Submitted:
06 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AMB-1 cultivation and characterization
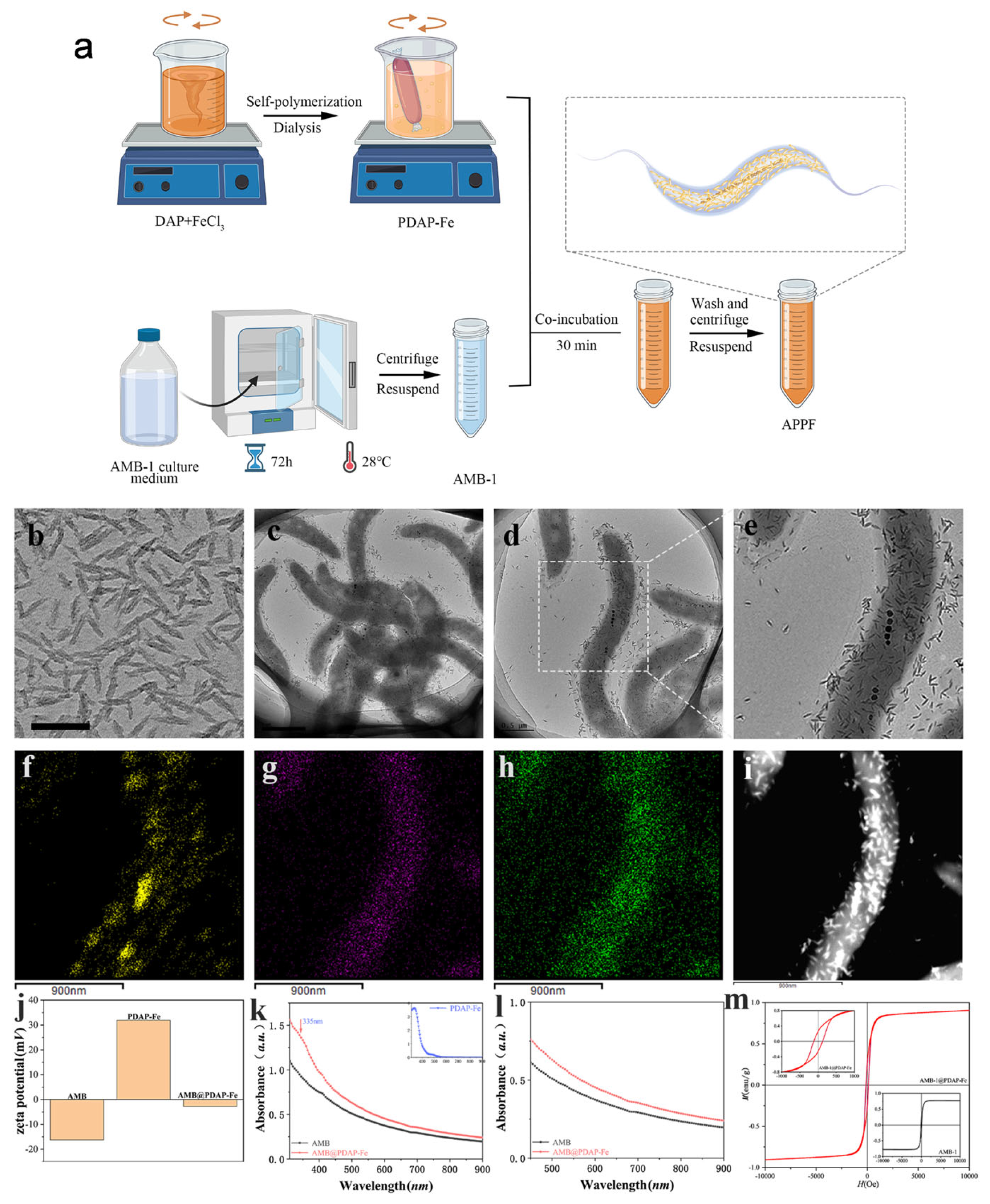
2.3. Preparation of APPF based on magnetotactic bacteria
2.4. In vitro photothermal performance testing
2.5. Measurement of GSH-dependent iron release
2.6. Intracellular reactive oxygen species (ROS) detection
2.7. In vitro photothermal therapy
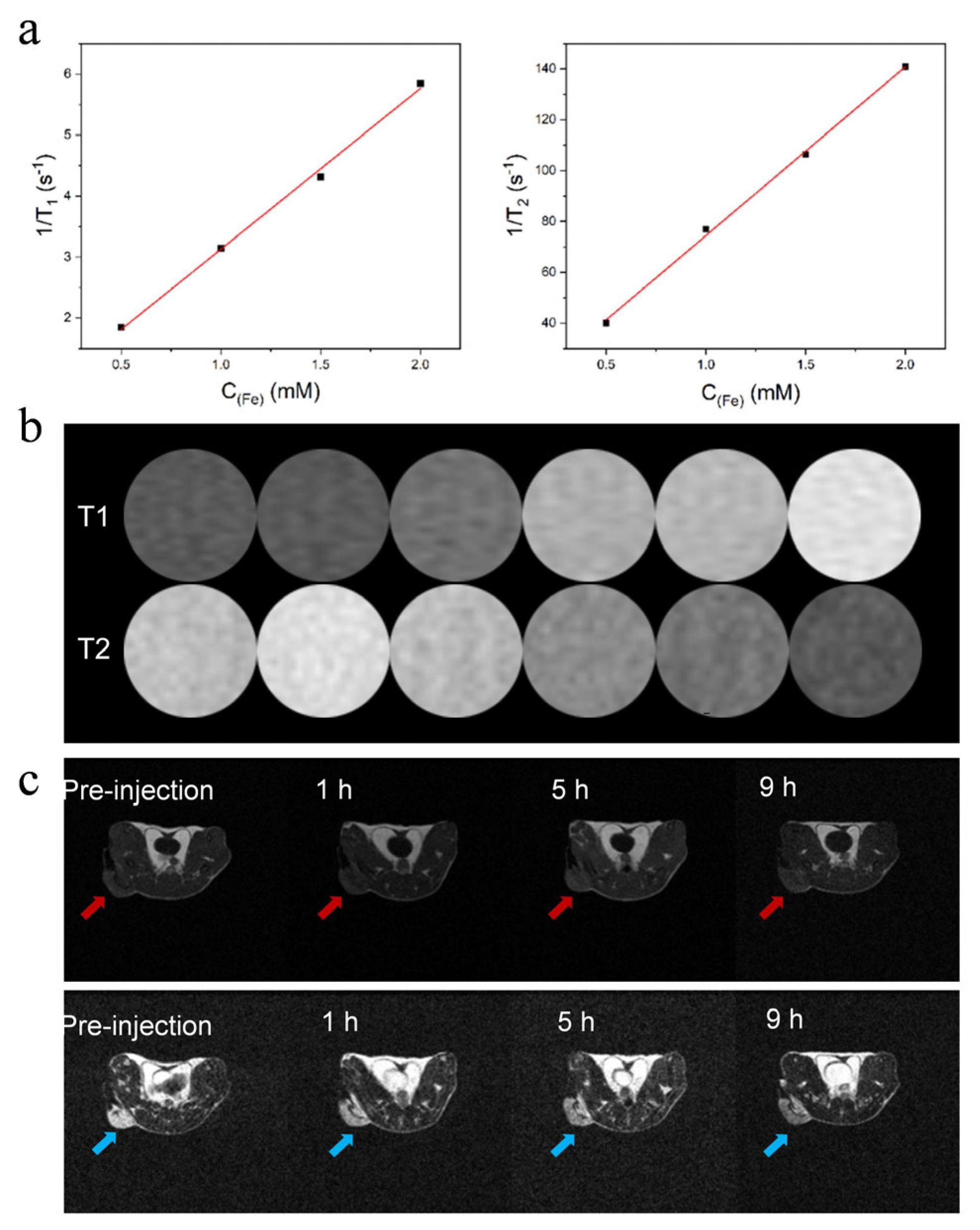
2.8. MRI measurement
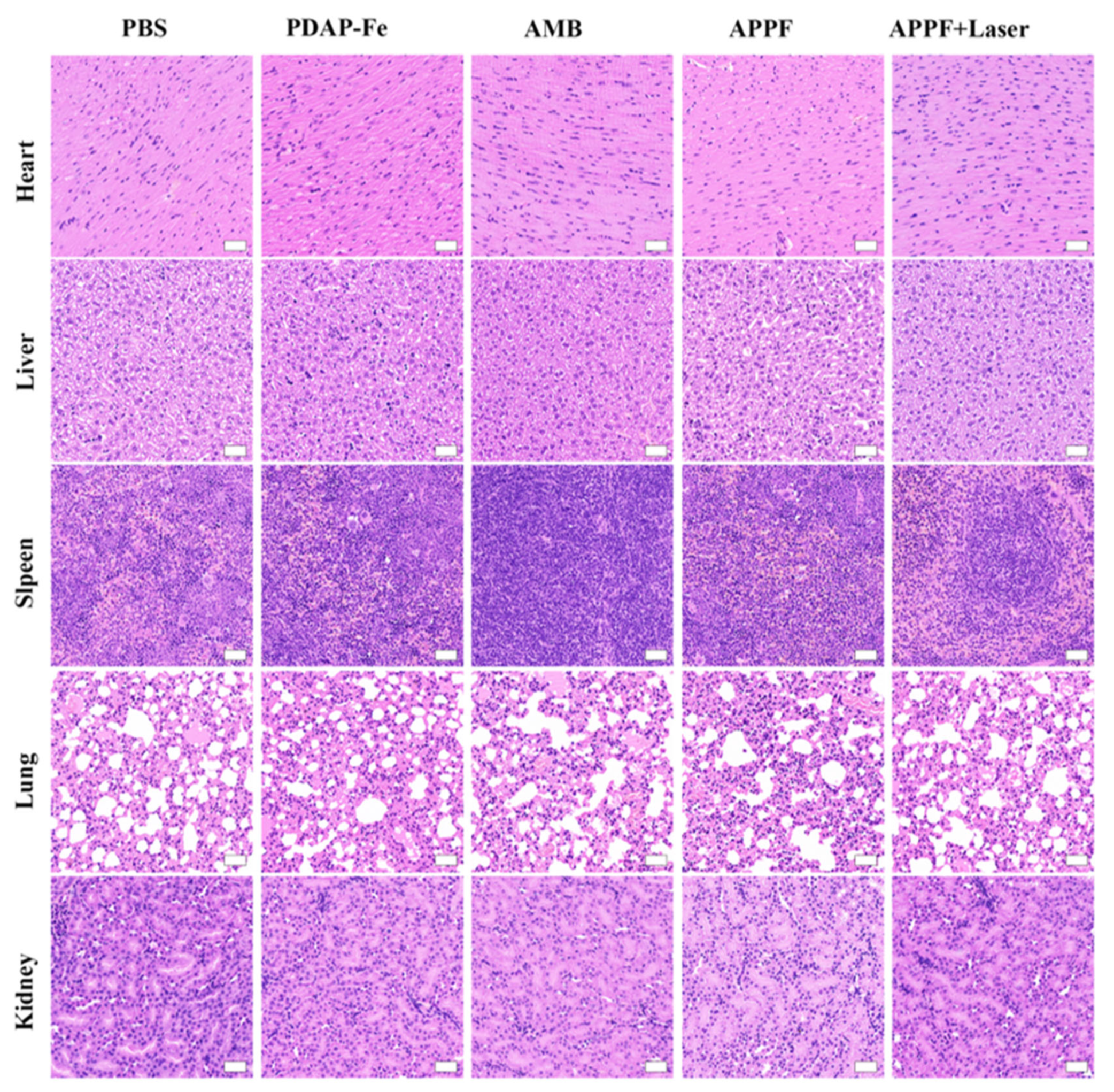
2.9. In vivo anti-tumor experiment
2.10. Statistical Analysis
2.11. Ethics
3. Results
3.1. Preparation and characterization of APPF
3.2. APPF demonstrates good photothermal effect and induces Fenton reaction in vitro
3.3. APPF demonstrates good MRI capability
3.4. Combined photothermal and chemodynamic anti-tumor effect of APPF
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahida, A.; Buschhorn, L.; Fröhling, S.; Jost, P.J.; Schneeweiss, A.; Lichter, P.; Kurzrock, R. The coming decade in precision oncology: six riddles. Nat. Rev. Cancer 2023, 23, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Du, J.; Chen, X.; Xue, Y.; Zhang, J.; Yang, X.; Chen, X.; Xie, J.; Ju, S. Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy. Nat. Commun. 2024, 15, 1–20. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Di Maio, M.; Chiodini, P.; Georgoulias, V.; Hatzidaki, D.; Takeda, K.; Wachters, F.M.; Gebbia, V.; Smit, E.F.; Morabito, A.; Gallo, C.; et al. Meta-Analysis of Single-Agent Chemotherapy Compared With Combination Chemotherapy As Second-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2009, 27, 1836–1843. [Google Scholar] [CrossRef]
- Datta, S.; Grant, D.J.W. Crystal structures of drugs: advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef]
- Yang, P.; Luo, Q.; Qi, G.; Gao, Y.; Li, B.; Zhang, J.; Wang, L.; Wang, H. Host Materials Transformable in Tumor Microenvironment for Homing Theranostics. Adv. Mater. 2017, 29, 1605869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Jiang, Y.; Park, J.; Gonzalez, K.M.; Wu, X.; Zhang, Q.-Y.; Lu, J. Cholesterol-modified sphingomyelin chimeric lipid bilayer for improved therapeutic delivery. Nat. Commun. 2024, 15, 2073. [Google Scholar] [CrossRef]
- Tsou, Y.; Zhang, X.; Zhu, H.; Syed, S.; Xu, X. Drug Delivery to the Brain across the Blood–Brain Barrier Using Nanomaterials. Small 2017, 13, 1701921. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Irfan, M.; Xu, B.; Li, J.; Zhang, L.; Qin, Z.; Yu, C.; Liu, H.; Su, X. Rational Design of DNA Framework-Based Hybrid Nanomaterials for Anticancer Drug Delivery. Small 2020, 16, e2002578. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.S.; Zhang, D.K.Y.; Koshy, S.T.; Mooney, D.J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 2018, 36, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, J.; Lang, T.; Kong, Y.; Rong, R.; Cai, Y.; Ran, W.; Xiong, F.; Zheng, C.; Wang, Y.; et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat. Nanotechnol. 2021, 16, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, D.; Huang, J.; He, S.; Yang, Z.; Zhang, Y.; Luo, Y.; Pu, K. Organic Semiconducting Pro-nanostimulants for Near-Infrared Photoactivatable Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 12680–12687. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Su, Z.; Han, S.; Huang, J.; Lin, L.; Shuai, X. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy. Sci. Adv. 2020, 6, eaay7785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, e1903060. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, L.; Li, N.; Wang, Y.; Liu, M.; Su, X.; Bao, X.; Yin, B.; Shen, S. Bacterial-Mediated Tumor Therapy: Old Treatment in a New Context. Adv. Sci. 2023, 10, 2205641. [Google Scholar] [CrossRef]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; De Lanauze, D.; Xu, Y.Z.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; LaFleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef]
- Faivre, D. and Schueler D. Magnetotactic bacteria and magnetosomes. Chem. Rev., 2008, 108, 11, 4875–4898. [Google Scholar] [CrossRef]
- Park, B.-W.; Zhuang, J.; Yasa, O.; Sitti, M. Multifunctional Bacteria-Driven Microswimmers for Targeted Active Drug Delivery. ACS Nano 2017, 11, 8910–8923. [Google Scholar] [CrossRef]
- Felfoul, O.; Martel, S. Assessment of navigation control strategy for magnetotactic bacteria in microchannel: toward targeting solid tumors. Biomed. Microdevices 2013, 15, 1015–1024. [Google Scholar] [CrossRef]
- Wang, P.; Chen, C.; Wang, Q.; Chen, H.; Chen, C.; Xu, J.; Wang, X.; Song, T. Tumor inhibition via magneto-mechanical oscillation by magnetotactic bacteria under a swing MF. J. Control. Release 2022, 351, 941–953. [Google Scholar] [CrossRef]
- Alphandéry, E.; Chebbi, I.; Guyot, F.; Durand-Dubief, M. Use of bacterial magnetosomes in the magnetic hyperthermia treatment of tumours: A review. Int. J. Hyperth. 2013, 29, 801–809. [Google Scholar] [CrossRef]
- Li, T.; Li, C.; Ruan, Z.; Xu, P.; Yang, X.; Yuan, P.; Wang, Q.; Yan, L. Polypeptide-Conjugated Second Near-Infrared Organic Fluorophore for Image-Guided Photothermal Therapy. ACS Nano 2019, 13, 3691–3702. [Google Scholar] [CrossRef]
- Bai, J.; Jia, X.; Zhen, W.; Cheng, W.; Jiang, X. A Facile Ion-Doping Strategy To Regulate Tumor Microenvironments for Enhanced Multimodal Tumor Theranostics. J. Am. Chem. Soc. 2018, 140, 106–109. [Google Scholar] [CrossRef]
- Li, J.; Ghoshal, S.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Halevi, B.; McKinney, S.; McCool, G.; Ma, C.; Yuan, X.; et al. Structural and mechanistic basis for the high activity of Fe–N–C catalysts toward oxygen reduction. Energy Environ. Sci. 2016, 9, 2418–2432. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Hu, Y.-R.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Multimodal Synergistic Cancer Therapy. Adv. Mater. 2020, 31, e1808325. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Feng, X.; Yu, C.; Feng, F.; Chai, Y.; Lu, P.; Song, T.; Wang, X.; Yao, L. Nanoparticle-Regulated Semiartificial Magnetotactic Bacteria with Tunable Magnetic Moment and Magnetic Sensitivity. Small 2019, 15, e1900427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, P.; Jana, D.; Wang, D.; Wang, M.; Yu, P.; Zhu, W.; Zhao, Y. Glutathione-Depleting Organic Metal Adjuvants for Effective NIR-II Photothermal Immunotherapy. Adv. Mater. 2022, 34, e2201706. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, H.; Liu, Y.; Ni, D.; Zhang, H.; Zhang, J.; Yao, Z.; He, M.; Shi, J.; Bu, W. Antiferromagnetic Pyrite as the Tumor Microenvironment-Mediated Nanoplatform for Self-Enhanced Tumor Imaging and Therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yuan, Y.; Jiang, C.; Blum, N.T.; He, J.; Huang, P.; Lin, J. Light-Triggered Transformable Ferrous Ion Delivery System for Photothermal Primed Chemodynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 6047–6054. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Qiao, B.; Luo, Y.; Cheng, C.; Yang, A.; Wang, M.; Yuan, X.; Fan, K.; Li, M.; Wang, Z. A multimodal imaging-guided nanoreactor for cooperative combination of tumor starvation and multiple mechanism-enhanced mild temperature phototherapy. Biomater. Sci. 2020, 8, 6561–6578. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.K.; Polepalli, S.; Rao, C.P. Binding of Fe(ii)-complex of phenanthroline appended glycoconjugate with DNA, plasmid and an agglutinin protein. New J. Chem. 2020, 44, 11727–11738. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Bai, J.; Jia, X.; Zhen, W.; Cheng, W.; Jiang, X. A Facile Ion-Doping Strategy To Regulate Tumor Microenvironments for Enhanced Multimodal Tumor Theranostics. J. Am. Chem. Soc. 2018, 140, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Nijiati, S.; Tang, L.; Ye, J.; Zhou, Z.; Chen, X. Ferroptosis Detection: From Approaches to Applications. Angew. Chem. Int. Ed. 2023, 62, e202300379. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Tan, P.; Chen, X.; Kopytynski, M.; Pan, D.; Zheng, X.; Gu, L.; Gong, Q.; Tian, X.; Gu, Z.; et al. Stimuli-Sensitive Linear–Dendritic Block Copolymer–Drug Prodrug as a Nanoplatform for Tumor Combination Therapy. Adv. Mater. 2022, 34, 2108049. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Dai, H.; Tan, X.; Li, H.; Liang, H.; Hu, C.; Huang, M.; Lee, J.Y.; Zhao, J.; Zhou, L.; et al. A Facile, Protein-Derived Supramolecular Theranostic Strategy for Multimodal-Imaging-Guided Photodynamic and Photothermal Immunotherapy In Vivo. Adv. Mater. 2022, 34, 2109111. [Google Scholar] [CrossRef]
- Xu, W.; Kattel, K.; Park, J.Y.; Chang, Y.; Kim, T.J.; Lee, G.H. Paramagnetic nanoparticle T1 and T2 MRI contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 12687–12700. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




