1. Introduction
The energy sector has been facing substantial challenges related to sustainable economic development and global climate change, which require the transition from the use of fossil fuels to renewable energy sources. Therefore, the oil and gas sector is expected to be the most affected in the coming decades, undergoing important structural changes, as a result of the end of the activities of many production systems. This scenario requires operators to plan platform decommissioning using the safest and suitable approaches, at the lowest cost possible [
1,
2,
3].
Decommissioning involve many stages, and the most expensive one is plug & abandonment (P&A) of wells, which corresponds to around 40 to 60% of total costs [
4,
5]. This activity avoids environmental and operational catastrophes by preventing well leaks, which result in contamination or extensive damage to the marine ecosystem, soils, groundwater and methane emissions into the atmosphere [
6].
In P&A operations, well barrier elements are settled into the well to prevent the unintentional flow of formation fluids to the external environment and between well intervals, restoring isolation between the different permeable intervals [
7,
8,
9,
10]. The most common materials used as well barrier element are cement plugs [
11]. However, the integrity of this material for this application has often been questioned due to the possibility of crack formation, even during curing, as well as mechanical or chemical degradation, which can cause leaks in adjacent regions or to the surface [
6,
12,
13].
Several materials have been studied as an alternative or supplement to cement in plugging hydrocarbon reservoirs [
12,
14,
15,
16,
17]. Some recent research presented the most used materials for plugging wells, as well as the new technologies studied, highlighting, for example, the application of blast furnace slag, bentonite, low melting point metal alloys, geopolymers, thermites and sand pastes [
11,
17,
18,
19].
Liquid barriers, which consist of fluid columns exerting enough hydrostatic pressure to contain the fluids in the permeable intervals and prevent their flow, are considered in the requirements of international guidelines as alternatives to plugging petroleum wells [
9,
20]. This type of barrier presents environmental and operational advantages, since the fluid formulation may be low toxic and biodegradable, and its settlement inside the well can be rigless, through the stationary production unit or platform supply vessel, reducing operating time and costs. On the other hand, the use of liquid barriers fluids in well abandonment imposes new technical challenges, related to the lack of regulatory standards for its qualification and the need for strategies to demonstrate and guarantee its long-term stability, since its properties can be severely impacted due to the continuous exposure to downhole conditions.
In this sense, this study proposes the use of a water-based fluid as a liquid well barrier element for temporary abandonment, based on estimates of its lifespan and survival probability on downhole conditions acquired through accelerated life tests.
2. Materials and Methods
2.1. Fluid Formulation
This work uses a water-based fluid formulation, developed at the Leopoldo Américo Miguez de Mello Research and Development Center - CENPES/PDDP/FCE. The amount of each additive, presented in
Table 1, was used to prepare samples of 350mL with a density of 9.8ppg.
The fluid was prepared on the high-shear mixer Silverson L5MA. The defoamer, so-dium bicarbonate, CMC LV, sodium bentonite, sodium hydroxide and magnesium oxide were added to the volume of deionized water, keeping a homogenization time of 5 minutes between the addition of each additive. Then, the fluid remained at rest for 16 hours at room temperature, to adequately hydrate the products added. The preparation of the fluid was completed after pre-hydration, with the addition of brine, CaCO3 2-44 and glutaraldehyde.
2.2. Fluid Properties
To investigate the rheological behavior of the fluid, flow curves were obtained by a controlled shear rate method (0.1 to 1000s-1) at room temperature, on the Haake Mars 60 rheometer (Thermo Scientific) equipped with the parallel plates with a 35mm sandblaster surface (P35/TI/SB), using a gap of 1mm between the plates. The rheological parameters of the fluid, including yield stress (τ0), consistency index (K) and behavior index (n), were determined by adjusting the flow curves to the Herschel-Bulkley model, in the RheoWin Data Manager software.
The chemical stability of the fluid was verified by measuring the pH, using the pH meter Plus (LineLAB). Filtration control was evaluated at high pressure and high temperature (300 psi/ 150°F), using the HPHT filter press (FANN series 387).
2.3. Accelerated Life Tests
Accelerated life tests use high-stress levels associated with an acceleration variable to observe failures and determine potential failure modes for a product in short periods of time [
21,
22].
In this work, accelerated life tests were carried out with constant stress, using temperature as the acceleration variable to evaluate the life characteristics of the water-based fluid under downhole conditions. The fluid samples were exposed to high temperatures in ovens with air circulation, according to the stress levels (temperature) and exposure times presented in the sampling plan in
Table 2. For temperature exposure, the fluid sample was placed in a stainless steel cells with a Teflon liner, pressurized to 100psi with nitrogen gas.
After the inspection time had been completed, the samples were removed from the oven and tested to obtain the rheological parameters, filtrate volume and pH, following the procedures presented previously.
Fluid failure was recorded for samples that presented a reduction in the rheological parameter consistency index (K) and/or increases of at least 40% in the filtrate volume.
2.4. Lifespan Prediction
Based on the failure information extracted from accelerated tests, the accelerated life model parameters are estimated and these estimators are used to make statistical inferences for the lifespan distribution. Some basic assumptions considered for this analysis are presented as follows.
2.4.1. Basic Assumptions
For analysis of fluid failure data, T (temperature level) was assumed to be a random variable that follows a Weibull distribution, with probability density function (f
W), cumulative distribution function (F
W) and reliability function (R
W) given by:
Where β corresponds to the scale parameter, which determines the smoothness of the distribution curve, while η corresponds to the shape parameter, which affects the geometrical shape of the probability density curve. Since the failure mechanism must remain the same for any accelerated stress, it is assumed that the shape parameter (η) is constant, while the β parameter correlates with the stress level (temperature), following the inverse power law, which derives from Arrhenius' model. These relationships are specified in Equations 4 and 5.
2.4.2. Estimation Method
The parameters r, s
0 and s
1 were estimated using the maximum likelihood method, based on failure data from accelerated fluid life tests. This estimation was performed in a function implemented in the RStudio 4.1.3 software, using the EM (Expectation-Maximization) algorithm [
23], which consists of a widely used alternative when the model depends on unobserved variables. It is applicable to the data obtained from the accelerated life tests of the water-based fluid, since some tests used to verify its properties are destructive, obtaining an incomplete data set. In this case, only the success or failure of the tested sample at a specified time can be observed, instead of their actual time to failure. Such data is therefore of either left or right censored.
Once the estimated values of these parameters were obtained, represented by , , it was possible to estimate the model parameters ( and ).
To estimate the parameter β, the values assigned to the temperature considered the usual conditions of typical abandonment operations, as well as the temperature levels at which accelerated life tests were carried out: 60, 70, 80, 85, 90, 95, 100, 110, 120, 130, 140 and 150°C.
Once the model parameters were estimated, the function implemented in the R software also estimated the number of failures (
) expected at each temperature level for a given inspection time, which follows a binomial distribution with parameters K
i and
, as shown in Equations 6 and 7:
2.4.3. Model Validation
The goodness-of-fit test was used to validate the model, based on M statistic, which is given by the distance between the observed (
) and estimated (
) failure numbers for each condition of temperature and inspection time (Equation 8):
When the assumed distribution does not fit the observed data well, a large value for the M statistic should be expected. The descriptive level of the test is given by Equation 9:
In this work, a significance level of 5% was considered, therefore, the p-value needs to be greater than 0.05 so that the hypothesis that the lifespan data follows the Weibull model is not rejected, and, therefore, the model is considered adequate.
Once the model was validated, estimates of the average lifespan (
) and survival probability (
), estimated by the reliability function, for a given time t, were obtained from the estimated parameters. Also, the estimated lifespan (
) for a given level of reliability, p, that is, R(
)=p was obtained. The equations that describe the estimation of these parameters, relating them to the model parameters, are presented in Equations 10, 11 and 12:
where
is the gamma function, given by
To estimate the probability of survival using the reliability function (
), times of 6 months (183 days) to 3 years (1095 days) were considered, which corresponds to the maximum time that the well can remain in a condition of temporarily abandoned without monitoring, following Brazilian Legislation [
10]. Regarding the estimation of the percentile (
), reliabilities were established from 0.9 to 0.9999999.
3. Results and Discussion
3.1. Rheological Behavior
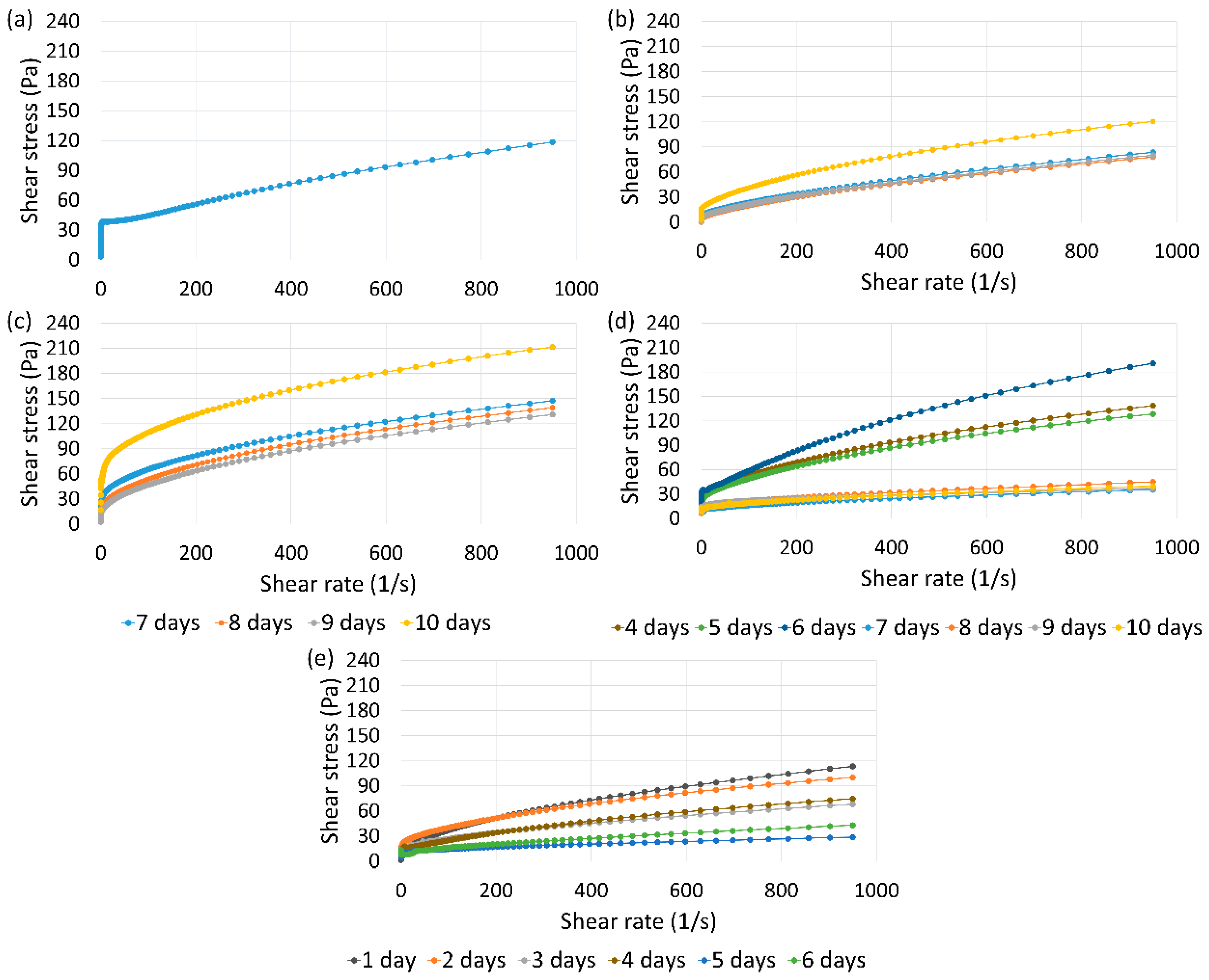
The graphs in
Figure 1 show the flow curves of the water-based fluid, considering the average shear stress values for the samples tested at the same time and temperature conditions.
As observed in the flow curves, the exposure to temperature impacts the rheological behavior of the fluid, resulting in more pronounced reductions in the flow profile for temperatures of 140 and 150°C, at longer exposure times.
The average values for the rheological parameters and the correlation index (R2), obtained from the adjustment of the flow curves to the Herschel Bulkley model, are presented in
Table 3.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
The average value of the consistency index at room temperature is 0.44mPa.s, so failure was recorded for samples exposed to temperature that showed values lower than that, observed only under the following conditions: 140°C/ 7 days (one sample), 140°C/8 days (one sample), 150°C/4 days (two samples), and 150°C/6 days (two samples). As the failure rate is low and only observed at temperatures of 140 and 150°C, it can be inferred that the fluid does not present a significant tendency to modify the rheological properties and, consequently, to the sedimentation of solids. This behavior suggests maintenance of the hydrostatic configuration of the fluid column inside the well during the abandonment operation.
Furthermore, there is a tendency for gradual increases in the average consistency index up to a temperature of 110°C, with a maximum value of 14.90mPa.s for the exposure time of 10 days. For 140 and 150°C, a significant decrease in these values is observed, reaching the minimum average value of 0.81mPa.s, for the condition of 150°C for 4 days.
This tendency may be related to the behavior assumed by the interactions between bentonite clay particles in the fluid with the increase in temperature. First, at room temperature, there is a predominance of dispersed particles. With the increase in temperature to 110°C, the clay particles tend to flocculate due to face-to-edge interactions, resulting in an increase in viscosity. Finally, the more pronounced increase in temperature, with the exposure of samples to 140 and 150°C, causes the aggregation of particles by face-to-face interactions, resulting in the presence of a greater volume of free water in the system and, consequently, reducing viscosity.
Additionally, the reduction in the fluid consistency index with increasing temperature may also be influenced by the degradation of CMC, which guarantees structural rigidity to the system at room temperature [
24], once its degradation in the presence of NaCl can be observed at temperatures close to 150°C [
25].
The behavior index (n) presents values below 1 in all conditions, indicating maintenance of pseudoplastic behavior. However, there is a reduction of this with exposure to temperature. At room temperature, the average value of this parameter is 0.78, while a variation from 0.36 (110°C/10 days) to 0.66 (150°C/4 days) is observed for samples exposed to temperature. A similar behavior was observed by Ahmad, Kamal and Al-Harthi (2018), when analyzing the effect of temperature on bentonite clay suspensions added with polymers [
26].
Analyzing the yield stress helps predict the appropriate operational procedure and tension needed for circulating the fluid. This is particularly important when the fluid needs replacement during the abandonment period or for re-entry into the well after temporary abandonment. The results for this rheological parameter also indicate a reduction with the exposure to temperature, however, as observed for the consistency index, after exposure to 110°C for 10 days, a high value of yield stress is observed, possibly related to the high degree of flocculation of clay particles in this condition. On the other hand, the reductions observed for the higher temperatures were attributed to the significant decrease in the reticulated structure due to high temperatures and high salinity, which impacts viscosity and yield strength [
27].
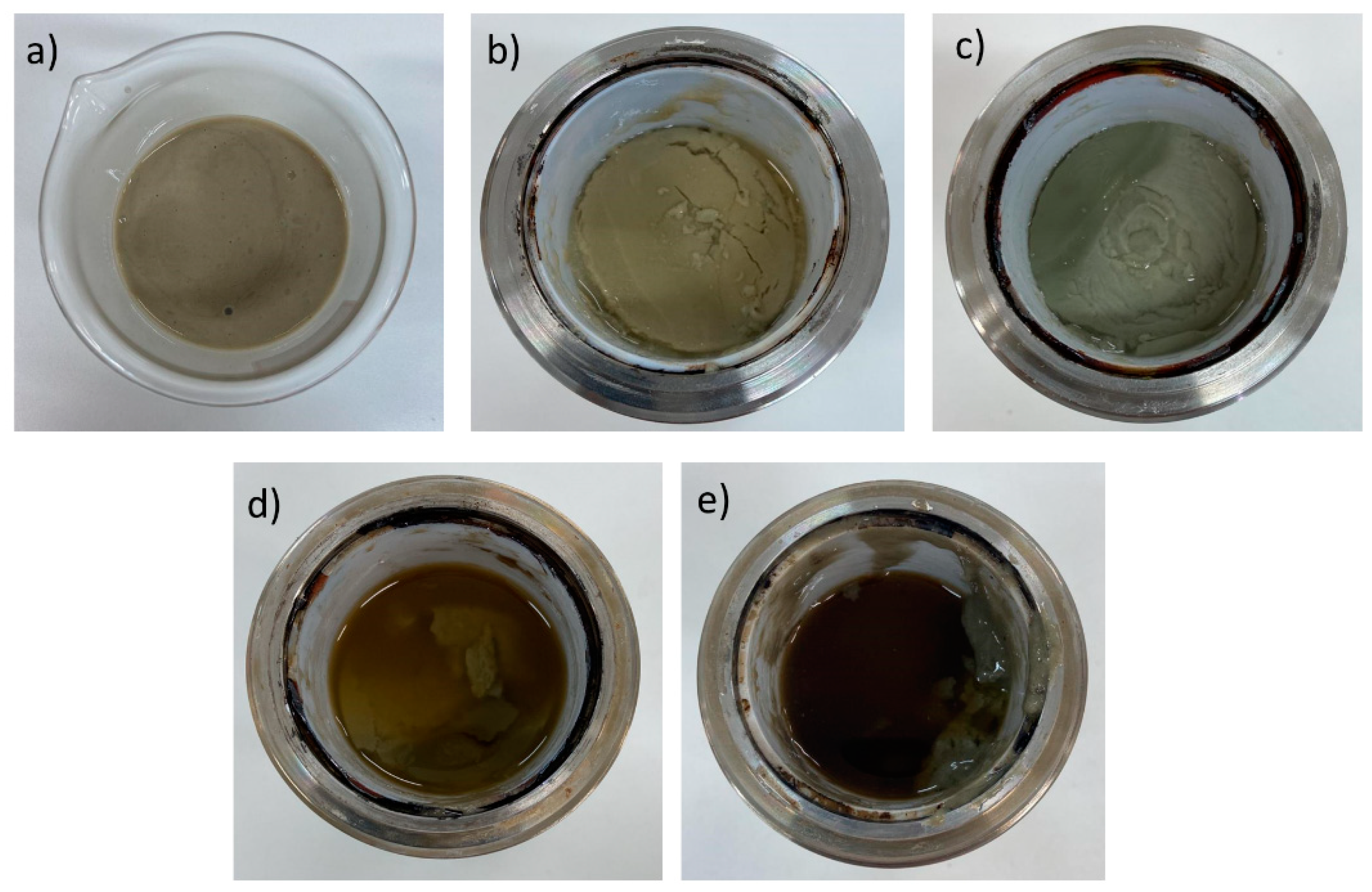
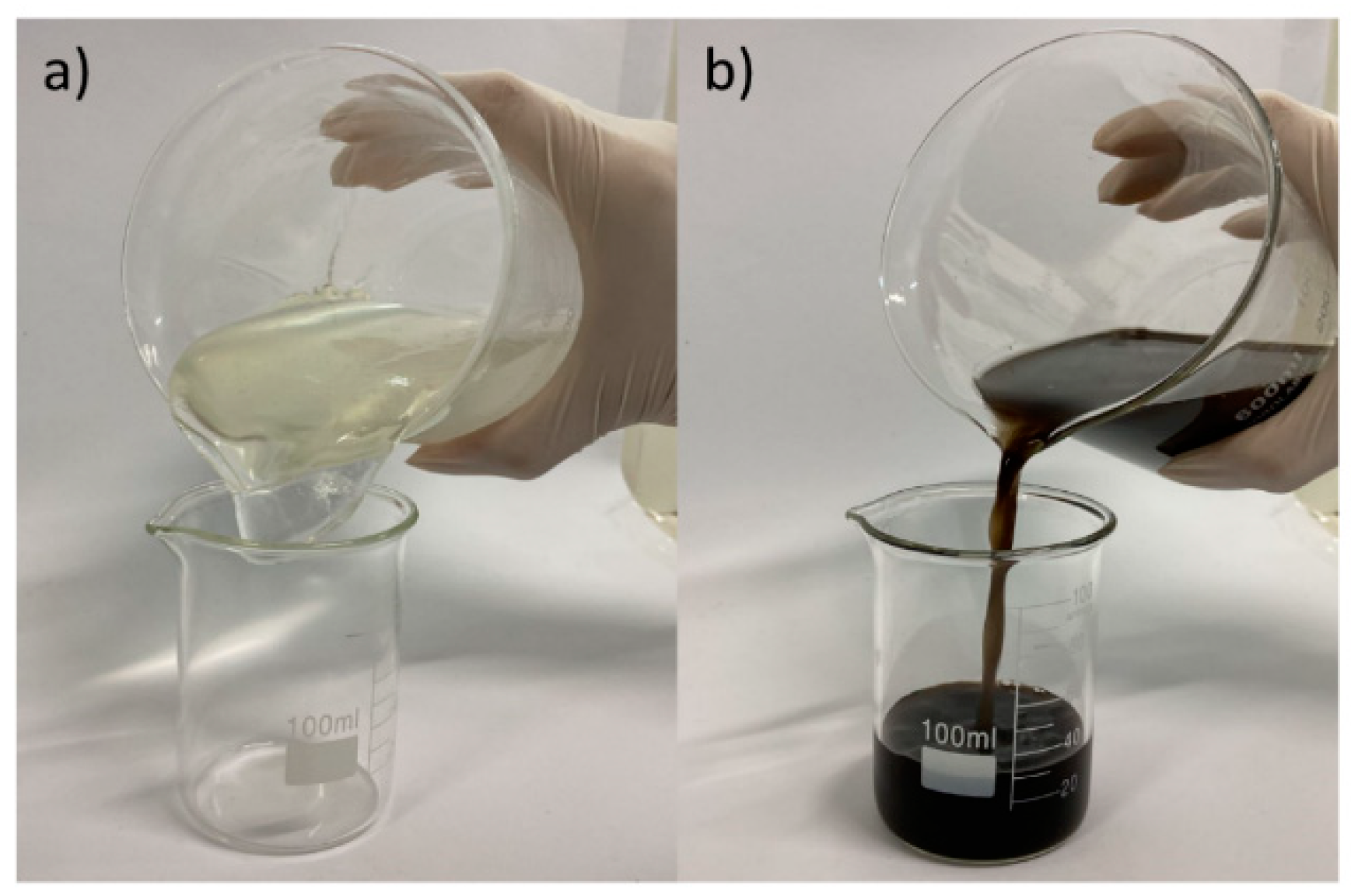
In addition to the variation in the rheological behavior, the effect of increasing temperature on fluid stability is also evidenced by the change in physical aspect, as shown in
Figure 2, in which a significant supernatant liquid phase is observed, for 140 and 150°C.
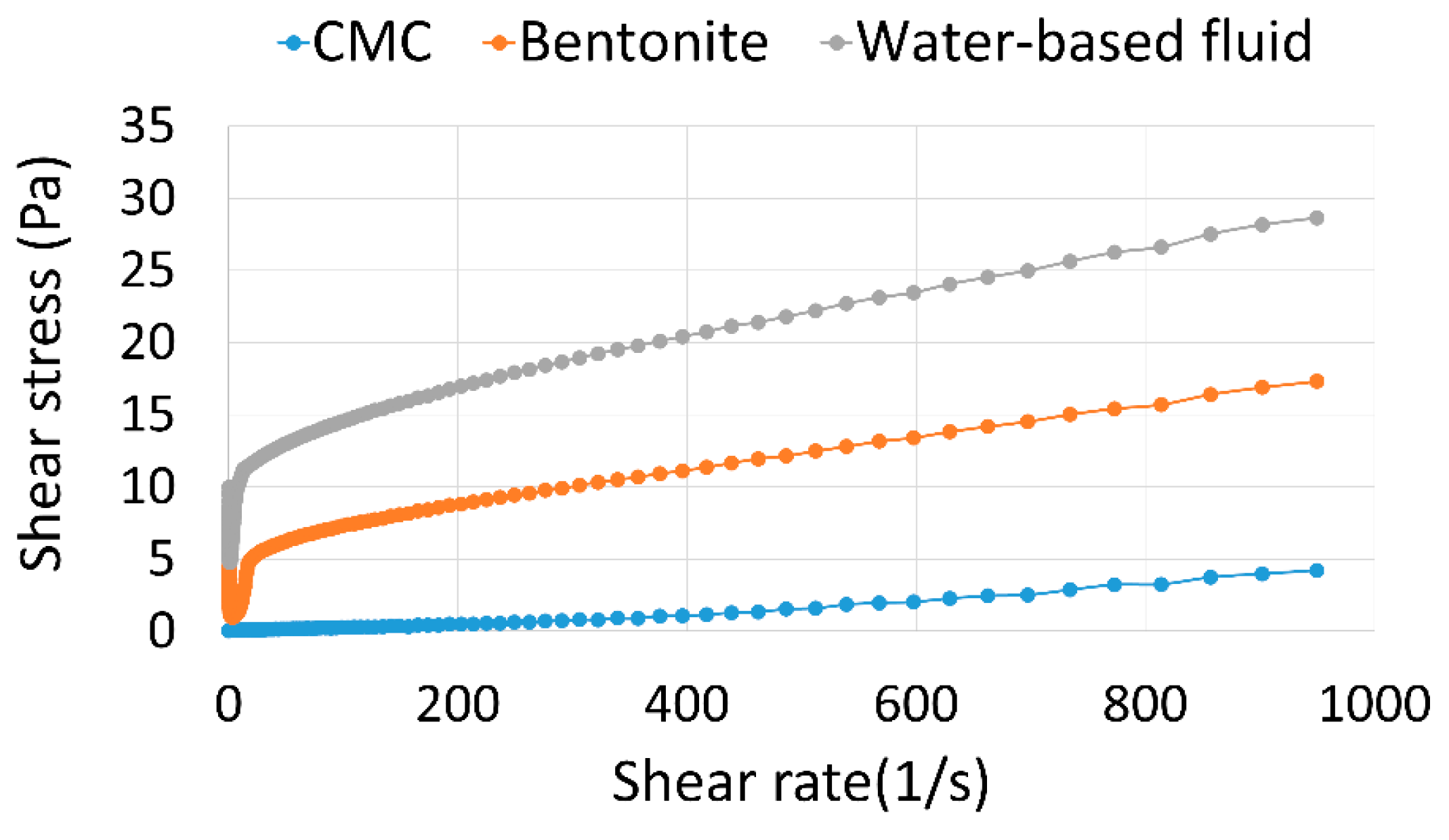
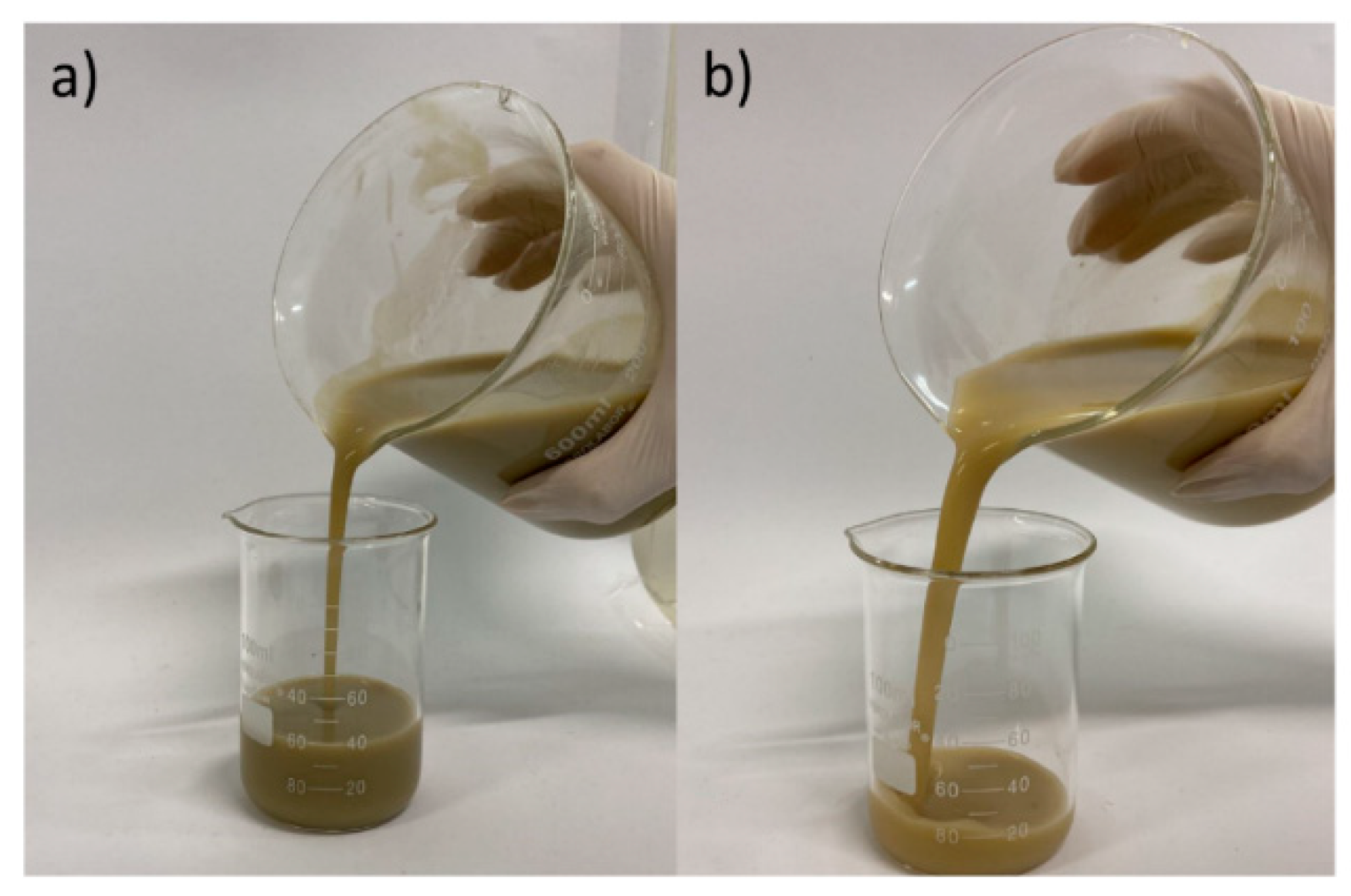
The change in the aspect of the fluid, as well as the changes in the flow profile, were compared to the effects of temperature on bentonite clay and CMC, which influences the rheological behavior of the fluid. It was done through saline dispersions/solutions for which these components were individually added and then exposed to the temperature of 150°C, for 5 days. The results obtained are presented in the flow curves in
Figure 3 and images in
Figure 4 and
Figure 5.
As shown in
Figure 3,
Figure 4 and
Figure 5, the effect of exposure to the temperature of 150°C after 5 days is much more severe for the CMC solution than for the bentonite clay dispersion. This is noted by a significant reduction in the flow profile, loss of viscous nature, extremely dark color and characteristic burning smell of the polymeric solution, which highlights the degradation of the polymer. This aspect is similar to that presented by the aqueous phase supernatant to the fluid sample after exposure to higher temperatures. Thus, this comparison suggests that the effect of temperature to the CMC chain is the main agent causing the degradation of the fluid.
The decrease in viscosity of CMC solutions subjected to temperature was also observed by Zheng, Wu and Huang (2020), who recorded percentage decreases that ranged from 74 to 94%, depending on the polymer concentration, for temperature increases from 40 to 190°C [
28]. According to the authors, this phenomenon is due to the fact that in-creasing temperature tends to increase molecular activity and reduce the interaction of molecules through hydrogen bonds, which leads to a decrease in the system's viscosity. Furthermore, it should be noted that the long time exposure in this study intensify the effects related to this variable and may also enable degradation of the polymer chain.
3.2. Filtrate Volume
Table 4 presents the average filtrate volume values for each temperature and time condition.
Based on the results, the fluid presented an average filtrate volume value at room temperature of 8.3mL. Thus, according to the failure criteria defined for this parameter, which establishes a maximum increase of 40% of this value, samples exposed to temperature fail once the filtrate volume exceeds 11.6mL. For 140°C, this condition is only observed in the following inspection times: 5 days (1 sample), 6 days (three samples), and for 7,8, 9 and 10 days (all samples tested). For 150°C, failure is observed for the following inspection times: 2 days (one sample), 3 days (one sample), 4 days (five samples), 6 and 7 days (all samples).
The filtrate volume shows gradual increases with exposure to higher temperatures and longer inspection times, which become more pronounced at temperatures of 140 and 150°C. As observed for rheological behavior, this increase in filtrate volume may be associated with the progressive thermal degradation of CMC, which acts primarily as a filtration reducer [
29]. Additionally, increasing temperature and changing in the electrochemical balance may interfere in the degree of flocculation and aggregation of bentonite clay
particles, causing changes in the permeability of the mudcake [
30], in addition to resulting in the presence of free water in the system, favoring the filtration of a greater volume of liquid phase.
Obtaining greater volumes of filtrate directly impacts the hydrostatic configuration of the well, increasing fluid density, due to the loss of water from the fluid column to the formation [
31]. Furthermore, a greater volume of aqueous phase in the formation may be related to the occurrence of damage to the formation, which could impact the reservoir's production curve. However, significant increases in this parameter were observed only for temperatures of 140 and 150°C, which are not usually found. In addition, it's important to note that damage to the formation would only be a concern for wells that are temporarily abandoned with the intention of resuming production activities in the future.
3.3. pH
Table 5 presents the average pH values of the water-based fluid for each temperature and time condition.
The fluid is basic at room temperature with an average pH of 10.92. For samples ex-posed to temperature, a reduction in this parameter is observed as the temperature and exposure time increase, exhibiting minimum values for 150°C after 6 days, whose average value is 7.80.
The decrease in pH, particularly at 140°C and 150°C, offers additional evidence of the thermal degradation of CMC. This degradation involves decarboxylation, which produces carbon dioxide (CO
2). The produced CO
2 then forms carbonic acid (H
2CO
3), leading to an increase in hydrogen ions (H
+), which results in the acidification of the aqueous medium [
32]. This reinforces the correlation between the polymer's degradation and the previously discussed rheological behavior, filtrate volume, and physical characteristics.
To mitigate adverse effects from contaminating electrolytes, minimize corrosion rates, and curb bacterial action on organic components, it is advisable to maintain a weakly alkaline medium with a pH between 8 and 11 for fluids containing clay [
33]. However, some samples exhibited a pH below 8 after exposure to temperatures of 140°C for 7 days or more and 150°C for 5 and 6 days. These conditions align with the failure of all samples tested instances and the highest filtrate volumes, indicating advanced degradation of the CMC and significant impairment of the formulation.
3.4. Estimation of the Life Characteristics of the Water-Based Fluid
3.4.1. Fluid Failures and Validation of the Statistical Model
Table 6 presents the results of accelerated life tests regarding the occurrence of failure obtained after testing fluids exposed to temperature, where ꚍ represents the inspection time in days; K is the number of samples tested, and n is the number of failures observed.
Based on the data in
Table 6, fluid failure occurred only in samples exposed to 140°C starting from the fifth day of inspection and to 150°C from the second day of inspection. No failure was observed during inspections at temperatures of 95°C and 110°C.
Based on the data set, the following estimates for the model parameters were obtained: s0= -5.5295, s1= 1029.2737 and r= 1.9017. The test statistic obtained from these estimates was 0.9038 (with a p-value of 0.6383), indicating that the Weibull model is suitable at the 5% significance level. After the model was validated, the parameter estimates were used to calculate the average lifespan, survival probability, and estimated lifespans for different reliability levels (percentiles).
3.4.2. Average Lifespan
The estimated average lifespan of the fluid for different temperature levels are shown in
Table 7.
According to the estimated average lifespan, the water-based fluid can be used as a barrier element for the maximum time allowed by Brazilian legislation for temporary abandonment without monitoring, which is 3 years [
10], for wells with a downhole temperature of up to 80°C. For temperatures above 80°C, where the average lifespan is significantly reduced, the use of the fluid must consider the expected duration of temporary abandonment and the possibility of periodic monitoring.
The lifespans estimated for the temperatures of 140°C and 150°C were compared to failure conditions observed in laboratory tests. At 140°C, the estimated average lifespan was 5.77 days. In accelerated life tests at this temperature, one failure occurred on the fifth day and three failures on the sixth day. At 150°C, the estimated average lifespan was 3.54 days. In laboratory tests, a significant number of failures occurred only after the fourth day, with only one failure for the inspections after 2 and 3 days. This comparison shows that the estimated values align with experimental results and are effective for predicting operational safety related to well maintenance.
3.4.3. Survival Probability
The estimated survival probability, that is, the estimated probability that the water-based fluid survives a given time T (in days) is presented in
Table 8.
Based on the requirements set by Brazilian legislation for well abandonment, it is noted that the probability of the fluid not failing during the maximum allowed time for temporary abandonment without monitoring (3 years - 1095 days) is 100% for temperatures of 60 and 70°C, and greater than 90% for 80°C.
Downhole temperatures are considered normal up to 80°C [
34]. Thus, these results demonstrate that the application of the water-based fluid as a well barrier element represents a viable and safe alternative in these conditions, making it possible to considerably reduce operational costs, since there is no need to monitor these component until the end of the operation.
If the temperature exceeds 80°C, it is recommended to use the water-based fluid for operations where the well will be temporarily abandoned for a duration less than the maximum allowed time. For temperatures of 85°C and 90°C, operational safety is guaranteed with fluid survival probabilities close to 100% for time intervals of 1 year (365 days) and 6 months (183 days) respectively. However, this probability decreases as the specified time increases, with a significant decline observed especially at a temperature of 90°C, where the fluid survival probability becomes zero after 1.5 years (548 days).
The application of the water-based fluid is limited by the high probability of failure for operations with an expected duration of more than 6 months for wells where the downhole temperature is greater than 95°C. In these cases, the operation design must consider shorter operating times, based on the estimated values for the other metrics obtained in this study, with well-defined predictions of reentry into the well, whether for monitoring, permanent abandonment or resumption of operations.
3.4.4. Estimated Lifespans According to Reliability Level
Table 9 presents the estimated lifespan of the water-based fluid for each specified temperature, given the reliability level (R(t)).
As observed for the average lifespan of the fluid, there is compatibility between the estimated lifespans and the results obtained in the laboratory. Considering a reliability level of 0.95, for example, lifetimes of 3.97 and 2.43 days are obtained, for 140 and 150°C, respectively, and the first failures at these temperature levels are observed on the fifth and second day of inspection. Thus, this comparison indicates that the survival study carried out is an adequate and satisfactory mechanism for anticipating the failure mechanism and qualifying the fluid as a well barrier element in abandonment operations.
The estimates presented in
Table 5 reaffirm that the application of the water-based fluid in the temporary abandonment of wells is a highly promising alternative, especially for temperatures of up to 70°C for which is possible to extend the duration of operation to the maximum time allowed, if necessary, since the lifespans are higher than three years (1095 days).
For temperatures above 80°C, the feasibility of applying this fluid as a barrier element must be guided by factors such as the degree of risk considered acceptable by the operator when planning the operation, the expected operating time, and the possibility of monitoring during the period of abandonment. In these situations, it is necessary to analyze all the estimated service life characteristics at the predicted downhole temperature. Additionally, for wells with high downhole temperatures where the use of water-based fluid may raise questions about its service life characteristics, the reliability of the barrier element acting in conjunction with the fluid should be considered in the operational design. These considerations should be based on technical knowledge and industry best practices, and may also involve the establishment of guidelines and requirements related to monitoring practices to ensure early detection of potential failure modes.
4. Conclusion
This study proposes the use of a water-based fluid as a liquid well barrier element for temporary abandonment, based on estimates of its lifespan and survival probability on downhole conditions, acquired through accelerated life tests.
Based on the results obtained, it is concluded that, for the inspection times outlined in the sampling plan, the performance of the water-based fluid is compromised only at temperatures of 140 and 150°C. This is evidenced by physical changes such as reduction of flow profile, increase in filtrate volume, separation of the aqueous phase, reduction in pH, and primarily relates to the degradation of CMC.
Additionally, the survival analysis of the water-based fluid conducted in this work based on accelerated life tests was considered adequate for the study, qualification and validation of liquid barriers used in the temporary abandonment of petroleum wells and attested the use of the water-based fluid for this application. Therefore, the reliability metrics estimated for this formulation, based on the application of this methodology, should guide the design of projects for abandonment operations that use this type of barrier, based on the expected temperature and time conditions, contributing to the selection of monitoring strategies that mitigate risks related to fluid degradation.
5. Patents
The work reported in this manuscript resulted in a patent deposited to the National Institute of Intellectual Property (INPI –Brazil) by the number BR 10 2023 019391-9.
Author Contributions
Conceptualization, Souza, E.A. and Barros, M.; methodology, Costa, W.R.P.; Costa, A.C.A; Nóbrega, K.C.; software, Oliveira, T.A. and Barros, M. validation, Oliveira, T.A. and Barros, M.; formal analysis, Oliveira, T.A. and Amorim, L.V.; investigation, Costa, W.R.P.; Costa, A.C.A.; Nascimento, R.C.A.M and Nóbrega, K.C.; resources, Nascimento, R.C.A.M and Souza, E.A. data curation, Nascimento, R.C.A.M. and Barros, M.; writing—original draft preparation, Costa, W.R.P. and Nóbrega, K.C..; writing—review and editing, Nascimento, R.C.A.M. and Amorim, L.V.; visualization, Costa, W.R.P. and Costa, A.C.A.; supervision, Amorim, L.V.; project administration, Amorim, L.V.; funding acquisition, Souza, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Petrobras, grant number 0050.0120134.21.9.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that this study received funding from Petrobras. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Leporini, M.; Marchetti, B.; Corvaro, F.; Polonara, F. Reconversion of offshore oil and gas platforms into renewable energy sites production: Assessment of different scenarios. Renew Energ 2019, 135, 1121–1132. [Google Scholar] [CrossRef]
- Colaleo, G.; Nardo, F.; Azzellino, A.; Vicinanza, D. Decommissioning of offshore platforms in Adriatic Sea: The total removal option from a life cycle assessment perspective. Energies 2022, 15, 9325. [Google Scholar] [CrossRef]
- Partridge, T.; Barandiaran, J.; Triozzi, N.; Valtierra, V. T. Decommissioning: another critical challenge for energy transitions. Glob Sol Challeng J 2023, 2, 188–202. [Google Scholar] [CrossRef]
- Giskemo, J. T.; Marcancola, F. How technology and an integrated project approach help reduce well liability costs during the decommissioning phase. In Offshore Technology Conference Brasil, Rio de Janeiro, Brazil, Oct. 2017.
- Dahi Taleghani, A.; Santos, L. (2023). Well Plugging and Abandonment. In: Wellbore Integrity. Dahi Taleghani, A.; Santos, L.; Springer Cham, 2023, pp.209-231.
- Achang, M.; Yanyao, L.; Radonjic, M. A review of past, present and future technologies for permanent plugging and abandonment of wellbores and restoration of subsurface geologic barriers. Environ Eng Sci 2020, 37, 396–407. [Google Scholar] [CrossRef]
- Trudel, E.; Bizhani, M.; Zare, M.; Frigaard, I. A. Plug and abandonment practices and trends: A British Columbia perspective. J Petrol Sci Eng 2019, 183, 106417. [Google Scholar] [CrossRef]
- Araujo, R.G.S.; da Silva, F. P. F.; de Sena Costa, B. L.; Moreira, P. H. S. S.; Rodrigues, M. A. F.; de Oliveira Freitas, J. C. Study of cement blend containing rice husk ash for oil well plug and abandonment operations. Constr Build Mater 2020, 254, 119217. [Google Scholar] [CrossRef]
- Agência Nacional do Petróleo, Gás Natural e Biocombustíveis - ANP. Regulamento de Abandono de Poços Perfurados com Vistas à Exploração ou Produção de Petróleo e/ou Gás (Regulation on the Abandonment of Wells Drilled for the Exploration or Production of Oil and/or Gas). Diário Oficial da União, Brasília, Brazil, 06 mar. 2002.
- Agência Nacional do Petróleo, Gás Natural e Biocombustíveis - ANP. Segurança Operacional para Integridade de Poços de Petróleo e/ou Gás (Operational Safety for the Integrity of Oil and/or Gas Wells). Diário Oficial da União, Brasília, Brazil, 03 nov. 2016.
- Chukwuemeka, A. O.; Oluyemi, G.; Mohammed, A. I.; Njuguna, J. Plug and abandonment of oil and gas wells–A comprehensive review of regulations, practices, and related impact of materials selection. Geoenergy Science and Engineering 2023, 226, 211718. [Google Scholar] [CrossRef]
- Willis, B. M.; Strutt, J. E.; Eden, R. D. Long Term Well Plug Integrity Assurance–A Probabilistic Approach. In: Offshore Technology Conference, Houston, USA, 6-9 May 2019.
- Hmadeh, L.; Jaculli, M. A.; Elahifar, B.; Sangesland, S. Development of bismuth-based solutions for well plugging and abandonment: A review. Petroleum Research 2024, 9, 250–264. [Google Scholar] [CrossRef]
- Vignes, B. Qualification of Well Barrier Elements-Test Medium, Test Temperatures and Long-term Integrity. In: SPE European Health, Safety and Environmental Conference and Exhibition, Vienna, Austria, 22-24 February 2011.
- Khalifeh, M.; Hodne, H., Saasen, A.; Vralstad, T. Techniques and materials for North Sea plug and abandonment operations. In Offshore Technology Conference, Houston, USA, 6-9 May 2013.
- Karapetov, R. V.; Mokhov, S. N.; Savelev, V. V. On implementation of process fluids to abandon wells in a wide range of densities (Russian). Oil Industry Journal 2017, 11, 122–125. [Google Scholar]
- Aslani, F.; Zhang, Y.; Manning, D.; Valdez, L. C.; Manning, N. Additive and alternative materials to cement for well plugging and abandonment: A state-of-the-art review. J Petrol Sci Eng 2022, 215, 110728. [Google Scholar] [CrossRef]
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J. D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J Petrol Sci Eng 2019, 173, 478–491. [Google Scholar]
- Pena, F. J.; de Souza, K. M.; de Lemos, M. J. Thermal behavior of aluminothermic thermite reaction for application in thermal sealing of oil wells. International Communications in Heat and Mass Transfer 2023, 149, 107113. [Google Scholar] [CrossRef]
- Norsok D-010, 2012, Well Integrity in Drilling and Well Operations, 4 ed. Lysaker, Standards Norway.
- Escobar, L. A.; Meeker, W. Q. A review of accelerated test models. Stat Sci 2006, 21, 552–577. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Y.; Wang, J.; Shiju, E.; Li, X. Accelerated thermal aging of grease-based magnetorheological fluids and their lifetime prediction. Mat. Res. Express 2018, 5, 085702. [Google Scholar] [CrossRef]
- Mclachlan, G. J.; Krishnan, T. The EM algorithm and extensions, 2nd ed.; John Wiley & Sons: New Jersey, USA, 2007. [Google Scholar]
- Hamad, B. A.; He, M.; Xu, M.; Liu, W.; Mpelwa, M.; Tang, S.; Jin, L.; Song, J. A novel amphoteric polymer as a rheology enhancer and fluid-loss control agent for water-based drilling muds at elevated temperatures. ACS Omega 2020, 5, 15, 8483–8495. [Google Scholar] [CrossRef]
- Plank, J. P.; Gossen, F. A. Visualization of fluid-loss polymers in drilling-mud filter cakes. SPE Drill Eng 1991, 6, 203–208. [Google Scholar] [CrossRef]
- Ahmad, H. M.; Kamal, M. S.; Al-Harthi, M. A. High molecular weight copolymers as rheology modifier and fluid loss additive for water-based drilling fluids. J. Mol. Liq. 2018, 252, 133–143. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Lv, K.; Liu, J.; Xiu, Z.; Wang, Z.; Jin, J. Synthesis of hydrophobic associative polymers to improve the rheological and filtration performance of drilling fluids under high temperature and high salinity conditions. J Petrol Sci Eng 2022, 209, 1–11. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, X.; Huang, Y. Impact of polymer addition, electrolyte, clay and antioxidant on rheological properties of polymer fluid at high temperature and high pressure. J Pet Explor. Prod. Technol. 2020, 10, 663–671. [Google Scholar] [CrossRef]
- Kumar, A. S.; Mahto, V.; Sharma, V. P. Behaviour of organic polymers on the rheological properties of Indian bentonite-water based drilling fluid system and its effect on formation damage. Indian J Chem Techn 2003, 10, 525–530. [Google Scholar]
- Nascimento, R. C. A. D. M.; Magalhães, J.; Pereira, E.; Amorim, L. V. Degradação térmica de fluidos de perfuração argilosos aditivados com polímeros e lubrificante. Matéria (Rio J.) 2013, 18, 1329–1339. [Google Scholar] [CrossRef]
- Patidar, A. K.; Sharma, A.; Joshi, D. Formulation of cellulose using groundnut husk as an environment-friendly fluid loss retarder additive and rheological modifier comparable to PAC for WBM. J Pet Explor. Prod. Technol. 2020, 10, 3449–3466. [Google Scholar] [CrossRef]
- Akar, E.; Altinişik, A.; Seki, Y. Preparation of pH-and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr Polym 2012, 90, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; León, O.; Urdaneta, J.; Munõz-Bonilla, A.; Fernandéz-García, M. Modified starch as a filter controller in water-based drilling fluids. Mater 2020, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dewprashad, B. T.; Nguyen, P. D.; Kuhlman, R. D.; Besler, M. Consolidation for screenless completions and proppant-flowback control in hot wells. In SPE International Oil and Gas Conference and Exhibition in China, Beijing, China, 2-6 November 1998.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).