Submitted:
06 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Case Report
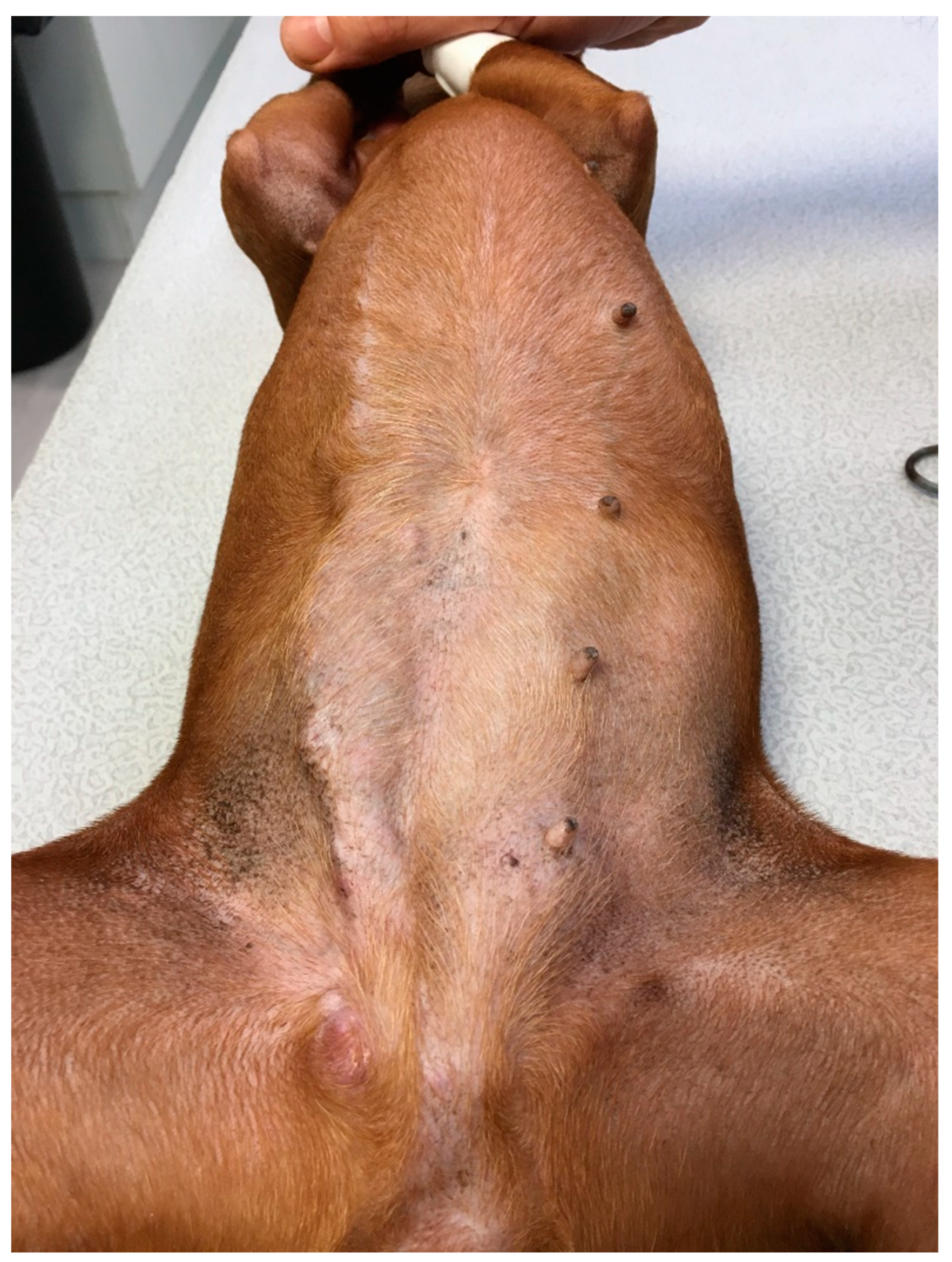
3.1. Clinical Presentation
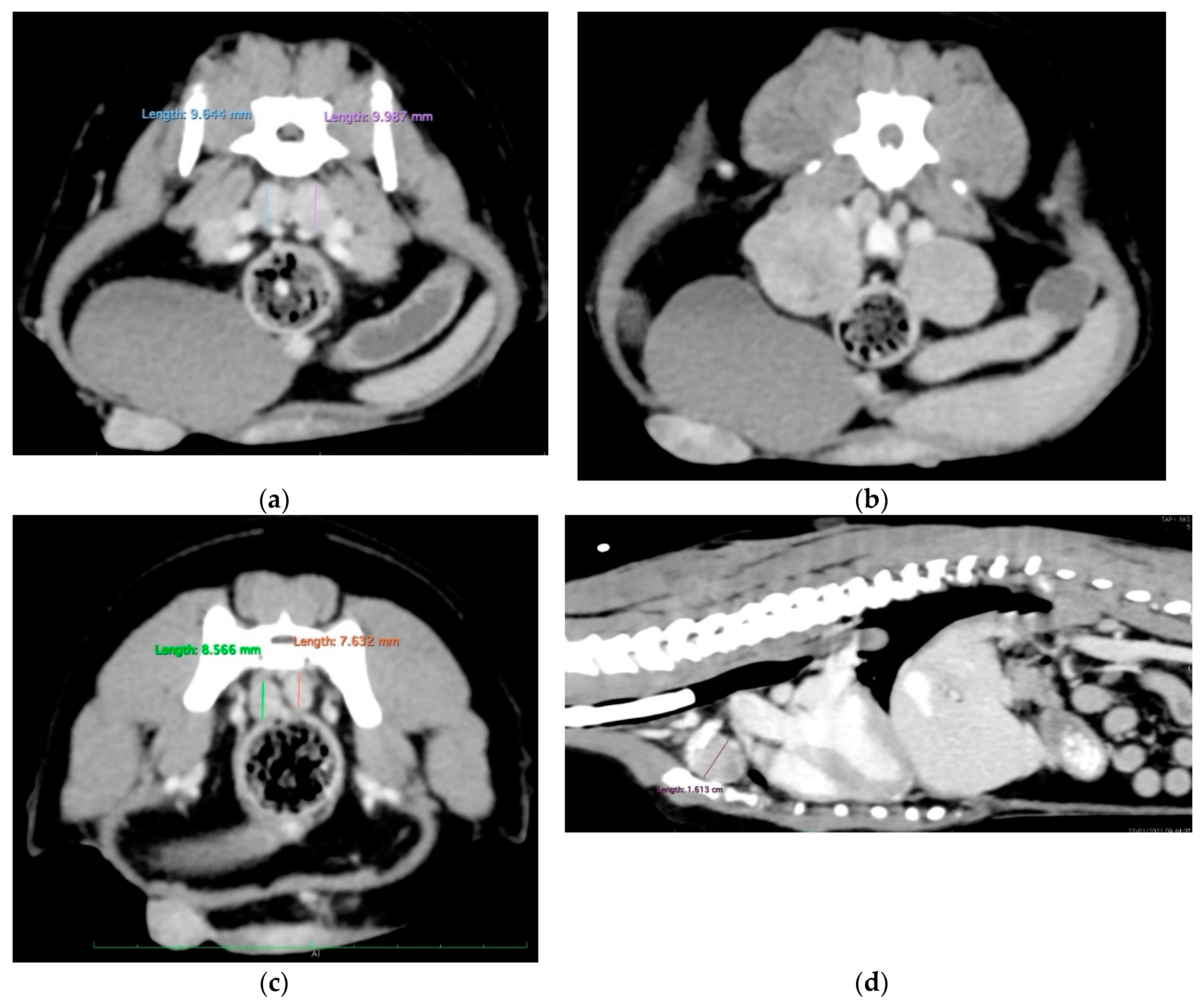
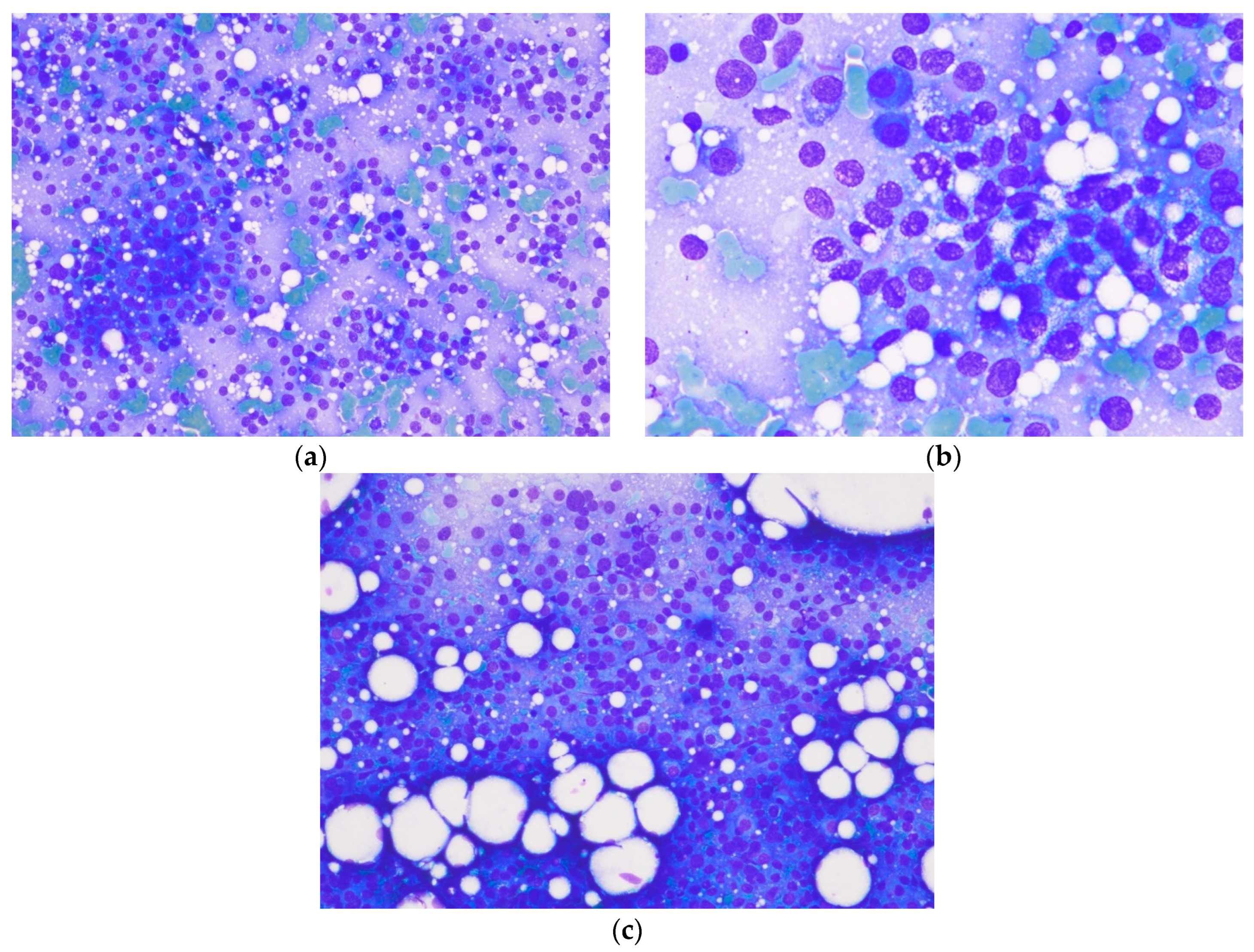
3.2. Additional Medical Tests
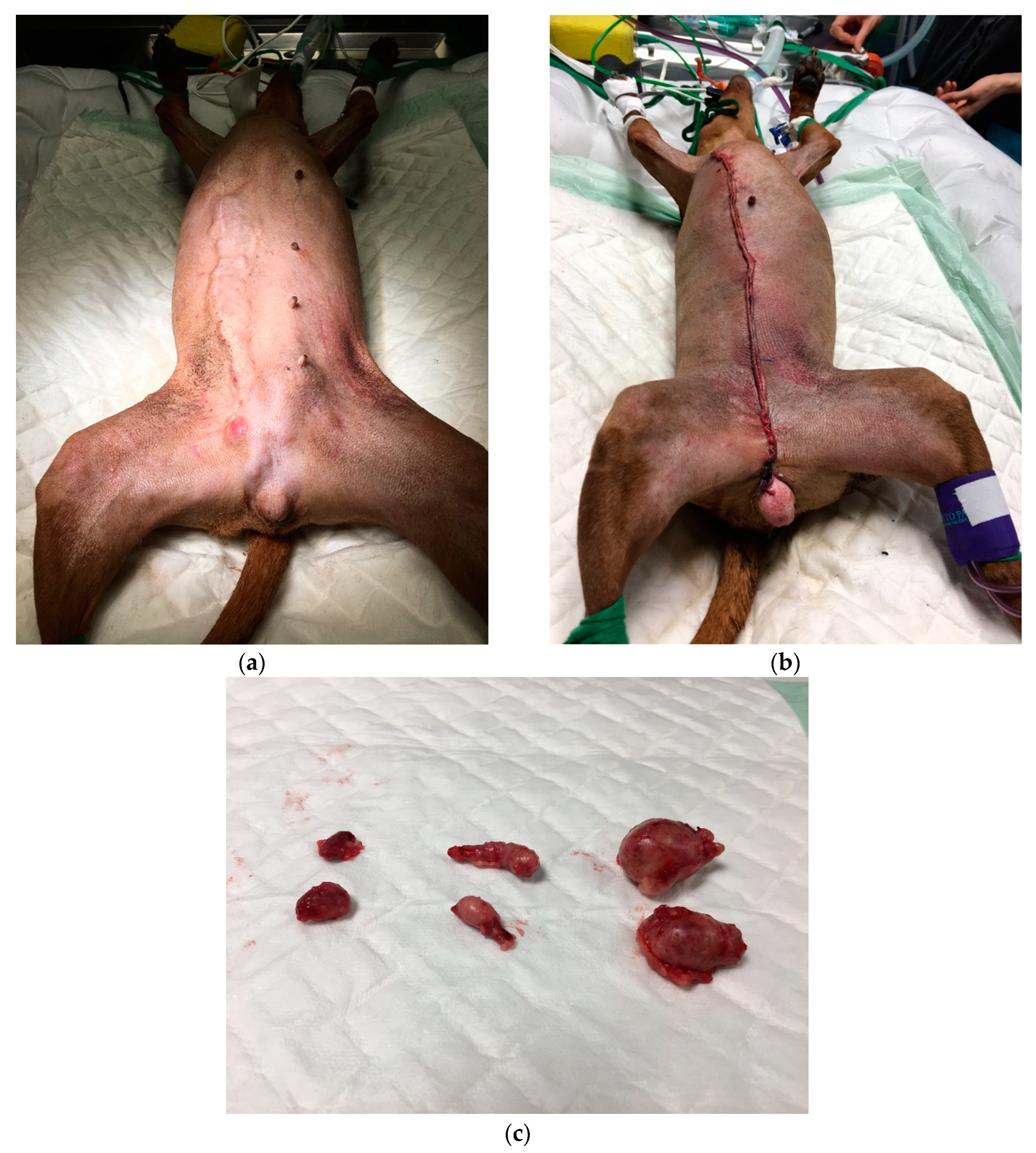
3.3. Surgery
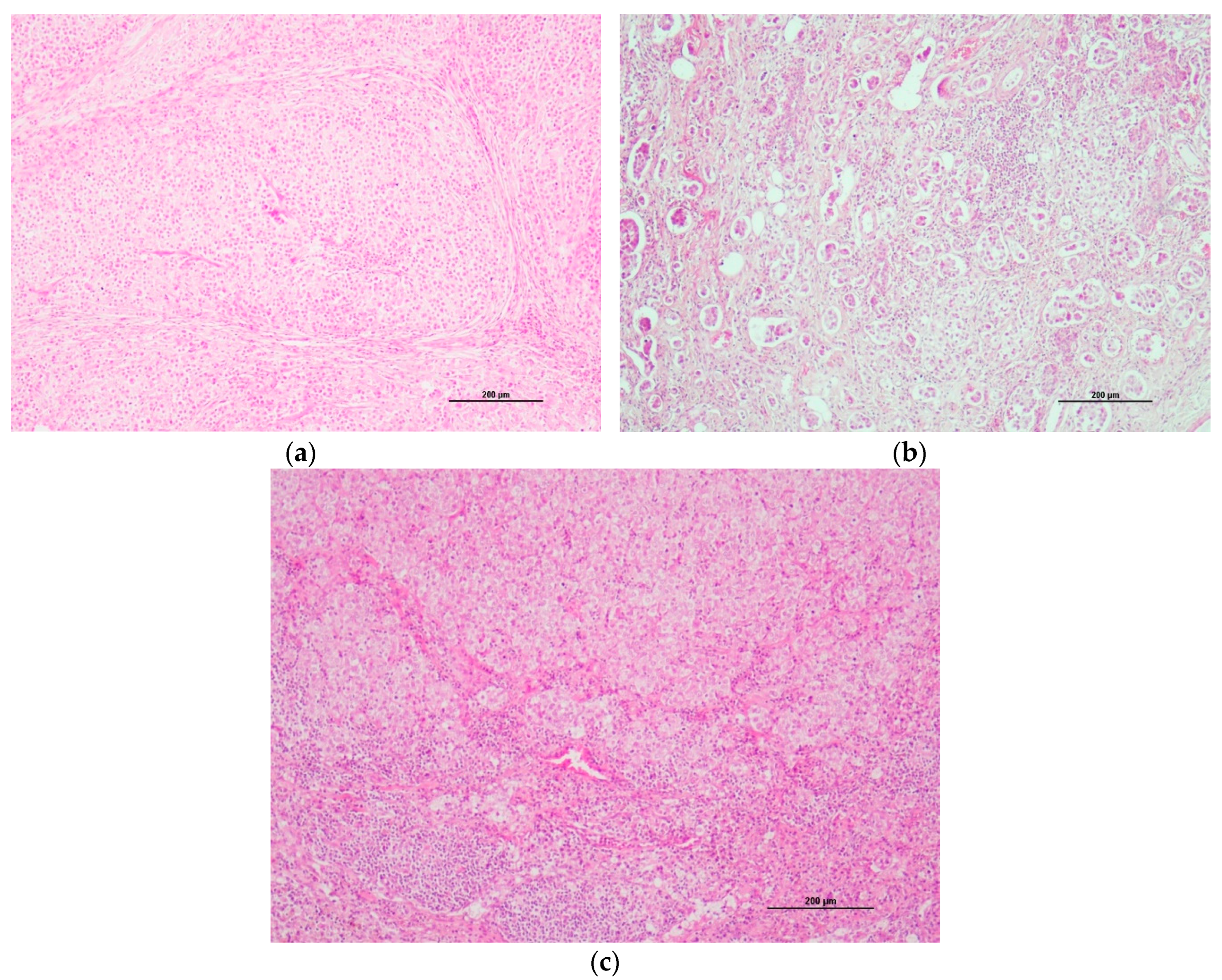
3.4. Histological Description
3.5. Adjuvant Treatment
3.6. Quality of Life and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suryawanshi, R.V. Assessment of Efficacy and Toxicity of Cyclophosphamide Chemotherapy in Canines with Malignant Mammary Tumor: A Retrospective Study. Vet. Med. Int. 2021, 2021, 5520603. [Google Scholar] [CrossRef] [PubMed]
- Karayannopoulou, M.; Kaldrymidou, E.; Constantinidis, T.C.; Dessiris, A. Adjuvant Post-operative Chemotherapy in Bitches with Mammary Cancer. J. Vet. Med. A 2001, 48, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, G.E.; De Campos, C.B.; Bertagnolli, C.; Cassali, G.D. Canine Malignant Mammary Gland Neoplasms with Advanced Clinical Staging Treated with Carboplatin and Cyclooxygenase Inhibitors. In Vivo 2012, 26, 375–380. [Google Scholar] [PubMed]
- Tran, C.M.; Moore, A.S.; Frimberger, A.E. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet. Comp. Oncol. 2014, 14, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Lorenzo, R.M.; Abramo, F.; Ratto, A.; Zini, E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet. Comp. Oncol. 2008, 6, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Schoenrock, D.; Baumgartner, W.; Nolte, I. Postoperative Adjuvant Treatment of Invasive Malignant Mammary Gland Tumors in Dogs with Doxorubicin and Docetaxel. J. Vet. Intern. Med. 2006, 20, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.C.; da Costa-Neto, J.M.; Portela, R.D.; D’Assis, M.J.M.H.; Martins-Filho, O.A.; Barrouin-Mello, S.; Ferreira Borges, N.; Silva, F.L.; Estrela-Lima, A. The effect of naltrexone as a carboplatin chemotherapy-associated drug on the immune response, quality of life and survival of dogs with mammary carcinoma. PLoS ONE 2018, 13, e0204830. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine invasive mammary carcinomas as models of human breastcancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat 2018, 167, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic Value of Histological Grading in Noninflammatory Canine Mammary Carcinomas in a Prospective Study with Two-Year Follow-Up: Relationship with Clinical and Histological Characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Canadas, A.; França, M.; Pereira, C.; Vilaça, R.; Vilhena, H.; Tinoco, F.; Silva, M.J.; Ribeiro, J.; Medeiros, R.; Oliveira, P.; et al. Canine Mammary Tumors: Comparison of Classification and Grading Methods in a Survival Study. Vet. Pathol. 2019, 56, 208–219. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, M.R.; Campos, L.C.; Ferreira, E.; Cassali, G.D. Quantitation of the Regional Lymph Node Metastatic Burden and Prognosis in Malignant Mammary Tumors of Dogs. J. Vet. Intern. Med. 2015, 29, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Chocteau, F.; Abadie, J.; Loussouarn, D.; Nguyen, F. Proposal for a Histological Staging System of Mammary Carcinomas in Dogs and Cats. Part 1: Canine Mammary Carcinomas. Front. Vet. Sci. 2019, 6, 388. [Google Scholar] [CrossRef]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: Natural history and prognostic factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Facchinetti, A.; Zanardello, C.; Rossi, E.; Vidotto, R.; Capello, K.; Melchiotti, E.; Laganga, P.; Zamarchi, R.; Vascellari, M. Detection and Prognostic Relevance of Circulating and Disseminated Tumour Cell in Dogs with Metastatic Mammary Carcinoma: A Pilot Study. Cancers 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Jaillardon, L.; Barthélemy, A.; Goy-Thollot, I.; Pouzot-Nevoret, C.; Fournel-Fleury, C. Mammary gland carcinoma in a dog with peripheral blood and bone marrow involvement associated with disseminated intravascular coagulation. Vet. Clin. Pathol. 2012, 41, 261–265. [Google Scholar] [CrossRef]
- Clemente, M.; Pérez-Alenza, M.D.; Peña, L. Metastasis of canine inflammatory versus non-inflammatory mammary tumours. J. Comp. Pathol. 2010, 143, 157–163. [Google Scholar] [CrossRef]
- Cooley, D.M.; Waters, D.J. Skeletal metastasis as the initial clinical manifestation of metastatic carcinoma in 19 dogs. J. Vet. Intern. Med. 1998, 12, 288–293. [Google Scholar] [CrossRef]
- Taylor, B.E.; Leibman, N.F.; Luong, R.; Loar, A.S.; Craft, D.M. Detection of carcinoma micrometastases in bone marrow of dogs and cats using conventional and cell block cytology. Vet. Clin. Pathol. 2013, 42, 85–91. [Google Scholar] [CrossRef]
- Flory, A.; Kruglyak, K.M.; Tynan, J.A.; McLennan, L.M.; Rafalko, J.M.; Fiaux, P.C.; Hernandez, G.E.; Marass, F.; Nakashe, P.; Ruiz-Perez, C.A.; et al. Clinical validation of a next-generation sequencing-based multi-cancer early detection “liquid biopsy” blood test in over 1000 dogs using an independent testing set: The CANcer Detection in Dogs (CANDiD) study. PLoS ONE 2022, 17, e0266623. [Google Scholar] [CrossRef] [PubMed]
- Arenas, C.; Peña, L.; Granados-Soler, J.L.; Pérez-Alenza, M.D. Adjuvant therapy for highly malignant canine mammary tumours: Cox-2 inhibitor versus chemotherapy: A case–control prospective study. Vet. Rec. 2016, 179, 125. [Google Scholar] [CrossRef]
- Machado, M.C.; Yamamoto, P.A.; Pippa, L.F.; de Moraes, N.V.; Neves, F.M.F.; Portela, R.D.; Barrouin-Melo, S.M.; Hielm-Björkman, A.; Godoy, A.L.P.C.; Estrela-Lima, A. Pharmacokinetics of Carboplatin in Combination with Low-Dose Cyclophosphamide in Female Dogs with Mammary Carcinoma. Animals 2022, 12, 3109. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.L.; Pires, I.; Lobo, L.; Lopes, C.S. The role of Cox-2 expression in the prognosis of dogs with malignant mammary tumours. Res. Vet. Sci. 2010, 88, 441–445. [Google Scholar] [CrossRef]
- Campos, C.B.D.E.; Lavalle, G.E.; Monteiro, L.N.; Pêgas, G.R.A.; Fialho, S.L.; Balabram, D. Adjuvant thalidomide and metronomic chemotherapy for the treatment of canine malignant mammary gland neoplasms. In Vivo 2018, 32, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.H.; Toledo-Piza, E.; Amorin, R.; Barboza, A.; Tobias, K.M. Inflammatory mammary carcinoma in 12 dogs: Clinical features, cyclooxygenase-2 expression, and response to piroxicam treatment. Can. Vet. J. 2009, 50, 506–510. [Google Scholar]
- Rossi, F.; Sabattini, S.; Vascellari, M.; Marconato, L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radi ation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet. Comp. Oncol. 2018, 16, 497–504. [Google Scholar] [CrossRef]
- Vieira, T.C.; Oliveira, E.A.; Dos Santos, B.J.; Souza, F.R.; Veloso, E.S.; Nunes, C.B.; Del Puerto, H.L.; Cassali, G.D. COX-2 expression in mammary invasive micropapillary carcinoma is associated with prognostic factors and acts as a potential therapeutic target in comparative oncology. Front. Vet. Sci. 2022, 12, 983110. [Google Scholar] [CrossRef]
- Brandi, A.; de Faria Lainetti, P.; Elias, F.; Rodrigues, M.M.P.; Fagundes Moraes, L.; Laufer-Amorim, R.; de Camargo, L.S.; Salles Gomes, C.O.M.; Fonseca-Alves, C.E. Firocoxib as a Potential Neoadjuvant Treatment in Canine Patients with Triple-Negative Mammary Gland Tumors. Animals 2022, 13, 60. [Google Scholar] [CrossRef]
- Alonso-Miguel, D.; Valdivia, G.; García-San José, P.; Alonso-Diez, Á.; Clares, I.; Portero, M.; Peña, L.; Pérez-Alenza, M.D. Clinical outcome of dogs diagnosed with canine inflammatory mammary cancer treated with metronomic cyclophosphamide, a cyclooxygenase-2 inhibitor and toceranib phosphate. Vet. Comp. Oncol. 2022, 20, 179–188. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. Principles of Medical Oncology. In Cancer: Principles and Practice of Oncology, 8th ed.; DeVita, V.T., Rosenberg, S.A., Eds.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 2008; pp. 340–342. [Google Scholar]
- London, C.A.; Gardner, H.L.; Mathie, T.; Stingle, N.; Portela, R.; Pennell, M.L.; Clifford, C.A.; Rosenberg, M.P.; Vail, D.M.; Williams, L.E.; et al. Impact of Toceranib/Piroxicam/Cyclophosphamide Maintenance Therapy on Outcome of Dogs with Appendicular Osteosarcoma following Amputation and Carboplatin Chemotherapy: A Multi-Institutional Study. PLoS ONE 2015, 10, e0124889. [Google Scholar] [CrossRef] [PubMed]
- Chon, E.; McCartan, L.; Kubicek, L.N.; Vail, D.M. Safety evaluation of combination toceranib phosphate (Palladia®) and piroxicam in tumour-bearing dogs (excluding mast cell tumours): A phase I dose-finding study. Vet. Comp. Oncol. 2012, 10, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Vargas Estrada, C.R.; Firmo, B.F.; Belchior Vela, D.; Maronezi, M.C.; Ramirez Uscategui, R.A.; Gasser, B.; Rossi Feliciano, M.A.; Pavan, L.; Nogueira Aires, L.P.; Piovan Lima, G.; De Nardi, A.B. Ultrasonographic assessment of renal perfusion in bitches with mammary carcinoma treated with long-term carprofen. Sci. Rep. 2021, 11, 23277. [Google Scholar] [CrossRef] [PubMed]
- Wouda, R.M.; Hocker, S.E.; Higginbotham, M.L. Safety evaluation of combination carboplatin and toceranib phosphate (Palladia) in tumour-bearing dogs: A phase I dose finding study. Vet. Comp. Oncol. 2018, 16, E52–E60. [Google Scholar] [CrossRef]
- Beata, C.; Beaudu-Lange, C.; Muller, C. Jusqu’où va-t-on dans les soins donnés à nos animaux de compagnie? Revue Vétérinaire Clinique 2021, 56, 157–169. [Google Scholar] [CrossRef]
| Date And carboplatin chemotherapy |
Chloraminophene 2 mg EOD |
Firocoxib 14,2 mg/d |
toceranib 10 mg | clinical examination and treatment steps | weight (kg) | RBCs (10^12/L) SV [5,6-8,8] | PCV (%) SV [37,3-61,7] | Hb (g/dL) SV [13,1-20,5] | WBCs (10^9/L) SV [5,05-16,7] | Neutrophils (10^9/L) SV [2,9-11,6] | Monocytes (10^9/L) SV [0,16-1,12] | Lymphocytes (10^9/L) SV [1-5,1] | Platelets (10^3/µL) SV [148-484] | ALT (IU/L) SV [10-125] | Blood Creatinine (mg/L) SV [5-18] |
| 21/01/28 | from 21/02/07 | from 21/02/09 3,56 mg/kg |
21/01/27-28 surgery in two steps 21/02/06 wound dehiscence (surgery on 21/02/08) |
4,0 | 7,85 | 49,4 | 17,3 | 9,33 | 7,83 | 0.,61 | 0,68 | 195 | 157 | 5 | |
| 21/02/11 chemotherapy N°1, 270 mg/m² | stop 21/02/11 restart 21/02/18 to 21/03/05 |
to 21/03/05 3,56 mg/kg |
4 | ||||||||||||
| 21/03/05 chemotherapy N°2, 202 mg/m² | stop 21/03/05 restart 21/03/13 to 21/04/23 |
stop | 4,1 | 7,27 | 46,8 | 18,3 | 3,6 | 1,85 grade 1 |
0,14 | 1,62 | 198 | 160 | 5 | ||
| 21/03/18 | vulvar edema | 7,36 | 46,5 | 15,9 | 6,16 | 2,94 grade 1 |
0,36 | 2,85 | 65 grade 2 |
||||||
| 21/03/26 | restart | 2,43 mg/kg EOD | vulvar edema, granulation of mammary tissue in left previous M4-M5 location increased Left (2cm) and right (3,5 cm) prescapular LN. |
4,1 | 7,09 | 44,9 | 15,4 | 6,77 | 4,41 | 0,23 | 2,12 | 175 | 88 | 6,4 | |
| 21/03/30 | surgical removal of Left and Right prescapular LN | ||||||||||||||
| 21/04/16 | correct surgical scar | 4,0 | |||||||||||||
| 21/04/23 chemotherapy N°3 162 mg/m² | stop 21/04/21 restart 21/04/26 2 mg one in 3 days |
stop 21/04/22 restart 21/05/01 3,39 mg/kg |
stop 21/04/22 restart 21/04/28 2,38 mg/kg |
4,2 | 6,92 | 44 | 15,1 | 4,69 | 3,94 | 0,3 | 0,19 | 314 | 56 | 5,8 | |
| 21/05/10 | normal clinical examination | 6,97 | 45,1 | 18,8 | 3,06 | 1,93 Grade 1 |
0,08 | 1,05 | 220 | 41 | 10,5 | ||||
| 21/05/20 chemo carboplatin N°4 151 mg/m² |
stop 21/05/18 restart 21/06/29 2 mg one in 3 days |
stop 21/05/19 restart 21/07/03 2,91 mg/kg |
stop 21/05/19 restart 21/07/01 2,04 mg/kg |
normal clinical examination | 4,89 | 7,75 | 50,3 | 16,8 | 4,18 | 3,52 | 0,27 | 0,17 | 266 | ||
| 21/06/05 | stop 21/06/13 | 7,61 | 49 | 17,0 | 2,75 | 1,61 Grade 1 |
0,06 | 1,08 | 145 | 32 | 7,3 | ||||
| 21/06/16 | 8,05 | 52,5 | 15,4 | 4,18 | 2,61 Grade 1 |
0,06 | 1,51 | 158 | |||||||
| 21/06/25 chemotherapy N°5 carboplatin 151 mg/m² |
restart 21/06/29 | stop 21/06/24 | stop 21/06/23 | normal clinical examination | 4,850 | 7,06 | 45,9 | 15,4 | 3,55 | 2,67 Grade 1 |
0,32 | 0,13 | 176 | 41 | 5,4 |
| 21/07/10 | normal clinical examination | 6,88 | 45,5 | 15,7 | 2,88 | 2,2 Grade 1 |
0,07 | 0,61 | 147 | 32 | 10 | ||||
| 21/07/23 chemotherapy n°6 carboplatin 151 mg/m² |
stop 21/07/21 restart 210727 |
stop 21/07/22 restart 21/07/31 |
stop 21/07/22 restart 21/07/29 2,03 mg/kg |
normal clinical examination | 4,930 | ||||||||||
| 21/08/07 | stopped 21/08/02 | stopped 21/08/02 | stopped 21/08/02 | left himb limb edema and ventral hematoma inguinal LN 18mm (not infiltrated by cancer cells) normal clotting time |
5,83 | 37,5 | 12,9 | 1,31 |
0 ,88 Grade 3 |
0,11 | 0,09 | 39 Grade 3 |
|||
| 21/08/16 | the hematoma is shrinking and the limb edema has disappeared | 6,94 | 44,9 | 14,6 | 3,83 | 3,32 | 0,08 | 0,42 | 35 Grade 3 |
||||||
| 21/08/25 | restart firocoxib | restart toceranib | 7,24 | 47,7 | 16,0 | 4,66 | 3,63 | 0,15 | 0,88 | 42 Grade 3 |
|||||
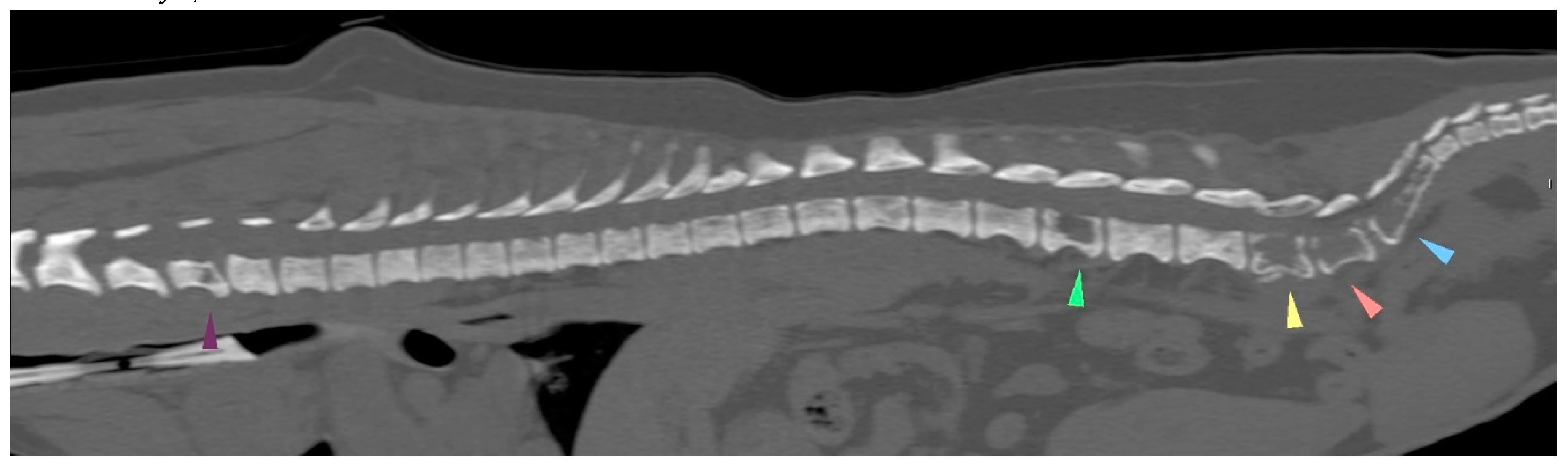
| 21/09/01 | flaccid tail, evolving into posterior paresis within 24 hours spinal metastases (CT examination) and death on 21/09/22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





