Submitted:
06 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Materials and Method:
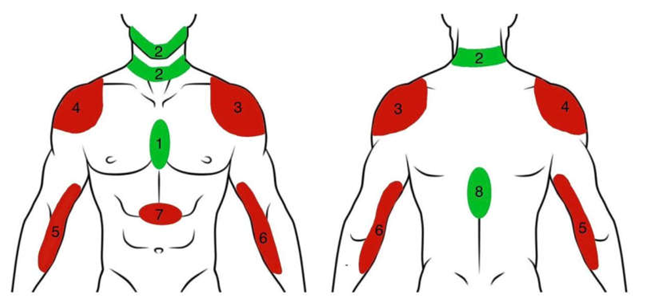
Statistical Analysis
Result:
Age, Sex Distribution, and Types of ACS
| Variable | Mean ±SD/ N (%) | |
|---|---|---|
| 1 | Age(years) | 57.03±11.28 |
| 2 | Weight(Kilograms) | 63.28±13.66 |
| 3 | Male | 337 (82.0%) |
| 4 | Female | 74(18.0%) |
| Pain Site | STEMI | NSTEMI | Total (%) | |
|---|---|---|---|---|
| 1 | Retrosternal | 273(60%) | 80(59.7%) | 353(59.9%) |
| 2 | Jaw and Neck | 13(2.8%) | 11(8.2%) | 24(4%) |
| 3 | Left Shoulder | 1(0.2%) | 1(0.7%) | 2(0.3%) |
| 4 | Right Shoulder | 0 | 0 | 0 |
| 5 | Right Arm | 0 | 0 | 0 |
| 6 | Left Arm | 0 | 0 | 0 |
| 7 | Epigastrium | 12(2.6%) | 4(2.9%) | 16(2.7%) |
| 8 | Backache | 0 | 0 | 0 |
| 9 | Retrosternal+ Jaw+ Neck | 65(14.3%) | 12(8.9%) | 77(13.07%) |
| 10 | Retrosternal + Jaw+ Neck +Backache | 2(0.4%) | 0 | 2(0.3%) |
| 11 | Retrosternal + Epigastrium | 2(0.4%) | 0 | 2(0.3%) |
| 12 | Retrosternal+ Epigastrium+ Backache | 1(0.2%) | 0 | 1(0.16%) |
| 13 | Retrosternal + Backache | 62(13.6%) | 10(7.5%) | 72(12.2%) |
| 14 | Retrosternal + Backache + Shoulder | 2(0.4%) | 1(0.7%) | 3(0.5%) |
| 15 | Retrosternal + Shoulder | 6(1.3%) | 2(1.5%) | 8(1.3%) |
| 16 | Jaw+ Neck + shoulder | 1(0.2%) | 0 | 1(0.16%) |
| 17 | Jaw+ Neck + Backache | 1(0.2%) | 0 | 1(0.16%) |
| 18 | Epigastrium + Backache | 1(0.2%) | 2(1.5%) | 3(0.5%) |
| 19 | Backache + Shoulder | 13(2.8%) | 11(8.2%) | 24(4%) |
| Vessel Involved | STEMI | NSTEMI | Total (%) | |
|---|---|---|---|---|
| 1 | LAD | 201(63.4%) | 49(53.3%) | 250(60.8%) |
| 2 | LCX | 33(10.4%) | 20(21.7%) | 53(12.9%) |
| 3 | RCA | 80(25.2%) | 15(16.3%) | 95(23.1%) |
| 4 | LMCA | 2(0.6%) | 0 | 2(0.5%) |
| LAD | LCX | RCA | LMCA | ||
|---|---|---|---|---|---|
| Retrosternal | Coefficient | 0.298 | -0.077 | -0.281 | 0.028 |
| Sig. | 0.001 | 0.121 | 0.001 | 0.571 | |
| Jaw + Neck | Coefficient | -0.310 | 0.647 | -0.137 | -0.017 |
| Sig. | 0.001 | 0.001 | 0.006 | 0.725 | |
| Retrosternal + Jaw + Neck | Coefficient | -0.496 | -0.129 | 0.713 | -0.034 |
| Sig. | 0.001 | 0.009 | 0.001 | 0.497 | |
| Retrosternal + Backache | Coefficient | -0.496 | -0.139 | 0.719 | -0.032 |
| Sig. | 0.001 | 0.005 | 0.001 | 0.518 | |
| Backache + Shoulder | Coefficient | -0.289 | 0.585 | -0.112 | -0.017 |
| Sig. | 0.001 | 0.001 | 0.023 | 0.725 |
Discussion:
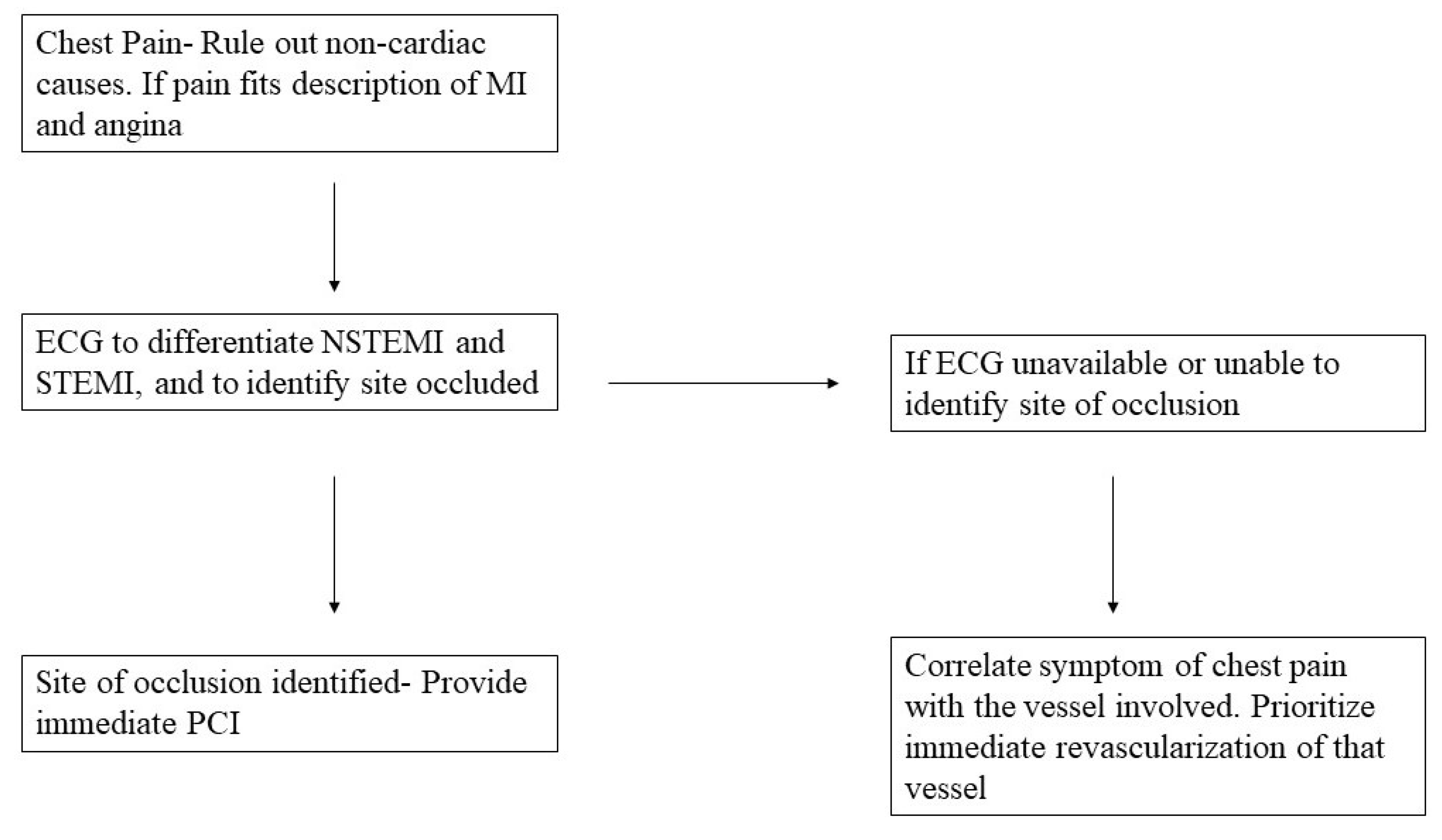
Conclusion:
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hickam, DH. Chest Pain or Discomfort. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations [Internet]. 3rd ed. Boston: Butterworths; 1990 [cited 2024 Mar 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK416/.
- Hickam, DH. Chest Pain or Discomfort. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations [Internet]. 3rd ed. Boston: Butterworths; 1990 [cited 2024 Mar 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK416/.
- Akbar H, Foth C, Kahloon RA, Mountfort S. Acute ST-Elevation Myocardial Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Mar 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK532281/.
- Fu LW, Longhurst JC. Regulation of Cardiac Afferent Excitability in Ischemia. In: Canning BJ, Spina D, editors. Sensory Nerves [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009 [cited 2024 Mar 15]. p. 185–225. (Handbook of Experimental Pharmacology; vol. 194). Available from: http://link.springer.com/10.1007/978-3-540-79090-7_6.
- Vance WH, Bowker RC. Spinal origins of cardiac afferents from the region of the left anterior descending artery. Brain Research. 1983 Jan;258(1):96–100.
- Crea F, Biasucci LM, Buffon A, Liuzzo G, Monaco C, Caligiuri G, et al. Role of Inflammation in the Pathogenesis of Unstable Coronary Artery Disease. The American Journal of Cardiology. 1997 Sep;80(5):10E-16E.
- Flores NA, Sheridan DJ. The pathophysiological role of platelets during myocardial ischaemia. Cardiovascular Research. 1994 Mar 1;28(3):295–302.
- Kohchi K, Takebayashi S, Hiroki T, Nobuyoshi M. Significance of adventitial inflammation of the coronary artery in patients with unstable angina: results at autopsy. Circulation. 1985 Apr;71(4):709–16.
- Van Der Wal AC, Becker AE, Van Der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994 Jan;89(1):36–44.
- Gaspardone A, Crea F, Tomai F, Iamele M, Crossman DC, Pappagallo M, et al. Substance P potentiates the algogenic effects of intraarterial infusion of adenosine. Journal of the American College of Cardiology. 1994 Aug;24(2):477–82.
- Hirsh PD, Campbell WB, Willerson JT, Hillis LD. Prostaglandins and ischemic heart disease. The American Journal of Medicine. 1981 Dec;71(6):1009–26.
- White JC. Cardiac Pain: Anatomic Pathways and Physiologic Mechanisms. Circulation. 1957 Oct;16(4):644–55.
- Lichstein E, Breitbart S, Shani J, Hollander G, Greengart A. Relationship between location of chest pain and site of coronary artery occlusion. American Heart Journal. 1988 Mar;115(3):564–8.
- de Silva RA, Bachman WR. Cardiac consultation in patients with neuropsychiatric problems. Cardiol Clin. 1995 May;13(2):225–39.
- Bösner S, Haasenritter J, Hani MA, Keller H, Sönnichsen AC, Karatolios K, et al. Gender differences in presentation and diagnosis of chest pain in primary care. BMC Fam Pract. 2009 Dec;10(1):79.
- Swap CJ. Value and Limitations of Chest Pain History in the Evaluation of Patients With Suspected Acute Coronary Syndromes. JAMA. 2005 Nov 23;294(20):2623.
- Leong K, Jones RH. Influence of the location of left anterior descending coronary artery stenosis on left ventricular function during exercise. Circulation. 1982 Jan;65(1):109–14.
- From AM, Best PJM, Lennon RJ, Rihal CS, Prasad A. Acute Myocardial Infarction Due to Left Circumflex Artery Occlusion and Significance of ST-Segment Elevation. The American Journal of Cardiology. 2010 Oct;106(8):1081–5.
- Karwowski J, Gierlotka M, Gąsior M, Poloński L, Ciszewski J, Bęćkowski M, et al. Relationship between infarct artery location, acute total coronary occlusion, and mortality in STEMI and NSTEMI patients. Polish Archives of Internal Medicine [Internet]. 2017 May 5 [cited 2024 Mar 15]; Available from: http://pamw.pl/en/node/4018.
- Basit H, Malik A, Huecker MR. Non–ST-Segment Elevation Myocardial Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Mar 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK513228/.
- Femia G, French JK, Juergens C, Leung D, Lo S. Right ventricular myocardial infarction: pathophysiology, clinical implications and management. Reviews in Cardiovascular Medicine. 2021;22(4):1229.
- Stähli BE, Varbella F, Linke A, Schwarz B, Felix SB, Seiffert M, et al. Timing of Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2023 Oct 12;389(15):1368–79.
- Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, et al. Door-to-Balloon Time and Mortality among Patients Undergoing Primary PCI. N Engl J Med. 2013 Sep 5;369(10):901–9.
- Ahmed SS, Gupta RC, Brancato RR. Significance of nausea and vomiting during acute myocardial infarction. Am Heart J. 1978 May;95(5):671–2.
- Aguiar Rosa S, Timóteo AT, Ferreira L, Carvalho R, Oliveira M, Cunha P, et al. Complete atrioventricular block in acute coronary syndrome: prevalence, characterisation and implication on outcome. Eur Heart J Acute Cardiovasc Care. 2018 Apr;7(3):218–23.
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. European Heart Journal. 2018 Jan 7;39(2):119–77.
- Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, et al. Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation [Internet]. 2015 Nov 3 [cited 2024 May 22];132(18_suppl_2). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000252.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





