Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Materials and Methods
2.1. Cell Culture and Chemical Treatments
2.2. Cell Viability Assays
2.3. Preparation of Molecular Cloning and Manipulation Cell Lines
2.3.1. Transient Overexpression System
2.3.2. Lentivirus-Based Stable shRNA System
2.4. Conditioned Media (CM) Isolation
2.5. Immunoblotting and One-Dimensional Gel Electrophoresis (1DE) Analysis
2.6. Secretome Analysis
2.6.1. Sample Preparation
2.6.2. Mass Spectrometry
2.7. Bioinformatic and Statistical Analysis
3. Results
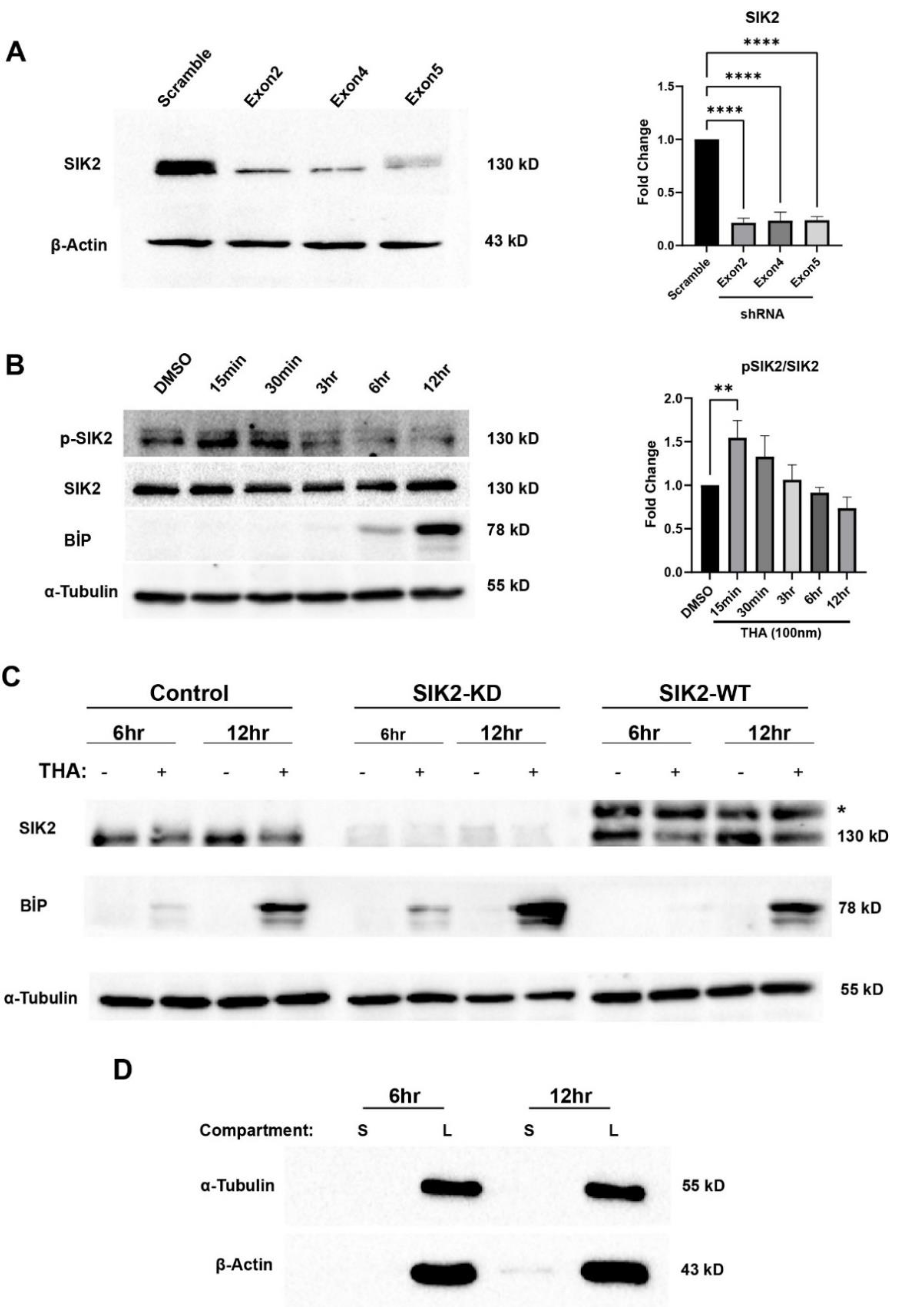
3.1. Establishment of the POMC Model for Comparative Secretome Analyses
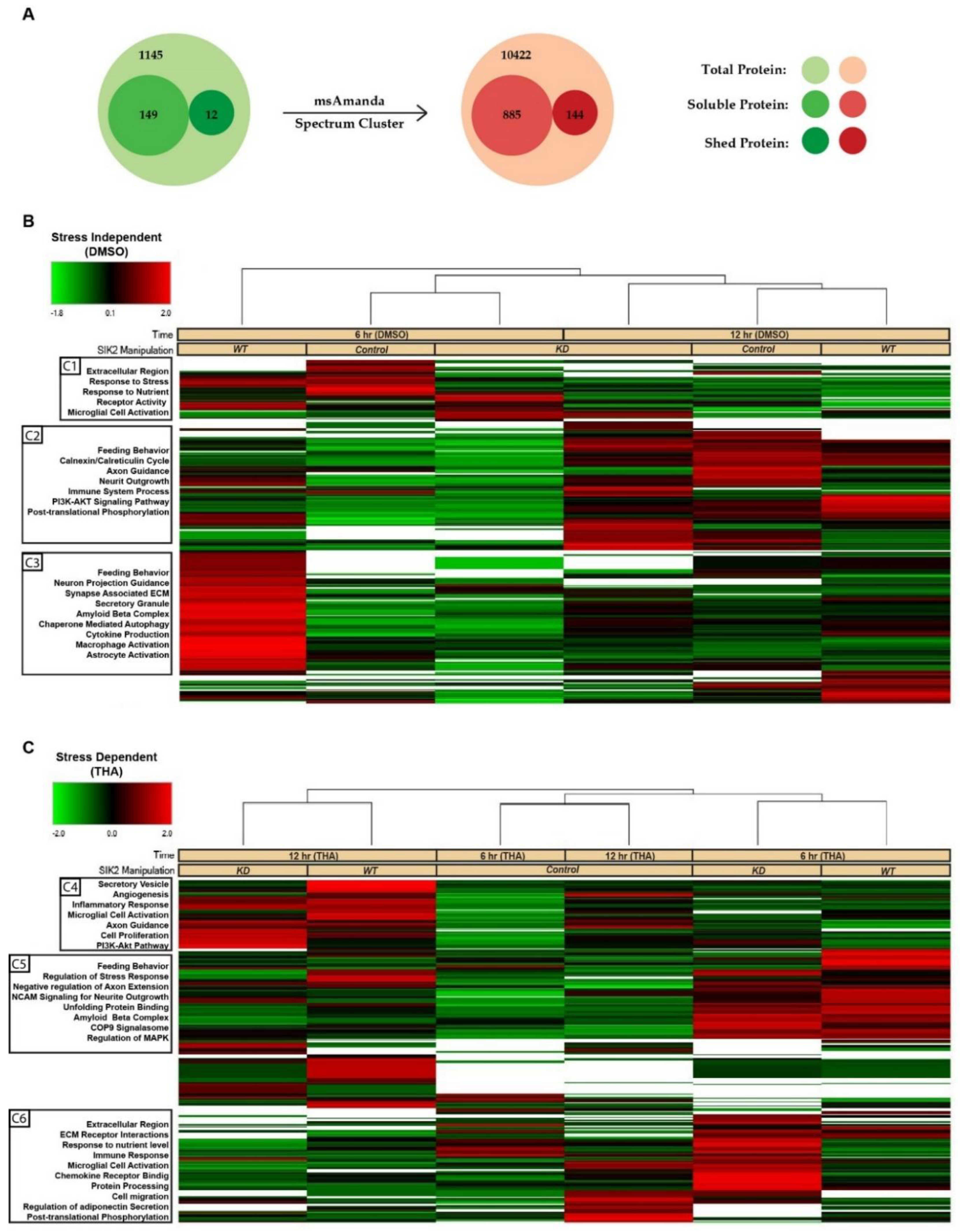
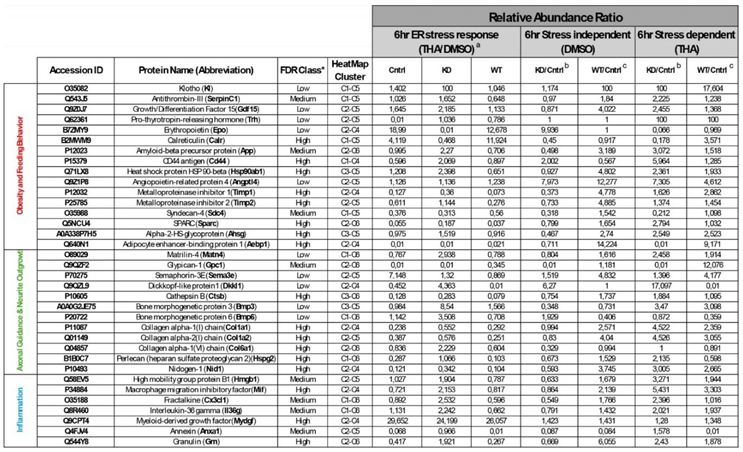
3.2. LC-MS/MS-Based Analysis of POMC Secretomes
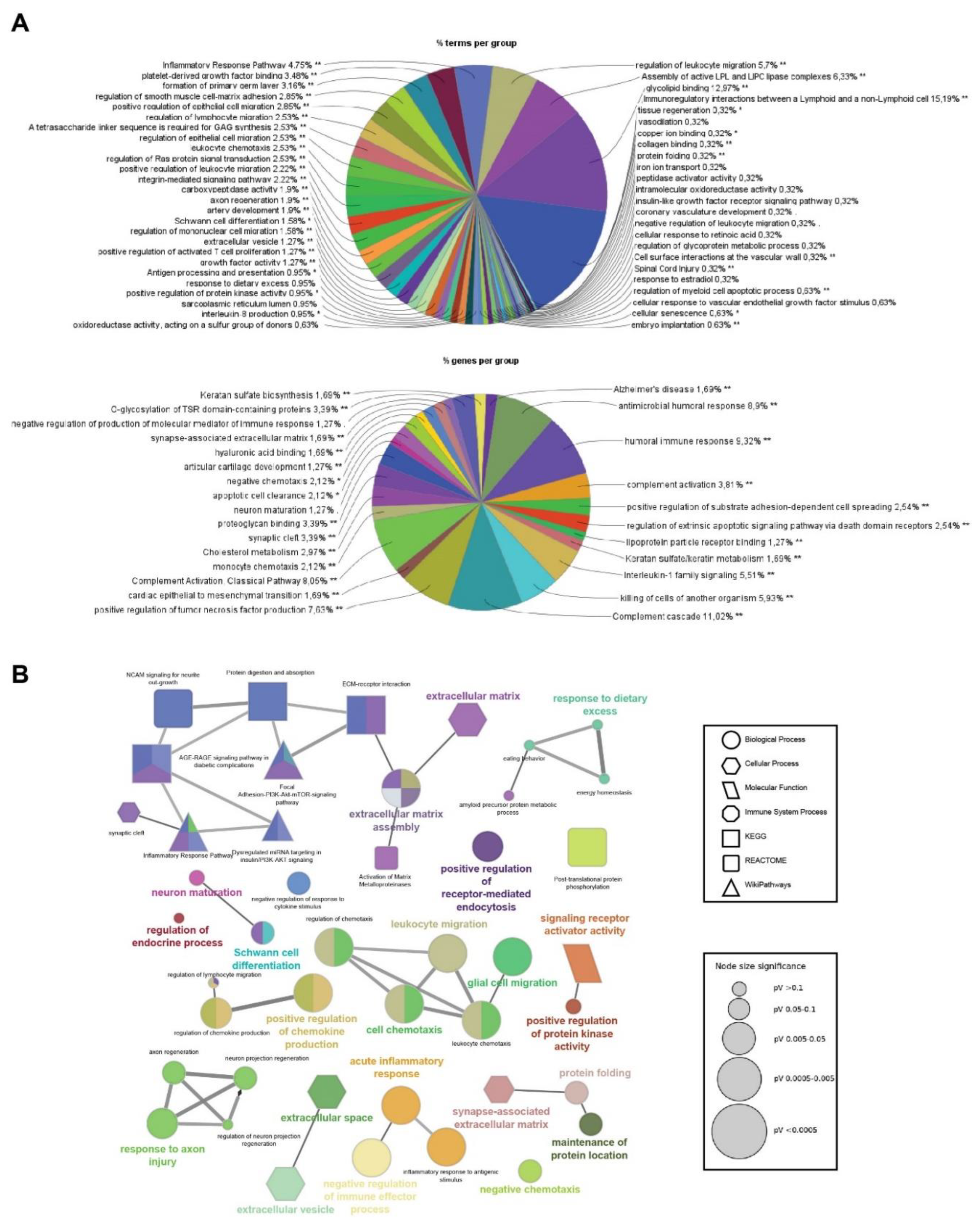
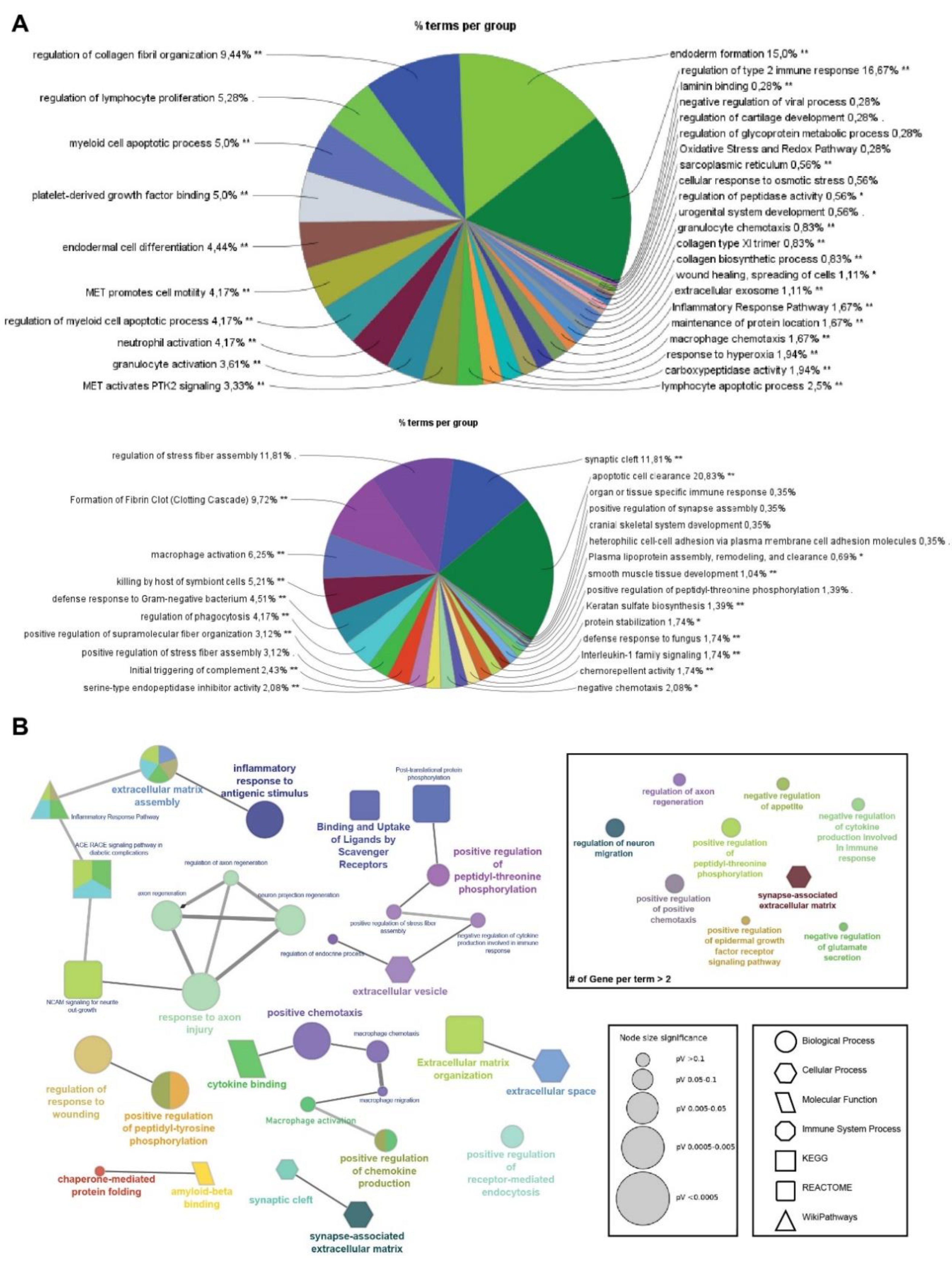
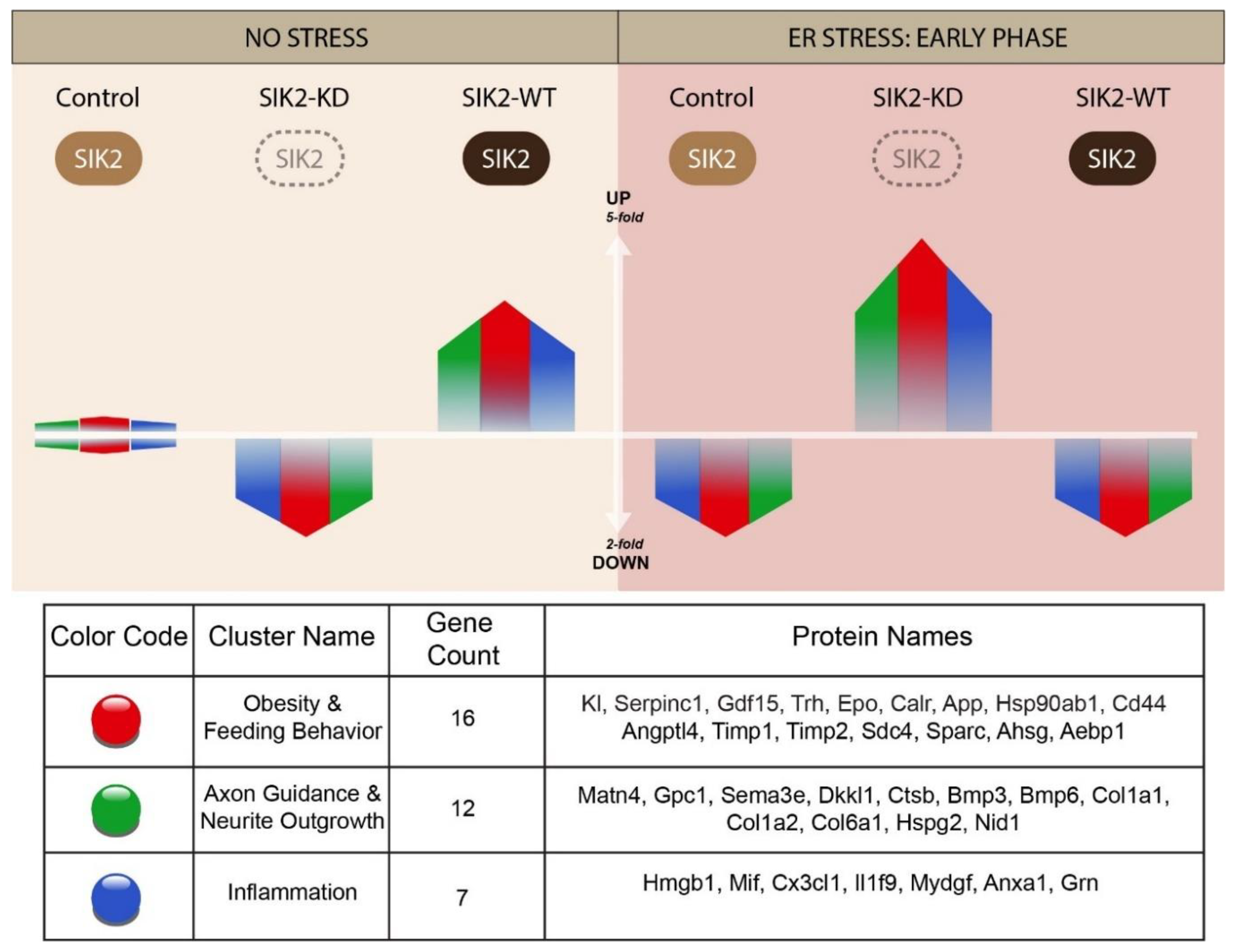
3.3. Functional Enrichment of the SIK2 and ER Stress-Derived Secretome
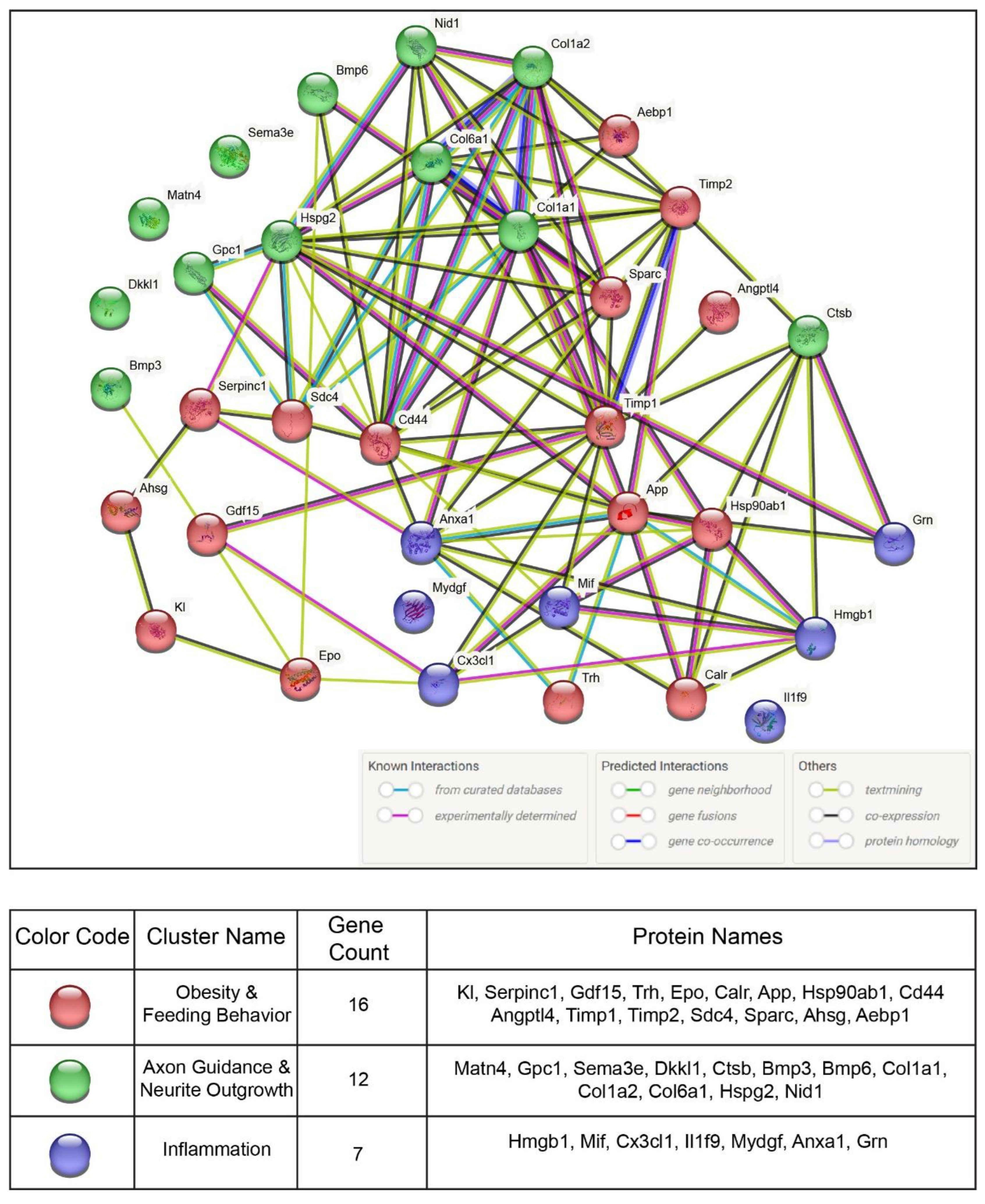
3.4. Protein-Protein Interaction Network Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| SIK2 | Salt inducible kinase 2 |
| POMCs | mHypoA-POMC/GFP-2 cells |
| DIO | Diet-induced obesity |
| ARC | Arcuate nucleus |
| CNS | Central nervous system |
| PVH | Paraventricular nuclei of the hypothalamus |
| DMH | Dorsomedial nucleus of the hypothalamus |
| LHA | Lateral hypothalamic area |
| ALS | Amyotrophic lateral sclerosis |
| LC‒MS/MS | Liquid chromatography‒mass spectrometry |
| IP-MS | Immunoprecipitation-based mass spectrometry |
| PSM | Peptide sequence mass |
| LFQ | Label-free quantification |
| GO | Gene Ontology |
| FDR | False discovery rate |
| 1DE | one-dimensional gel electrophoresis |
| DDA | Data-dependent acquisition |
| DGE | Differential gene expression |
References
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Shah, B.P.; Madara, J.C.; Olson, D.P.; Strochlic, D.E.; Garfield, A.S.; Vong, L.; Pei, H.; Watabe-Uchida, M.; Uchida, N.; et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 2014, 507, 238–242. [Google Scholar] [CrossRef]
- Tong, Q.; Ye, C.-P.; E Jones, J.; Elmquist, J.K.; Lowell, B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008, 11, 998–1000. [Google Scholar] [CrossRef]
- Quarta, C.; Fioramonti, X.; Cota, D. POMC Neurons Dysfunction in Diet-induced Metabolic Disease: Hallmark or Mechanism of Disease? Neuroscience 2019, 447, 3–14. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Dores, R.M.; Baron, A.J. Evolution of POMC: origin, phylogeny, posttranslational processing, and the melanocortins. Ann. New York Acad. Sci. 2011, 1220, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, X.; Zhao, Z.; Feng, Q.; Lin, R.; Sun, Y.; Ding, T.; Xu, F.; Luo, M.; Zhan, C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 2015, 9, 40–40. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Dorfman, M.D.; Fasnacht, R.; Douglass, J.D.; Wyse-Jackson, A.C.; Barria, A.; Thaler, J.P. CX3CL1 Action on Microglia Protects from Diet-Induced Obesity by Restoring POMC Neuronal Excitability and Melanocortin System Activity Impaired by High-Fat Diet Feeding. Int. J. Mol. Sci. 2022, 23, 6380. [Google Scholar] [CrossRef]
- A Natale, C.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O'Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W.; States, U. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5. [Google Scholar] [CrossRef]
- Nasif, S.; de Souza, F.S.J.; González, L.E.; Yamashita, M.; Orquera, D.P.; Low, M.J.; Rubinstein, M. Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc. Natl. Acad. Sci. 2015, 112, E1861–70. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef]
- Coupé, B.; Ishii, Y.; Dietrich, M.O.; Komatsu, M.; Horvath, T.L.; Bouret, S.G. Loss of Autophagy in Pro-opiomelanocortin Neurons Perturbs Axon Growth and Causes Metabolic Dysregulation. Cell Metab. 2012, 15, 247–255. [Google Scholar] [CrossRef]
- Kwon, E.; Jo, Y.-H. Activation of the ARCPOMC→MeA Projection Reduces Food Intake. Front. Neural Circuits 2020, 14. [Google Scholar] [CrossRef]
- Horike, N.; Takemori, H.; Katoh, Y.; Doi, J.; Min, L.; Asano, T.; Sun, X.J.; Yamamoto, H.; Kasayama, S.; Muraoka, M.; et al. Adipose-specific Expression, Phosphorylation of Ser794 in Insulin Receptor Substrate-1, and Activation in Diabetic Animals of Salt-inducible Kinase-2. J. Biol. Chem. 2003, 278, 18440–18447. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yoon, Y.-S.; Han, H.-S.; Kim, Y.-H.; Ogawa, Y.; Park, K.-G.; Lee, C.-H.; Kim, S.-T.; Koo, S.-H. SIK2 Is Critical in the Regulation of Lipid Homeostasis and Adipogenesis In Vivo. Diabetes 2014, 63, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Takemori, H.; Yagita, Y.; Terasaki, Y.; Uebi, T.; Horike, N.; Takagi, H.; Susumu, T.; Teraoka, H.; Kusano, K.-I.; et al. SIK2 Is a Key Regulator for Neuronal Survival after Ischemia via TORC1-CREB. Neuron 2011, 69, 106–119. [Google Scholar] [CrossRef]
- Yang, F.-C.; Tan, B.C.-M.; Chen, W.-H.; Lin, Y.-H.; Huang, J.-Y.; Chang, H.-Y.; Sun, H.-Y.; Hsu, P.-H.; Liou, G.-G.; Shen, J.; et al. Reversible Acetylation Regulates Salt-inducible Kinase (SIK2) and Its Function in Autophagy*. J. Biol. Chem. 2013, 288, 6227–6237. [Google Scholar] [CrossRef]
- Liu, Y.; Poon, V.; Sanchez-Watts, G.; Watts, A.G.; Takemori, H.; Aguilera, G. Salt-Inducible Kinase Is Involved in the Regulation of Corticotropin-Releasing Hormone Transcription in Hypothalamic Neurons in Rats. Endocrinology 2012, 153, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Iorio, C.; Rourke, J.L.; Wells, L.; Sakamaki, J.-I.; Moon, E.; Hu, Q.; Kin, T.; Screaton, R.A. Silencing the G-protein coupled receptor 3-salt inducible kinase 2 pathway promotes human β cell proliferation. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Darling, N.J.; Toth, R.; Arthur, J.S.C.; Clark, K. Inhibition of SIK2 and SIK3 during differentiation enhances the anti-inflammatory phenotype of macrophages. Biochem. J. 2017, 474, 521–537. [Google Scholar] [CrossRef]
- Darling, N.J.; Arthur, J.S.C.; Cohen, P. Salt-inducible kinases are required for the IL-33–dependent secretion of cytokines and chemokines in mast cells. J. Biol. Chem. 2021, 296, 100428. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-C.; Lin, Y.-H.; Chen, W.-H.; Huang, J.-Y.; Chang, H.-Y.; Su, S.-H.; Wang, H.-T.; Chiang, C.-Y.; Hsu, P.-H.; Tsai, M.-D.; et al. Interaction between Salt-inducible Kinase 2 (SIK2) and p97/Valosin-containing Protein (VCP) Regulates Endoplasmic Reticulum (ER)-associated Protein Degradation in Mammalian Cells. Journal of Biological Chemistry 2013, 288, 33861–33872. [CrossRef]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef]
- Ghasemi, M.; Roshandel, E.; Mohammadian, M.; Farhadihosseinabadi, B.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials. Stem Cell Res. Ther. 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Manadas, B.; Behie, L.A.; Salgado, A.J. Secretome of Undifferentiated Neural Progenitor Cells Induces Histological and Motor Improvements in a Rat Model of Parkinson's Disease. STEM CELLS Transl. Med. 2018, 7, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Santos, M.; de Sousa, N.; Duarte-Silva, S.; Vaz, A.R.; Salgado, A.J.; Brites, D. Intrathecal Injection of the Secretome from ALS Motor Neurons Regulated for miR-124 Expression Prevents Disease Outcomes in SOD1-G93A Mice. Biomedicines 2022, 10, 2120. [Google Scholar] [CrossRef]
- Yi, C.-X.; Walter, M.; Gao, Y.; Pitra, S.; Legutko, B.; Kälin, S.; Layritz, C.; García-Cáceres, C.; Bielohuby, M.; Bidlingmaier, M.; et al. TNFα drives mitochondrial stress in POMC neurons in obesity. Nat. Commun. 2017, 8, 15143. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y.; Yi, C.-X.; Tong, Q.; Cai, D. The hypothalamus for whole-body physiology: from metabolism to aging. Protein Cell 2021, 13, 394–421. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.F.P.; Solon, C.; Nascimento, L.F.; De-Lima-Junior, J.C.; Nogueira, G.; Moura, R.; Rocha, G.Z.; Fioravante, M.; Bobbo, V.; Morari, J.; et al. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Liu, S.; Klionsky, D.J.; Lip, G.Y.; Tuomilehto, J.; Kavalakatt, S.; Pereira, D.M.; Samali, A.; Ren, J. ER stress in obesity pathogenesis and management. Trends Pharmacol. Sci. 2021, 43, 97–109. [Google Scholar] [CrossRef]
- Rumora, A.E.; LoGrasso, G.; Hayes, J.M.; Mendelson, F.E.; Tabbey, M.A.; Haidar, J.A.; Lentz, S.I.; Feldman, E.L. The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. J. Neurosci. 2019, 39, 3770–3781. [Google Scholar] [CrossRef]
- Nazarians-Armavil, A.; A Chalmers, J.; Lee, C.B.; Ye, W.; Belsham, D.D. Cellular insulin resistance disrupts hypothalamic mHypoA-POMC/GFP neuronal signaling pathways. J. Endocrinol. 2013, 220, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.K.; Belsham, D.D. Palmitate induces neuroinflammation, ER stress, and Pomc mRNA expression in hypothalamic mHypoA-POMC/GFP neurons through novel mechanisms that are prevented by oleate. Mol. Cell. Endocrinol. 2018, 472, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jäntti, M.H.; Jackson, S.N.; Kuhn, J.; Parkkinen, I.; Sree, S.; Hinkle, J.J.; Jokitalo, E.; Deterding, L.J.; Harvey, B.K. Palmitate and thapsigargin have contrasting effects on ER membrane lipid composition and ER proteostasis in neuronal cells. Biochim. et Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 2022, 1867, 159219–159219. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Xie, K.; Mu, Y.; Lei, Y.; Zhang, B.; Xiong, S.; Chen, Y.; Qi, N. Salt ions and related parameters affect PEI–DNA particle size and transfection efficiency in Chinese hamster ovary cells. Cytotechnology 2013, 67, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Latek, R.; Hossbach, M.; Tuschl, T.; Lewitter, F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 2004, 32, W130–W134. [Google Scholar] [CrossRef] [PubMed]
- Addgene, ‘Addgene Plasmid 10878. Protocol Version 1.0.’, pLKO.1 - TRC Cloning Vector. Accessed: Jun. 06, 2024. [Online]. Available: https://www.addgene.org/protocols/plko/.
- Anton Posch, Proteomic Profiling, vol. 2261. in Methods in Molecular Biology, vol. 2261. New York, NY: Springer US, 2021. [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Griss, J.; Stanek, F.; Hudecz, O.; Dürnberger, G.; Perez-Riverol, Y.; Vizcaíno, J.A.; Mechtler, K. Spectral Clustering Improves Label-Free Quantification of Low-Abundant Proteins. J. Proteome Res. 2019, 18, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, V.; Pichler, P.; Stranzl, T.; Stadlmann, J.; Taus, T.; Winkler, S.; Mechtler, K. MS Amanda, a Universal Identification Algorithm Optimized for High Accuracy Tandem Mass Spectra. J. Proteome Res. 2014, 13, 3679–3684. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Nicholson, J.M.; Mordaunt, M.; Lopez, P.; Uppala, A.; Rosati, D.; Rodrigues, N.P.; Grabitz, P.; Rife, S.C. scite: A smart citation index that displays the context of citations and classifies their intent using deep learning. Quant. Sci. Stud. 2021, 2, 882–898. [Google Scholar] [CrossRef]
- J. Goodman, ‘Characterizing the secretome of adipose tissue in metabolic stress’, 2024. [CrossRef]
- Hamanaka, R.B.; Bobrovnikova-Marjon, E.; Ji, X.; A Liebhaber, S.; A Diehl, J. PERK-dependent regulation of IAP translation during ER stress. Oncogene 2008, 28, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Hyoda, K.; Hosoi, T.; Horie, N.; Okuma, Y.; Ozawa, K.; Nomura, Y. PI3K-Akt inactivation induced CHOP expression in endoplasmic reticulum-stressed cells. Biochem. Biophys. Res. Commun. 2006, 340, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lemaire, H.-G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.-H.; Multhaup, G.; Beyreuther, K.; Müller-Hill, B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Kralisch, S.; Lipfert, J.; Weise, S.; Krause, K.; Jessnitzer, B.; Lã¶Ssner, U.; Blã¼Her, M.; Stumvoll, M.; Fasshauer, M. Amyloid precursor protein expression is induced by tumor necrosis factor α in 3T3-L1 adipocytes. J. Cell. Biochem. 2009, 108, 1418–1422. [Google Scholar] [CrossRef]
- C. Zhang et al., ‘Activation of salt Inducible Kinases, IRE1 and PERK leads to Sec bodies formation in Drosophila S2 cells’, 2021.
- Wang, H.-H.; Lin, C.-Y.; Su, S.-H.; Chuang, C.-T.; Chang, Y.-L.; Lee, T.-Y.; Lee, S.-C.; Chang, C.-J. Activation of salt-inducible kinase 2 promotes the viability of peritoneal mesothelial cells exposed to stress of peritoneal dialysis. Cell Death Dis. 2016, 7, e2298–e2298. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chang, C.-C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Cimino, I.; Coll, A.P. The role of GDF15 in food intake and appetitive behaviour. Curr. Opin. Endocr. Metab. Res. 2021, 22, 100299. [Google Scholar] [CrossRef]
- Hale, C.; Véniant, M.M. Growth differentiation factor 15 as a potential therapeutic for treating obesity. Mol. Metab. 2020, 46, 101117. [Google Scholar] [CrossRef]
- Jeffery, E.; Peters, L.R.; Raghavan, M. The Polypeptide Binding Conformation of Calreticulin Facilitates Its Cell-surface Expression under Conditions of Endoplasmic Reticulum Stress. J. Biol. Chem. 2011, 286, 2402–2415. [Google Scholar] [CrossRef]
- Johnson, R.J.; Xiao, G.; Shanmugaratnam, J.; Fine, R.E. Calreticulin functions as a molecular chaperone for the-amyloid precursor protein, 2001. [Online]. Available: www.elsevier.com/locate/neuaging.
- Kang, H.S.; Liao, G.; DeGraff, L.M.; Gerrish, K.; Bortner, C.D.; Garantziotis, S.; Jetten, A.M. CD44 Plays a Critical Role in Regulating Diet-Induced Adipose Inflammation, Hepatic Steatosis, and Insulin Resistance. PLOS ONE 2013, 8, e58417. [Google Scholar] [CrossRef]
- Hasib, A.; Hennayake, C.K.; Bracy, D.P.; Bugler-Lamb, A.R.; Lantier, L.; Khan, F.; Ashford, M.L.J.; McCrimmon, R.J.; Wasserman, D.H.; Kang, L. CD44 contributes to hyaluronan-mediated insulin resistance in skeletal muscle of high-fat-fed C57BL/6 mice. Am. J. Physiol. Metab. 2019, 317, E973–E983. [Google Scholar] [CrossRef]
- Zhang, J.; Gan, W.; Peng, R.; Lu, L.; Lu, W.; Liu, J. Activation of the mTOR pathway promotes neurite growth through upregulation of CD44 expression. J. Int. Med Res. 2023, 51. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.L.; Kahle, M.P.; Roberts, J.M.; Marcelo, A.; de Hoog, L.; Boychuk, J.A.; Grupke, S.L.; Berretta, A.; Gowing, E.K.; Boychuk, C.R.; et al. Perlecan Domain-V Enhances Neurogenic Brain Repair After Stroke in Mice. Transl. Stroke Res. 2020, 12, 72–86. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sánchez, S.; García-Yagüe, Á.J.; López-Royo, T.; Casarejos, M.; Lanciego, J.L.; Lastres-Becker, I. Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of Parkinson’s disease. Glia 2018, 66, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W.G.M. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Feng, D.; Xu, L.; Li, F.; Liu, J.; Jin, X.; Qian, Z.; Kang, X.; Sun, H. PGRN exerts inflammatory effects via SIRT1-NF-κB in adipose insulin resistance. J. Mol. Endocrinol. 2020, 64, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Negoita, F.; Säll, J.; Morén, B.; Stenkula, K.; Göransson, O. Salt-inducible kinase 2 regulates TFEB and is required for autophagic flux in adipocytes. Biochem. Biophys. Res. Commun. 2019, 508, 775–779. [Google Scholar] [CrossRef]
- Wang, H.-H.; Lin, C.-Y.; Su, S.-H.; Chuang, C.-T.; Chang, Y.-L.; Lee, T.-Y.; Lee, S.-C.; Chang, C.-J. Activation of salt-inducible kinase 2 promotes the viability of peritoneal mesothelial cells exposed to stress of peritoneal dialysis. Cell Death Dis. 2016, 7, e2298–e2298. [Google Scholar] [CrossRef]
| Primer Name | Oligo Set |
| 2F_KpnI (Tm: 61) | AAAAAGGTACCATGGTCATGGCGGATG |
| 2R_KpnI (Tm: 64) | AAAAGGTACCCTAGGTCTCCCGGGCTAAG |
| mSIK2_Exon2_ shRNA_F | CCGGAATCTACCGAGAAGTACAGATCTCGAGATCTGTACTTCTCGGTAGATTTTTTTG |
| mSIK2_Exon2_ ShRNA_R | AATTCAAAAAAATCTACCGAGAAGTACAGATCTCGAGATCTGTACTTCTCGGTAGATT |
|
mSIK2_Exon4_ shRNA_F |
CCGGAATTCTGTCTGCTGTTGATTACTCGAGTAATCAACAGCAGACAGAATTTTTTTG |
|
mSIK2_Exon4_ ShRNA_R |
AATTCAAAAAAATTCTGTCTGCTGTTGATTACTCGAGTAATCAACAGCAGACAGAATT |
|
mSIK2_Exon5_ shRNA_F |
CCGGAAGGACCACAGCTGGATATATCTCGAGATATATCCAGCTGTGGTCCTTTTTTTG |
|
mSIK2_Exon5_ shRNA_R |
AATTCAAAAAAAGGACCACAGCTGGATATATCTCGAGATATATCCAGCTGTGGTCCTT |
|
Scrambled_ shRNA_F |
CCGGCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG |
|
Scrambled_ shRNA_R |
AATTCAAAAACCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





