Submitted:
05 August 2024
Posted:
06 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1 Chemicals and Materials
2.2 Defluorination of Fluorinated Carbon Nanotubes
2.3 Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit Rev Solid State Mater Sci 2016, 41, 257–317. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat Mater 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, L.; Xia, Z.; Li, C.M.; Dai, L. Hole-punching for enhancing electrocatalytic activities of 2D graphene electrodes: Less is more. J Chem Phys 2020, 153. [Google Scholar] [CrossRef] [PubMed]
- Ovid’ko, I.A. Review on Grain Boundaries in Graphene. Curved Poly- and Nanocrystalline Graphene Structures as New Carbon Allotropes. Rev Adv Mater Sci 2012, 30, 201–224. [Google Scholar]
- Zhang, S.H.; Zhou, J.; Wang, Q.; Chen, X.S.; Kawazoe, Y.; Jena, P. Penta-graphene: A new carbon allotrope. PNAS 2015, 112, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhou, X.F.; Zhang, X.M.; Zhu, Q.; Dong, H.F.; Zhao, M.W.; Oganov, A.R. Phagraphene: A Low-Energy Graphene Allotrope Composed of 5-6-7 Carbon Rings with Distorted Dirac Cones. Nano Lett 2015, 15, 6182–6186. [Google Scholar] [CrossRef] [PubMed]
- Chalifoux, W.A.; Tykwinski, R.R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat Chem 2010, 2, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, L.; Mao, H.K.; Chow, P.; Xiao, Y.M.; Baldini, M.; Shu, J.F.; Mao, W.L. Amorphous Diamond: A High-Pressure Superhard Carbon Allotrope. Phys Rev Lett 2011, 107. [Google Scholar] [CrossRef]
- Khabashesku, V.N.; Gu, Z.N.; Brinson, B.; Zimmerman, J.L.; Margrave, J.L.; Davydov, V.A.; Kashevarova, L.S.; Rakhmanina, A.V. Polymerization of single-wall carbon nanotubes under high pressures and high temperatures. J Phy Chem B 2002, 106, 11155–11162. [Google Scholar] [CrossRef]
- Terrones, M.; Terrones, H.; Banhart, F.; Charlier, J.C.; Ajayan, P.M. Coalescence of single-walled carbon nanotubes. Science 2000, 288, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Csanyi, G.; Salvetat, J.P.; Lee, T.N.; Couteau, E.; Kulik, A.J.; Benoit, W.; Brugger, J.; Forro, L. Reinforcement of single-walled carbon nanotube bundles by intertube bridging. Nat Mater 2004, 3, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Jaroenapibal, P.; Luzzi, D.E.; Evoy, S. Tuning the resonant frequency of single-walled carbon nanotube bundle oscillators through electron-beam-induced cross-link formations. Appl Phys Lett 2007, 90, 081912. [Google Scholar] [CrossRef]
- Peng, B.; Locascio, M.; Zapol, P.; Li, S.Y.; Mielke, S.L.; Schatz, G.C.; Espinosa, H.D. Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvements. Nat Nanotechnol 2008, 3, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Ma, B.; Bunker, C.E.; Liu, B. All-carbon polymers (polyfullerenes) from photochemical reactions of fullerene clusters in room-temperature solvent mixtures. J Am Chem Soc 1995, 117, 12705–12711. [Google Scholar] [CrossRef]
- Lin, T.; Zhang, W.-D.; Huang, J.; He, C. A DFT Study of the Amination of Fullerenes and Carbon Nanotubes: Reactivity and Curvature. J Phys Chem B 2005, 109, 13755–13760. [Google Scholar] [CrossRef]
- Galano, A. Carbon nanotubes: promising agents against free radicals. Nanoscale 2010, 2, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Green, M.J.; Behabtu, N.; Pasquali, M.; Adams, W.W. Nanotubes as polymers. Polymer 2009, 50, 4979–4997. [Google Scholar] [CrossRef]
- Yao, Y.B.; Luo, S.D.; Liu, T. Determination of the Length, Diameter, Molecular Mass, Density and Surfactant Adsorption of SWCNTs in Dilute Dispersion by Intrinsic Viscosity, Sedimentation, and Diffusion Measurements. Macromolecules 2014, 47, 3093–3100. [Google Scholar] [CrossRef]
- Parra-Vasquez, A.N.G.; Duque, J.G.; Green, M.J.; Pasquali, M. Assessment of length and bundle distribution of dilute single-walled carbon nanotubes by viscosity measurements. AIChE J 2014, 60, 1499–1508. [Google Scholar] [CrossRef]
- Tsentalovich, D.E.; Ma, A.W.K.; Lee, J.A.; Behabtu, N.; Bengio, E.A.; Choi, A.; Hao, J.; Luo, Y.M.; Headrick, R.J.; Green, M.J.; et al. Relationship of Extensional Viscosity and Liquid Crystalline Transition to Length Distribution in Carbon Nanotube Solutions. Macromolecules 2016, 49, 681–689. [Google Scholar] [CrossRef]
- Shen, Z.; Roding, M.; Kroger, M.; Li, Y. Carbon Nanotube Length Governs the Viscoelasticity and Permeability of Buckypaper. Polymers 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Chen, H.W.; Ge, J.; Zhao, J.N.; Li, Q.W.; Tang, J.X.; Cui, Y.; Chen, L.W. Direct Intertube Cross-Linking of Carbon Nanotubes at Room Temperature. Nano Lett 2016, 16, 6541–6547. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, H.F. Experimental and Computational Investigations of the Properties of Fluorinated Single-Walled Carbon Nanotubes. ChemPhysChem 2003, 4, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Porfyrakis, K.; Sambrook, M.R.; Ardavan, A.; Briggs, G.A.D. Determination of the thermal stability of the fullerene dimers C120, C120O, and C120O2. J Phys Chem B 2006, 110, 16979–16981. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam Relat Mater 2022, 127, 109180. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; He, K.; Wang, F. Raman Study of 532-Nanometer Laser-Induced Degradation of Red Lead. Materials 2024, 17, 770. [Google Scholar] [CrossRef]
- Restelli, S.; Albini, B.; Bonomi, S.; Bini, M.; Mozzati, M.C.; Galinetto, P. Raman study of the laser-induced decomposition of ZnFe2O4 nanoparticles. Mater Today Commun 2023, 35. [Google Scholar] [CrossRef]
- Kato, R.; Miyazawa, K.i. Raman Laser Polymerization of C60 Nanowhiskers. J Nanotechnol 2012, 2012, 101243. [Google Scholar] [CrossRef]
- Liao, M.; Shan, B.; Li, M. In Situ Raman Spectroscopic Studies of Thermal Stability of All-Inorganic Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 1217–1225. [Google Scholar] [CrossRef]
- Mases, M.; Noel, M.; Dossot, M.; McRae, E.; Soldatov, A. V. Laser-induced damage and destruction of HiPCO nanotubes in different gas environments. Phys. Status Solidi B 2011, 248, 2540–2543. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.C.; Wang, Z.M.; Lai, W.C.; Zhang, X.J.; Wang, X.; Liu, X.Y. Investigation of the dispersion behavior of fluorinated MWCNTs in various solvents. Phys Chem Chem Phys 2017, 19, 21565–21574. [Google Scholar] [CrossRef]
- Mickelson, E.T.; Huffman, C.B.; Rinzler, A.G.; Smalley, R.E.; Hauge, R.H.; Margrave, J.L. Fluorination of single-wall carbon nanotubes. Chem Phys Lett 1998, 296, 188–194. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: synthesis, properties and engineering applications. Carbon Lett 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Sakai, M.; Ichida, M.; Nakamura, A. Photopolymerization and thermal decomposition of polymerized phase in C60 crystals under strong laser illumination. Fullerene Sci Techn 2001, 9, 351–361. [Google Scholar] [CrossRef]
- Kneipp, K.; Perelman, L.T.; Kneipp, H.; Backman, V.; Jorio, A.; Dresselhaus, G.; Dresselhaus, M.S. Coupling and scattering power exchange between phonon modes observed in surface-enhanced Raman spectra of single-wall carbon nanotubes on silver colloidal clusters. Phys Rev B 2001, 63, 193411. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth Met 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Huang, H.J.; Maruyama, R.; Noda, K.; Kajiura, H.; Kadono, K. Preferential destruction of metallic single-walled carbon nanotubes by laser irradiation. J Phys Chem B 2006, 110, 7316–7320. [Google Scholar] [CrossRef] [PubMed]
- Maultzsch, J.; Telg, H.; Reich, S.; Thomsen, C. Radial breathing mode of single-walled carbon nanotubes: Optical transition energies and chiral-index assignment. Phys Rev B 2005, 72, 205438. [Google Scholar] [CrossRef]
- Corio, P.; Santos, P.S.; Pimenta, M.A.; Dresselhaus, M.S. Evolution of the molecular structure of metallic and semiconducting carbon nanotubes under laser irradiation. Chem Phys Lett 2002, 360, 557–564. [Google Scholar] [CrossRef]
- Zhang, W.; Dubois, M.; Guerin, K.; Bonnet, P.; Kharbache, H.; Masin, F.; Kharitonov, A.P.; Hamwi, A. Effect of curvature on C-F bonding in fluorinated carbons: from fullerene and derivatives to graphite. Phys Chem Chem Phys 2010, 12, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, H.F.; Peng, H. Thermolysis of Fluorinated Single-Walled Carbon Nanotubes: Identification of Gaseous Decomposition Products by Matrix Isolation Infrared Spectroscopy. J Phys Chem B 2005, 109, 23218–23224. [Google Scholar] [CrossRef] [PubMed]
- Bulusheva, L.G.; Fedoseeva, Y.V.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Koroteev, V.O.; Yaya, A.; Ewels, C.P.; Chuvilin, A.L.; Felten, A.; et al. Stability of Fluorinated Double-Walled Carbon Nanotubes Produced by Different Fluorination Techniques. Chem Mater 2010, 22, 4197–4203. [Google Scholar] [CrossRef]
- Judek, J.; Jastrzebski, C.; Malolepszy, A.; Mazurkiewicz, M.; Stobinski, L.; Zdrojek, M. Laser induced temperature effects in multi-walled carbon nanotubes probed by Raman spectroscopy. Phys Status Solidi A 2012, 209, 313–316. [Google Scholar] [CrossRef]
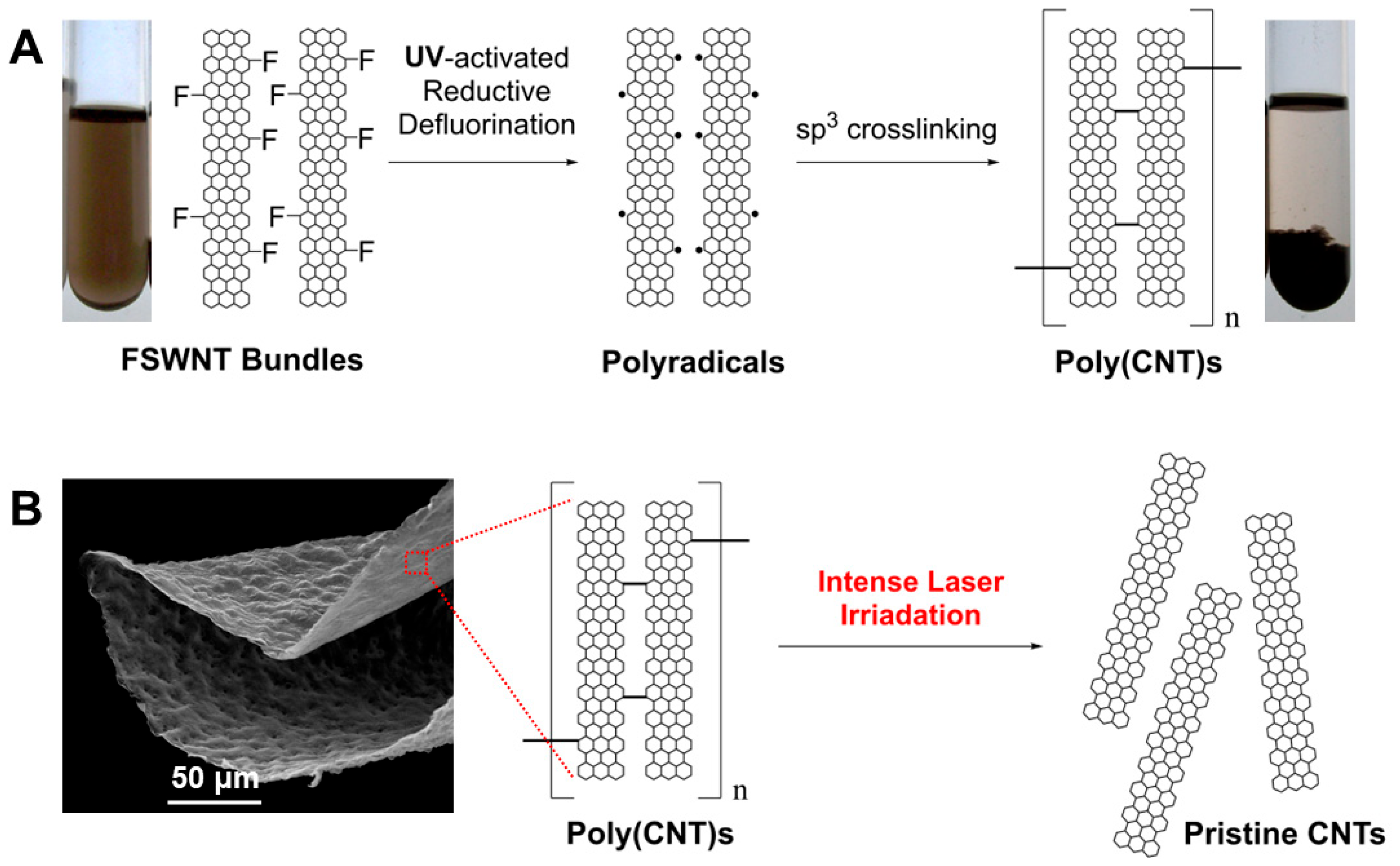
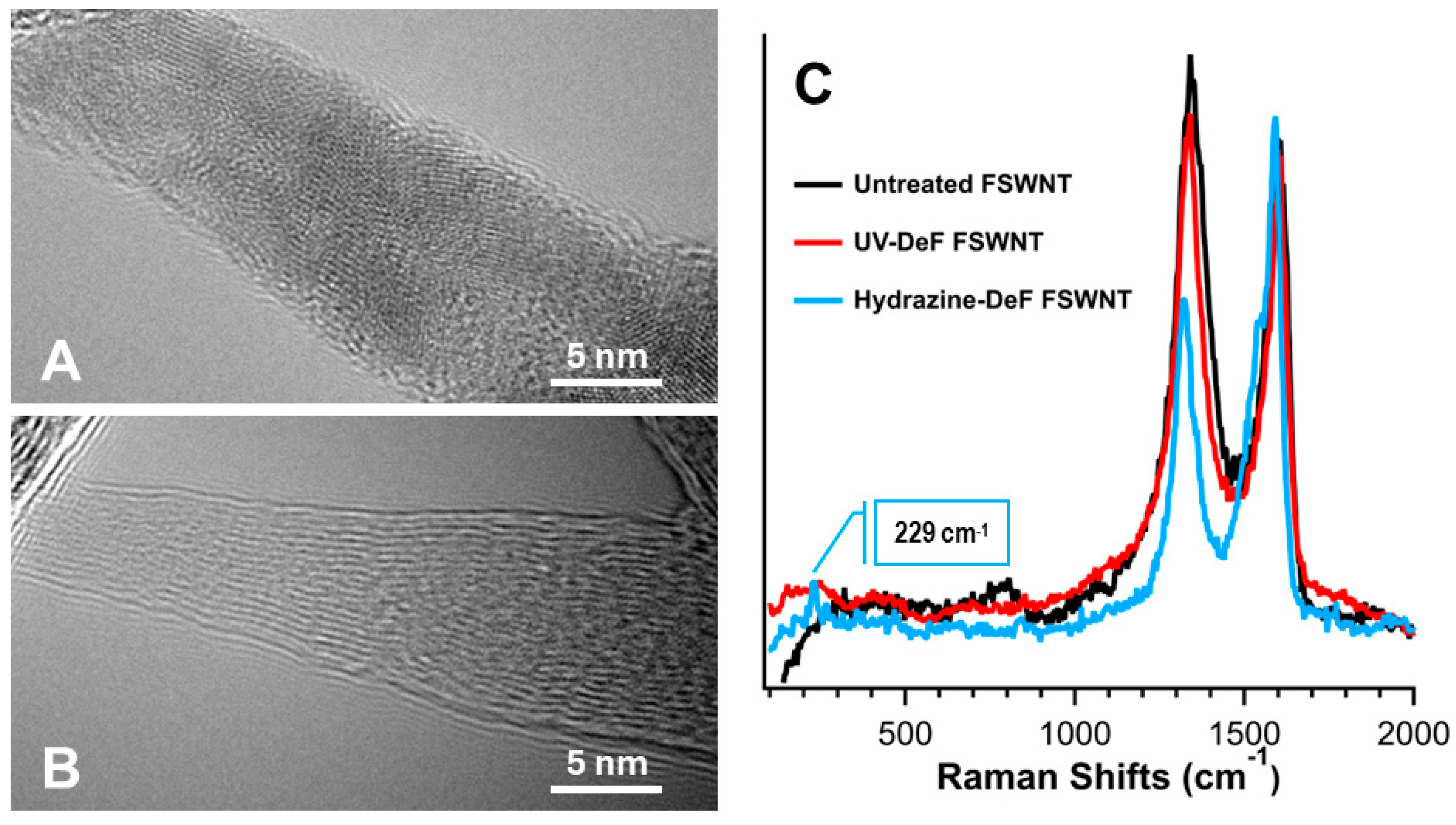
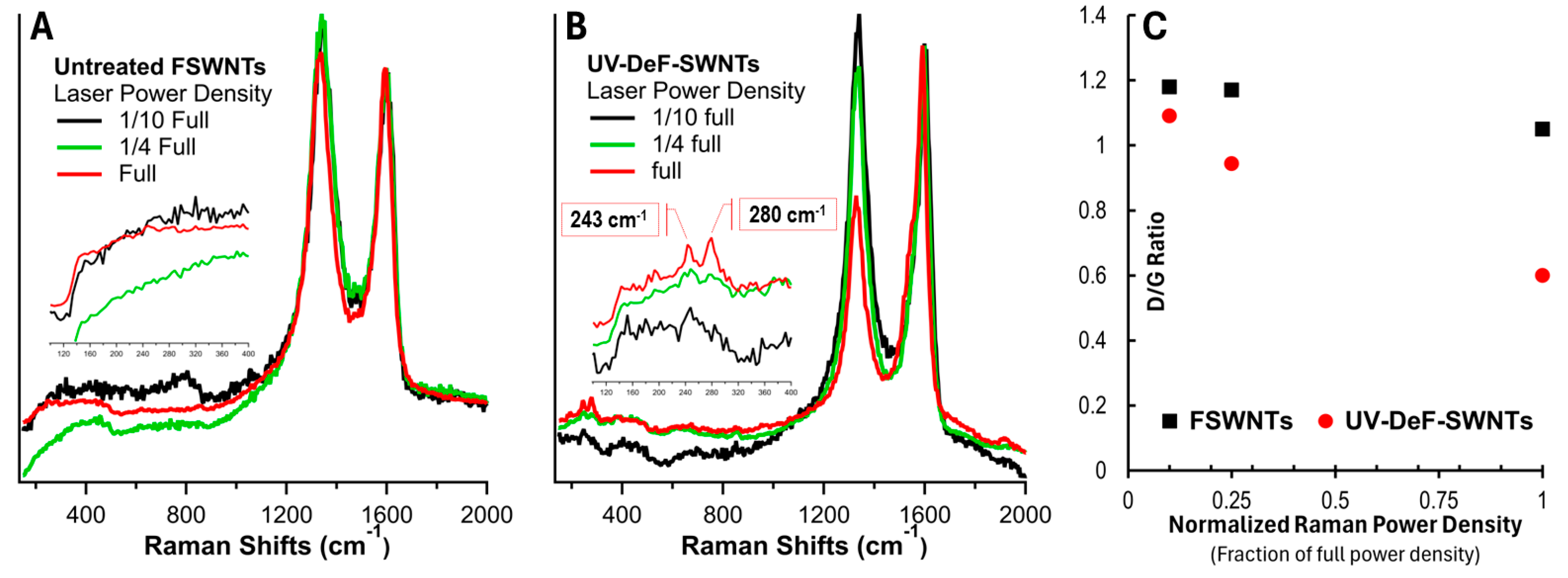
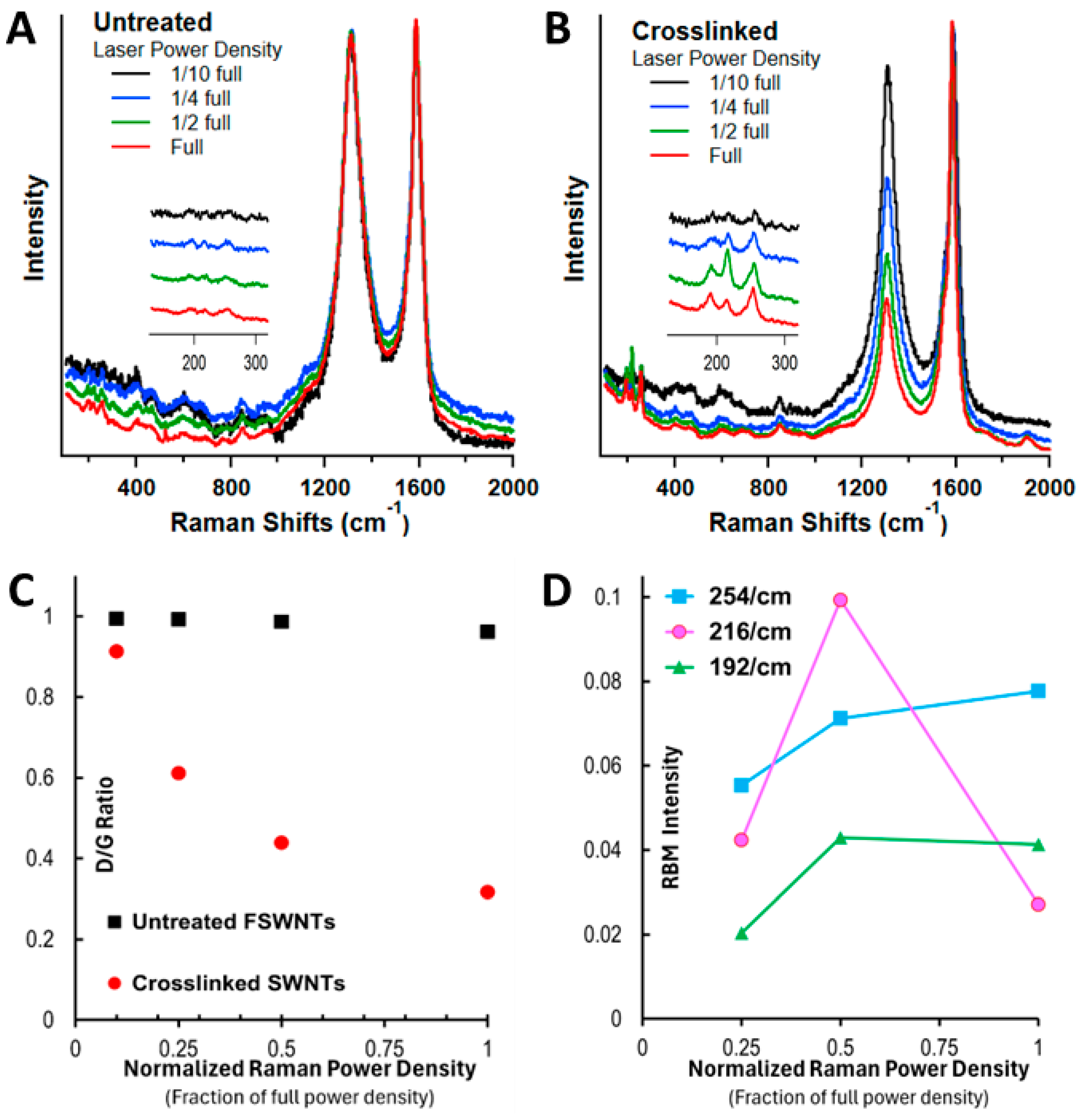
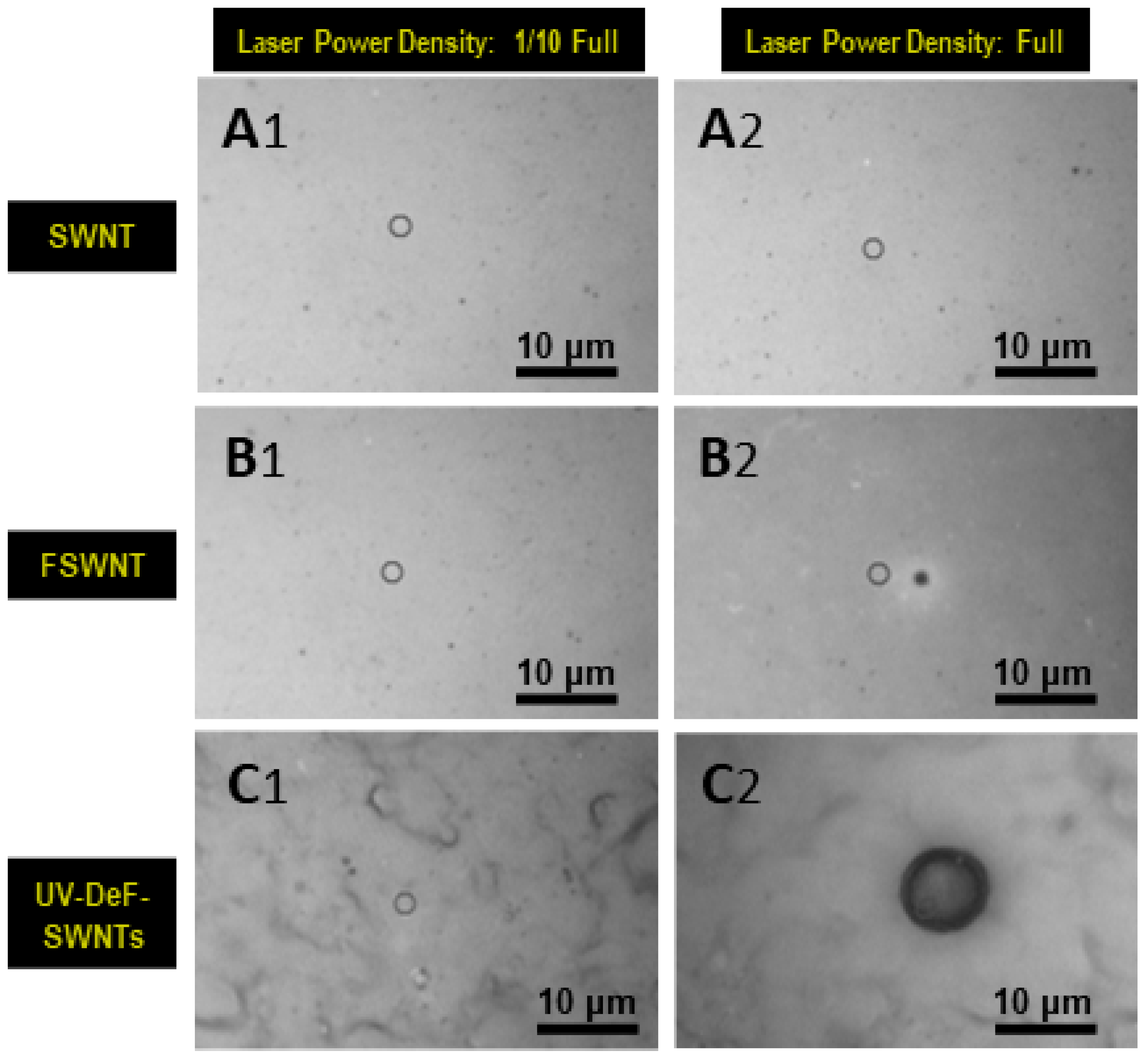
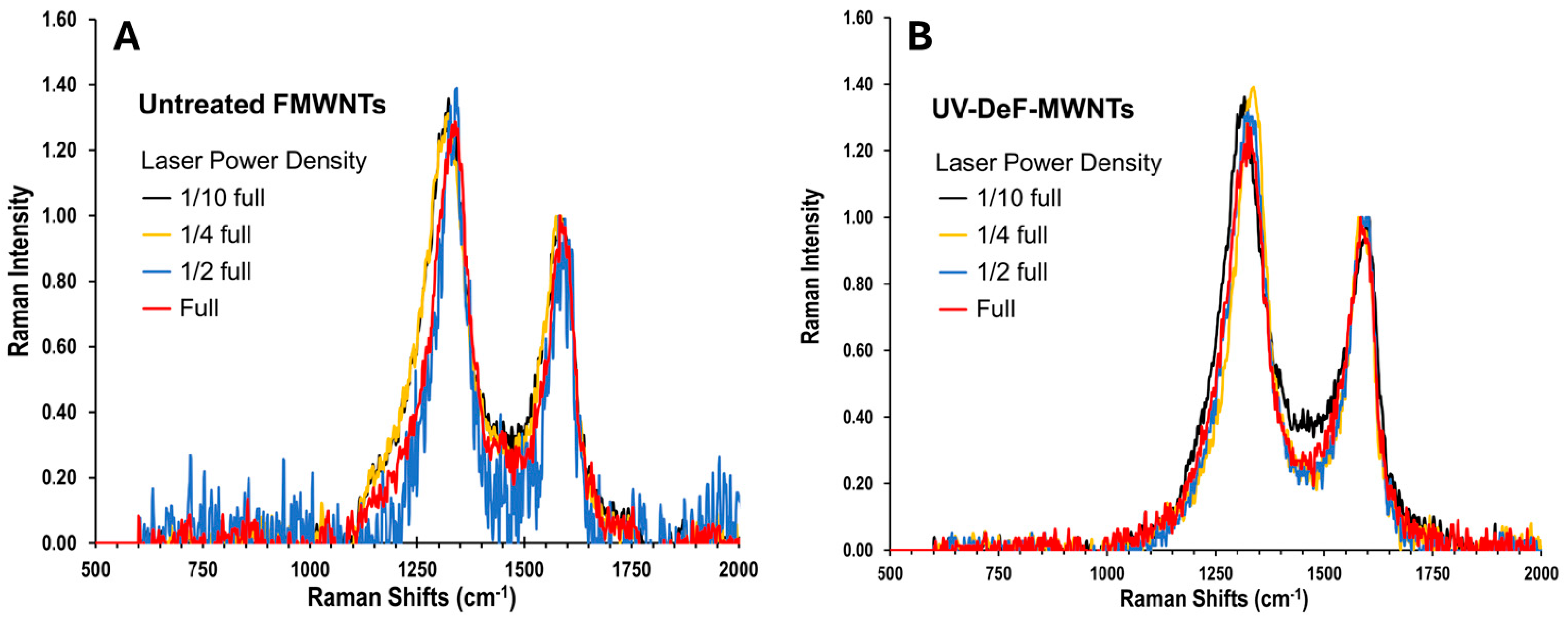
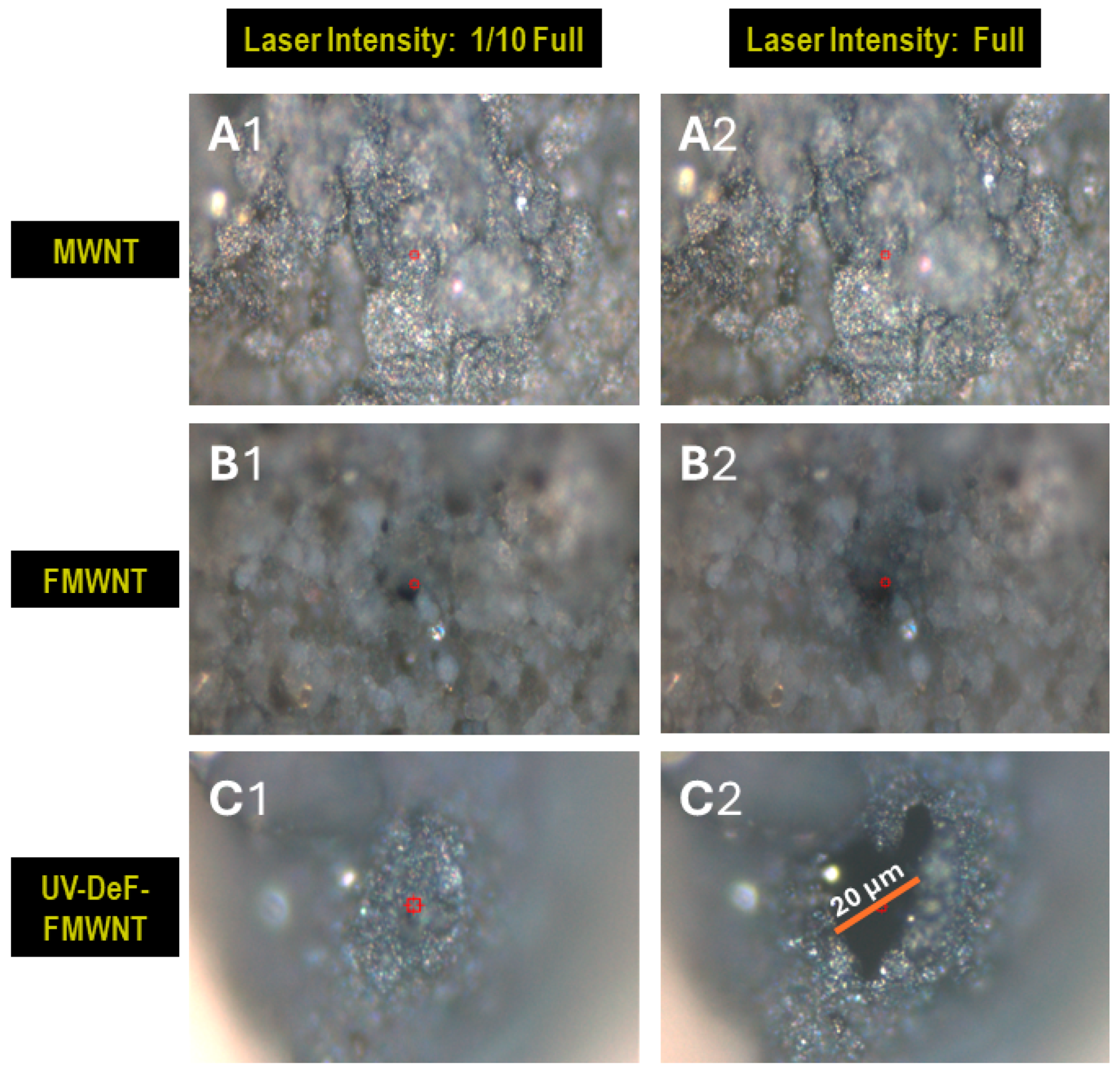
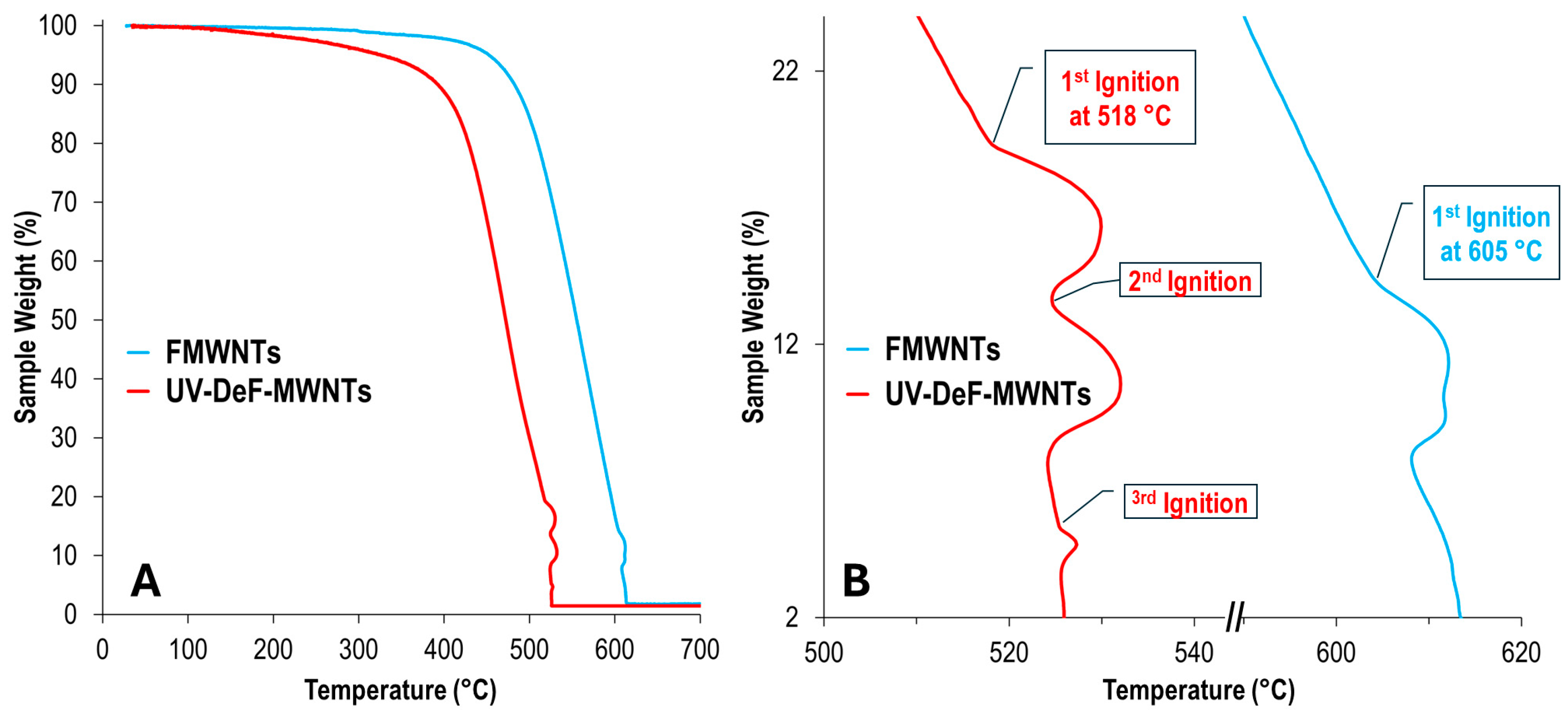
| Samples | Conditions | Composition (atom%) | |||
| C | O | F | N | ||
| FSWNTs | Untreated | 68.23 | 3.98 | 27.79 | 0.00 |
| UV-DeF-SWNTs | UV, 2 hrs. | 92.66 | 5.11 | 2.23 | 0.00 |
| HDZ-DeF-SWNTs | Hydrazine, 2 hr. | 85.37 | 4.43 | 5.71 | 4.50 |
| Samples | Conditions | Composition (atom%) | ||
| C | O | F | ||
| FMWNTs | Untreated | 54.15 | 1.67 | 44.18 |
| UV-DeF-MWNTs | UV, 16 hrs. | 66.59 | 4.99 | 28.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





