviral innate immunity. Science. 2015 Nov 13;350(6262):826–30. [CrossRef]
- Tan S, Li D, Zhu X. Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother. 2020 Apr;124:109821. [CrossRef]
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021 Jan;19(1):55–71. [CrossRef]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015 Nov 27;350(6264):1084–9. [CrossRef]
- The Interplay between Immunity and Microbiota at Intestinal Immunological Niche: The Case of Cancer - PubMed [Internet]. [cited 2024 Jun 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/30682772/.
- Maslowski KM. Metabolism at the centre of the host-microbe relationship. Clin Exp Immunol. 2019 Aug;197(2):193–204. [CrossRef]
- Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol. 2013 Jul;14(7):646–53. [CrossRef]
- Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692. [CrossRef]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015 Apr 3;348(6230):56–61. [CrossRef]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013 Jul 11;369(2):134–44. [CrossRef]
- Chai Y, Huang Z, Shen X, Lin T, Zhang Y, Feng X, et al. Microbiota Regulates Pancreatic Cancer Carcinogenesis through Altered Immune Response. Microorganisms. 2023 May 8;11(5):1240. [CrossRef]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996 Mar 22;271(5256):1734–6. [CrossRef]
- Barbari C, Fontaine T, Parajuli P, Lamichhane N, Jakubski S, Lamichhane P, et al. Immunotherapies and Combination Strategies for Immuno-Oncology. Int J Mol Sci. 2020 Jul 15;21(14):5009. [CrossRef]
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018 Mar 23;359(6382):1361–5. [CrossRef]
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021 Apr 6;11(4):69. [CrossRef]
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017 Dec 28;377(26):2531–44. [CrossRef]
- Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017 Dec 28;377(26):2545–54. [CrossRef]
- Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. 2014 Oct;16(10):406. [CrossRef]
- Xia P, Wu Y, Lian S, Yan L, Meng X, Duan Q, et al. Research progress on Toll-like receptor signal transduction and its roles in antimicrobial immune responses. Appl Microbiol Biotechnol. 2021 Jul;105(13):5341–55. [CrossRef]
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018 Feb;57(1):1–24. [CrossRef]
- Modrego J, Ortega-Hernández A, Goirigolzarri J, Restrepo-Córdoba MA, Bäuerl C, Cortés-Macías E, et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int J Mol Sci. 2023 Sep 9;24(18):13892. [CrossRef]
- Keku TO, Dulal S, Deveaux A, Jovov B, Han X. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015 Mar 1;308(5):G351-363. [CrossRef]
- Lai YR, Chang YF, Ma J, Chiu CH, Kuo ML, Lai CH. From DNA Damage to Cancer Progression: Potential Effects of Cytolethal Distending Toxin. Front Immunol. 2021;12:760451. [CrossRef]
- Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. 2015;6:157. [CrossRef]
- Cox LM, Weiner HL. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics. 2018 Jan;15(1):135–45. [CrossRef]
- Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022 Jul 14;21(1):144. [CrossRef]
- Asseri AH, Bakhsh T, Abuzahrah SS, Ali S, Rather IA. The gut dysbiosis-cancer axis: illuminating novel insights and implications for clinical practice. Front Pharmacol. 2023;14:1208044. [CrossRef]
- Dey P, Ray Chaudhuri S. Cancer-Associated Microbiota: From Mechanisms of Disease Causation to Microbiota-Centric Anti-Cancer Approaches. Biology (Basel). 2022 May 16;11(5):757. [CrossRef]
- Zhang M, Liu J, Xia Q. Role of gut microbiota in cancer immunotherapy: from predictive biomarker to therapeutic target. Exp Hematol Oncol. 2023 Sep 28;12(1):84. [CrossRef]
- The microbiota and Its Implications in Cancer Immunotherapy - PubMed [Internet]. [cited 2024 Jun 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/33401586/.
- Dadgar N, Edlukudige Keshava V, Raj MS, Wagner PL. The Influence of the microbiota on Immunotherapy for Gastroesophageal Cancer. Cancers (Basel). 2023 Sep 5;15(18):4426. [CrossRef]
- Wardill HR, Chan RJ, Chan A, Keefe D, Costello SP, Hart NH. Dual contribution of the gut microbiota to immunotherapy efficacy and toxicity: supportive care implications and recommendations. Support Care Cancer. 2022 Aug;30(8):6369–73. [CrossRef]
- Alon-Maimon T, Mandelboim O, Bachrach G. Fusobacterium nucleatum and cancer. Periodontol 2000. 2022 Jun;89(1):166–80. [CrossRef]
- Najmi M, Tran T, Witt RG, Nelson KC. Modulation of the Gut microbiota to Enhance Immunotherapy Response in Metastatic Melanoma Patients: A Clinical Review. Dermatol Ther (Heidelb). 2022 Nov;12(11):2489–97. [CrossRef]
- Khan MAW, Ologun G, Arora R, McQuade JL, Wargo JA. Gut microbiota Modulates Response to Cancer Immunotherapy. Dig Dis Sci. 2020 Mar;65(3):885–96. [CrossRef]
- Rajapakse J, Khatiwada S, Akon AC, Yu KL, Shen S, Zekry A. Unveiling the complex relationship between gut microbiota and liver cancer: opportunities for novel therapeutic interventions. Gut Microbes. 2023 Dec;15(2):2240031. [CrossRef]
- Viswanathan S, Parida S, Lingipilli BT, Krishnan R, Podipireddy DR, Muniraj N. Role of Gut Microbiota in Breast Cancer and Drug Resistance. Pathogens. 2023 Mar 16;12(3):468. [CrossRef]
- Li Q, Jin M, Liu Y, Jin L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front Cell Infect Microbiol. 2020;10:572492. [CrossRef]
- Smet A, Kupcinskas J, Link A, Hold GL, Bornschein J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse? Cell Mol Gastroenterol Hepatol. 2022;13(3):857–74. [CrossRef]
- Huang X, Pan J, Xu F, Shao B, Wang Y, Guo X, Zhou S. Bacteria-based cancer immunotherapy. Adv Sci (Weinh). 2021 Feb 10;8(7):2003572. PMCID: PMC8025040. [CrossRef] [PubMed]
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020 May;77(9):1745–70. [CrossRef]
- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:481651. [CrossRef]
- Rezasoltani S, Yadegar A, Asadzadeh Aghdaei H, Reza Zali M. Modulatory effects of gut microbiota in cancer immunotherapy: A novel paradigm for blockade of immune checkpoint inhibitors. Cancer Med. 2021 Feb;10(3):1141–54. [CrossRef]
- Sankarapandian V, Venmathi Maran BA, Rajendran RL, Jogalekar MP, Gurunagarajan S, Krishnamoorthy R, et al. An Update on the Effectiveness of Probiotics in the Prevention and Treatment of Cancer. Life (Basel). 2022 Jan 2;12(1):59. [CrossRef]
- Noor S, Ali S, Riaz S, Sardar I, Farooq MA, Sajjad A. Chemopreventive role of probiotics against cancer: a comprehensive mechanistic review. Mol Biol Rep. 2023 Jan;50(1):799–814. [CrossRef]
- Wan L, Wu C, Wu Q, Luo S, Liu J, Xie X. Impact of probiotics use on clinical outcomes of immune checkpoint inhibitors therapy in cancer patients. Cancer Med. 2023 Jan;12(2):1841–9. [CrossRef]
- Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, et al. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers (Basel). 2019 Jan 3;11(1):38. [CrossRef]
- Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019 Nov 22;11(12):2862. [CrossRef]
- Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C, et al. Antibiotics for cancer treatment: A double-edged sword. J Cancer. 2020;11(17):5135–49. [CrossRef]
- Manipulating Gut Microbiota Composition to Enhance the Therapeutic Effect of Cancer Immunotherapy - PubMed [Internet]. [cited 2024 Jun 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/31517538/.
- Quaranta G, Guarnaccia A, Fancello G, Agrillo C, Iannarelli F, Sanguinetti M, Masucci L. Fecal microbiota transplantation and other gut microbiota manipulation strategies. Microorganisms. 2022 Dec 7;10(12):2424. PMCID: PMC9781458. [CrossRef] [PubMed]
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013 Jan 31;368(5):407–15. [CrossRef]
- Li W, Deng Y, Chu Q, Zhang P. Gut microbiota and cancer immunotherapy. Cancer Lett. 2019 Apr 10;447:41–7.
- Bultman SJ. The microbiota and its potential as a cancer preventive intervention. Semin Oncol. 2016 Feb;43(1):97–106. [CrossRef]
- Ghafar A, Khan A, Cabezas-Cruz A, Gauci CG, Niaz S, Ayaz S, et al. An Assessment of the Molecular Diversity of Ticks and Tick-Borne Microorganisms of Small Ruminants in Pakistan. Microorganisms. 2020 Sep 17;8(9):1428. [CrossRef]
- The Use of Bacteriophages in the Poultry Industry - PubMed [Internet]. [cited 2024 Jun 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/32443410/.
- Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiota and human cancer. Science. 2021 Mar 26;371(6536):eabc4552.
- Chrysostomou D, Roberts LA, Marchesi JR, Kinross JM. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology. 2023 Feb;164(2):198–213. [CrossRef]
- Ma Y, Liu J, Rhodes C, Nie Y, Zhang F. Ethical Issues in Fecal Microbiota Transplantation in Practice. Am J Bioeth. 2017 May;17(5):34–45. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).