Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
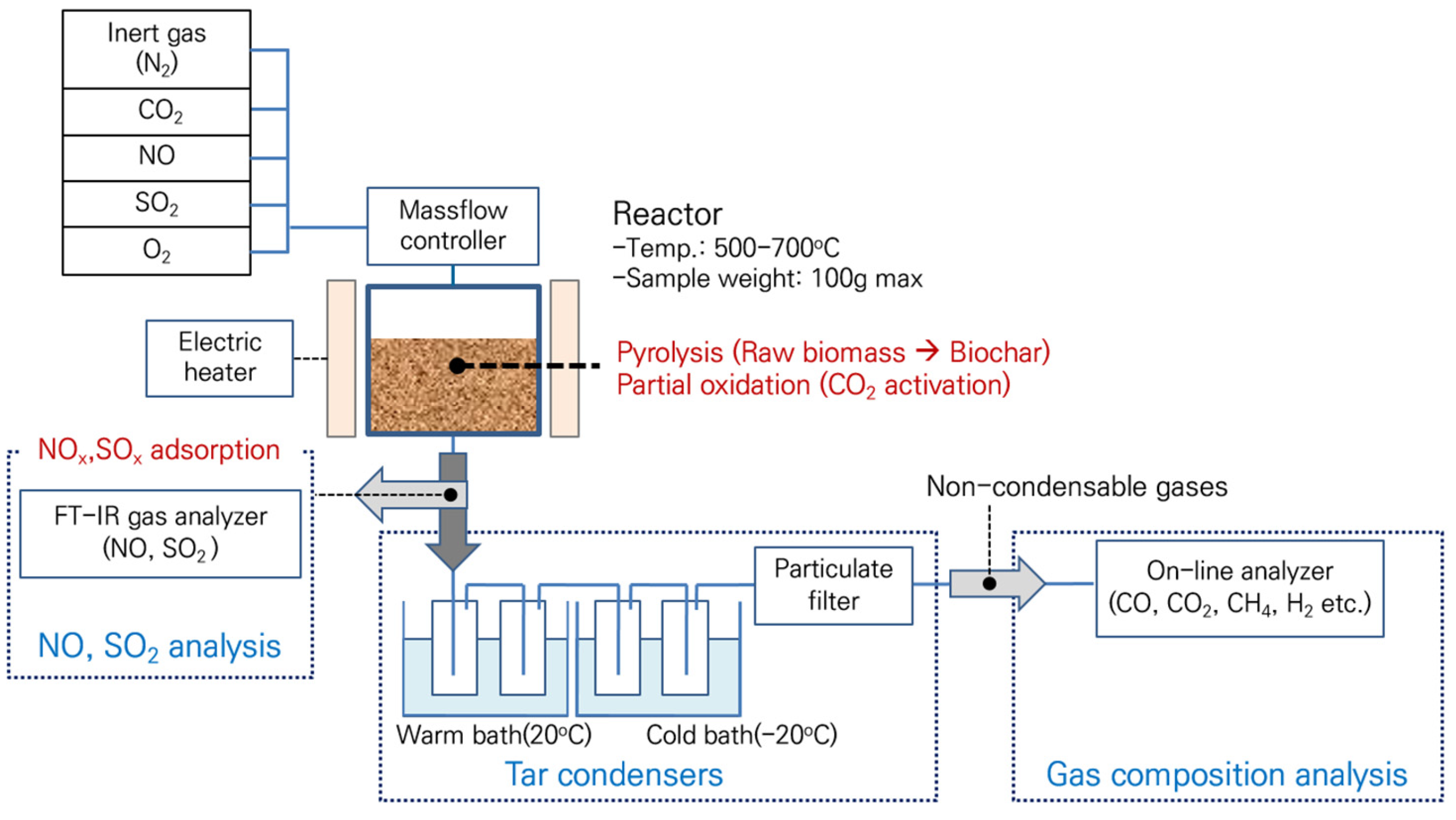
2. Materials and Methods
2.1. Materials
Slow pyrolysis
2.2. CO2 Partial Gasification for Biochar Activation
2.3. Analysis of Biomass and Biochar
2.4. Characteristics of NOx/ SOx Adsorption from Biochar and Activated Biochar
3.1. Biomass Characterization
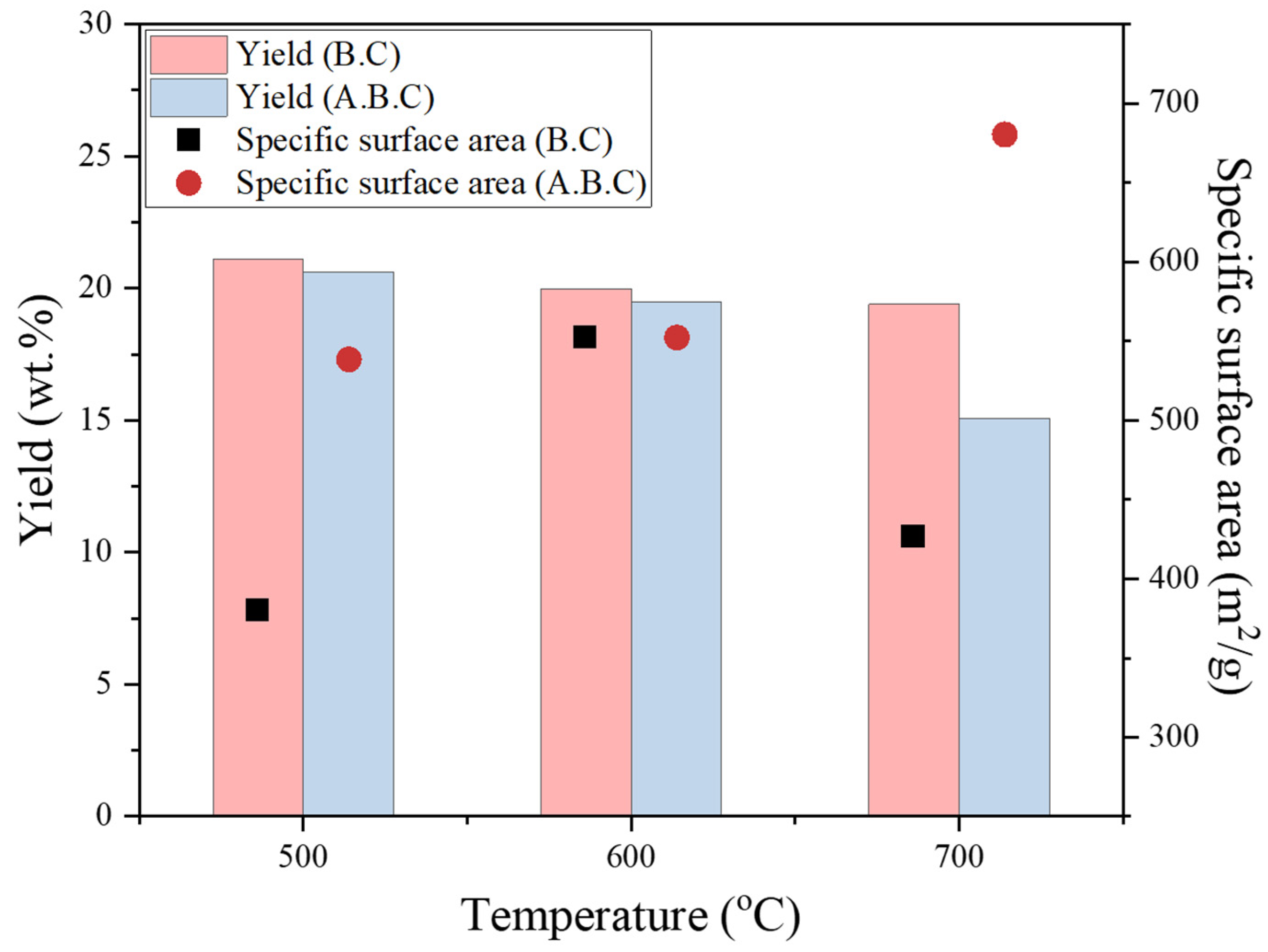
3.2. Biochar Properties under Slow Pyrolysis
3.3. Activated Biochar Properties under CO2 Partial Gasification
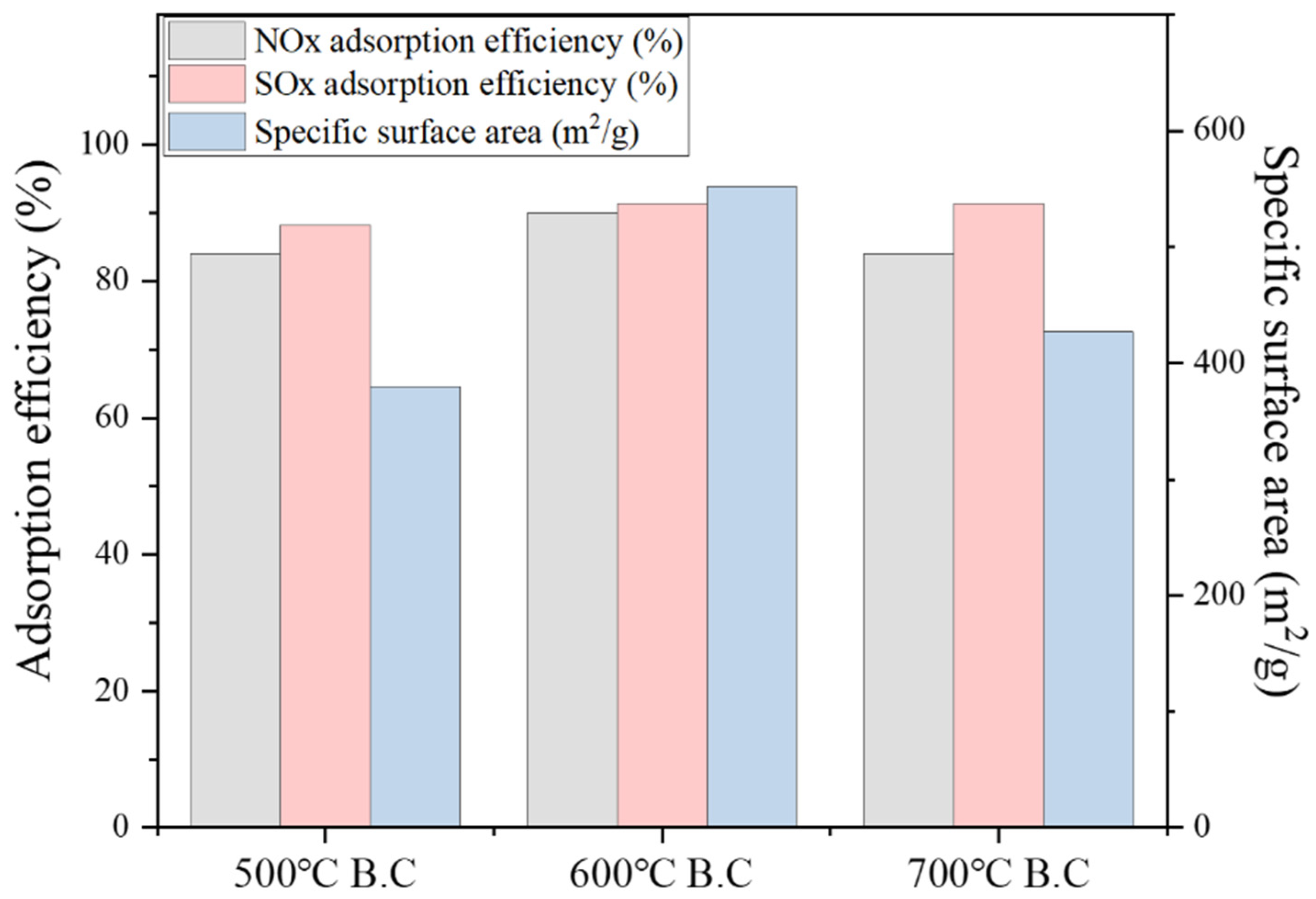
3.4. Results of NOx/ SOx Adsorption under Biochar and Activated Biochar
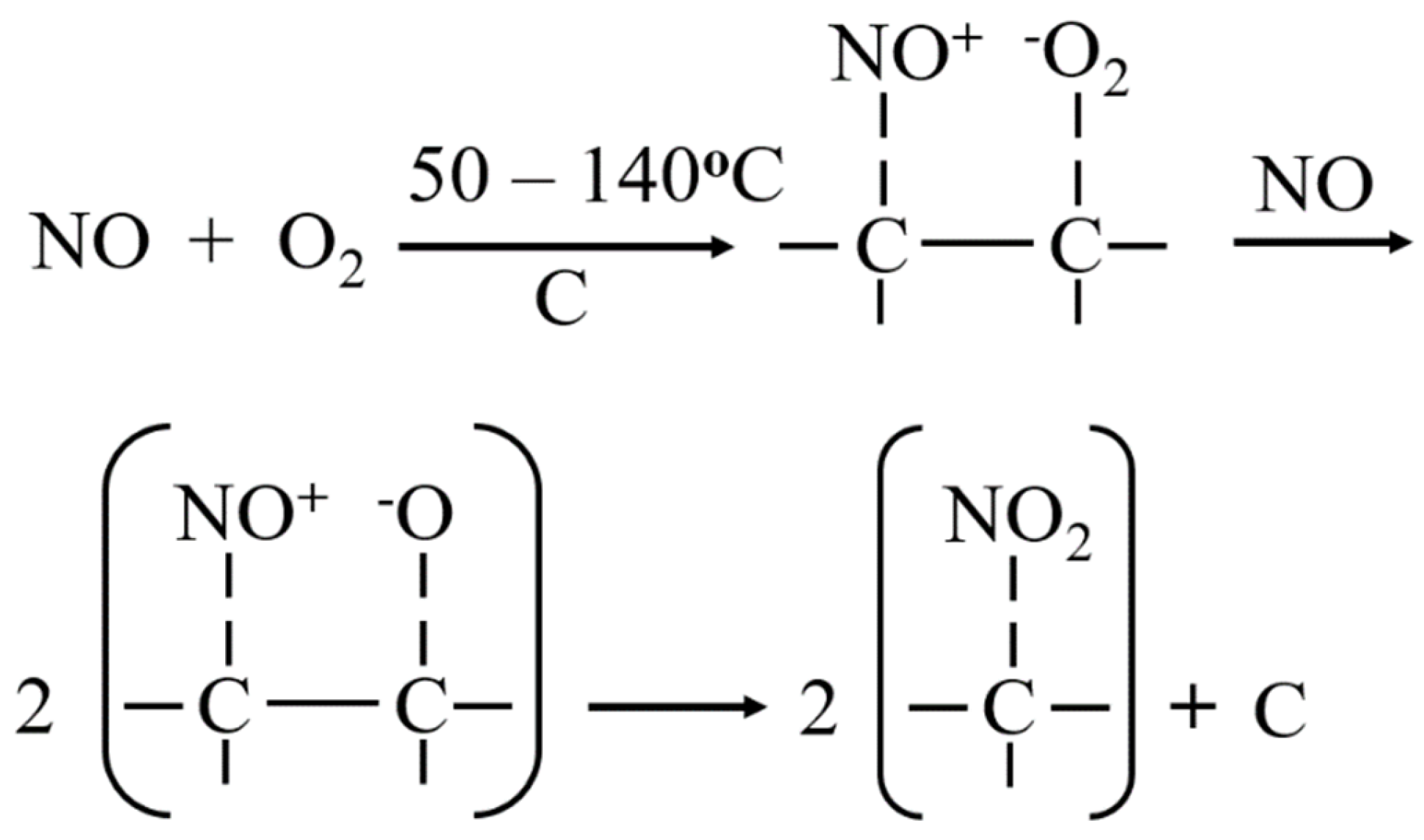
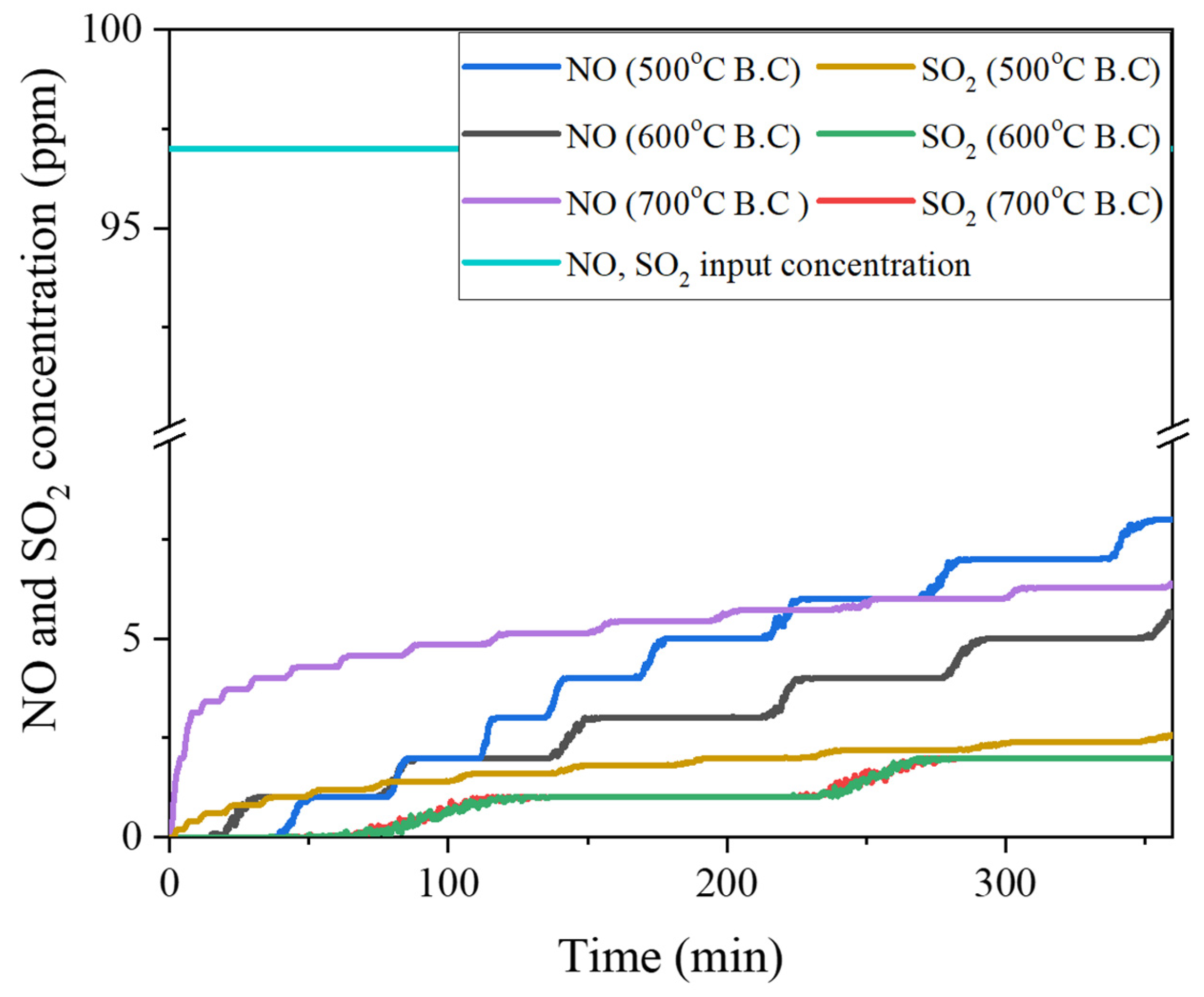
3.4.1. Characterization of NO/ SO2 Adsorption by Biochar
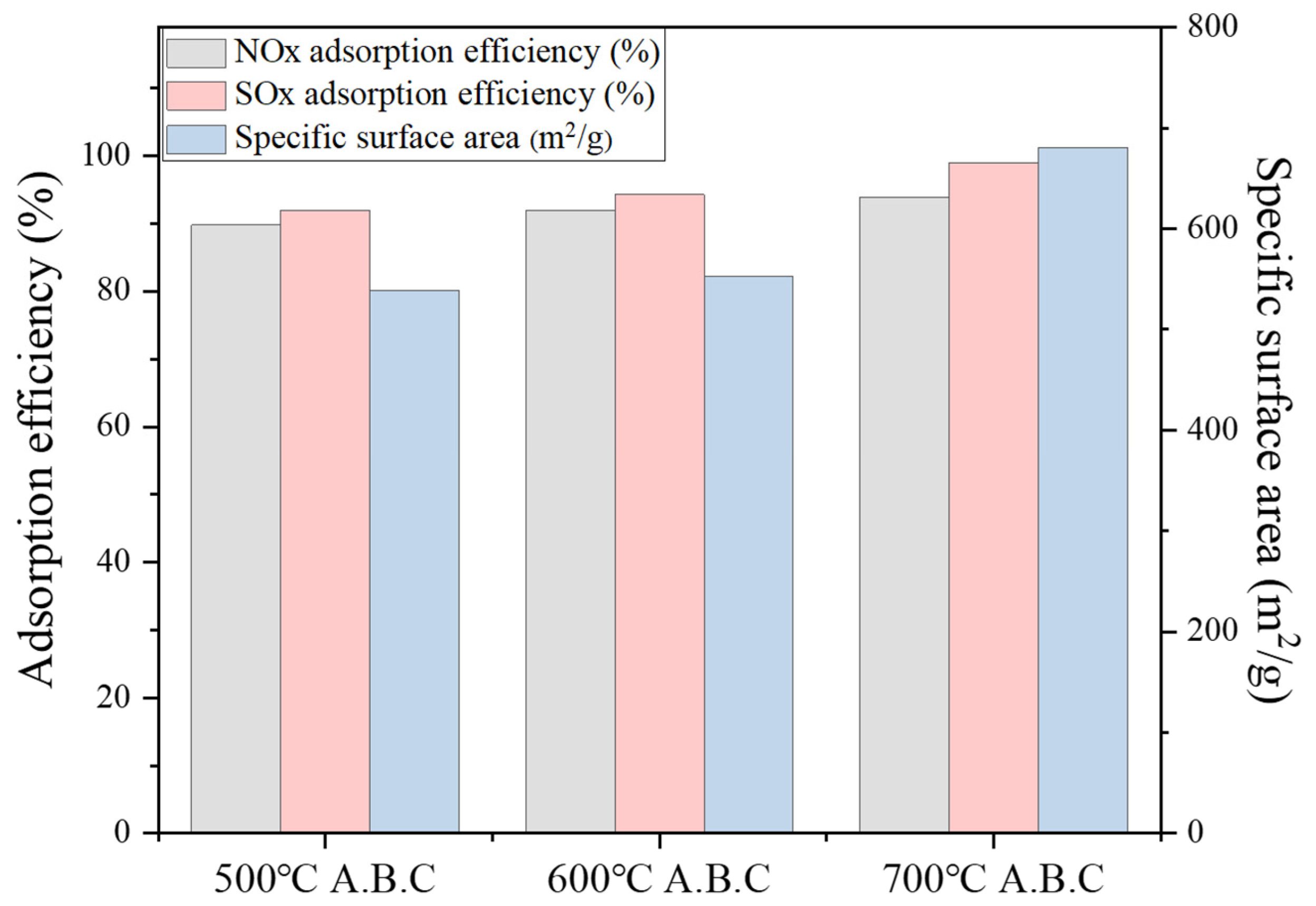
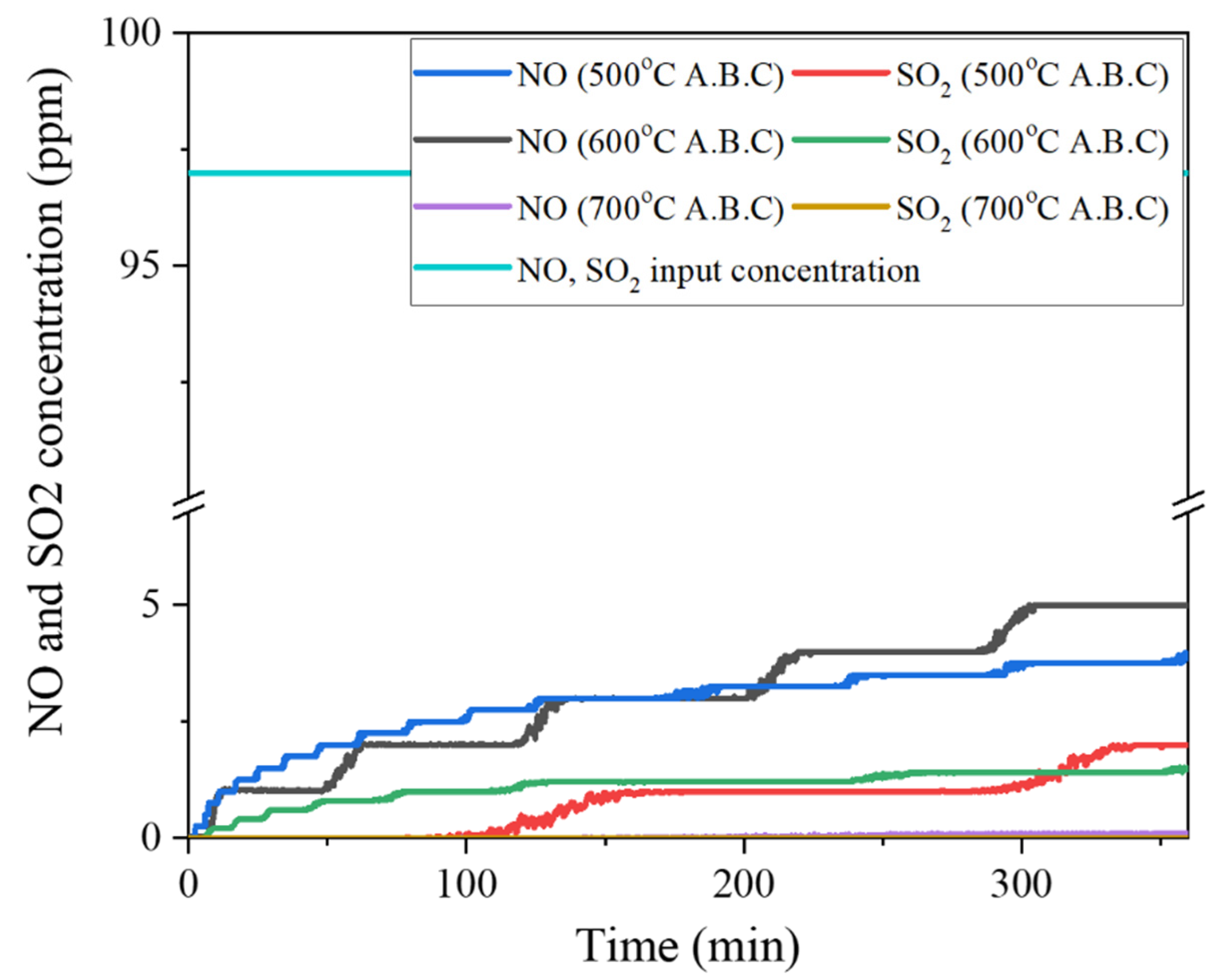
3.4.2. Characterization of NO/ SO2 Adsorption by Activated Biochar
4. Conclusions
Acknowledgement
References
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Shelef, M. Selective catalytic reduction of NOx with N-free reductants. Chem. Rev. 1995, 95, 209–225. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Yang, Q.; Xiong, S.; Li, J.; Crittenden, J. Performance of modified LaxSr1–xMnO3 perovskite catalysts for NH3 oxidation: TPD, DFT, and kinetic studies. Environ. Sci. Technol. 2018, 52, 7443–7449. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, F.; Xie, L.; Shan, W.; He, H. NH3 -SCR performance of fresh and hydrothermally aged Fe-ZSM-5 in standard and fast selective catalytic reduction reactions. Environ. Sci. Technol. 2013, 47, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Park, R.; Jeon, J.K.; Kim, D.H.; Jung, S.C.; Kim, S.C.; Park, Y.K. Effect of surfactant, HCl and NH3 treatments on the regeneration of waste activated carbon used in selective catalytic reduction unit. J. Ind. Eng. Chem. 2015, 32, 109–112. [Google Scholar] [CrossRef]
- Pacciani, R.; Torres, J.; Solsona, P.; Coe, C.; Quinn, R.; Hufton, J.; Golden, T.; Vega, L.F. Influence of the concentration of CO2 andSO2 on the absorption of CO2 by a lithium orthosilicate-Based Absorbent. Environ. Sci. Technol. 2011, 45, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Jozewicz, W.; Singer, C. SO2 scrubbing technologies: a review. Environ. Prog. 2001, 20, 219–228. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption—desorption on activated carbons with varying pore structure. J. Environ. Sci. (China) 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Chang, J.; Hu, X.; Tian, H.; Yuan, F.; Xu, J.; Guo, Q. Simulation and experimental study on smelter off-gas desulfurization using calcium-based desulfurizer. Environ. Sci. Pollut. Res. 2018, 69, 2233–2241. [Google Scholar] [CrossRef]
- Hanif, M.A.; Ibrahim, N.; Abdul Jalil, A.A. Sulfur dioxide removal: an overview of regenerative flue gas desulfurization and factors affecting desulfurization capacity and sorbent regeneration. Environ. Sci. Pollut. Res. Int. 2020, 27, 27515–27540. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Song, S.; Li, J.; Tang, Z.; Ye, J.; Yang, J. Preparation and CO2 adsorption properties of porous carbon by hydrothermal carbonization of tree leaves. J. Mater. Sci. Technol. 2019, 35, 875–884. [Google Scholar] [CrossRef]
- Rao, L.; Liu, S.; Wang, L.; Ma, C.; Wu, J.; An, L.; Hu, X. N-doped porous carbons from low temperature and single-step sodium amide activation of carbonized water chestnut shell with excellent CO2 capture performance. Chem. Eng. J. 2019, 359, 428–435. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, B.; Majumder, A.; Gupta, A.K.; Nimbhorkar, S.K. A comprehensive review on the synthesis, performance, modifications, and regeneration of activated carbon for the adsorptive removal of various water pollutants. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Mobasser, S.; Wager, Y.; Dittrich, T.M. Indoor air purification of volatile organic compounds (VOCs) using activated carbon, zeolite, and Organosilica sorbents. Ind. Eng. Chem. Res. 2022, 61, 6791–6801. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Gao, J.; Sun, S. Changes of biochar physiochemical structures during tar H2O and CO2 heterogeneous reforming with biochar. Fuel Process. Technol. 2017, 165, 72–79. [Google Scholar] [CrossRef]
- Shim, T.; Yoo, J.; Ryu, C.; Park, Y.-K.; Jung, J. Effect of Steam Activation of Biochar Produced from a Giant Miscanthus on Copper Sorption and Toxicity, Bioresour. Technol. 2015, 197, 85–90. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Zhang, X.; Feng, Y.; Zhang, H.; Zhang, S.; Chen, H. Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation, Fuel 224 (2018). Fuel 2018, 224, 138–146. [Google Scholar] [CrossRef]
- Pallaŕes, J.; Gonzalez-Cencerrado, A. I. Arauzo, Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Xu, Z.; He, M.; Xu, X.; Cao, X.; Tsang, D.C.W. Impacts of different activation processes on the carbon stability of biochar for oxidation resistance. Bioresour. Technol. 2021, Oct, 125555. [Google Scholar] [CrossRef] [PubMed]
- Ahiduzzaman, Md.; Sadrul Islam, A.K.M. Preparation of porous bio-char and activated carbon from rice husk by leaching ash and chemical activation. SpringerPlus 2016, 5, 1248. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Qi, Y.; Yang, L.; Wu, L.; He, L.; Li, P.; Qi, X.; Gao, F.; Ding, Y.; et al. An efficient, green and sustainable potassium hydroxide activated magnetic corn cob biochar for Imidacloprid removal, Chemosphere. Chemosphere 2022, 291, 132707. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, J.S. Production of biochars by intermediate pyrolysis and activated carbons from oak by three activation methods using CO2. J. Anal. Appl. Pyrol. 2014, 107, 116–122. [Google Scholar] [CrossRef]
- Mulabagal, V.; Baah, D.A.; Egiebor, N.O.; Sajjadi, B.; Chen, W.-Y.; Viticoski, R.L.; Hayworth, J.S. Biochar from biomass: A strategy for carbon dioxide sequestration, soil amendment, power generation, CO2 utilization, and removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in the environment. In Handbook of Climate Change Mitigation and Adaptation, , Leal Filho, W., Ed., 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1023–1085. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, H.; Chen, S.; Jiang, B. A microwave-assisted Boudouard reaction: A highly effective reduction of the greenhouse gas CO2 to useful CO feedstock with semi-coke. Molecules 2021, 26, 1507. [Google Scholar] [CrossRef]
- Farzaneh, A.; Richards, T.; Sklavounos, E.; van Heiningen, A. A kinetic study of CO2 and steam gasification of char from lignin produced in the SEW process. Biol. Res. 2014, 9, 3052–3063. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Feng, Y.; Chen, Y.; Wang, X.; Chen, H. Nitrogen enriched biochar modified by high temperature CO2–ammonia treatment: characterization and adsorption of CO2. Chem. Eng. J. 2014, 257, 20–27. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Yun, C.H.; Park, Y.H.; Park, C.R. Effects of pre-carbonization on porosity development of activated carbons from rice straw. Carbon 2001, 39, 559–567. [Google Scholar] [CrossRef]
- Lee, Y.; Eum, P.-R.-B.; Ryu, C.; Park, Y.-K.; Jung, J.-H.; Hyun, S. Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour. Technol. 2013, 130, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Neathery, J.K.; Rubel, A.M.; Stencel, J.M. Uptake of NOx by activated carbons: bench scale and pilot-plant testing. Carbon NY. Carbon 1997, 35, 1321–1327. [Google Scholar] [CrossRef]
- Kong, Y.; Cha, C.Y. NOx adsorption on char in presence of oxygen and moisture. Carbon 1996, 34, 1027–1033. [Google Scholar] [CrossRef]
- Chen, M.; Xie, B.; He, F.; Deng, X. Efficient inhibition of S(IV) oxidation in a novel basic aluminum sulfate regenerative flue gas desulfurization process by ethylene glycol: kinetics and reaction mechanism. Energy Fuels 2019, 33, 1383–1391. [Google Scholar] [CrossRef]
- Jin, B.; Zhao, H.; Zheng, C. Dynamic simulation and control design for pulverized-coal-fired oxy-combustion power plants. In Clean Coal Technology and Sustainable Development. ISCC 2015, Yue, G., Li, S., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Chiu, C.H.; Lin, H.P.; Kuo, T.H.; Chen, S.S.; Chang, T.C.; Su, K.H.; Hsi, H.C. Simultaneous control of elemental mercury/sulfur dioxide/nitrogen monoxide from coal-fired flue gases with metal oxide-impregnated activated carbon. Aerosol Air Qual. Res. 2015, 15, 2094–2103. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Ryu, C. Reduction of primary tar vapor from biomass by hot char particles in fixed bed gasification. Biomass Bioenergy 2016, 90, 114–121. [Google Scholar] [CrossRef]
| Condition | Inlet concentration | Flow rate | |||||
| NO (ppm) |
SO2 (ppm) |
O2 (vol.%) |
NO (L/min) |
SO2 (L/min) |
O2 (L/min) |
Total (L/min) |
|
| NO adsorption | 50 | - | 10 | 0.6 | - | 1.2 | 1.8 |
| SO2 adsorption | - | 97 | - | - | 1.2 | - | 1.2 |
| NO+ SO2* | 97 | 97 | 10 | 0.13 | 0.13 | 1 | 1.26 |
| Proximate analysis (wt.%) | Elemental analysis (wt.%) | Higher heating value (MJ/kg) | BET-Surface area (m2/g) | ||||||
| M | VM | FC | ASH | C | H | O | N | ||
| 11.4 | 76.6 | 11.8 | 0.2 | 46.32 | 5.84 | 47.6 | 0.04 | 18.1 | 0.759 |
| Sample | Mass yields of slow pyrolysis products | Properties of biochar | |||||
| Biochar (wt.%) |
Tar (wt.%) |
Gases (wt.%) |
VM (wt.%) |
FC (wt.%) |
BET surface area (m2/g) | ||
| Raw biomass | - | - | - | 88.65 | 13.35 | 0.76 | |
| Biochar | 500℃ | 21.10 | 48.80 | 30.10 | 25.22 | 74.78 | 380.04 |
| 600℃ | 19.98 | 49.65 | 30.37 | 20.88 | 79.12 | 552.33 | |
| 700℃ | 19.39 | 49.75 | 30.86 | 14.9 | 85.1 | 427.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





