1. Introduction
Zika virus (ZIKV), an RNA arbovirus belonging to the Flaviviridae family, is transmitted by mosquitoes of the genus
Aedes and was initially isolated from rhesus macaques in the Zika forest of Uganda in 1947 [
1,
2]. The most common symptoms of infections in humans are rash, fever, arthralgia, and conjunctivitis. Most patients present only mild and transient disease, but severe neurological complications have been described, including congenital Zika syndrome (CZS) [
3] and Guillain-Barré syndrome (GBS) [
4]. Newborns with CZS present a distinct clinical phenotype of microcephaly: abnormalities of skull shape and redundancy of the scalp. In addition, these infants may present fetal immobility, distal contractures of the hands and fingers, and feet misplacements [
5]. GBS is a rare autoimmune neurological disease, which is increased in frequency among people who have been infected with ZIKV; it causes progressive paralysis of the limbs and muscle weakness [
4].
From 1947 to 2016, ZIKV spread to 60 countries and territories in which active ZIKV infection had been reported [
6]. In 2016, a significant increase in the number of neonates with microcephaly and other serious disorders was observed in Brazil and believed to be associated with ZIKV infections in utero [
7,
8], prompting WHO to declare the microcephaly epidemic a “public health emergency of international concern” [
9,
10]. By November of 2018, 2,819 cases related to ZIKV involving microcephaly and other congenital growth and developmental defects were confirmed in Brazil, of which 1,843 were concentrated in the Northeast region, including 424 in the state of Pernambuco [
11]
The rapid development of this epidemic elicited an immediate and strong commitment to the development of animal models that could be used to develop a better understanding of ZIKV disease, anti-ZIKV immune response, pathologies caused by infection, potential routes of transmission, and efficacy of candidate drugs and vaccines. The primary models that were developed were non-human primates (NHPs) and mice [
12,
13,
14,
15,
16,
17,
18]. Both model species have enormous scientific value and potential for research on ZIKV, but they also have severe limitations. The greatest limitations to using NHPs are cost, which prohibits large-scale experimental studies; and the long life cycle and the long gestation period, which do not allow results from developmental and longitudinal studies to be obtained quickly [
19]. A major limitation of mice is the fact that wild-type immunocompetent mice are resistant to infection by the virus after the neonatal stage, and neonatal mice experimentally infected with the virus die within days [
20]. Furthermore, data obtained from genetically modified immunodeficient mice do not adequately represent normal human subjects for translating experimental results to understanding the biological sequelae of ZIKV infection in humans [
21]. Finally, these genetically modified animals do not serve as fully valid models for vaccine development or efficacy testing [
21,
22]. Although the chicken embryo [
23] and guinea pig models [
24,
25] have been used with limited utility, no animal model that obviates the above-mentioned limitations has been established previously.
In response to the deficiencies of animal models suitable for research on ZIKV, we have established
Monodelphis domestica, the genetic stocks and strains of which are known as laboratory opossums [
26] as a model that complements the NHP and mouse models. This model is especially advantageous for research on infection in the embryonic and fetal stages of development [
27], as well as for research on ZIKV infection in fully immunocompetent juveniles and adults.
M. domestica adults weigh from 80-150g versus 20-30g for mice, so four to five times more blood volume can be safely removed from an opossum compared to a mouse on a single occasion or over a given timeframe. In addition, a substantial amount of blood can be safely removed from the opossums at an earlier stage of development than in mice. By comparison with monkeys, opossums have a short gestation period (14 days, even shorter than mice) and produce large litters (a mean of ten and as many as 13 pups at weaning for the most fecund stock), breed continuously (capable of rearing four litters per year if the litters survive, and many more if the pups are harvested or die pre-weaning), are inexpensive to maintain (in mouse cages; fed commercial pelleted opossum chow), are economical to produce and to use experimentally in large numbers, and reach sexual maturity in 6 months [
26].
While these favorable characteristics make laboratory opossums ideal, and in some instances unique, laboratory animals for experimental research in many fields of biology, they also have some additional characteristics that make them unique as animal models for research on ZIKV. Their developmental stage at birth is similar to that of a 5-6 week human embryo and to that of a 12-day mouse embryo [
28], so they complete most of embryonic and all of the fetal development outside of the mother’s uterus. Moreover, female
M. domestica do not have a pouch, so the neonates (each of which attaches to a teat shortly after birth) are easily accessible for experimental manipulation when the mother is anesthetized. In addition, the neonates are extraordinarily robust to experimental manipulation throughout development. They can be inoculated directly with ZIKV beginning on the day of birth, enabling controlled experiments that are not confounded by maternal and placental physiology or by virus interactions with the mother’s immune system. ZIKV can even be inoculated directly into the brain on the day of birth [
27]. In addition,
M. domestica are susceptible to ZIKV infection at all life stages, and they support the persistence of the virus. Moreover, a variety of genetic stocks and strains are available for research on host-virus interactions and on the genetics of susceptibility to ZKV infection and consequent pathologies.
The characteristics of the laboratory opossum make this species uniquely suited as a third important model, together with NHPs and mice, for investigating the biological aspects of ZIKV infection and the pathological sequelae. The validation of this model makes its use feasible in large-scale experimental protocols of types that are not practical with NHPs or rodents. This capacity may transform fundamental experimental research on ZIKV infection and its consequent pathologies and can exert a sustained and powerful influence in this field.
Toward that goal, we developed and validated an indirect ELISA for measuring antibodies to ZIKV in laboratory opossums, as well as an immunohistochemical method to detect ZIKV NS1 protein in tissue samples and a PCR method to detect ZIKV RNA in tissue samples. We used these methods to determine some basic characteristics of ZIKV infection in this species, including susceptibility to infection by ZIKV lineages form Puerto Rico and Brazil, persistence of infection in animals inoculated at different ages and via different routes, effects of multiple sequential inoculations of individual animals, tissue tropism, the relative efficiencies of single vs. several sequential inoculations and varied routes of inoculation to elicit an antibody response, the competence of oral transmission, and anti-ZIKV antibody kinetics up to 38 weeks after exposure to the virus.
2. Materials and Methods
2.1. Animals
The laboratory opossums were produced in the breeding colony at The University of Texas Rio Grande Valley (UTRGV). The opossums in this study were maintained under standard conditions in individually ventilated cages [
26]. Blood samples were collected by cardiac puncture under isoflurane anesthesia. The animals were checked daily for signs of illness. Although infection by ZIKV did not cause overt signs of discomfort or illness, the few animals in the study that became ill were promptly euthanized by CO2 inhalation.
2.2. Antigens and Preparation of Virus
Four antigens were evaluated for their capacity to capture antibodies against ZIKV. Two of them were the recombinant antigens ZIKV ENV (ZENV16-R-10) and NS1 (ZNS117-R-10) purchased from Alpha Diagnostic. The other two were ZIKV lineages PRV (Puerto Rican Virus, PRVABC059) and BZV (Brazilian Virus, BR1911), produced in the laboratory of one of the authors (JMT) and inactivated by UV irradiation before use.
Live PRV and BZV were used for inoculations. As per [
27] for preparation of the virus, Vero cells (CCL-81; ATCC, USA) were used for virus titration, whereas C6/36 cells (CRL-1660; ATCC, USA) were used to amplify the lyophilized virus. Following a single passage in C6/36 cells, the supernatant was clarified and purified using a sucrose cushion. For plaque assays, Vero cells were seeded in six-well plates the night before. A 90% confluent monolayer was infected with tenfold serial dilutions of PRV, incubated for 96 hours, fixed with 4% PFA, and stained with crystal violet. Virus supernatants were quantified by plaque assay and stored at -80°C.
2.3. Inoculations and Sample Collection
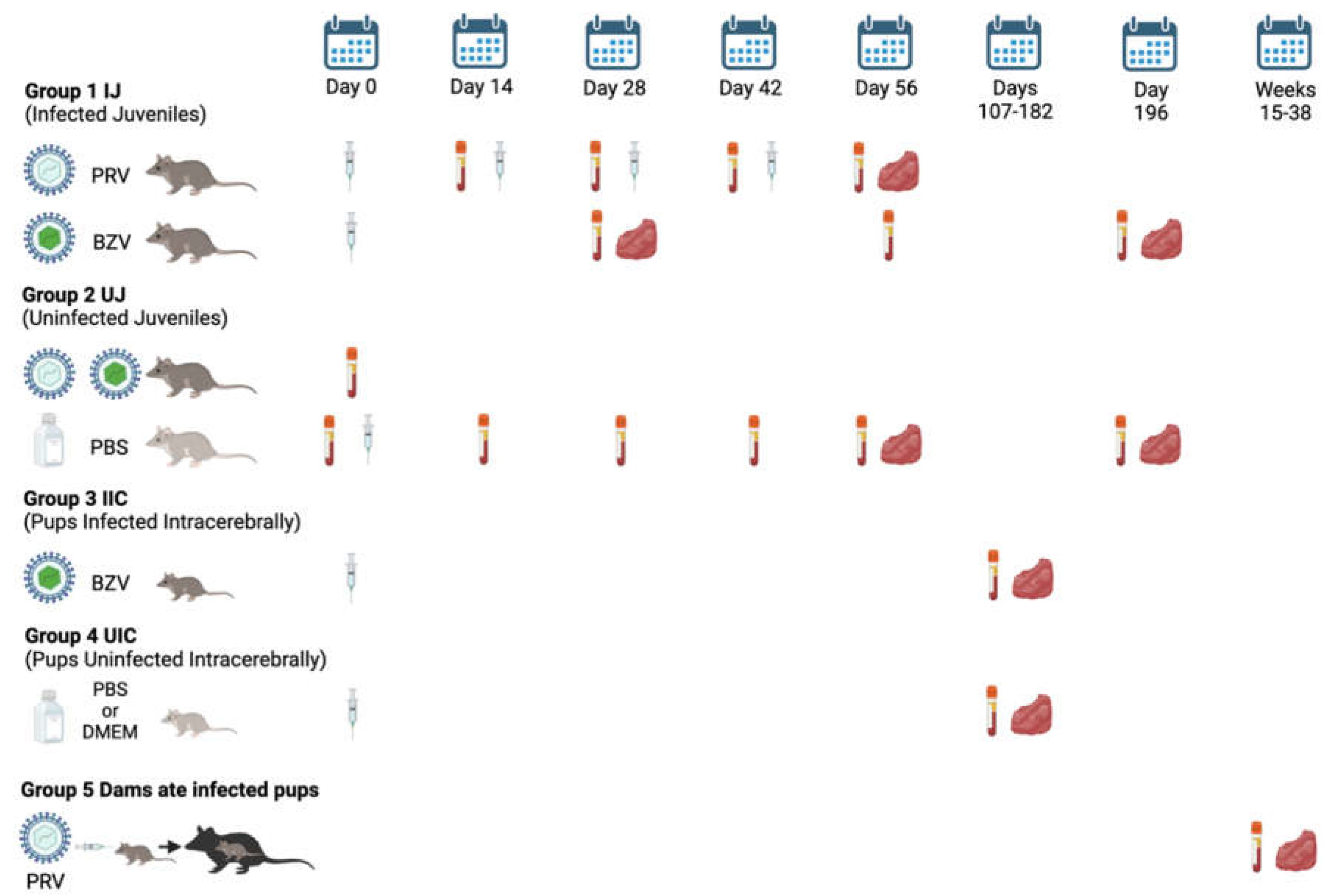
The study design is shown in
Figure 1. Juvenile opossums were inoculated using a 27g needle with PRV via five routes, including intraheart (IH), intramuscular (IM), intraperitoneal (IP), intratesticular (IT), or subcutaneous (SC); or with BZV via the IM, IP, or SC routes. Each inoculum contained 10
5 PFU in 50µL of Dulbecco’s Modified Eagle Medium (DMEM). Control animals were injected with 50µL of sterile phosphate-buffered saline (PBS) or DMEM.
There were five groups of animals: 1. Presumed Infected Juveniles (IJ), which were inoculated with PRV or BZV; 2. Uninfected Juveniles (UJ), which were injected with PBS; 3. Pups presumed to be Infected Intracerebrally (IIC), by inoculation of BZV; 4. Pups inoculated intracerebrally with PBS or DMEM, Uninfected Intracerebrally (UIC); and 5. Dams (Dams) that ate infected pups. The timeline is represented by days after the opossums were injected (syringe) with ZIKV or PBS or DMEM, or, for Group 5, by weeks after they ate infected pups. The vacutainer tubes indicate dates of blood collection, and the red symbols indicate dates of tissue harvest.
For PRV, animals were injected starting at 18 weeks of age, which was designated as Day 0 of the experiment, given three booster shots of ZIKV at 2-week intervals (Days 14, 28, 42), and necropsied for tissue collection at 26 weeks of age (Day 56). Tissue samples were placed in 10% formalin or flash frozen at -80°C. Serum samples were collected on Day 0 before the first injection, immediately before each booster, and at the time of necropsy, and stored at -80°C.
For BZV, animals were injected at 18 weeks of age only, and serum samples were collected on Days 28, 56, and/or 196 after inoculation. Tissue samples were collected at 22 weeks of age (Day 28) or 46 weeks of age (Day 196).
Suckling pups were injected intracerebrally with 5,000 PFU of BZV in 2μL of DMEM. Control pups were inoculated with sterile PBS (16 animals) or DMEM (16 animals). Inoculations were conducted when the animals were 3-8 days of age, and the animals were euthanized at 22, 26, 29, or 52 weeks of age. One animal, P1967, was euthanized early at 19 weeks because it had an inflamed, bleeding scrotum. Another animal, P1968 was euthanized at 21 weeks of age as an approximately age-matched control for P1967. For data analysis, both animals were placed in the 22-week-age group. At the time of animal processing, blood was collected for isolation of serum, and tissue samples were harvested and placed in 10% formalin or flash-frozen at -80°C until they were analyzed.
Ten dams that cannibalized infected suckling pups that had been inoculated with 1,000 PFU of PRV were euthanized between 18 and 38 weeks after they ate their pups, and blood and tissue samples were collected. An exception was an additional dam (P1110) whose pups were inoculated with a high dose of PRV (105PFU); that dam was euthanized 15 weeks after she ate nine of her pups.
2.4. Serum Samples
The 503 serum samples available for testing by ELISA to detect antibodies against ZIKV were divided among five groups, each of which represented a different route of potential exposure. Group 1, Infected Juveniles (IJ): Two hundred thirty-nine (239) samples were available from 102 potentially infected juveniles that had been inoculated with ZIKV lineage BZV BR1911 (n = 59) or ZIKV lineage PRV ABC059 (n = 43). Group 2, Uninfected Juveniles (UJ): The negative control group comprised 188 samples collected from the same animals on Day 0 of the study (before inoculation of ZIKV) (n = 107) or that were inoculated with placebo (n = 81). Group 3, Suckling Pups Infected Intracerebrally (IIC): Thirty-three samples were available, one from each of 33 animals that had been inoculated intracerebrally with ZIKV (BZV) at an embryonic stage of development (3 to 8 days after birth). Group 4, Suckling Pups Inoculated with Placebo (designated Uninfected Intracerebrally, UIC): The negative control group comprised 32 samples, one from each of 32 animals inoculated with placebo. Group 5. Mothers that Ate ZIKV-Infected Suckling Pups (designated Dams): Eleven samples were available, one from each of 11 mothers that ate one or more sucking pups that had been inoculated with ZIKV.
2.5. ELISA Optimization
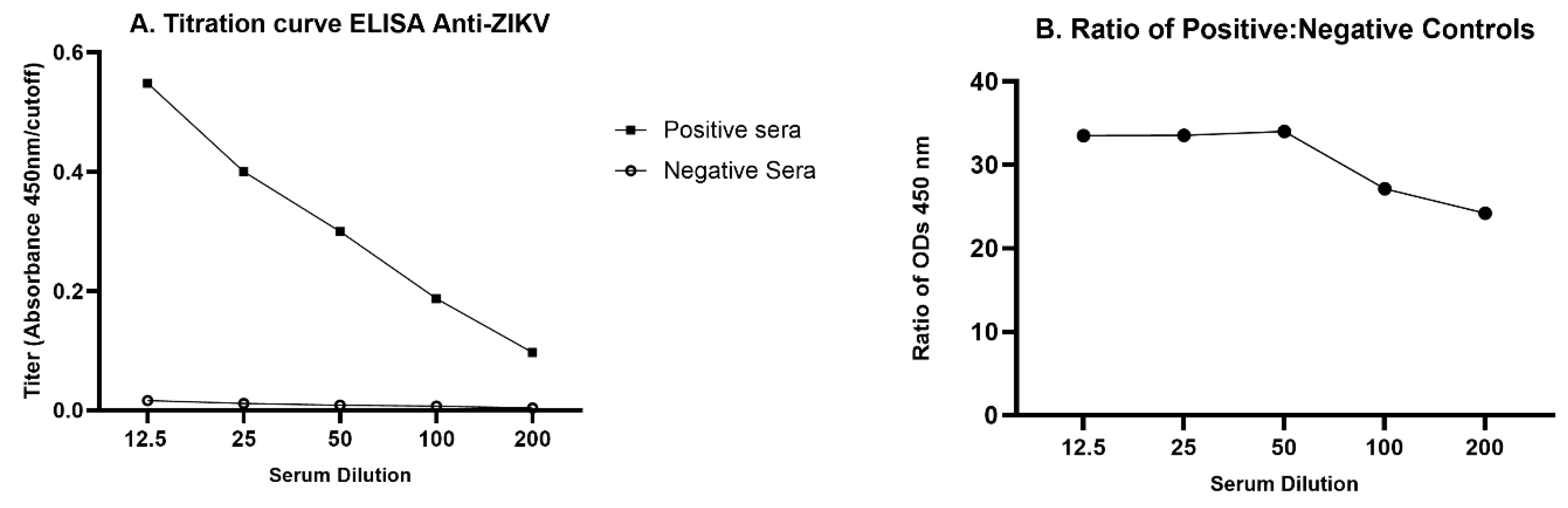
The process of optimization involved testing many different combinations of assay variables on samples selected with the a priori expectation that they would have antibody levels ranging from none to high. The objective was to identify the optimal combination of conditions, i.e., the conditions that provided the greatest power to discriminate between samples from infected and non-infected animals. After the ELISA was optimized, validation was accomplished by performing the ELISA on the 503 samples that were available in the five categories defined above. These included animals injected with saline and believed to be uninfected, animals inoculated with ZKV and believed to be infected (some of which were documented by immunohistochemistry to be infected; see below), and animals that might potentially have become infected via a route other than inoculation of ZIKV. The factors that were evaluated during optimization were as follows. 1. Concentrations of capture antigens: Four concentrations of the recombinant antigens ZIKV NS1 and E (from 6.25 ng/well to 50 ng/well) and two dilutions of the inactivated virus PRV and BZV (100 and 1,000 PFU/well) were evaluated for coating the ELISA plates. 2. Dilutions of opossum serum: Five serial dilutions of opossum serum, ranging from 1:12.5 to 1:200, were evaluated. 3. Dilutions of secondary antibodies: Three secondary antibody dilutions (1:1000, 1:5000, and 1:10000) were evaluated. 4. Incubation times: Two incubation times (1h and 2h) were evaluated both for binding of the opossum antibodies to the capture antigens and for binding of the secondary antibodies to the opossum antibodies.
After the preliminary results were obtained, a titration curve was developed with five opossum serum dilutions (1:12.5, 1:25, 1:50, 1:100, 1:200) using pools of presumed positive and presumed negative samples. The dilution that showed the highest ratio of optical densities for the positive pool versus the negative pool, 1:50, was chosen for use in the optimized protocol.
2.6. Indirect ELISA Anti-ZIKV
For the optimized protocol, 96-well plates (Thermo Nunc MaxiSorp® flat-bottom) were coated with 100 PFU of ZIKV PRV lineage per well in carbonate–bicarbonate buffer (0.1M pH 9.6; Sigma SRE0034) and incubated at 4°C overnight (12-24h). Then the plates were washed once with washing buffer (PBS 1x, pH 7.2, supplemented with 0.05% Tween 20—PBS-T). Blocking was performed for 1h at room temperature using PBS 1x, pH 7.2, plus 1% BSA (Fisher BP1600), followed by three washes with PBS-T. Then, 100 μL of opossum serum diluted 1:50 in PBS-T supplemented with 1% BSA (PBS-T/B) was loaded into each well, followed by incubation at 37ºC for 2h. The plates were wrapped with plastic wrap before incubation. After three washes, 100μL of goat anti-opossum IgG (H+L)-HRP conjugate (Alpha Diagnostic) diluted 1:1,000 in PBS-T/B was added to each well, followed by 2h incubation at 37°C. Following the final three washes, the TMB substrate (Sigma T5525) was added, and the plates were incubated for 15 min at room temperature. Then, the reaction was stopped with 50 μL of 2M H2SO4 (Sigma 258105). Finally, the plates were read at 450 nm using the Thermo Multiskan FC, and the results were expressed as optical density (OD).
2.7. Serum Pools and Quality Control
Negative and positive serum pools were included on each ELISA plate. The negative pool was used to calculate the cutoff value that discriminates negative from positive samples, while the purpose of the positive pool was to ensure that the assay had functioned properly in its ability to detect positive samples.
The negative pool was created from equal volumes of serum collected on Day 0 just before ZIKV inoculation from 48 naïve animals at the time the serum was collected; serum from these animals had tested negative during the optimization process. One hundred microliters of this pool, diluted 1:50, was assayed in nine wells of each plate. The positive pool was created from equal volumes of 29 aliquots of serum from animals that had been inoculated as juveniles on one occasion with ZIKV (BZV) 196 days before blood collection and that tested positive during the optimization process. The positive pool was composed of samples that tested in the low range of positivity, to make it a more robust validator of the ELISA capacity to detect low positive samples. One hundred microliters of this pool, diluted 1:50, was assayed in three wells of each plate.
To test the reproducibility of the ELISA, the coefficient of variability [%CV = (σ/mean OD) x 100] was calculated (σ = standard deviation; OD = optical density). Four groups, spanning the range of ELISA values that had been observed, were used for this calculation: 1, high positive; 2, medium positive; 3, low positive; 4, negative. Groups 1, 2, and 3 were formed by creating a pool from 10 positive samples each, while Group 4 was the same negative pool described above. Five assays were conducted on different days, using four replicates for each group. The intra-assay %CV was calculated based on the mean OD of the five replicates [
29].
2.8. Immunohistochemistry (IHC)
Tissues analyzed by IHC included brain, eye, heart, spleen, and reproductive organs (testis, epididymis, vagina, ovary). Seventy-six animals from the five groups (
IJ,
UC,
IIC,
UIC, and
Dams) were examined. The IHC method follows [
27] with some modifications. Tissue sections were incubated in PBTB (PBS + 0.01% Tween20 + 0.2% BSA) for 1 hour, followed by incubation with a 1:500 dilution of primary antibody (Arigo Biolaboratories, Taiwan) for 1 hour at room temperature or overnight at 4°C. The primary antibody targeted the ZIKV NS1 protein. After removing the primary antibody with three quick washes and three 10-minute washes in PBTB, the slides were incubated in a 1:200 dilution of AlexaFluor 546-conjugated secondary antibody (Thermo Fisher Scientific, USA) for 1 hour. The secondary antibody was removed similarly, with the addition of DAPI and AlexaFluor 488-conjugated phalloidin during the first 10-minute wash at 1:1000 and 1:200 dilutions, respectively. Imaging was performed using an Olympus FV10i confocal microscope or a Motic BA410E with a fluorescent upgrade.
Validation of the anti-NS1 antibody signal was conducted by analyzing two tissue slides from each sample: one with the primary antibody and the other without it. The ovary from a ZIKV-infected animal, P2141, served as a positive control, whereas tissues from animals injected with PBS were used as negative controls.
2.9. Viral RNA Extraction from Tissue of ICC Animals, and RT-PCR Analysis
The TissueLyser II (QIAGEN) was used to lyse tissues from ten 22-week-old animals inoculated intracerebrally as pups (IIC group). Viral RNA was extracted using two different kits: the QIAGEN RNeasy® Fibrous Tissue Mini Kit for the spleen and reproductive organs, and the QIAGEN RNeasy® Lipid Tissue Mini Kit for the brain, which is considered fatty tissue.
RNA was quantified using a Nanodrop, and qRT-PCR was performed with the SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Scientific, Carlsbad, CA, USA) on a 7500 Fast RT-PCR machine (Thermo Scientific, Carlsbad, CA, USA). Primers and probes for ZIKV-NS5 were used, following the cycle settings described by [
30]. Reactions were carried out in duplicate in a MicroAmp™ Fast Optical 96-Well Reaction Plate with Barcode, 0.1 mL (Thermo Scientific, Carlsbad, CA, USA). CT values were used to evaluate the samples, with positive signal CT values ranging between 12 and 38, as recommended by the CDC and manufacturer’s guidelines under the Triplex Real-time RT-PCR Assay. DH20 served as a negative and NTC control, and ZIKA NS5 RNA was used as a positive control.
2.10. Statistical Analysis
The mean absorbance value for each sample, assayed in triplicate, was used as the measure of serum antibody level against ZIKV. Eight candidate calculations of cutoff values were evaluated for their ability to discriminate between known positive and known negative samples. The one that was best able to discriminate between positive and negative samples was empirically determined to be, for each ELISA plate, the mean OD of the nine wells containing the negative serum pool plus 3x the standard deviation of that mean, multiplied by 1.75. The formula [(Mean OD + 3xSD of mean OD) x 1.75] defined the cutoff described above. The titer was calculated by dividing the test sample mean absorbance by the cutoff value. A serum sample with a titer between 0.900 and 0.999, inclusive, was considered to be indeterminate, while below 0.900 was designated as negative, and equal to or higher than 1.000 was designated as positive [
31]. The sensitivity, specificity, and positive and negative predictive values also were determined.
In addition, a receiver operating characteristic (ROC) curve was made to determine the accuracy of the test for juveniles and pups that were inoculated with the virus. In addition, the mean titers of the routes of injection were compared between animals inoculated with BZV or PRV, and the antibody kinetics were evaluated for the days following the inoculations. The variables were compared among the groups using a one-way ANOVA analysis with false discovery rate (FDR) correction with p values less than 0.05 were considered statistically significant. Graphs were made using the GraphPad Prism software version 6.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. ELISA Optimization
To determine the highest sensitivity of the ELISA for detecting anti-ZIKV in opossums, we first compared serially diluted serum pools from inoculated and presumed infected juvenile opossums and presumed uninfected juvenile controls (
Figure 2A). The 1:50 dilution yielded the highest ratio of positive:negative OD (
Figure 2B) and was chosen to be used in the optimized protocol. Other optimized parameters were documented to be inactivated PRV as the capture antigen at 100 PFU/well, blocking with 1% BSA, and a secondary antibody dilution of 1:1,000.
3.2. Indirect Anti-ZIKV ELISA
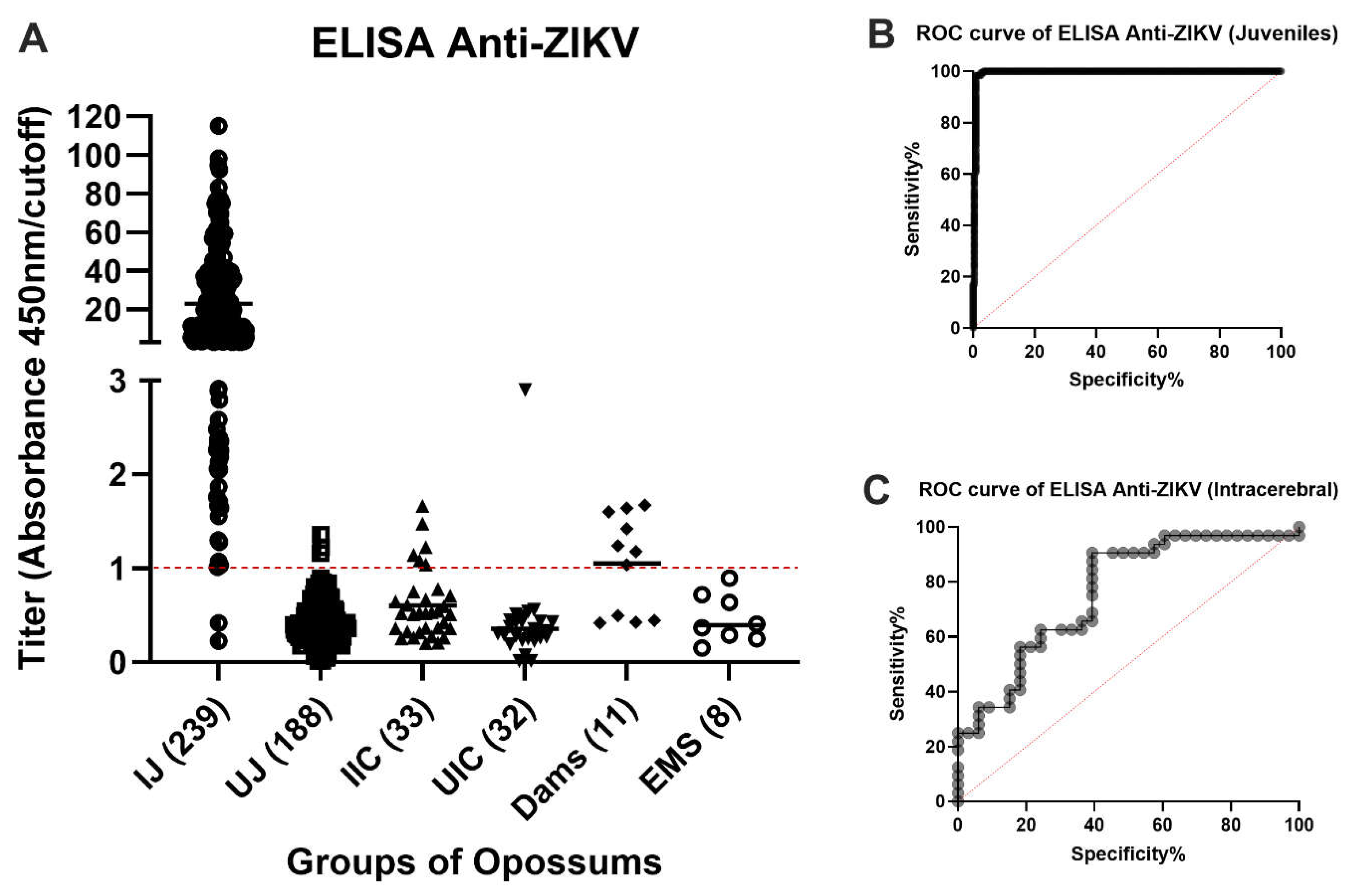
Two hundred thirty-seven of the 239 available samples from virus-inoculated juveniles were positive by ELISA and two were negative (
Figure 3A). Both negative samples were from animals that had received a subcutaneous inoculation of ZIKV 2 weeks (O9460) or 4 weeks (P1397) before blood collection, respectively. It had been entered into the record of O9460 that some (or all) of the inoculum was observed to leak out of the injection site immediately after inoculation. The animal O9460 was inoculated subcutaneously again at Days 14, 28 and 42 after the first ZIKV injection and had converted to positive by ELISA in samples collected at Days 28, 42 and 56. No animals inoculated by any other route failed to yield a positive ELISA result at the first or any subsequent blood collection.
One hundred eighty-five of the 188 samples collected from the same animals on Day 0 of the study (before inoculation of ZIKV) or that were inoculated with placebo gave negative ELISA results. However, three samples gave low positive results (
Figure 3A). We believe that these three results were false positives. The cutoff value was chosen to minimize false negatives, at the risk of having a higher (but still low) number of false positives. These data reflect a sensitivity of 99.14%, specificity of 98.40% (
Table 1), and accuracy of 99.45% (
Figure 3B).
Six of the 33 samples from animals that had been inoculated intracerebrally with ZIKV (BZV) at an embryonic stage of development (0 to 9 days after birth) were positive by ELISA (
Figure 3A); the other 27 samples were negative. Thirty-one of the 32 samples from the negative control group were all unequivocally negative by ELISA. The one positive sample was a high positive, suggesting exposure to a high dose of ZIKV. For the intracerebrally inoculated group, if it were assumed that all ZIKV-inoculated animals became infected and developed antibodies to the virus, the sensitivity of the test would be 18.18%, the specificity would be 96.88% (
Table 1), and the accuracy would be 78.61% (
Figure 3C).
Four of the serum samples from the 11 mothers that ate one or more sucking pups that had been inoculated intracerebrally with ZIKV, were negative by ELISA, and seven were positive (63.6%) (
Figure 3A).
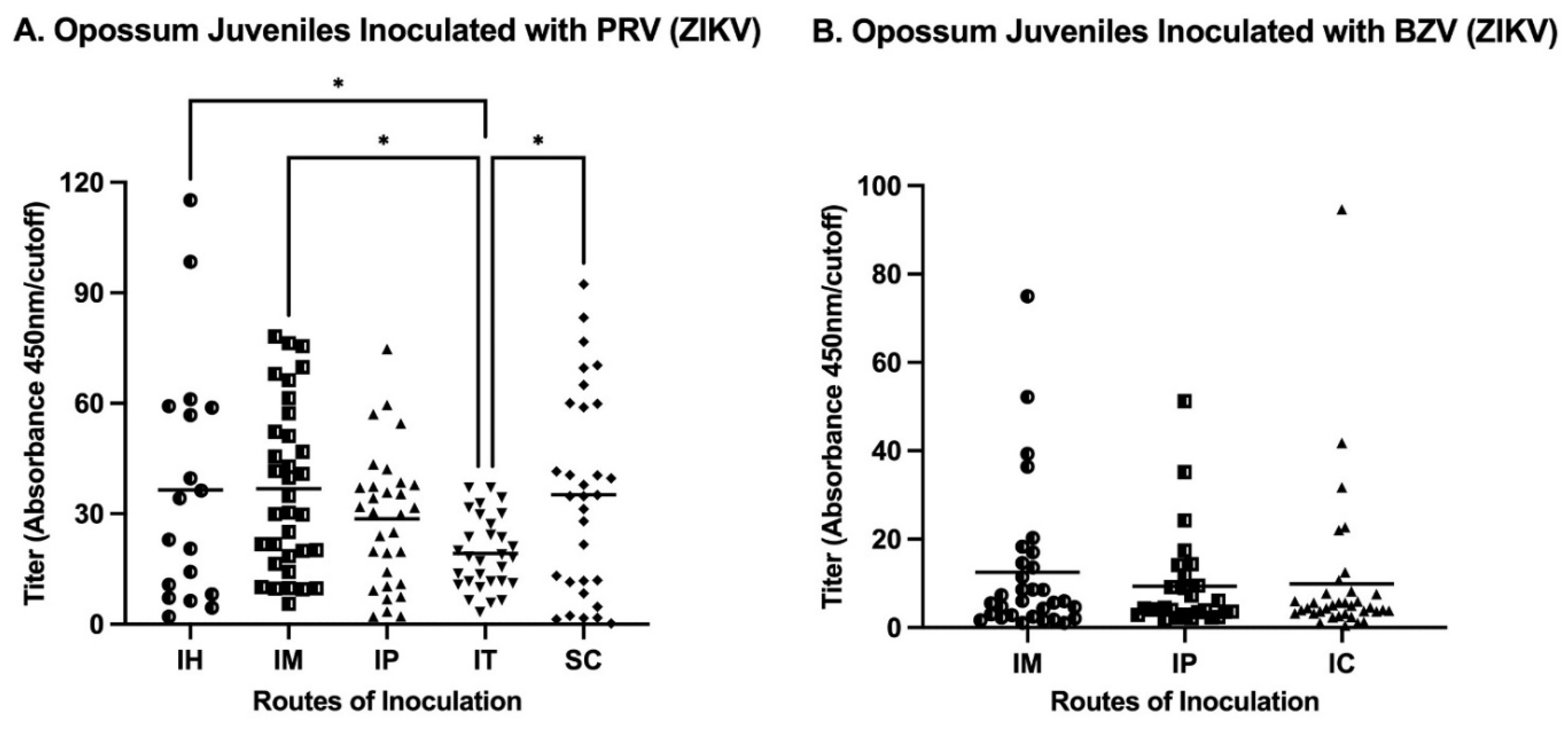
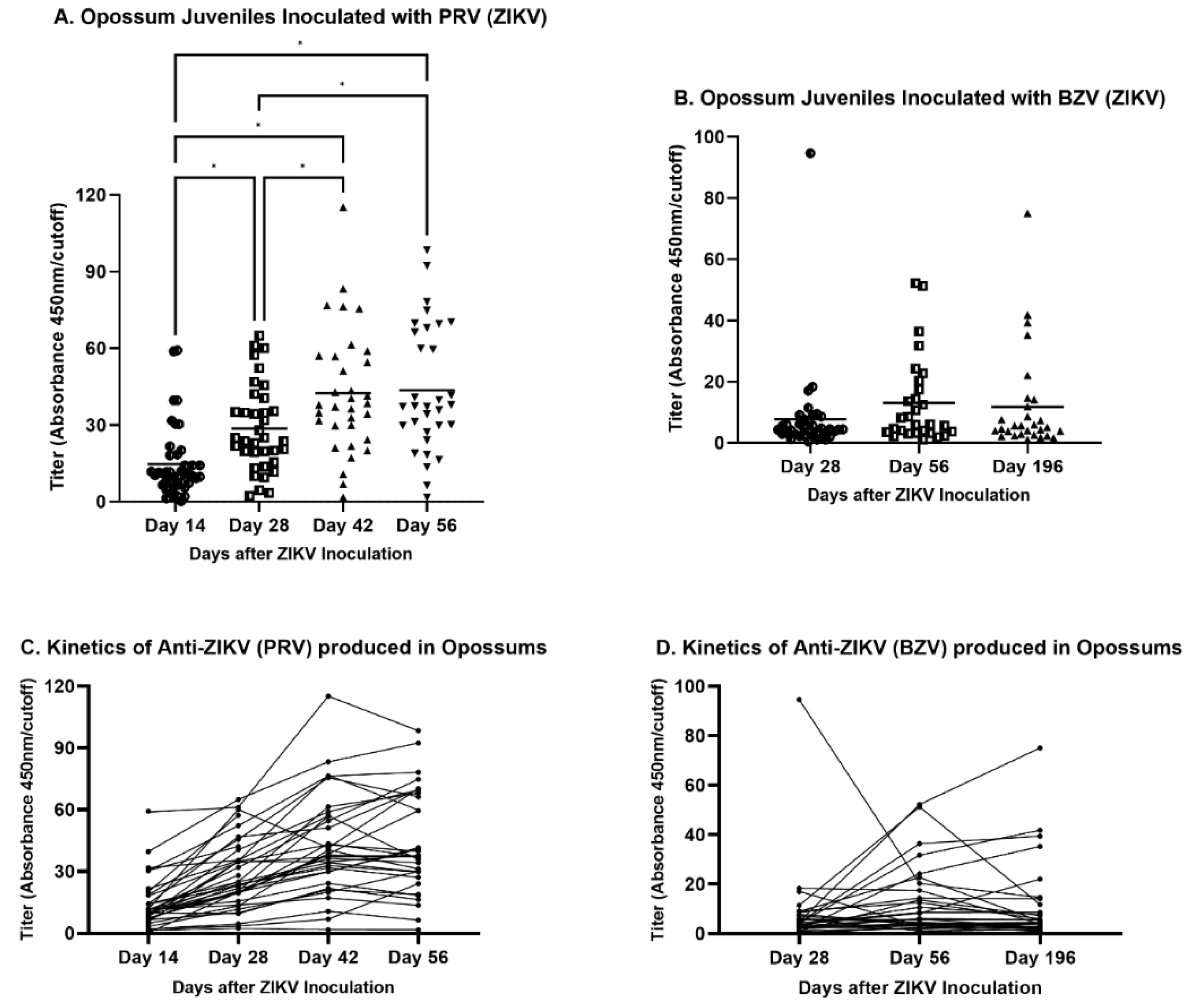
The analysis of the routes of injection of juveniles showed that IM inoculations resulted in the maximum mean titer in animals infected either with PRV or BZV (
Figure 4). Regarding the mean values for juveniles inoculated with PRV, the IM or SC routes resulted in statistically significantly higher titers than the mean titer of animals inoculated by the IT route (
Figure 4A). For BZV infection, although the IM route resulted in the higher mean titer, there was no statistically significant difference between animals inoculated by the IM, IP, or SC routes (
Figure 4A).
The inoculation schedules differed for PRV and BZV. Animals were inoculated with PRV on four sequential occasions (Days 0, 14, 28, and 42), while opossums were inoculated with BZV only on Day 0 of the study. The titers of animals inoculated with PRV increased sequentially from Day 14 to Day 42, but they did not increase further by Day 56 (
Figure 5A). However, BZV-inoculated opossums exhibited no significant differences in titers at 28, 56, or 196 days after inoculation (
Figure 5B), although the titers were higher on Days 56 and 196 than on Day 28. The lack of significance may have been a consequence of a single outlying titer at Day 28, with a value that was five-fold the next highest titer at that timepoint.
The antibody kinetics during the days after inoculation also were assessed for each animal (
Figure 5C and
5D). In PRV-infected animals, the titers increased from Day 14 to Day 42, as the animals received bi-weekly inoculations. After the final inoculation on Day 42, the titers of some animals continued to increase, whereas the titers of other animals decreased (
Figure 5C). The kinetic pattern of BZV-infected animals did not change from Day 56 to Day 196, with few exceptions (
Figure 5D).
3.3. Quality Control
To determine the coefficients of variability of the ELISA, one negative and three positive pools with high, medium, and low titers were tested in four replicates. The intra-assay CV was 16.7% while the inter-assay variability was 16.1% (
Table 2). Coefficients of variation with values less than 20% indicate adequate repeatability of the assay [
31].
3.4. IHC and PCR
3.4.1. IJ and UJ Groups
To determine to the extent of distribution and long-term persistence of ZIKV in infected animals, IHC was conducted on a subset of animals inoculated as juveniles with ZIKV (
IJ group) or PBS (
UJ) group. Twenty-six animals inoculated with PRV as juveniles were assessed for NS1 by IHC, and signal was detected in 24 of them in some tissues. NS1 protein was detected in brain, eye, heart, spleen, and/or reproductive organs (
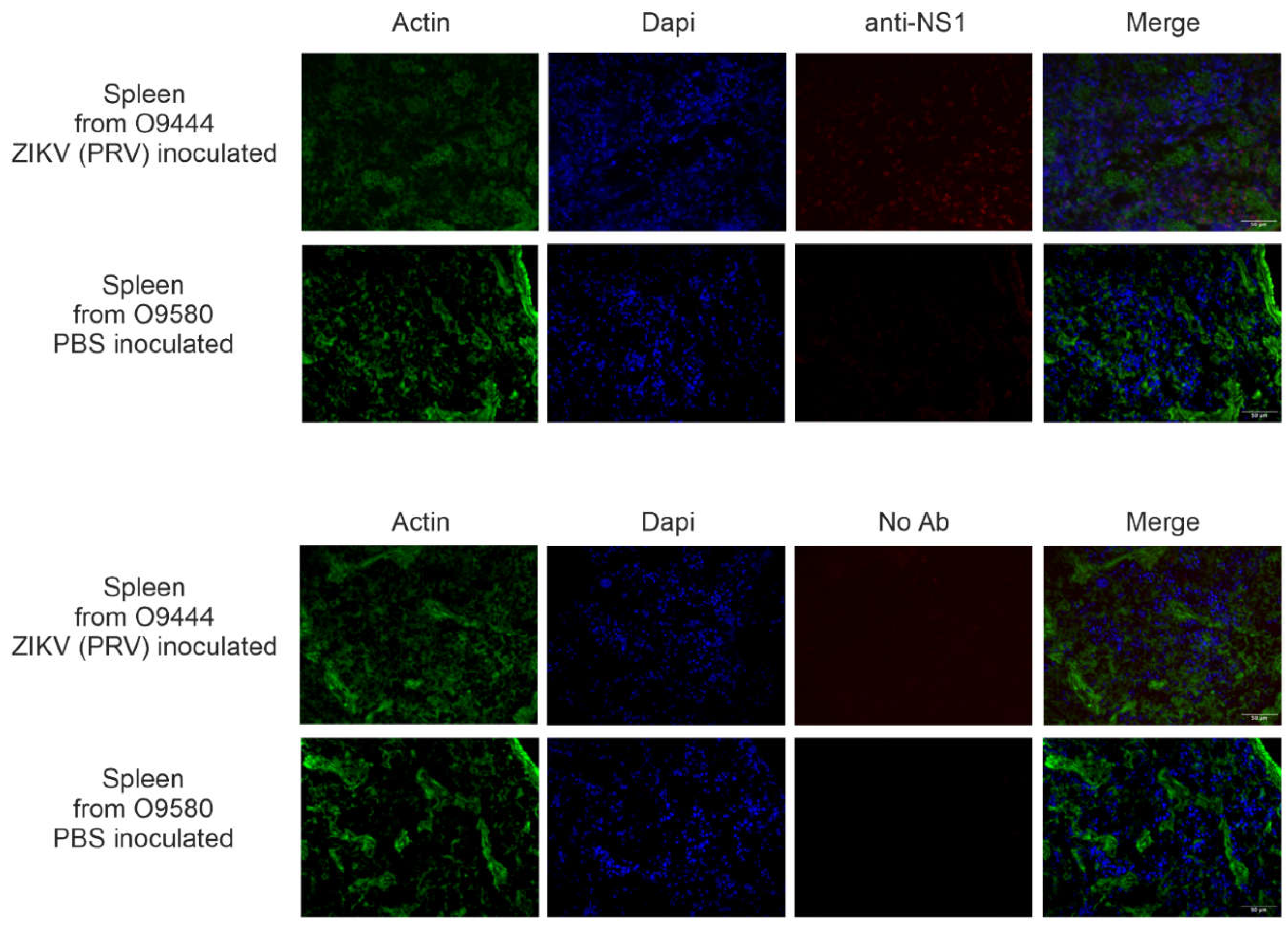
Table 3). Representative results from spleen of an IHC-positive animal (O9444) and an IHC-negative animal (O9580) are shown in
Figure 6. Note that slight background staining is visible on the right-hand side of the image of O9580 stained without primary antibody, and that diffuse background staining is visible throughout the image of O0444 stained with primary antibody. Those appearances of non-specific background staining are quite distinct from the true NS1 signal in spleen from O9444, which appears as distinct foci in the anti-NS1 panel and are localized primarily to the nuclei, as apparent from the merged panel.
The two animals in which no signal was detected in any tissue, O9374 and O9342, had been inoculated by IM and SC routes, respectively. Tissue samples from twelve of the juveniles inoculated with BZV were examined; ten of them displayed NS1 signals in reproductive organs and/or spleen (
Table 3). No signal was detected in two of the animals, P1351 and P1366, which had been inoculated via the IM route. Despite the failure to detect signal by IHC in any tissue of the four animals, three of them were positive by ELISA. P1397 was the only
IJ animal that gave a negative ELISA result. Eight juveniles injected with PBS were assessed for NS1 by IHC, and one of them (P1545) exhibited an unexpected signal in the reproductive system (uterus). Eight of these control animals were negative in the ELISA, and one was positive on Study Days 42 and 56
3.4.2. Suckling Pups Inoculated Intracerebrally with PRV (IIC Group) or PBS or DMEM (UIC Group)
Seven of 16 (43.8%) of the
IIC animals displayed viral NS1 signal in at least one tissue type, indicating persistent infection at the time of organ harvesting 26 weeks after inoculation (
Table 3). The reproductive organs showed the highest infection rate, followed by brain and spleen. No NS1 signal was found in any of the evaluated tissues of the other nine animals. The four
UIC animals analyzed did not exhibit any evidence of NS1 protein in the brain, spleen, testes, epididymis, vagina, or ovaries.
RT-PCR was conducted on RNA from tissue samples of ten
IIC animals. All ten of the animals gave positive RT-PCR signals in at least one tissue type (
Table 3). The tissues with the highest infection rate analyzed by RT-PCR were the spleen and reproductive organs, followed by the brain.
3.4.3. Dams
To assess the possibility that ZIKV could be transmitted in this species via the oral route, IHC was conducted on tissues from ten of the dams that ate suckling pups that had been inoculated with PRV. The dams had eaten their pups 18–38 weeks prior to the collection of tissue samples from the dams. All of the dams displayed NS1 signal in the spleen. Despite the confirmation of infection, anti-ZIKV antibodies were detected in serum by ELISA in only six of the eleven dams (
Table 4). The serum was collected at the same time as the tissues.
4. Discussion
Our results have established the laboratory opossum, M. domestica, as a unique animal model for research on ZIKV infection and transmission, and humoral immune response to ZIKV infection. The combination of characteristics that make it unique are the following: 1) unlike any other model, opossums are born at the developmental stage of a 5- or 6-week human embryo and of a 11.5-day mouse embryo, and can develop persistent infection and widespread dissemination of ZIKV after intracerebral inoculation at the embryonic stage, 2) unlike normal immunocompetent mice and NHPs, juvenile and adult opossums develop persistent infection after inoculation of ZIKV, 3) unlike mice and NHPs, opossums can easily be infected via the oral route.
For translating the results from opossums to humans,
Table 5 relates ages of the opossums to the approximate ages of developmental equivalency of humans and mice.
4.1. Humoral Response to ZIKV
Here, we describe the first indirect ELISA developed to capture IgG antibodies against ZIKV in
M. domestica. The ELISA results were almost 100% perfect in detecting true positives among animals inoculated as juveniles with ZIKV. From 102 infected juveniles with PRVABC059 or BR1911, 101 mounted a robust humoral immune response against ZIKV (
Figure 3). An exception was P1397, but that animal was positive for the NS1 signal by IHC. Although P1397, which was inoculated subcutaneously, might have received a low dose of virus because of leakage of the inoculated material, the detection of NS1 by IHC confirms that the animal had become infected. Either it failed to mount a humoral immune response, or the ELISA result was a false negative. In the uninfected juvenile group, three of 188 samples showed a low positive signal. We hypothesize that these spurious false positives might have been a consequence of a cross-reaction with a natural antibody of those animals.
Many routes of ZIKV inoculation have been used in ZIKV animal models, such as direct application to palatine tonsils, intraamniotic, intragastric, intranasal, intraperitoneal, intravenous, intrauterine, intravaginal, and subcutaneous [
12,
14,
15,
25,
32,
33] Subcutaneous is a well-established route used for ZIKV inoculations of guinea pigs, mice, and NHPs [
15,
19,
24]. Rhesus macaques are difficult to infect intranasally, but the application of high-dose ZIKV directly to the tonsils of rhesus macaques resulted in detectable viremia similar to that detected after inoculation by the subcutaneous route [
33]. Intranasal inoculation of ZIKV is capable of establishing infection in guinea pigs, A129 immunodeficient mice, and cynomolgus macaques, which were also infected intragastrically [
32]. Intravaginal ZIKV infection was successful in the guinea pig model [
25] Almost all of the routes cited above have been utilized to infect immunodeficient mice efficiently [
15,
32].
Regarding the elicitation of antibodies in mice and other small animal models [
34], AG129 mice, which lack interferon receptors a/b and g, produced IgA, IgM, and IgG antibodies against ZIKV. Wild-type C57BL/6 mice also mounted humoral adaptive immune responses to ZIKV, but the responses were not necessary to prevent disease [
35]. However, in mice in which the type I IFN pathway was suppressed, the adaptive immune response had an important role in regulating the infection [
35]. Virus-like particles (VLPs) containing ZIKV envelope protein domain III also induced potent neutralizing immune responses in C57BL/6 mice [
36]. Anti-ZIKV antibodies are documented to have an important response in combating the virus, since passive transfer of immune serum significantly reduced viral replication in AG129 mice [
37], and was able to prevent death of wild-type mice inoculated intracerebrally with a lethal dose of ZIKV [
16]. Anti-ZIKV neutralizing antibodies were detected in infected Dunkin-Hartley guinea pigs using the plaque reduction neutralization test [
24]. Also, ZIKV-specific immunoglobulin G (IgG) antibodies were detected at 14 dpi and sustained thereafter [
32]. However, New Zealand White (NZW) rabbits are not susceptible to ZIKV infection and sera from inoculated animals do not neutralize the virus, indicating lack of seroconversion [
38].
Many species of large mammals, such as goats, lions, sheep, water buffalos, and NHPs have been demonstrated to produce a humoral immune response against ZIKV [
12,
22]. Rhesus macaques presented neutralizing antibodies by 21 dpi, and they exhibited no detectable virus replication when challenged 10 weeks after the initial challenge with a homologous strain [
19]. In addition, rhesus macaques infected with East African ZIKV were completely protected from detectable viremia when subsequently reinjected with heterologous Asian ZIKV [
39]. Moreover, strong anti-ZIKV specific antibody responses were displayed in both the maternal and fetal/neonatal circulation of rhesus macaques after pregnant females were inoculated [
18]. However, adult sheep, cattle, pigs, and chickens are not susceptible to ZIKV infection and do not produce a humoral response against the virus [
40].
Our results from juvenile opossums established that all routes (IH: Intraheart; IM: Intramuscular; IP: Intraperitoneal; IT: Intratesticular; SC: Subcutaneous) were efficient in stimulating an antibody response. Anti-ZIKV antibodies were detected by 14 days after inoculation (the first timepoint tested) and were present until Day 196 (the last timepoint tested). In summary, the IM and SC routes of inoculation elicited the highest antibody levels (
Figure 4A), but the fact that the two samples from animals inoculated by the SC route (which resulted in leakage of the inoculum) were ELISA-negative, established that as the IM route is the best route of choice for future investigations involving opossums. The SC route has been predominantly used in NHPs to elicit a humoral response [
18,
19,
39], and may well be appropriate for NHPs and other species, including mice, that have much thicker skin than opossums and that are not prone to leakage of inoculum delivered by that route.
The ELISA identified antibodies in only 18% (6/33) pups inoculated intracerebrally, even though ZIKV NS1 protein was detected by IHC in the organs of some of the ELISA-negative animals. A recent study of mice inoculated intracerebrally at embryonic Day 13.5 (E13.5), developmentally similar to an opossum with 2 days of age (
Table 5) revealed the up-regulation of immune-related genes 3 days after infection (E16.5) [
41]. Similarly, Shao et al. observed that genes related to the immune system were upregulated at E17.5, three days after intracerebral inoculation in mice [
42]. These results suggest a strong immune response against ZIKV in mice infected intracerebrally as embryos, at which time infection with ZIKV is associated with abnormal brain development. Our results from opossums suggest that many of the pups inoculated intracerebrally at an embryonic stage were tolerized to ZIKV and were not able to mount an adaptive antibody immune response after their immune systems developed. However, despite the absence of antibodies and the widespread dissemination of virus to other organs, these animals displayed no signs of illness.
Seven of the 11 dams that cannibalized their pups showed positive ELISA signals (
Figure 4). To our knowledge, this was the first documentation in any animal model of ZIKV transmission by eating infected suckling pups, as was confirmed by IHC of 10 of the dams. The eleventh dam, P1110, was ELISA-positive, but no tissues were available for analysis by IHC.
4.2. Persistence of ZIKV Infection
Juvenile animals in Group
IJ were inoculated at 18 weeks of age with ZIKV isolates PRVABC059 (four bi-weekly inoculations) and BR1911 (one inoculation, only); persistent infection was confirmed by IHC in tissue samples collected at 22-46 weeks of age. The infection was demonstrated to persist for at least 28 weeks (when the animals that had been infected for the longest duration were euthanized), which is equivalent to 9 mouse weeks and 16 human years (
Table 5). ZIKV RNA was detected up to 414 days after symptom onset in human semen, indicating that ZIKV also can persist long term in humans [
43]
Intramuscular (IM), intraperitoneal (IP), and subcutaneous (SC) routes of inoculation were conducted with both viral isolates (PRV and BZV); the IHC results established that all three routes were efficient in infecting the animals, that the infections spread to many organs, and that they persisted long term. Not all animals were assayed by IHC for all organs in the panel used in our study, but among those that were assayed for all organs, a female (O9344) inoculated IP, exhibited NS1 in brain, heart, reproductive organs, and spleen, but not eye; indicating the widespread dissemination and long-term persistence of the virus. All eight control animals injected with PBS (the UJ group) were negative in the IHC assay.
The intracerebral route has been used to determine if ZIKV has the potential to infect the nervous system of mice that are immunocompromised either because they are pre-term or newborn and without a well-developed immune system, or are genetically modified [
41,
42,
44,
45]. Detection of negative-sense strand viral RNA and isolation of infectious virus confirmed low levels of replicating ZIKV in small foci in the brains of convalescent mice 1 year after they had been inoculated subcutaneously at 1 day of age (before the establishment of a well-developed immune system), but the virus was absent from all other organs [
46]. That result is consistent with our IHC and PCR results, which established that infant
M. domestica (i.e., exteriorized embryos and fetuses) inoculated intracerebrally at 3–8 days of age can develop persistent infection in the brain; but it is different form our results in that and that the virus spreads from the opossum brain to other organs, where it also persists long term. IHC detected an NS1 signal only in 44% of the 16 animals analyzed, whereas PCR showed 100% sensitivity, having detected amplification in all ten investigated opossums (
Table 3). The positive tissues were brain, reproductive organs, and spleen. The fact that organs other than the brain showed positive signals validates that the animals were infected, that the virus had disseminated, and that it persisted until 26 weeks of age (the endpoint of the study), similar to the developmental stage of mice at 8 weeks of age and humans at 15 years of age (
Table 5).
Of the 33 pups infected intracerebrally, 16 animals had evidence of ZIKV infection or humoral immune responses. Based on IHC, PCR and ELISA data, we suggest that the 17 samples that were negative in all assays were from animals that never became infected with ZIKV (and did not mount an immune response) (
Figure 3 and
Table 3). This interpretation is consistent with previous data from our laboratory indicating that not all intracerebrally inoculated opossum pups become infected [
27]
IHC detected viral NS1 protein in the spleens (the only tissue analyzed) of all 10 analyzed dams that ate suckling pups that had been inoculated intracerebrally; the virus had persisted long after the dams had eaten the pups (
Table 3). Based on the IHC results, we believe that all of these mothers became infected and that the four that tested negative by ELISA did not mount an antibody response after becoming infected via the oral route.
Because
M. domestica is endemic to northeastern Brazil, where the height of the ZIKV occurred, it is possible, and perhaps likely, that this species serves as a natural reservoir for ZIKV. If that is the case,
M. domestica (and possibly other of the many marsupial species that are native to northeastern Brazil) might be much more important reservoirs than NHPs or other eutherian species, because the opossums are easily infected with ZIKV at every life stage, because the infection disseminates to many organs, and because the infection persists long term. Moreover, we have demonstrated frequent vertical transmission of ZIKV from mothers to offspring in this species (unpublished data), so infected mothers in the wild would be likely to transmit ZIKV to their pups, expanding the reservoir of infected animals. Cannibalism of opossum pups by mothers occurs frequently, so infected mothers might receive additional boluses of viral challenge by cannibalizing infected pups from sequential litters. Although the sylvatic cycle of ZIKV in South America has been discussed with regard to wild animals, such as NHPs and mosquitos [
47], the concept of opossums (and other marsupials) serving as reservoirs has not previously been suggested.
5. Conclusion
Taken together, our results establish M. domestica as a valid animal model that mimics many aspects of ZIKV infection in humans, but that also displays some significant differences from humans. The use of opossums is viable in a variety of procedures and protocols that are not possible or practical with other models. We demonstrated that inoculated pups and juveniles, could become infected by various routes, that dams that ate inoculated pups became infected, that ZIKV spreads widely among the body’s organs, and that a robust humoral immune response is elicited. Our results have established this model as an alternative to other models for research on ZIKV infection, and as a unique model for research on some aspects of ZIKV infection. It also may serve as an alternative model for testing therapeutics and vaccines. They also suggest that opossums, and perhaps other marsupials native to northeastern Brazil might be important reservoirs that contribute to human ZIKV infections; and that, via vertical transmission to pups as well as serial infections of dams that cannibalize pups, they might create a long-term risk of new ZIKV epidemics in Brazil.
Future initiatives may include studies to determine viral kinetics, the innate immune response, and the long-term physical, behavioral, and sexual health of infected animals. In addition, determining how opossum genes are regulated during the ZIKV infection could provide insight into the pathways that confer protection from or susceptibility to flavivirus infection. Moreover, additional research on inoculated pups could establish a valuable model of CZS.
The results have established that laboratory opossums not only can serve as an important third animal model, in addition to mice and NHPs, for research on ZIKV infection, but that they can be used experimentally to address questions about host-pathogen relationships that cannot be easily addressed, or addressed at all, in mouse or monkey models.
Author Contributions
(authors listed in order of authorship) Conceptualization: André Filipe Pastor, Susan M. Mahaney, John M. Thomas III, John L. VandeBerg. Data curation: André Filipe Pastor, Susan M. Mahaney. Formal analysis: André Filipe Pastor, Susan M. Mahaney, Juan Garcia Jr., Marisol Morales, Oscar Quintanilla. Funding acquisition: John M. Thomas III, John L. VandeBerg. Investigation: André Filipe Pastor, Susan M. Mahaney, Juan Garcia Jr., Marisol Morales, Oscar Quintanilla, Marco A. Arriaga, John M. Thomas III, John L. VandeBerg. Methodology: André Filipe Pastor, Susan M. Mahaney, Juan Garcia Jr., Marisol Morales, Oscar Quintanilla, Marco A. Arriaga, John M. Thomas III, John L. VandeBerg. Project administration: John M. Thomas III, John L. VandeBerg. Resources: John M. Thomas III, John L. VandeBerg. Supervision: John M. Thomas III, John L. VandeBerg. Validation: André Filipe Pastor, Susan M. Mahaney, John M. Thomas III, John L. VandeBerg. Visualization: André Filipe Pastor. Writing—original draft: André Filipe Pastor. Writing—revising & editing: André Filipe Pastor, Susan M. Mahaney, John M. Thomas III, John L. VandeBerg.
Funding
Part of this research was conducted in facilities constructed at UTRGV under support from NIH grant C06 RR020547.
Ethics Statement
The research was conducted under protocols that had been approved by the UTRGV Institutional Animal Care and Use Committee: 2016-005 (approved August 28, 2016) and AUP-19-32 (approved July 9, 2019). The study complied with all relevant legislation.
Data Availability Statement
The original contributions from the study are included in the article; inquiries may be directed to the corresponding authors.
Acknowledgments
We thank Alejandro Reyes and his team of animal care technicians for managing the opossum research resource and for conducting the technical procedures with the animals. We thank Mario Patlan for assistance with technical procedures with the animals and with IHC and ELISA; and Dr. Matthew D. Terry for providing the IHC protocol. Some of the methods and results reported here were presented in M.S. theses by three of the authors (JGJr., MM, and OQ).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dick, G.W. Epidemiological Notes on Some Viruses Isolated in Uganda; Yellow Fever, Rift Valley Fever, Bwamba Fever, West Nile, Mengo, Semliki Forest, Bunyamwera, Ntaya, Uganda S and Zika Viruses. Trans R Soc Trop Med Hyg 1953, 47, 13–48. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika Virus. I. Isolations and Serological Specificity. Trans R Soc Trop Med Hyg 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M. de F.; Araújo, T.V.B.; Andreza Barkokebas, L.; Caroline A. Bezerra, Cynthia Braga, S.P.B.-F.; Carlos Alexandre A. Brito, Renata G. Cabral, Adriana R. Carneiro, Maria Durce C.G. Carvalho, Marli T. Cordeiro, I.C.-J.; Adriana S.C. Cunha, Danielle D.C.S. Cruz, R.D. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis 2016, 22, 1090. [Google Scholar] [CrossRef]
- Barbi, L.; Coelho, A.V.C.; Alencar, L.C.A. de; Crovella, S. Prevalence of Guillain-Barré Syndrome among Zika Virus Infected Cases: A Systematic Review and Meta-Analysis. Brazilian Journal of Infectious Diseases 2018, 22, 137–141. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Feitosa, I.M.L.; Ribeiro, E.M.; Horovitz, D.D.G.; Pessoa, A.L.S.; França, G.V.A.; García-Alix, A.; Doriqui, M.J.R.; Wanderley, H.Y.C.; Sanseverino, M.V.T.; et al. The Phenotypic Spectrum of Congenital Zika Syndrome. Am J Med Genet A 2017, 173, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika Virus-Spread, Epidemiology, Genome, Transmission Cycle, Clinical Manifestation, Associated Challenges, Vaccine and Antiviral Drug Development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. New England Journal of Medicine 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- França, G.V.A.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.P.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika Virus Syndrome in Brazil: A Case Series of the First 1501 Livebirths with Complete Investigation. The Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, O.T.; Souza, J.P.; De Mucio, B.; de León, R.G.P.; Perea, W.; Gülmezoglu, A.M. WHO Interim Guidance on Pregnancy Management in the Context of Zika Virus Infection. Lancet Glob Health 2016, 4, e510–e511. [Google Scholar] [CrossRef]
- World Health Organization WHO Statement on the First Meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. Available online: https://www.who.int/news/item/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-(2005)-(ihr-2005)-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations (accessed on 8 July 2021).
- BRASIL Monitoramento Integrado de Alterações No Crescimento e Desenvolvimento Relacionadas à Infecção Pelo Vírus Zika e Outras Etiologias Infecciosas, Até a Semana Epidemiológica 45 de 2018. Boletim Epidemiológico | Secretaria de Vigilância em Saúde | Ministério da Saúde 2018, 49, 8.
- Bradley, M.P.; Nagamine, C.M. Animal Models of Zika Virus. Comp Med 2017, 67, 242–252. [Google Scholar]
- Duggal, N.; Ritter, J.; Pestorius, S.; Zaki, S.; Davis, B.; Chang, G.; Bowen, R.; Brault, A. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep 2017, 18, 1751–1760. [Google Scholar] [CrossRef]
- Krause, K.K.; Azouz, F.; Shin, O.S.; Kumar, M. Understanding the Pathogenesis of Zika Virus Infection Using Animal Models. Immune Netw 2017, 17, 287. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, H.; Chudnovets, A.; Burd, I.; Pekosz, A.; Klein, S.L. Animal Models of Congenital Zika Syndrome Provide Mechanistic Insight into Viral Pathogenesis during Pregnancy. PLoS Negl Trop Dis 2020, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nazerai, L.; Schøller, A.S.; Rasmussen, P.O.S.; Buus, S.; Stryhn, A.; Christensen, J.P.; Thomsen, A.R. A New in Vivo Model to Study Protective Immunity to Zika Virus Infection in Mice with Intact Type I Interferon Signaling. Front Immunol 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Pawitwar, S.S.; Dhar, S.; Tiwari, S.; Ojha, C.R.; Lapierre, J.; Martins, K.; Rodzinski, A.; Parira, T.; Paudel, I.; Li, J.; et al. Overview on the Current Status of Zika Virus Pathogenesis and Animal Related Research. Journal of Neuroimmune Pharmacology 2017, 12, 371–388. [Google Scholar] [CrossRef]
- Steinbach, R.J.; Haese, N.N.; Smith, J.L.; Colgin, L.M.A.; MacAllister, R.P.; Greene, J.M.; Parkins, C.J.; Kempton, J.B.; Porsov, E.; Wang, X.; et al. A Neonatal Nonhuman Primate Model of Gestational Zika Virus Infection with Evidence of Microencephaly, Seizures and Cardiomyopathy; 2020; Vol. 15; ISBN 1111111111.
- Dudley, D.M.; Aliota, M.; Mohr, E.; Weile, A.; Lehrer-Brey, G.; Weisgrau, K.; Mohns, M.; Breitbach, M.; Rasheed, M.; Newman, C.; et al. A Rhesus Macaque Model of Asian-Lineage Zika Virus Infection. Nat Commun 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Ireland, D.D.C.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T Cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog 2016, 12, 1–20. [Google Scholar] [CrossRef]
- Morrison, T.E.; Diamond, M.S. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J Virol 2017, 91, 1–15. [Google Scholar] [CrossRef]
- Nazerai, L.; Christensen, J.P.; Thomsen, A.R. A ‘Furry-Tale’ of Zika Virus Infection: What Have We Learned from Animal Models? Viruses 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Goodfellow, F.T.; Tesla, B.; Simchick, G.; Zhao, Q.; Hodge, T.; Brindley, M.A.; Stice, S.L. Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev 2016, 25, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Krause, K.K.; Azouz, F.; Nakano, E.; Nerurkar, V.R. A Guinea Pig Model of Zika Virus Infection. Virol J 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Saver, A.E.; Crawford, S.A.; Joyce, J.D.; Bertke, A.S. Route of Infection Influences Zika Virus Shedding in a Guinea Pig Model. Cells 2019, 8. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. The Laboratory Opossum. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals; Golledge, H., Richardson, C., Eds.; Wiley: West Sussex, 2024; pp. 301–323. [Google Scholar]
- Thomas, J.; Garcia, J.; Terry, M.; Mahaney, S.; Quintanilla, O.; Silva, D.C.; Morales, M.; VandeBerg, J.L. Monodelphis Domestica as a Fetal Intra-Cerebral Inoculation Model for Zika Virus Pathogenesis. Pathogens 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene Expression across Mammalian Organ Development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Almeida, I.C.; Covas, D.T.; Soussumi, L.M.T.; Travassos, L.R. A Highly Sensitive and Specific Chemiluminescent Enzyme-Linked Immunosorbent Assay for Diagnosis of Active Trypanosoma Cruzi Infection. Transfusion (Paris) 1997, 37, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Pabbarajua, K.; Wonga, S.; Gill, K.; Fonseca, K.; Tipplesa, G.A.; Tellier, R. Simultaneous Detection of Zika, Chikungunya and Dengue Viruses by a Multiplex Real-Time RT-PCR Assay. Journal of Clinical Virology 2016, 83, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.H. Validation of Serological Assays for Diagnosis of Infectious Diseases. OIE Revue Scientifique et Technique 1998, 17, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Zhang, N.N.; Li, X.F.; Wang, Y.Q.; Tian, M.; Qiu, Y.F.; Fan, J.W.; Hao, J.N.; Huang, X.Y.; Dong, H.L.; et al. Intranasal Infection and Contact Transmission of Zika Virus in Guinea Pigs. Nat Commun 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly Efficient Maternal-Fetal Zika Virus Transmission in Pregnant Rhesus Macaques. PLoS Pathog 2017, 13, 1–22. [Google Scholar] [CrossRef]
- Julander, J.G.; Siddharthan, V. Small-Animal Models of Zika Virus. Journal of Infectious Diseases 2017, 216, S919–S927. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive Immune Responses To Zika Virus Are Important For Controlling Virus Infection And Preventing Infection In Brain And Testes. J Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like Particles That Display Zika Virus Envelope Protein Domain III Induce Potent Neutralizing Immune Responses in Mice. Sci Rep 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.; Mendy, J.; Manayani, D.; Vang, L.; Wang, C.; Richard, T.; Guenther, B.; Aruri, J.; Avanzini, J.; Garduno, F.; et al. Passive Transfer of Immune Sera Induced by a Zika Virus-Like Particle Vaccine Protects AG129 Mice Against Lethal Zika Virus Challenge. EBioMedicine 2018, 27, 61–70. [Google Scholar] [CrossRef]
- Miller, M.R.; Fagre, A.C.; Clarkson, T.C.; Markle, E.D.; Foy, B.D. Three Immunocompetent Small Animal Models That Do Not Support Zika Virus Infection. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Dudley, D.M.; Newman, C.M.; Mohr, E.L.; Gellerup, D.D.; Breitbach, M.E.; Buechler, C.R.; Rasheed, M.N.; Mohns, M.S.; Weiler, A.M.; et al. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS Negl Trop Dis 2016, 10, 1–22. [Google Scholar] [CrossRef]
- Ambagala, A.; Truong, T.; Cottam-Birt, C.; Berhane, Y.; Gerdts, V.; Karniychuk, U.; Safronetz, D.; Babiuk, S. Susceptibility of Chicken Embryos, Sheep, Cattle, Pigs, and Chickens to Zika Virus Infection. Front Vet Sci 2020, 7. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.; Ye, Q.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Herrlinger, S.; Yang, S.-L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.-F. Zikus Virus Infection Disrupts Neurovascular Development and Results in Postnatal Microcephaly with Brain Damage. Stem Cells and Regeneration 2016, 143, 4127–4136. [Google Scholar]
- Bujan, L.; Mansuy, J.M.; Hamdi, S.; Pasquier, C.; Joguet, G. 1 Year after Acute Zika Virus Infection in Men. Lancet Infect Dis 2020, 20, 25–26. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.A.; Nogueira, J.S.; Réssio, R.A.; Cirqueira, C.S.; Kimura, L.M.; Fernandes, K.R.; Cunha, M.S.; Souza, R.P.; Guerra, J.M. Experimental Zika Virus Infection Induces Spinal Cord Injury and Encephalitis in Newborn Swiss Mice. Experimental and Toxicologic Pathology 2017, 69, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Zuo, G.L.; Li, X.F.; Ye, Q.; Deng, Y.Q.; Huang, X.Y.; Cao, W.C.; Qin, C.F.; Luo, Z.G. Vertical Transmission of Zika Virus Targeting the Radial Glial Cells Affects Cortex Development of Offspring Mice. Cell Res 2016, 26, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.D.C.; Manangeeswaran, M.; Lewkowicz, A.P.; Engel, K.; Clark, S.M.; Laniyan, A.; Sykes, J.; Lee, H.N.; McWilliams, I.L.; Kelley-Baker, L.; et al. Long-Term Persistence of Infectious Zika Virus: Inflammation and Behavioral Sequela in Mice. PLoS Pathog 2020, 16, 1–26. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M. Human Urban Arboviruses Can Infect Wild Animals and Jump to Sylvatic Maintenance Cycles in South America. Front Cell Infect Microbiol 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Study design and timeline.
Figure 1.
Study design and timeline.
Figure 2.
Titration curve for ELISA anti-ZIKV in opossum. Serum pools were diluted serially from 1:12.5 to 1:200. A. The positive serum pool was made from equal volumes from 29 inoculated juveniles, whereas the negative serum pool was made from equal volumes from 48 uninfected juveniles. B. Ratio of OD mean positive/ OD mean negative pool at each dilution (means derived from four replicates).
Figure 2.
Titration curve for ELISA anti-ZIKV in opossum. Serum pools were diluted serially from 1:12.5 to 1:200. A. The positive serum pool was made from equal volumes from 29 inoculated juveniles, whereas the negative serum pool was made from equal volumes from 48 uninfected juveniles. B. Ratio of OD mean positive/ OD mean negative pool at each dilution (means derived from four replicates).
Figure 3.
ELISA evaluation of the response of anti-ZIKV antibodies in different groups of infected opossums. A. Summary of the anti-ZIKV ELISA results. The points represent the results of the serum samples from the animals in the various groups evaluated: IJ: Inoculated Juveniles; UJ: Unexposed Juveniles; IIC: Infected Intracerebrally; UIC: Unexposed Intracerebrally; Dams: Ate inoculated pups; EMS: Exposed by Mate or Sire. Titers between 0.900 and 0.999, inclusive, are considered indeterminate, whereas those below 0.900 are negative, and those equal to or higher than 1.000 are positive. B and C. Receiver operating characteristic (ROC) curves evaluating the sensitivity of the ELISA with serum from IJ (B) and IIC (C) groups. For the IJ group, the area under the curve was 0.9945 (99.45% accuracy), whereas it was 0.7861 (78.61%) for the IIC group.
Figure 3.
ELISA evaluation of the response of anti-ZIKV antibodies in different groups of infected opossums. A. Summary of the anti-ZIKV ELISA results. The points represent the results of the serum samples from the animals in the various groups evaluated: IJ: Inoculated Juveniles; UJ: Unexposed Juveniles; IIC: Infected Intracerebrally; UIC: Unexposed Intracerebrally; Dams: Ate inoculated pups; EMS: Exposed by Mate or Sire. Titers between 0.900 and 0.999, inclusive, are considered indeterminate, whereas those below 0.900 are negative, and those equal to or higher than 1.000 are positive. B and C. Receiver operating characteristic (ROC) curves evaluating the sensitivity of the ELISA with serum from IJ (B) and IIC (C) groups. For the IJ group, the area under the curve was 0.9945 (99.45% accuracy), whereas it was 0.7861 (78.61%) for the IIC group.
Figure 4.
Comparison of titers elicited by different routes of inoculation of juveniles. A. Routes of inoculation with PRV (IH: Intraheart; IM: Intramuscular; IP: Intraperitoneal; IT: Intratesticular; SC: Subcutaneous). B. Routes of inoculations with BZV (IM: Intramuscular; IP: Intraperitoneal; SC: Subcutaneous). A one way ANOVA analysis with false discovery rate (FDR) correction was performed. * p<0.05.
Figure 4.
Comparison of titers elicited by different routes of inoculation of juveniles. A. Routes of inoculation with PRV (IH: Intraheart; IM: Intramuscular; IP: Intraperitoneal; IT: Intratesticular; SC: Subcutaneous). B. Routes of inoculations with BZV (IM: Intramuscular; IP: Intraperitoneal; SC: Subcutaneous). A one way ANOVA analysis with false discovery rate (FDR) correction was performed. * p<0.05.
Figure 5.
ELISA results for anti-ZIKV antibodies in opossums inoculated with PRV bi-weekly on four occasions or BZV on one occasion. A and B. A one-way ANOVA analysis with false discovery rate (FDR) correction was performed. * p<0.05. C and D. Kinetics of viral titers. Each line represents a single animal.
Figure 5.
ELISA results for anti-ZIKV antibodies in opossums inoculated with PRV bi-weekly on four occasions or BZV on one occasion. A and B. A one-way ANOVA analysis with false discovery rate (FDR) correction was performed. * p<0.05. C and D. Kinetics of viral titers. Each line represents a single animal.
Figure 6.
IHC results from spleens of O9444, an animal inoculated with PRV by the IT (intratesticular) route, and O9580 and animal injected with PBS by the IH (intraheart) route, and necropsied at 26 weeks of age. Images depict tissue stained with and without primary antibody for detection of viral non-structural protein 1 (NS1). Images were photographed at 40X magnification on three separate channels (green for cytoskeleton, blue for DAPI, and red for NS1 signal) and merged for the composite photo.
Figure 6.
IHC results from spleens of O9444, an animal inoculated with PRV by the IT (intratesticular) route, and O9580 and animal injected with PBS by the IH (intraheart) route, and necropsied at 26 weeks of age. Images depict tissue stained with and without primary antibody for detection of viral non-structural protein 1 (NS1). Images were photographed at 40X magnification on three separate channels (green for cytoskeleton, blue for DAPI, and red for NS1 signal) and merged for the composite photo.
Table 1.
Summary of ELISA data from the juveniles inoculated by various routes and the pups inoculated intracerebrally. The numbers of positive sera are shown for each group, as well as the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy derived from the data based on the comparison between the true positive and true negatives.
Table 1.
Summary of ELISA data from the juveniles inoculated by various routes and the pups inoculated intracerebrally. The numbers of positive sera are shown for each group, as well as the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy derived from the data based on the comparison between the true positive and true negatives.
| Juveniles (IJ and UJ groups) |
n |
Pups (IIC and UIC groups) |
n |
|
IJ positives (true positives) |
237 |
IIC positives (true positives) |
6 |
|
UJ positives (false positives) |
3 |
UIC positives (false positives) |
1 |
|
IJ negatives (false negatives) |
2 |
IIC negatives (false negative) |
27 |
|
UJ negatives (true negatives) |
184 |
UIC negatives (true negatives) |
31 |
| Statistical analysis |
% |
Statistical analysis |
% |
| Sensitivity |
99.16 |
Sensitivity |
18.18 |
| Specificity |
98.40 |
Specificity |
96.85 |
| PPV |
98.75 |
PPV |
85.71 |
| NPV |
98.93 |
NPV |
53.45 |
| Accuracy |
99.45 |
Accuracy |
78.61 |
Table 2.
Results of assessment of intra- and inter-assay variability.
Table 2.
Results of assessment of intra- and inter-assay variability.
| |
Intra-assay CV % |
Inter-assay CV% |
|
|
| |
Mean (%) |
SD/Mean OD (%) |
Mean OD |
SD (σ) |
| Positive |
|
|
|
|
| High |
8.5 |
2.7 |
0.970 |
0.026 |
| Medium |
11.0 |
6.8 |
0.831 |
0.056 |
| Low |
9.9 |
22.3 |
0.296 |
0.066 |
| Negative |
37.6 |
32.7 |
0.007 |
0.002 |
| Mean |
16.7 |
16.1 |
|
|
Table 3.
ELISA, IHC, and PCR results from all animals for which IHC results are available and that comprise the five groups of opossums: IJ, UJ, IIC, UIC, and Dams. IM: Intramuscular; IP: Intraperitoneal; IC: Intracerebral; SC: Subcutaneous; Oral. ELISA results are indicated as positive (+) or negative (-). IHC and PCR are designed as (Y) meaning a strong immunofluorescent signal; (W) meaning a weak signal; (-) meaning no signal; ( / ) not analyzed. Organs are designated by letters in the IHC and PCR columns: B (brain), E (eye), H (heart), R (reproductive organ), and S (spleen). * Animals tested for five organs by IHC.
Table 3.
ELISA, IHC, and PCR results from all animals for which IHC results are available and that comprise the five groups of opossums: IJ, UJ, IIC, UIC, and Dams. IM: Intramuscular; IP: Intraperitoneal; IC: Intracerebral; SC: Subcutaneous; Oral. ELISA results are indicated as positive (+) or negative (-). IHC and PCR are designed as (Y) meaning a strong immunofluorescent signal; (W) meaning a weak signal; (-) meaning no signal; ( / ) not analyzed. Organs are designated by letters in the IHC and PCR columns: B (brain), E (eye), H (heart), R (reproductive organ), and S (spleen). * Animals tested for five organs by IHC.
| ID number |
Sex |
Group |
Route |
Treatment |
Age of Animal at Necropsy (weeks) |
Experimental Study Day |
ELISA |
IHC |
PCR |
| P1366 |
F |
IJ |
IM |
BZV |
22 |
28 |
+ |
- |
/ |
| P1349 |
F |
IJ |
IM |
BZV |
46 |
196 |
+ |
W (S) |
/ |
| P1350 |
F |
IJ |
IM |
BZV |
46 |
196 |
+ |
Y (R) |
/ |
| P1351 |
F |
IJ |
IM |
BZV |
46 |
196 |
+ |
- |
/ |
| P1424 |
F |
IJ |
IM |
BZV |
46 |
196 |
+ |
Y (R) |
/ |
| P1465 |
F |
IJ |
IM |
BZV |
22 |
28 |
+ |
Y (R) |
/ |
| P1341 |
F |
IJ |
IP |
BZV |
46 |
196 |
+ |
W (S) |
/ |
| P1475 |
F |
IJ |
IP |
BZV |
22 |
28 |
+ |
Y (R) |
/ |
| P1477 |
F |
IJ |
IP |
BZV |
46 |
196 |
+ |
Y (R) |
/ |
| P1397 |
F |
IJ |
SC |
BZV |
22 |
28 |
- |
W (R) |
/ |
| P1426 |
F |
IJ |
SC |
BZV |
46 |
196 |
+ |
Y (R) |
/ |
| P1493 |
F |
IJ |
SC |
BZV |
22 |
28 |
+ |
W (R) |
/ |
| O9343 |
F |
IJ |
IM |
PRV |
26 |
56 |
+ |
Y (H, R, S) |
/ |
| O9347 |
F |
IJ |
IM |
PRV |
22 |
28 |
+ |
Y (B, S) |
/ |
| O9374 |
M |
IJ |
IM |
PRV |
26 |
56 |
+ |
- |
/ |
| O9344 |
F |
IJ |
IP |
PRV |
22 |
28 |
+ |
Y (B, H, R, S) |
/ |
| O9348 |
F |
IJ |
IP |
PRV |
26 |
56 |
+ |
Y (B, R, S) |
/ |
| O9375 |
M |
IJ |
IP |
PRV |
26 |
56 |
+ |
Y (E, S) |
/ |
| O9342 |
F |
IJ |
SC |
PRV |
26 |
56 |
+ |
- |
/ |
| O9346 |
F |
IJ |
SC |
PRV |
26 |
56 |
+ |
Y (E) |
/ |
| O9373 |
M |
IJ |
SC |
PRV |
26 |
56 |
+ |
Y (E, S) |
/ |
| O9523 |
F |
IJ |
IH |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9530 |
M |
IJ |
IH |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9534 |
F |
IJ |
IH |
PRV |
26 |
56 |
+ |
Y (S) |
/ |
| O9455 |
F |
IJ |
IM |
PRV |
26 |
56 |
+ |
Y (S) |
/ |
| O9461 |
M |
IJ |
IM |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9525 |
F |
IJ |
IM |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9526 |
F |
IJ |
IP |
PRV |
26 |
56 |
+ |
Y (S) |
/ |
| O9443 |
M |
IJ |
IP |
PRV |
26 |
56 |
+ |
Y (S) |
/ |
| O9353 |
M |
IJ |
IT |
PRV |
26 |
56 |
+ |
Y(S) |
/ |
| O9354 |
M |
IJ |
IT |
PRV |
26 |
56 |
+ |
Y(S) |
/ |
| O9376 |
M |
IJ |
IT |
PRV |
26 |
56 |
+ |
Y(S) |
/ |
| O9444 |
M |
IJ |
IT |
PRV |
26 |
56 |
+ |
Y(S) |
/ |
| O9463 |
M |
IJ |
IT |
PRV |
26 |
56 |
+ |
Y(S) |
/ |
| O9524 |
F |
IJ |
SC |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9460 |
M |
IJ |
SC |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9524 |
F |
IJ |
SC |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9539 |
M |
IJ |
SC |
PRV |
26 |
56 |
+ |
W (S) |
/ |
| O9580 |
M |
UJ |
IH |
PBS |
26 |
56 |
- |
- |
/ |
| O9572 |
F |
UJ |
IM |
PBS |
26 |
56 |
- |
- |
/ |
| P1542 |
F |
UJ |
IM |
PBS |
46 |
196 |
- |
- |
/ |
| P1330 |
F |
UJ |
IP |
PBS |
22 |
28 |
- |
- |
/ |
| P1521 |
F |
UJ |
IP |
PBS |
46 |
196 |
- |
- |
/ |
| P1540 |
F |
UJ |
IP |
PBS |
46 |
196 |
- |
- |
/ |
| P1541 |
F |
UJ |
IP |
PBS |
46 |
196 |
- |
- |
/ |
| O9575 |
F |
UJ |
SC |
PBS |
26 |
56 |
+ |
- |
/ |
| P1937 |
M |
IIC |
IC |
BZV |
26 |
177 |
- |
Y (R) |
/ |
| P1965 |
F |
IIC |
IC |
BZV |
26 |
177 |
- |
- |
Y (R, S) |
| P1967 |
M |
IIC |
IC |
BZV |
19 |
129 |
- |
Y (B, R, S) |
Y (S) |
| P1968 |
M |
IIC |
IC |
BZV |
21 |
148 |
- |
Y (R) |
Y (R) |
| P2087 |
F |
IIC |
IC |
BZV |
24 |
163 |
- |
- |
Y (B, S) |
| P2090 |
M |
IIC |
IC |
BZV |
24 |
163 |
- |
- |
Y (B, R, S) |
| P2133 |
F |
IIC |
IC |
BZV |
22 |
149 |
- |
- |
Y (R) |
| P2138 |
M |
IIC |
IC |
BZV |
22 |
149 |
- |
- |
Y (B, R) |
| P2141 |
F |
IIC |
IC |
BZV |
22 |
149 |
+ |
Y (B, R) |
/ |
| P2142 |
F |
IIC |
IC |
BZV |
22 |
149 |
+ |
Y (B, R) |
/ |
| P2146 |
M |
IIC |
IC |
BZV |
22 |
149 |
- |
W (S) |
Y (B, R, S) |
| P2241 |
F |
IIC |
IC |
BZV |
22 |
146 |
+ |
- |
Y (S) |
| P2246 |
M |
IIC |
IC |
BZV |
22 |
146 |
- |
W (S) |
Y (R, S) |
| P2275 |
F |
IIC |
IC |
BZV |
26 |
177 |
- |
- |
/ |
| P2276 |
F |
IIC |
IC |
BZV |
26 |
176 |
- |
- |
/ |
| P2279 |
M |
IIC |
IC |
BZV |
26 |
176 |
- |
- |
/ |
| P1945 |
F |
UIC |
IC |
PBS |
26 |
175 |
- |
- |
/ |
| P2296 |
M |
UIC |
IC |
PBS |
26 |
176 |
- |
- |
/ |
| P2300 |
F |
UIC |
IC |
PBS |
22 |
149 |
- |
- |
/ |
| P2306 |
M |
UIC |
IC |
PBS |
22 |
149 |
- |
- |
/ |
| P1456 |
F |
DAM |
ate 1 pup |
PRV |
87 |
NA |
+ |
Y (S) |
/ |
| P1571 |
F |
DAM |
ate 8 pups |
PRV |
84 |
NA |
+ |
Y (S) |
/ |
| P1624 |
F |
DAM |
ate 2 pups |
PRV |
83 |
NA |
+ |
Y (S) |
/ |
| P2217 |
F |
DAM |
ate 4 pups |
PRV |
70 |
NA |
+ |
Y (S) |
/ |
| P2344 |
F |
DAM |
ate 2 pups |
PRV |
68 |
NA |
- |
Y (S) |
/ |
| P2355 |
F |
DAM |
ate 10 pups |
PRV |
67 |
NA |
+ |
Y (S) |
/ |
| P2375 |
F |
DAM |
ate 2 pups |
PRV |
67 |
NA |
- |
Y (S) |
/ |
| P2451 |
F |
DAM |
ate 10 pups |
PRV |
67 |
NA |
- |
Y (S) |
/ |
| P2452 |
F |
DAM |
ate 6 pups |
PRV |
67 |
NA |
- |
Y (S) |
/ |
| P3132 |
F |
DAM |
ate 4 pups |
PRV |
57 |
NA |
+ |
Y (S) |
/ |
Table 4.
ELISA results from 11 dams whose pups had each been inoculated with either 105 PFU (ID P1110) or 1,000 PFU (all other dams) of PRV. IM: Intramuscular; SC: Subcutaneous; IC: Intracerebral. ELISA results are indicated as positive (+) or negative (-), with titers in parentheses. All dams exhibited a strong positive IHC result in spleen samples, except P1110 which was not tested by IHC.
Table 4.
ELISA results from 11 dams whose pups had each been inoculated with either 105 PFU (ID P1110) or 1,000 PFU (all other dams) of PRV. IM: Intramuscular; SC: Subcutaneous; IC: Intracerebral. ELISA results are indicated as positive (+) or negative (-), with titers in parentheses. All dams exhibited a strong positive IHC result in spleen samples, except P1110 which was not tested by IHC.
| Litter Inoculation Date |
Dam ID |
ELISA Results |
Route |
Age of Pups (Days) at Time of Inoculation |
Number of Pups Eaten |
Number of Pups and Days Between Inoculation and Being Eaten |
Number of Weeks Prior to Dam’s Necropsy |
| 2018-11-06 |
P2451 |
- (0.419) |
IM |
4 |
10 |
10 pups at 14 days |
34 |
| 2018-11-26 |
P2344 |
- (0.425) |
SC |
4 |
2 |
1 pup at 17 days, 1 at 45 days |
27–31 |
| 2018-12-03 |
P2375 |
- (0.446) |
IM |
5 |
2 |
1 pup at 11 days, 1 at 38 days |
27–31 |
| 2019-02-04 |
P2452 |
- (0.496) |
SC |
4 |
6 |
2 pups at 15 days, 3 at 25 days, 1 at 30 days |
19–21 |
| 2018-11-06 |
P1456 |
+ (1.036) |
IC |
5 |
1 |
1 pup at 20 days |
34 |
| 2019-02-11 |
P3132 |
+ (1.180) |
IC |
4 |
4 |
2 pups at 12 days, 1 at 17 days, 1 at 23 days |
18–21 |
| 2018-10-19 |
P1571 |
+ (1.241) |
IC |
1 |
8 |
2 pups at 6 days, 3 at 17 days, 3 at 32 days |
34–38 |
| 2019-02-01 |
P1624 |
+ (1.421) |
IC |
3 |
2 |
1 pup at 18 days, 1 at 33 days |
19–21 |
| 2018-12-03 |
P2355 |
+ (1.601) |
IM |
3 |
10 |
5 pups at 14 days, 5 at 18 days |
30–31 |
| 2018-10-22 |
P2217 |
+ (1.640) |
IM |
3 |
4 |
2 pups at 23 days, 2 at 29 days |
34–36 |
| 2018-05-09 |
P1110 |
+ (1.674) |
IC |
1 |
9 |
1 pup at 1 day, 1 at 2 days, 1 at 5 days, and 6 at 8 days |
14–15 |
Table 5.
Developmental equivalencies of laboratory opossums, laboratory mice, and humans (adapted from [
26] E = Embryonic age in days (d), months (m), or years (y).
Table 5.
Developmental equivalencies of laboratory opossums, laboratory mice, and humans (adapted from [
26] E = Embryonic age in days (d), months (m), or years (y).
| Opossums |
Mice |
Humans |
Life History Event |
| E13.5d |
E10.5d |
E4-5w |
|
| 0d |
E11.5d |
E5-6w |
Birth of opossum |
| 2d |
E13d |
E7w |
|
| 4d |
E15d |
E8-11w |
|
| 6d |
E16.5d |
E12w |
|
| 14d |
0d |
E16w |
Detachment of opossums from nipples; birth of mouse |
| 21d |
3d |
E20w |
Opossum fur growth well-initiated |
| 30d |
6d |
0d |
Birth of human |
| 8w |
3.5w |
3y |
Natural weaning, toddler stage |
| 12w |
4w |
6y |
|
| 18w |
5w |
9y |
|
| 22w |
6w |
12y |
Onset of puberty |
| 26w |
8w |
15y |
Adolescence |
| 52w |
17w |
25y |
Physically and reproductively prime |
| 2y |
1y |
50y |
Loss of female fertility |
| 3y |
23m |
75y |
Elderly |
| 4y |
3y |
100y |
Near maximum lifespan |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).