Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Intervention and Monitoring
2.3. Selection Criteria Inclusion/Exclusion Criteria
2.4. Clinical Parameters and Biomarkers of Gingival Crevicular Fluid (GCF)
2.5. Safety Analysis
2.6. Data Set Characterization
2.7. Statistical Analysis
3. Results
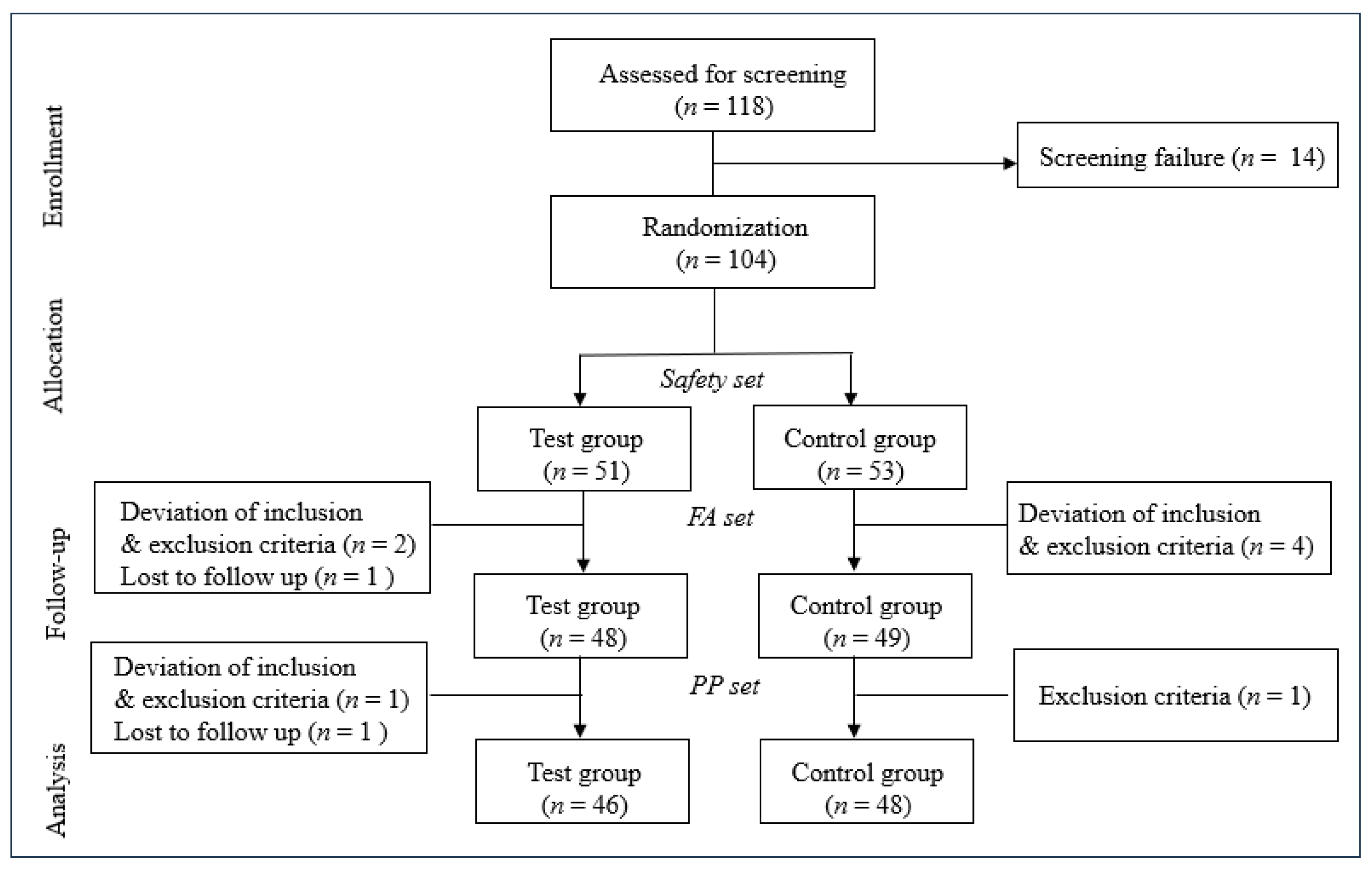
3.1. Participant Flow and Baseline Characteristics
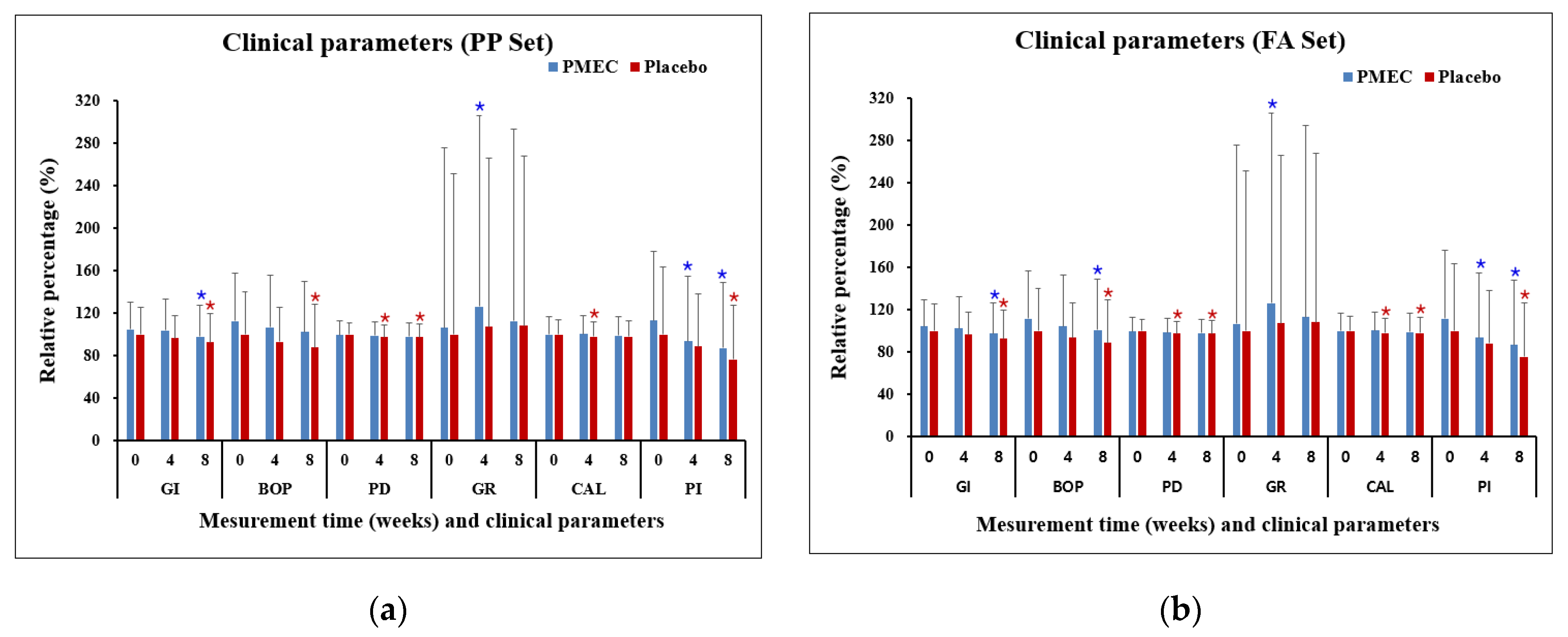
3.2. Clinical Parameters
3.3. Biomarkers of Gingival Crevicular Fluid (GCF)
3.4. Safety Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.K.; Reddy, N.R.; Babu, M.; Kumar, P.M.; Reddy, V.S.; Chavan, C.V. Estimation of prostaglandin E2 levels in gingival crevicular fluid in periodontal health, disease and after treatment. Contemp Clin. Dent 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- He, W.; You, M.; Wan, W.; Xu, F.; Li, F.; Li, A. Point-of-Care Periodontitis Testing: Biomarkers, Current Technologies, and Perspectives. Trends Biotechnol. 2018, 36, 1127–1144. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Tabeta, K.; Sugita, N.; Yoshie, H. Profiling Biomarkers in Gingival Crevicular Fluid Using Multiplex Bead Immunoassay. Arch. Oral Biol. 2013, 58, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing. An indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Newbrun, E. Indices to measure gingival bleeding. J. Periodontol. 1996, 67, 555–561. [Google Scholar] [CrossRef]
- Buduneli, N.; Kinane, D.F. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J. clin. periodontol. 2011, 38 (Suppl. 11), 85–105. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules. 2021, 26, 1208. [Google Scholar] [CrossRef]
- Delima, A.J.; Van Dyke, T.E. Origin and function of the cellular components in gingival crevice fluid. Periodontol. 2000. 2003, 31, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Baeza, M.; Garrido, M.; Hernández-Ríos, P.; Dezerega, A.; García-Sesnich, J.; Strauss, F.; Aitken, J. P.; Lesaffre, E.; Vanbelle, S.; Gamonal, J.; Brignardello-Petersen, R.; Tervahartiala, T.; Sorsa, T.; Hernández, M. Diagnostic accuracy for apical and chronic periodontitis biomarkers in gingival crevicular fluid: an exploratory study. J. clin. periodontol. 2016, 43, 34–45. [Google Scholar] [CrossRef]
- Offenbacher, S.; Heasman, P.A.; Collins, J.G. Modulation of Host PGE2 Secretion as a Determinant of Periodontal Disease Expression. J. Periodontol. 1993, 64, 432–444. [Google Scholar] [CrossRef]
- Kinney, J.S.; Morelli, T.; Oh, M.; Braun, T.M.; Ramseier, C.A.; Sugai, J.V.; Giannobile, W.V. Crevicular fluid biomarkers and periodontal disease progression. J. Clin. Periodontol. 2014, 41, 113–120. [Google Scholar] [CrossRef]
- Hernández, M.; Baeza, M.; Räisänen, I.T.; Contreras, J.; Tervahartiala, T.; Chaparro, A.; Sorsa, T.; Hernández-Ríos, P. Active MMP-8 Quantitative Test as an Adjunctive Tool for Early Diagnosis of Periodontitis. Diagnostics 2021, 11(8), 1503. [Google Scholar] [CrossRef]
- Champagne, C.M.; Buchanan, W.; Reddy, M.S.; Preisser, J.S.; Beck, J.D.; Offenbacher, S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontol. 2000. 2003, 31, 167–180. [Google Scholar] [CrossRef]
- Loos, B.G.; Tjoa, S. Host-derived diagnostic markers for periodontitis: do they exist in gingival crevice fluid? Periodontol. 2000. 2005, 39, 53–72. [Google Scholar] [CrossRef]
- Beck, J.D.; Offenbacher, S. Systemic Effects of Periodontitis: Epidemiology of Periodontal Disease and Cardiovascular Disease. J. Periodontol. 2005, 76, 2089–2100. [Google Scholar] [CrossRef]
- Mealey, B.L. Periodontal Disease and Diabetes: A Two-Way Street. J. Am. Dent. Assoc. 2006, 137 (Suppl.), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Saviuc, C.M.; Carmen Chifiriuc, M. Periodontitis and Periodontal Disease - Innovative Strategies for Reversing the Chronic Infectious and Inflammatory Condition by Natural Products. Curr. Pharm. Design. 2015, 22, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, S.P.; Casati, M.Z.; Ribeiro, F.V.; Corrêa, M.G.; Franck, F.C.; Benatti, B.B.; Cirano, F.R. Impact of Natural Curcumin on the Progression of Experimental Periodontitis in Diabetic Rats. J. Periodontal Res. 2020, 55, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yuanita, T.; Ristyawati, D.; Samadi, K. Cytoxicity test of NaOCl and Mangosteen (Garcinia Mangostin L.) peel extract used as an irrigation solution in human periodontal ligament fibroblast cells (HPdLFc). Dent. J 2018, 51, 133–137. [Google Scholar] [CrossRef]
- Lee, H.N.; Jang, H.Y.; Kim, H.J.; Moon, H.K.; Kim, J.H. Antitumor and apoptosis-inducing effects of α-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int. J. Mol. Med. 2016, 37, 939–948. [Google Scholar] [CrossRef]
- Pietta, P.G.; Gardana, C.; Pietta, A.M. Analytical Methods for Quality Control of Propolis. Fitoterapia. 2002, 73, S7–S20. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic Acid Phenethyl Ester (CAPE), Derived from a Honeybee Product Propolis, Exhibits a Diversity of Anti-Tumor Effects in Pre-Clinical Models of Human Breast Cancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar] [CrossRef]
- Campos, J.F.; Dos Santos, U.P.; da Rocha, P.dosS.; Damião, M.J.; Balestieri, J.B.; Cardoso, C.A.; Paredes-Gamero, E.J.; Estevinho, L.M.; de Picoli Souza, K.; Dos Santos, E.L. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless BeeTetragonisca fiebrigi(Jataí). Evid. Based Complement. Altern. Med. 2015, 1–11. [CrossRef]
- Lim, Y.K.; Cho, E.J.; Jeong, H.J.; Choi, D.S.; Yoo, M.A.; Lee, M.G.; Lee, H.S. Anti-inflammatory and in vitro bone formation effects of Garcinia mangostana L. and propolis extracts. Food Sci. Biotechnol. 2020, 29, 539–548. [Google Scholar] [CrossRef]
- Sung, S.J. Effect of Garcinia Mangostana L. and Propolis Extracts on the Inhibition of Inflammation and Alveolar Bone Loss in Ligature-Induced Periodontitis in Rats. Int. J. Oral Biol. 2019, 44, 55–61. [Google Scholar] [CrossRef]
- Lee, K.H.; Yoo, S.Y.; Kook, J.K.; Sung, S.J.; Lee, K.W.; Lim, Y.K.; Lee, D.S.; Yu, S.J. Inhibitory effect of mangosteen peel and propolis ethanol extracts on alveolar bone loss against increased treatment amount of Porphyromonas gingivalis lipopolysaccharide in rat. Korean J. Dent. Mater. 2021, 48, 71–78. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal Disease in Pregnancy I. Prevalence and Severity. Acta. Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced Plaque Formation by the Chloromethyl Analogue of Vitamin C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Romero-Castro, N.S.; Vázquez-Villamar, M.; Muñoz-Valle, J.F.; Reyes-Fernández, S.; Serna-Radilla, V.O.; García-Arellano, S.; Castro-Alarcón, N. Relationship Between TNF-α, MMP-8, and MMP-9 Levels in Gingival Crevicular Fluid and the Subgingival Microbiota in Periodontal Disease. Odontology. 2020, 108, 25–33. [Google Scholar] [CrossRef]
- Kuehl, F.A. Jr.; Egan, R.W. Prostaglandins, arachidonic acid, and inflammation. Science 1980, 210, 978–984. [Google Scholar] [CrossRef]
- Dziak, R. Biochemical and molecular mediators of bone metabolism. J. Periodontol. 1993, 64 (Suppl. 5), 407–415. [Google Scholar] [PubMed]
- Beklen, A.; Tüter, G.; Sorsa, T.; Hanemaaijer, R.; Virtanen, I.; Tervahartiala, T.; Konttinen, Y. T. Gingival tissue and crevicular fluid co-operation in adult periodontitis. J. dent. res. 2006, 85, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Syam, S.; Abdelwahab, S.I.; Thangavel, N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: an in silico, in vitro and in vivo approach. Food Funct. 2018, 9, 3860–3871. [Google Scholar] [CrossRef]
- Ali, K.M.; Saleh, Z.; Jalal, J. Effect of local propolis irrigation in experimental periodontitis in rats on inflammatory markers (IL-1β and TNF-α) and oxidative stress. Indian J. Dent. Res. 2020, 31, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Eghbali Zarch, R.; Askari, M.; Boostani, H.; Mirzaii-Dizgah, I. Effect of propolis extract on clinical parameters and salivary level of matrix metalloproteinase 8 in periodontitis patients: A randomized controlled clinical trial. J. Adv. Periodontol. Implant Dent. 2021, 13, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N. Biomarkers in Periodontal Health and Disease: Rationale, Benefits, and Future Directions. Springer; Cham, Switzerland: Biomarkers in periodontal health and disease: Rationale, benefits, and future directions. 2019. Volume 90.
- Marks, R.G.; Magnusson, I.; Taylor, M.; Clouser, B.; Maruniak, J.; Clark, W.B. Evaluation of reliability and reproducibility of dental indices. J. Clin. Periodontol. 1993, 20(1), 54–58. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Manual periodontal probing in supportive periodontal treatment. Periodontol. 2000. 1996, 12, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Osborn, J.B.; Stoltenberg, J.L.; Huso, B.A.; Aeppli, D.M.; Pihlstrom, B.L.; Adams, D.F. Comparison of Measurement Variability in Subjects with Moderate Periodontitis Using a Conventional and Constant Force Periodontal Probe. J. Periodontol. 1992, 63, 283–289. [Google Scholar] [CrossRef]
- Nazar Majeed, Z.; Philip, K.; Alabsi, A.M.; Pushparajan, S.; Swaminathan, D. Identification of gingival crevicular fluid sampling, analytical methods, and oral biomarkers for the diagnosis and monitoring of periodontal diseases: A systematic review. Dis. Markers 2016, 1804727. [Google Scholar] [CrossRef]
- Al-Majid, A.; Alassiri, S.; Rathnayake, N.; Tervahartiala, T.; Gieselmann, D.R.; Sorsa, T. Matrix Metalloproteinase-8 as an Inflammatory and Prevention Biomarker in Periodontal and Peri-Implant Diseases. Int. J. Dent. 2018, 2018, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kardeşler, L.; Buduneli, N.; Biyikoğlu, B.; Cetinkalp, S.; Kütükçüler, N. Gingival crevicular fluid PGE2, IL-1beta, t-PA, PAI-2 levels in type 2 diabetes and relationship with periodontal disease. Clin. Biochem. 2008, 41, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Jain, R.; Anand, S.C. Levels of Gingival Crevicular Metalloproteinases-8 and -9 in Periodontitis. Saudi Dent. J. 2010, 22, 129–131. [Google Scholar] [CrossRef] [PubMed]

| Test | Placebo | |||
| Raw Material | Compounding Ratio (%) |
Content (mg) |
Compounding Ratio (%) |
Content (mg) |
| Propolis Mangosteen Extract Complex (PMEC) | 41.28 | 194.00 | - | - |
| Lactose Powder | 30.00 | 141.00 | 62.70 | 294.69 |
| Microcrystalline cellulose | 24.22 | 113.85 | 34.27 | 161.07 |
| Sucrose esters of fatty acids | 2.00 | 9.10 | - | - |
| Caramel color | - | - | 2.00 | 9.40 |
| Magnesium stearate | 1.50 | 7.05 | 1.00 | 4.70 |
| silicon dioxide | 1.00 | 4.70 | - | - |
| Food blue NO.1 | - | - | 0.03 | 0.14 |
| Total | 100 | 470 | 100 | 470 |
| Variables | Test Group (n = 46) |
Control Group (n = 48) |
P-Value |
|---|---|---|---|
| Age (years), mean ± SD | 43.17±11.01 | 42.02±11.40 | 0.6194(T) |
| Gender, n (%) | |||
| Male | 16 (34.78) | 16 (33.33) | 0.8822(C) |
| Female | 30 (65.22) | 32 (66.67) | |
| Smoking status, n (%) | |||
| No | 46 (100.00) | 48 (100.00) | - |
| Yes | 0 (0.00) | 0 (0.00) | |
| Physical activity, n (%) | |||
| None | 8 (17.39) | 14 (29.17) | 0.5364(C) |
| 1-2 times/week | 12 (26.09) | 10 (20.83) | |
| 3 times/week | 14 (30.43) | 14 (29.17) | |
| 4-5 times/week | 5 (10.87) | 2 (4.17) | |
| 7 times/week | 7 (15.22) | 8 (16.67) | |
| Drinking status, n (%) | |||
| No | 16 (34.78) | 16 (33.33) | 0.8822(C) |
| Yes | 30 (65.22) | 32 (66.67) | |
| Weight (kg) | |||
| Mean±SD | 64.09±11.82 | 65.79±12.88 | 0.4984(W) |
| Median | 61.70 | 63.65 | |
| Min, Max | 48.00, 97.60 | 46.10, 101.30 | |
| Height (cm) | |||
| Mean±SD | 165.14±7.86 | 164.64±7.97 | 0.8146(W) |
| Median | 164.85 | 163.95 | |
| Min, Max | 154.00, 178.00 | 149.00, 183.00 |
| Clinical parameters |
Measurement time | Test | Control | Control |
| GI | Baseline | 1.70±0.41 | 1.62±0.42 | 0.1780 (W) |
| 4 weeks | 1.68±0.48 | 1.57±0.34 | 0.0671 (W) | |
| p-value [1] | 0.6560 | 0.1895 | ||
| 8 weeks | 1.59±0.47 | 1.50±0.44 | 0.1512 (W) | |
| p-value [1] | 0.0267 | 0.0027 | ||
| BOP (%) | Baseline | 47.26±18.90 | 41.93±16.69 | 0.1502 (T) |
| 4 weeks | 44.70±20.43 | 39.03±13.60 | 0.1319 (W) | |
| p-value [1] | 0.2396 | 0.0772 | ||
| 8 weeks | 42.98±20.02 | 36.88±16.95 | 0.1140 (T) | |
| p-value [1] | 0.0577 | 0.0019 | ||
| PD | Baseline | 2.58±0.35 | 2.59±0.28 | 0.6497 (W) |
| 4 weeks | 2.56±0.34 | 2.53±0.31 | 0.7477 (W) | |
| p-value [1] | 0.4485 | 0.0010 | ||
| 8 weeks | 2.54±0.33 | 2.52±0.32 | 0.9879 (W) | |
| p-value [1] | 0.1115 | 0.0047 | ||
| GR | Baseline | 0.18±0.28 | 0.17±0.25 | 0.9262 (W) |
| 4 weeks | 0.21±0.30 | 0.18±0.27 | 0.5198 (W) | |
| p-value [1] | 0.0026 | 0.3165 | ||
| 8 weeks | 0.19±0.30 | 0.18±0.27 | 0.7699 (W) | |
| p-value [1] | 0.4090 | 0.2733 | ||
| CAL | Baseline | 2.76±0.46 | 2.76±0.37 | 0.7477 (W) |
| 4 weeks | 2.77±0.47 | 2.71±0.38 | 0.6884 (W) | |
| p-value [1] | 0.5222 | 0.0212 | ||
| 8 weeks | 2.73±0.49 | 2.71±0.42 | 0.9608 (W) | |
| p-value [1] | 0.2707 | 0.0628 | ||
| PI | Baseline | 0.60±0.34 | 0.53±0.34 | 0.2438(W) |
| 4 weeks | 0.50±0.33 | 0.47±0.26 | 0.7990(W) | |
| p-value [1] | 0.0023 | 0.1564 | ||
| 8 weeks | 0.46±0.33 | 0.41±0.27 | 0.4641 (W) | |
| p-value [1] | 0.0003 | 0.0036 |
| Clinical parameters |
Measurement time | Test | Control | p-value [2] |
| GI | Baseline | 1.69±0.41 | 1.62±0.41 | 0.2145 (W) |
| 4 weeks | 1.66±0.49 | 1.57±0.33 | 0.0976 (W) | |
| p-value [1] | 0.4961 | 0.1752 | ||
| 8 weeks | 1.58±0.47 | 1.51±0.44 | 0.1848 (W) | |
| p-value [1] | 0.0164 | 0.0020 | ||
| BOP (%) | Baseline | 46.83±18.71 | 41.87±16.52 | 0.1696 (T) |
| 4 weeks | 43.76±20.63 | 39.31±13.60 | 0.2305 (W) | |
| p-value [1] | 0.1495 | 0.1185 | ||
| 8 weeks | 42.23±20.00 | 37.26±16.99 | 0.0874 (W) | |
| p-value [1] | 0.0351 | 0.0051 | ||
| PD | Baseline | 2.59±0.35 | 2.60±0.29 | 0.7127 (W) |
| 4 weeks | 2.57±0.33 | 2.54±0.32 | 0.7208 (W) | |
| p-value [1] | 0.3886 | 0.0009 | ||
| 8 weeks | 2.55±0.33 | 2.54±0.32 | 0.9453 (W) | |
| p-value [1] | 0.0919 | 0.0031 | ||
| GR | Baseline | 0.18±0.28 | 0.17±0.25 | 0.9971 (W) |
| 4 weeks | 0.21±0.30 | 0.18±0.26 | 0.4564 (W) | |
| p-value [1] | 0.0026 | 0.3164 | ||
| 8 weeks | 0.19±0.30 | 0.18±0.27 | 0.8488 (W) | |
| p-value [1] | 0.4088 | 0.2732 | ||
| CAL | Baseline | 2.77±0.45 | 2.77±0.37 | 0.7866 (W) |
| 4 weeks | 2.78±0.46 | 2.72±0.38 | 0.6861 (W) | |
| p-value [1] | 0.5885 | 0.0198 | ||
| 8 weeks | 2.74±0.48 | 2.71±0.42 | 0.9798 (W) | |
| p-value [1] | 0.2341 | 0.0483 | ||
| PI | Baseline | 0.60±0.34 | 0.54±0.34 | 0.2773 (W) |
| 4 weeks | 0.50±0.32 | 0.48±0.26 | 0.7774 (W) | |
| p-value [1] | 0.0024 | 0.1348 | ||
| 8 weeks | 0.47±0.33 | 0.41±0.27 | 0.4452 (W) | |
| p-value [1] | 0.0002 | 0.0021 |
| Biomarkers | measurement time | Test | Control | p-value [2] |
| PGE2 (ng/mL) |
Baseline | 602.38±178.15 | 526.36±172.55 | 0.0244 (W) |
| 4 weeks | 455.80±113.81 | 518.74±141.58 | 0.0202 (W) | |
| p-value [1] | <.0001 | 0.7403 | ||
| 8 weeks | 345.43±69.02 | 576.08±186.17 | <.0001 (T) | |
| p-value [1] | <.0001 | 0.0791 | ||
| IL-1ß (pg/mL) |
Baseline | 135.45±93.70 | 114.86±65.96 | 0.4075 (W) |
| 4 weeks | 102.83±62.54 | 124.84±71.32 | 0.1096 (W) | |
| p-value [1] | 0.0042 | 0.2406 | ||
| 8 weeks | 70.89±65.46 | 141.95±78.02 | <.0001 (W) | |
| p-value [1] | <.0001 | 0.0062 | ||
| MMP-8 (ng/mL) |
Baseline | 36.21±18.33 | 32.74±16.13 | 0.4205 (W) |
| 4 weeks | 28.30±14.29 | 34.36±15.41 | 0.0511 (T) | |
| p-value [1] | <.0001 | 0.3191 | ||
| 8 weeks | 21.76±14.65 | 36.76±16.15 | <.0001 (W) | |
| p-value [1] | <.0001 | 0.0390 | ||
| MMP-9 (ng/mL) |
Baseline | 65.19±25.20 | 60.51±19.81 | 0.3180 (T) |
| 4 weeks | 49.47±19.97 | 63.55±20.47 | 0.0011 (T) | |
| p-value [1] | <.0001 | 0.2065 | ||
| 8 weeks | 35.55±20.79 | 66.46±23.25 | <.0001 (W) | |
| p-value [1] | <.0001 | 0.0701 |
| Biomarkers | measurement time | Test | Control | p-value [2] |
| PGE2 (ng/mL) |
Baseline | 590.38±183.91 | 523.20±172.16 | 0.0492 (W) |
| 4 weeks | 456.83±111.49 | 517.22±140.49 | 0.0209 (W) | |
| p-value [1] | <.0001 | 0.7910 | ||
| 8 weeks | 353.76±80.08 | 575.77±184.23 | <.0001 (T) | |
| p-value [1] | <.0001 | 0.0600 | ||
| IL-1ß (pg/mL) |
Baseline | 131.99±93.75 | 113.65±65.81 | 0.4728 (W) |
| 4 weeks | 101.19±62.07 | 124.06±70.79 | 0.0866 (W) | |
| p-value [1] | 0.005 | 0.2125 | ||
| 8 weeks | 70.26±64.66 | 141.07±77.45 | <.0001 (W) | |
| p-value [1] | <.0001 | 0.0047 | ||
| MMP-8 (ng/mL) |
Baseline | 35.51±18.58 | 32.55±16.02 | 0.4019 (T) |
| 4 weeks | 27.90±14.30 | 34.31±15.25 | 0.0354 (T) | |
| p-value [1] | <.0001 | 0.2716 | ||
| 8 weeks | 21.69±14.47 | 36.78±15.98 | <.0001 (W) | |
| p-value [1] | <.0001 | 0.0278 | ||
| MMP-9 (ng/mL) |
Baseline | 64.67±26.64 | 59.71±20.38 | 0.3045 (T) |
| 4 weeks | 49.49±20.78 | 62.79±20.94 | 0.0023 (T) | |
| p-value [1] | <.0001 | 0.1916 | ||
| 8 weeks | 36.46±21.54 | 65.85±23.41 | <.0001 (W) | |
| p-value [1] | <.0001 | 0.0573 |
|
Test n=51 |
Control n=53 |
p-value [2] | ||||
| n | Mean±SD | n | Mean±SD | |||
| WBC (103/μL) |
Baseline | 51 | 6.09±1.42 | 53 | 6.07±1.15 | 0.9344[T] |
| 8 weeks | 47 | 5.91±1.42 | 49 | 6.17±1.58 | 0.3963[T] | |
| p-value [1] | 0.2759 | 0.9625 | ||||
| RBC (106/μL) |
Baseline | 51 | 4.48±0.36 | 53 | 4.48±0.39 | 0.9940[T] |
| 8 weeks | 47 | 4.45±0.37 | 49 | 4.51±0.39 | 0.2821[T] | |
| p-value [1] | 0.2801 | 0.6985 | ||||
| Hb (g/dL) |
Baseline | 51 | 13.57±1.11 | 53 | 13.52±1.22 | 0.8289[T] |
| 8 weeks | 47 | 13.45±1.25 | 49 | 13.61±1.30 | 0.2633[W] | |
| p-value [1] | 0.2572 | 0.7399 | ||||
| Hct (%) |
Baseline | 51 | 40.77±3.29 | 53 | 40.64±3.46 | 0.8391[T] |
| 8 weeks | 47 | 40.41±3.50 | 49 | 40.72±3.50 | 0.5704[T] | |
| p-value [1] | 0.3200 | 0.7725 | ||||
| Platelet (103/μL) |
Baseline | 51 | 250.53±45.59 | 53 | 253.45±38.99 | 0.5324[W] |
| 8 weeks | 47 | 241.49±45.37 | 49 | 249.22±40.50 | 0.9633[T] | |
| p-value [1] | 0.0942 | 0.1531 | ||||
| ALT (GPT) (IU/L) |
Baseline | 51 | 17.80±9.36 | 53 | 19.74±12.93 | 0.6463[W] |
| 8 weeks | 47 | 17.85±9.91 | 49 | 19.24±11.61 | 0.9384[W] | |
| p-value [1] | 0.7919 | 0.5569 | ||||
| BUN (mg/dL) |
Baseline | 51 | 12.99±3.62 | 53 | 13.09±3.55 | 0.8785[W] |
| 8 weeks | 47 | 13.09±3.70 | 49 | 12.58±3.48 | 0.4591[T] | |
| p-value [1] | 0.7682 | 0.4535 | ||||
| Creatinine (mg/dL) |
Baseline | 51 | 0.78±0.17 | 53 | 0.77±0.16 | 0.6303[W] |
| 8 weeks | 47 | 0.75±0.15 | 49 | 0.75±0.15 | 0.5729[T] | |
| p-value [1] | 0.0939 | 0.1186 | ||||
| Ca (mg/dL) |
Baseline | 51 | 9.46±0.34 | 53 | 9.52±0.37 | 0.3455[T] |
| 8 weeks | 47 | 9.47±0.37 | 49 | 9.50±0.30 | 0.6974[T] | |
| p-value [1] | 0.9454 | 0.6203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





