Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Sample Collection
2.3. Chemical Analysis
2.3.1. Monoterpene Analysis
2.3.2. Diterpene Analysis
2.3.3. Non-Structural Carbohydrate (Soluble Sugars and Starch) Analyses
2.4. Anatomical Defence and Growth Characteristics
2.5. Data Analysis
2.5.1. Gradient Analysis
2.5.2. Linear Mixed-Effect Models
2.5.3. Comparative Analyses
3. Results
3.1. Do Historical Resin Duct Characteristics Relate to the Current Year’s Terpenes?
3.1.1. Monoterpenes
| Outbreak Years | BAI | Total monoterpenes | Total diterpenes | ||||
| Coefficient | t-value | P-value | Coefficient | t-value | P-value | ||
| 2014 | Post-outbreak | 532.4 | 2.7 | 0.015 | -0.8 | -0.5 | 0.608 |
| 5-yr pre-outbreak | 549.9 | 2.6 | 0.015 | -0.8 | -0.5 | 0.658 | |
| 10-yr pre-outbreak | 665.1 | 3.2 | 0.004 | -1.2 | -0.7 | 0.516 | |
| 2015 | Post-outbreak | 485.9 | 2.6 | 0.013 | 4.9 | 2.7 | 0.012 |
| 5-yr pre-outbreak | 686.8 | 3.7 | 0.002 | 4.9 | 2.4 | 0.024 | |
| 10-yr pre-outbreak | 791.7 | 3.7 | 0.001 | 6.3 | 2.6 | 0.013 | |
3.2. Does NSCs Relate to the Terpenes?
3.3. Do the Radial Growth Characteristics Relate to the Terpenes?
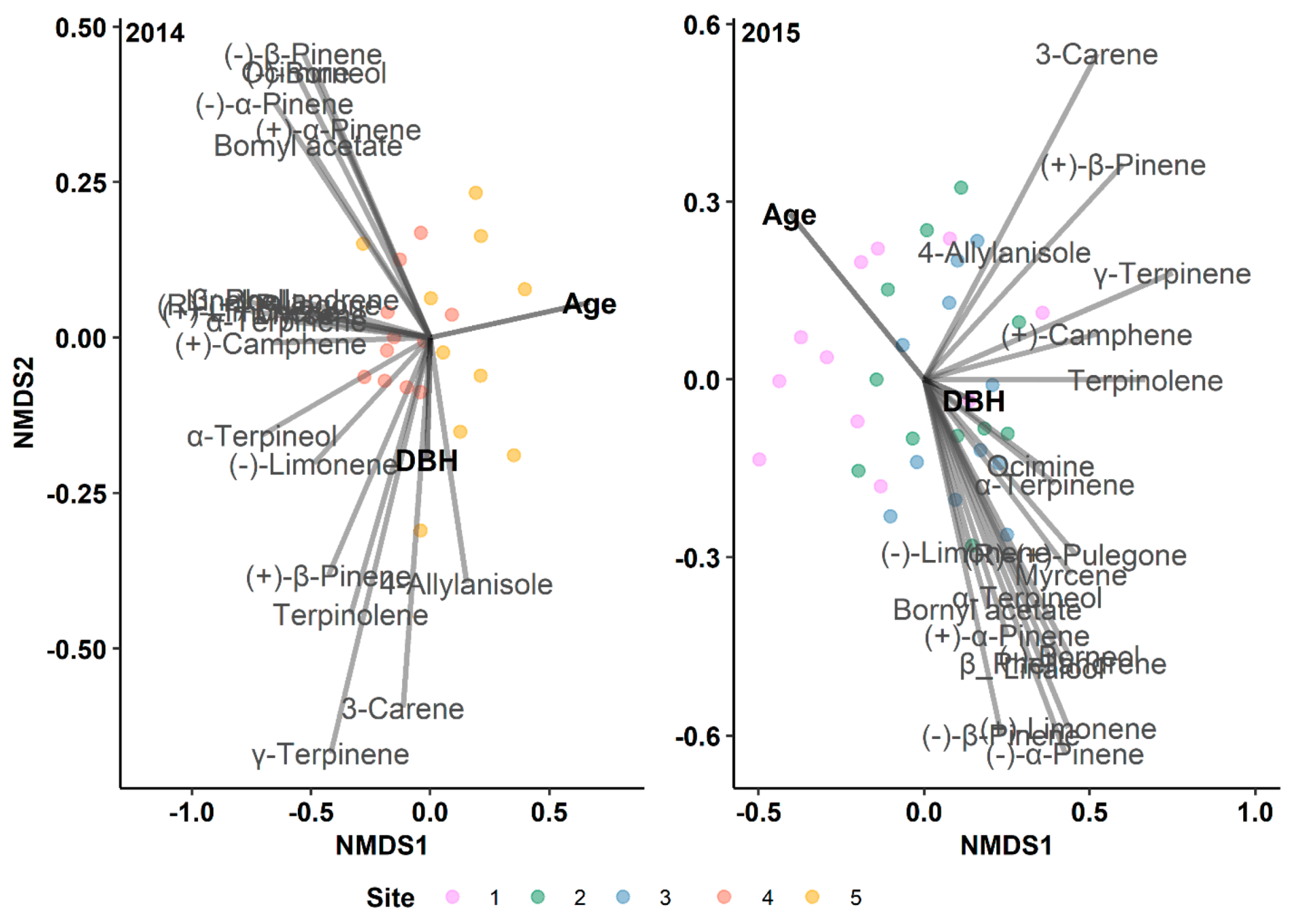
3.4. Do tree DBH and Age Relate to the Terpenes?
3.5. Do NSCs Relate to Anatomical Defence Traits, Radial Growth Rate, and Tree Growth Traits?
3.6. Is There Any Trade-Off between Monoterpenes and Diterpenes?
3.7. Additional Results
4. Discussions
Supplementary Materials
Author Contributions
Funding
Data availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
References
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale Drivers of Natural Disturbances Prone to Anthropogenic Amplification: The Dynamics of Bark Beetle Eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Kausrud K, Økland B, Skarpaas O, Grégoire JC, Erbilgin N, Stenseth NC (2012) Population dynamics in changing environments: the case of an eruptive forest pest species. Biol Rev. 87: 34-51.
- Seidl R, Schelhaas MJ, Rammer W, Verkerk PJ (2014) Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Chang. 4:806–810.
- Ghimire, B.; Williams, C.A.; Collatz, G.J.; Vanderhoof, M.; Rogan, J.; Kulakowski, D.; Masek, J.G. Large carbon release legacy from bark beetle outbreaks across Western United States. Glob. Chang. Biol. 2015, 21, 3087–3101. [Google Scholar] [CrossRef] [PubMed]
- Aldea J, Dahlgren J, Holmström E, Löf M (2023) Current and future drought vulnerability for three dominant boreal tree species. Glob Change Biol. 30: e17079.
- Erbilgin N, Cale JA, Hussain A, Ishangulyyeva G, Klutsch JG, Najar A, Zhao S (2017) Weathering the storm: how lodgepole pine trees survive mountain pine beetle outbreaks. Oecologia. 184:469–478.
- Zhao SY, Erbilgin N (2019) Larger resin ducts are linked to the survival of lodgepole pine trees during mountain pine beetle outbreak. Front Plant Sci. 10:1459 https://doiorg/103389/fpls201901459.
- Zhao SY, Klutsch JG, Cale JA, Erbilgin N (2019) Mountain pine beetle outbreak enhanced resin duct-defenses of lodgepole pine trees. For Ecol Manag. 441: 271-279.
- Amoroso MM, Coates KD, Astrup R (2013) Stand recovery and self-organization following large-scale mountain pine beetle induced canopy mortality in northern forests. For Ecol Manag. 310: 300-311.
- Perovich C, Sibold JS (2016) Forest composition change after a mountain pine beetle outbreak, Rocky Mountain National Park, CO, USA. For Ecol Manag. 1: 366.
- Morris JE, Buonanduci MS, Agne MC, Battaglia MA, Harvey BJ (2022) Does the legacy of historical thinning treatments foster resilience to bark beetle outbreaks in subalpine forests? Ecol Appl. 32 (1): e02474.
- Rodman, K.C.; Andrus, R.A.; Carlson, A.R.; Carter, T.A.; Chapman, T.B.; Coop, J.D.; Fornwalt, P.J.; Gill, N.S.; Harvey, B.J.; Hoffman, A.E.; et al. Rocky Mountain forests are poised to recover following bark beetle outbreaks but with altered composition. J. Ecol. 2022, 110, 2929–2949. [Google Scholar] [CrossRef]
- Lieffers VJ, Benedik J, Stadt K, Macdonald SE (2024) Poor regeneration of pine after mountain pine beetle attack in colder boreal regions of Canada. Can J For Res. 54:168-191.
- Kichas NE, Trowbridge AM, Raffa KF et al (2021) Growth and defense characteristics of whitebark pine (Pinus albicaulis) and lodgepole pine (Pinus contorta var latifolia) in a high-elevation, disturbance-prone mixed-conifer forest in northwestern Montana, USA. For Ecol Manag. 493: 119286.
- Marini L, Økland B, Jönsson AM et al (2017) Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography. 40:1426–1435.
- Thom D, Rammer W, Seidl R (2017) The impact of future forest dynamics on climate: interactive effects of changing vegetation and disturbance regimes. Ecol Monog. 87:665–684.
- Sommerfeld A, Rammer W, Heurich M, Hilmers T, Müller T, Seidl R (2021) Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? J Ecol. 109: 737-749.
- Raffa KF, Aukema BH, Erbilgin N, Klepzig KD, Wallin KF (2005) Interactions among conifer terpenoids and bark beetles across multiple levels of scale: an attempt to understand links between population patterns and physiological processes In T R John (Ed), Recent Advances in Phytochemistry (pp 79–118) Kidlington, Oxford, UK: Elsevier.
- Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 167: 353–76.
- Erbilgin N, Krokene P, Christiansen E, Zeneli G, Gershenzon J (2006) Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia. 148: 426–436.
- Vazquez-Gonzalez C, Zas R, Erbilgin N, Ferrenberg S, Rozas V, Sampedro L (2020) Resin ducts as resistance traits in conifers: linking dendrochronology and resin-based defenses. Tree Physiol. 40:1313-1326.
- Erbilgin N (2019) Phytochemicals as mediators for host range expansion of a native invasive forest insect herbivore. New Phytol. 221: 1268–1278.
- Mason CJ, Keefover-Ring K, Villari C, Klutsch JG, Cook S, Bonello P, Erbilgin N, Raffa KF, Townswend PA (2019) Anatomical defenses against bark beetles related to degree of historical exposure between species and are allocated independently of chemical defenses within trees. Plant Cell Environ. 42: 633–646.
- Nagel R, Hammerbacher A, Kunert G, Phillips MA, Gershenzon J, Schmidt A (2022) Bark beetle attack history does not influence the induction of terpene and phenolic defenses in mature Norway spruce (Picea abies) trees by the bark beetle-associated fungus Endoconidiophora polonica. Front Plant Sci. 6:13:892907.
- Mageroy MH, Nagy NE, Steffenrem A, Krokene P, Hietala AM (2023) Conifer defences against pathogens and pests - mechanisms, breeding, and management. Curr For Rep. 9: 429–443.
- Erbilgin N, Ma C, Whitehouse C, Shan B, Najar A, Evenden M (2014) Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. New Phytol. 201: 940–950.
- Chiu CC, Keeling CI, Bohlmann J (2017) Toxicity of pine monoterpenes to mountain pine beetle. Sci Reps. 7: 8858 (7). [CrossRef]
- Ullah A, Klutsch JG, Erbilgin N (2021) Production of complementary defense metabolites reflects a co-evolutionary arms race between a host plant and a mutualistic bark beetle-fungal complex. Plant Cell Environ. 44: 3064-3077.
- Chiu CC, Bohlmann J (2022) Mountain pine beetle epidemic: an interplay of terpenoids in host defense and insect pheromones. Ann Rev Plant Biol. 73: 475-494.
- Schiebe C, Hammerbacher A, Witzell J et al (2012) Inducibility of chemical defenses in Norway spruce bark is correlated with unsuccessful mass attacks by the spruce bark beetle. Oecologia. 170:183–198.
- Goodsman DW, Lusebrink I, Landhäusser SM, Erbilgin N, Lieffers VJ (2013) Variation in carbon availability, defense chemistry and susceptibility to fungal invasion along the stems of mature trees New Phytol 197: 586–594.
- Roth M, Hussain A, Cale JA, Erbilgin N (2018) Successful colonization of lodgepole pine trees by mountain pine beetle increased monoterpene production and exhausted carbohydrate reserves. J Chem Ecol. 44: 209–214.
- Erbilgin, N.; Zanganeh, L.; Klutsch, J.G.; Chen, S.; Zhao, S.; Ishangulyyeva, G.; Burr, S.J.; Gaylord, M.; Hofstetter, R.; Keefover-Ring, K.; et al. Combined drought and bark beetle attacks deplete non-structural carbohydrates and promote death of mature pine trees. Plant, Cell Environ. 2021, 44, 3866–3881. [Google Scholar] [CrossRef] [PubMed]
- Huang JB, Kautz M, Trowbridge AM et al (2020) Tree defense and bark beetles in a drying world: carbon partitioning, functioning and modeling. New Phytol. 225: 26-36.
- Zas R, Moreira X, Ramos M et al (2015) Intraspecific variation of anatomical and chemical defensive traits in Maritime pine (Pinus pinaster) as factors in susceptibility to the pinewood nematode (Bursaphelenchus xylophilus). Trees-Struct Func. 29:663–673.
- Blanche CA, Lorio P L, Sommers RA, Hodges JD, Nebeker T E (1992) Seasonal cambial growth and development of loblolly pine: xylem formation, inner bark chemistry, resin ducts, and resin flow. For Ecol Manag. 49: 151–65.
- Lombardero MJ, Ayres MP, Lorio Jr, PL, Ruel JJ (2000) Environmental effects on constitutive and inducible resin defences of Pinus taeda. Ecol Let. 3: 329–339.
- Rodríguez-García A, López R, Martín JA, Pinillos F, Gil L (2014) Resin yield in Pinus pinaster is related to tree dendrometry, stand density and tapping-induced systemic changes in xylem anatomy. For Ecol Manag. 313: 47–54.
- Hood S, Sala A (2015) Ponderosa pine resin defenses and growth: metrics matter. Tree Physiol. 35: 1223–1235.
- Westbrook JW, Walker AR, Neves LG et al (2015) Discovering candidate genes that regulate resin canal number in Pinus taeda stems by integrating genetic analysis across environments, ages, and populations. New Phytol. 205: 627–641.
- Kane JM, Kolb TE (2010) Importance of resin ducts in reducing ponderosa pine mortality from bark beetle attack. Oecologia. 164: 601–609.
- Ferrenberg S, Kane JM, Mitton JB (2014) Resin duct characteristics associated with tree resistance to bark beetles across lodgepole and limber pines. Oecologia. 174: 1283–92.
- Hood S, Sala A, Heyerdahl EK, Boutin M (2015) Low-severity fire increases tree defense against bark beetle attacks. Ecology. 96: 1846–1855.
- Cale AC, Klutsch GJ, Dykstra BC, Peters B, Erbilgin N (2019) Pathophysiological responses of pine defensive metabolites largely lack differences between pine species but vary with eliciting ophiostomatoid fungal species. Tree Physiol. 39: 1121–1135.
- Kersten PJ, Kopper BJ, Raffa KF, Illman BL (2006) Rapid analysis of abietanes in conifers J Chem Ecol. 22: 1367–1388.
- R Core Team, R, 2018 R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria https://wwwR-projectorg/.
- Harrell, F.E., Jr. HMISC: Harrell Miscellaneous. R Package Version 4.1-1. 2018. Available online: http://CRAN.R-project.org/package=Hmisc (accessed on 21 March 2022).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H.; Vegan: Community Ecology Package. R Package Version 2.4-6. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 27 April 2018).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67(1): 1-48.
- Klutsch JG, Erbilgin N (2018) Dwarf mistletoe infection in jack pine alters growth-defense relationships. Tree Physiol. 38:1538–1547.
- Erbilgin N (2019) Phytochemicals as mediators for host range expansion of a native invasive forest insect herbivore. New Phytol. 221: 1268–1278.
- Moles AT, Peco B, Wallis IR et al (2013) Correlations between physical and chemical defences in plants: tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytol. 198: 252–263.
- Gaylord ML, Kolb TE, McDowell NG (2015) Mechanisms of piñon pine mortality after severe drought: a retrospective study of mature trees. Tree Physiol. 35: 806–816.
- Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plans. Ann Rev Plant Biol. 65: 667–687.
- Mullin M, Klutsch JG, Cale JA, Hussain A, Zhao S, Whitehouse C, Erbilgin N (2021) Primary and secondary metabolite profiles of lodgepole pine trees change with elevation, but not with latitude. J Chem Ecol. 47: 280–293.
- Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. The Quar Rev Biol. 67: 283–335.
- Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol. 20: 1281–1328.
- Wiley E, Rogers BJ, Hodgkinson R, Landhäusser SM (2016) Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytol. 209: 550–562.
- Kolb TE, Keefover-Ring K, Burr SJ, Hofstetter R, Gaylord M, Raffa K F (2019) Drought-mediated changes in tree physiological processes weaken tree defenses to bark beetle attack. J Chem Ecol. 45:888–900.
- Swihart RK, Bryant JP (2001) Importance of biogeography and ontogeny of woody plants in winter herbivory by mammals. J Mammal. 82: 1–21.
- Erbilgin N, Colgan LJ (2012) Differential effects of plant ontogeny and damage type on phloem and foliage monoterpenes in jack pine (Pinus banksiana). Tree Physiol. 32: 946–957.
- Shrimpton DM (1973) Age- and size-related response of lodgepole pine to inoculation with Europhium clavigerum. Can J Bot. 51:1155–1160.
- Hartmann H, Moura CF, Anderegg WR et al (2018) Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 218:15–28.
- Seybold SJ, Huber DPW, Lee JC, Graves AD, Bohlmann J (2006) Pine monoterpenes and pine bark beetles: a marriage of convenience for defense and chemical communication. Phytochem Rev. 5: 143–178.
- Reid ML, Purcell JRC (2011) Condition-dependent tolerance of monoterpenes in an insect herbivore. Arthropod-Plant Inter. 5: 331–337.
- Shrimpton DM, Reid RW (1973) Change in resistance of lodgepole pine to mountain pine beetle between 1965 and 1972. Can J For Res. 3:430–43.

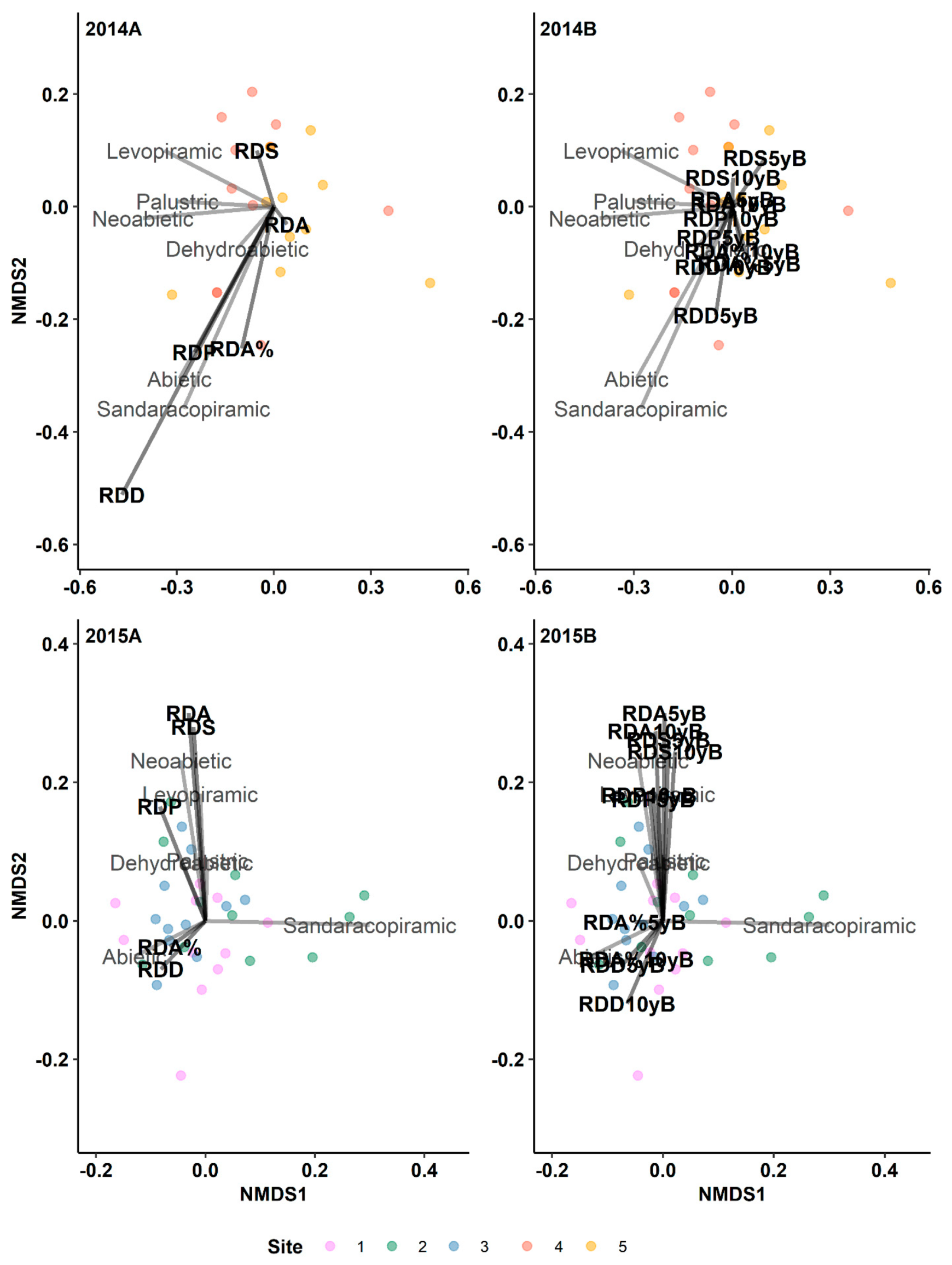
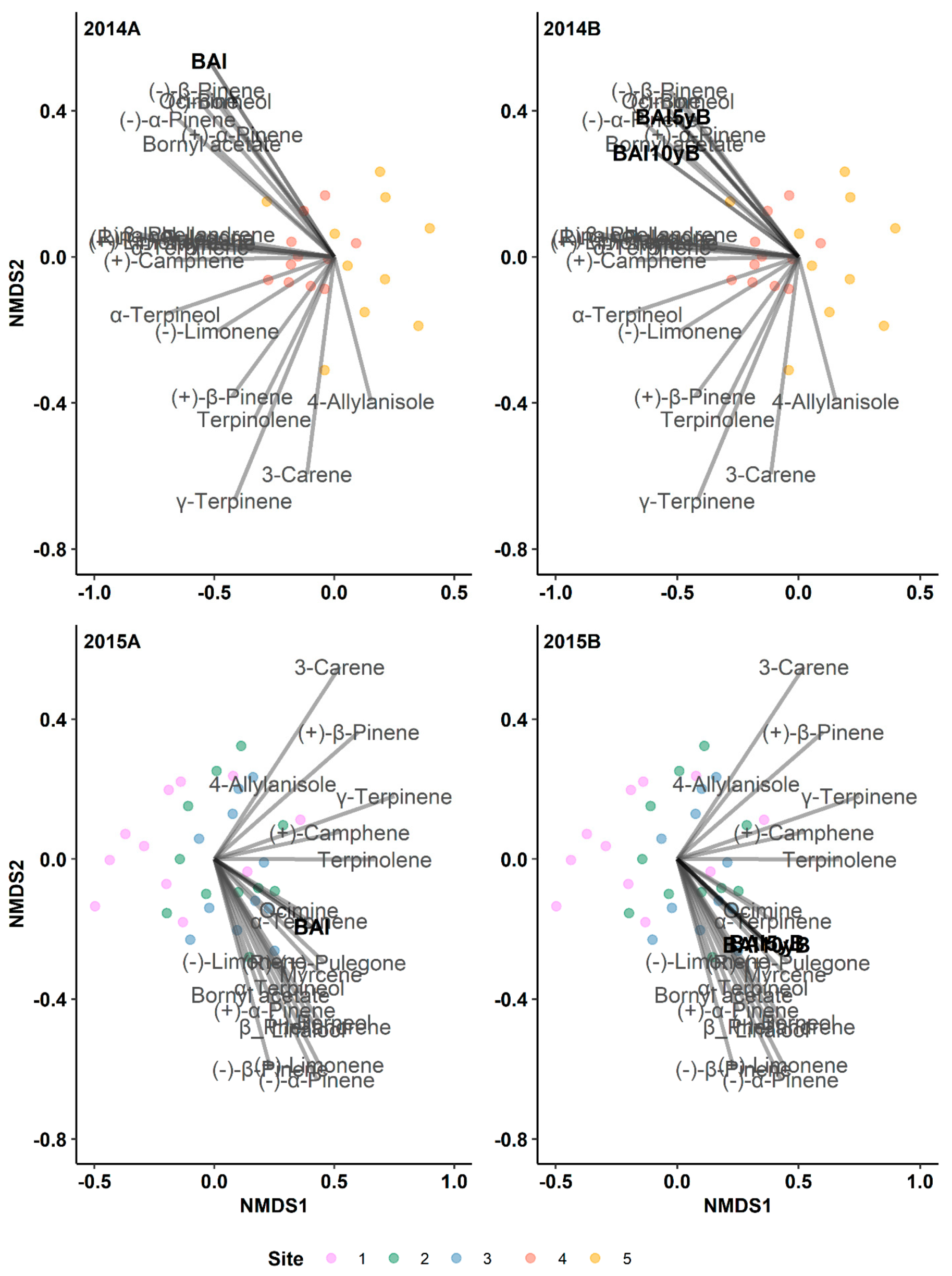
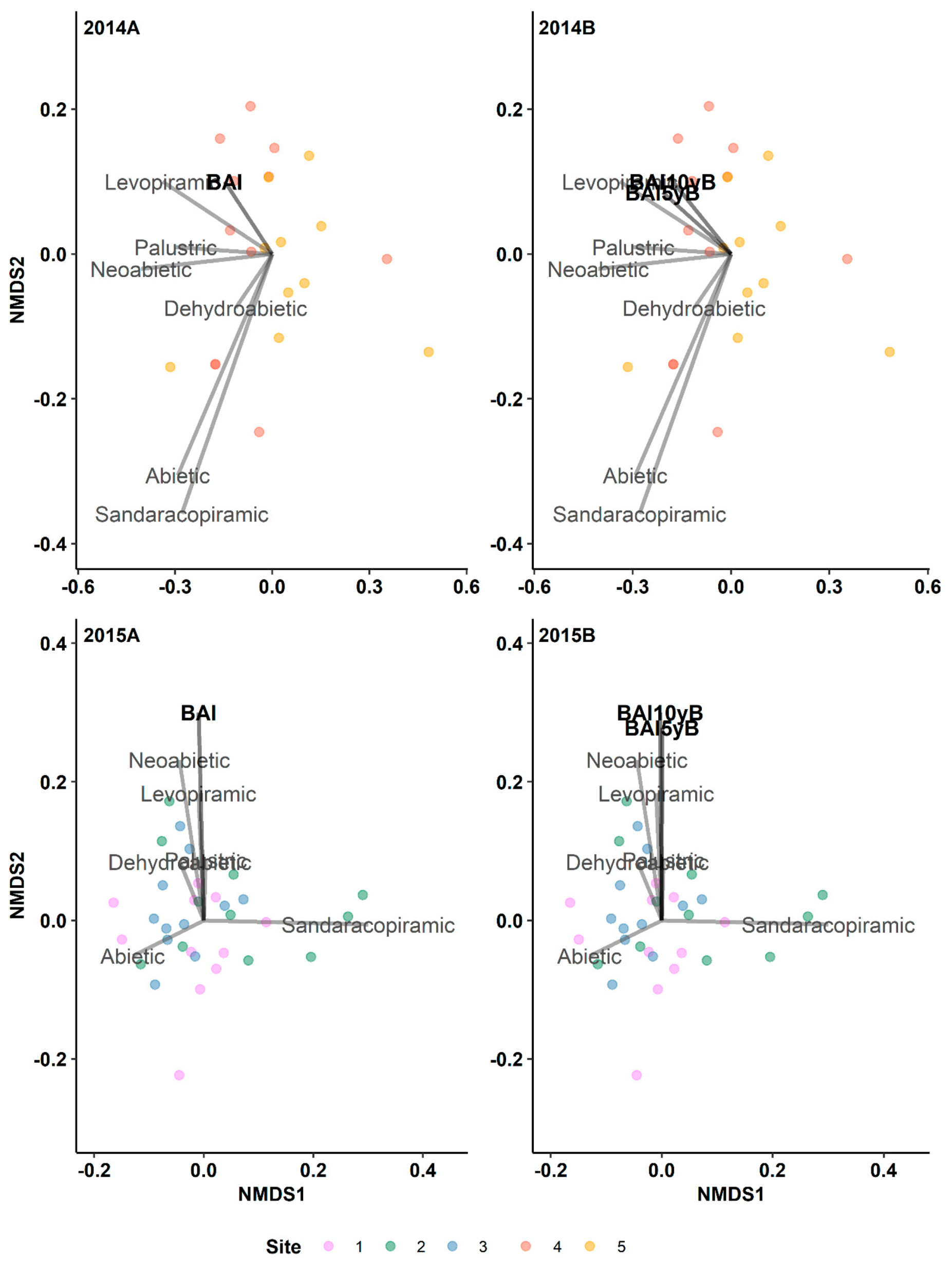
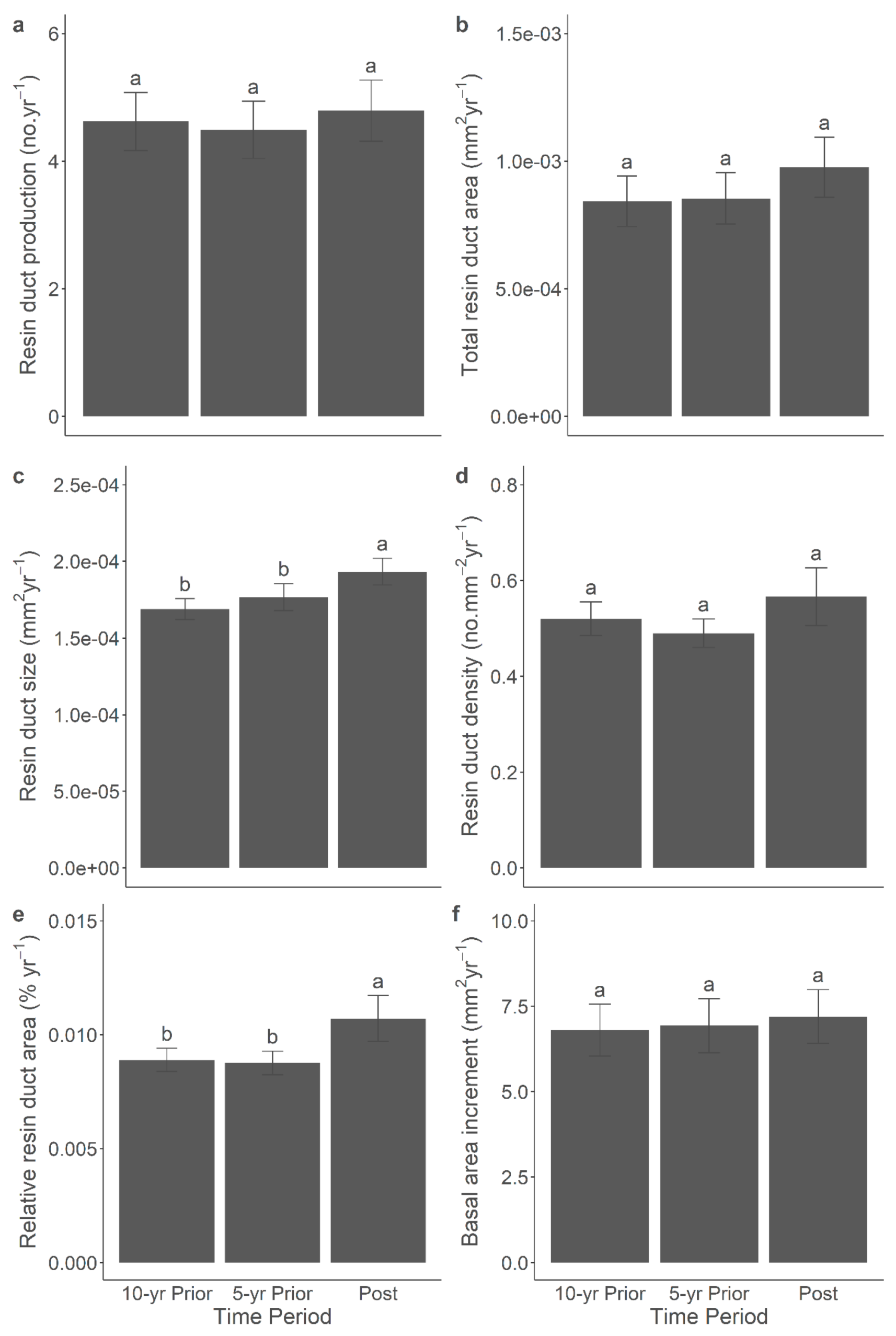
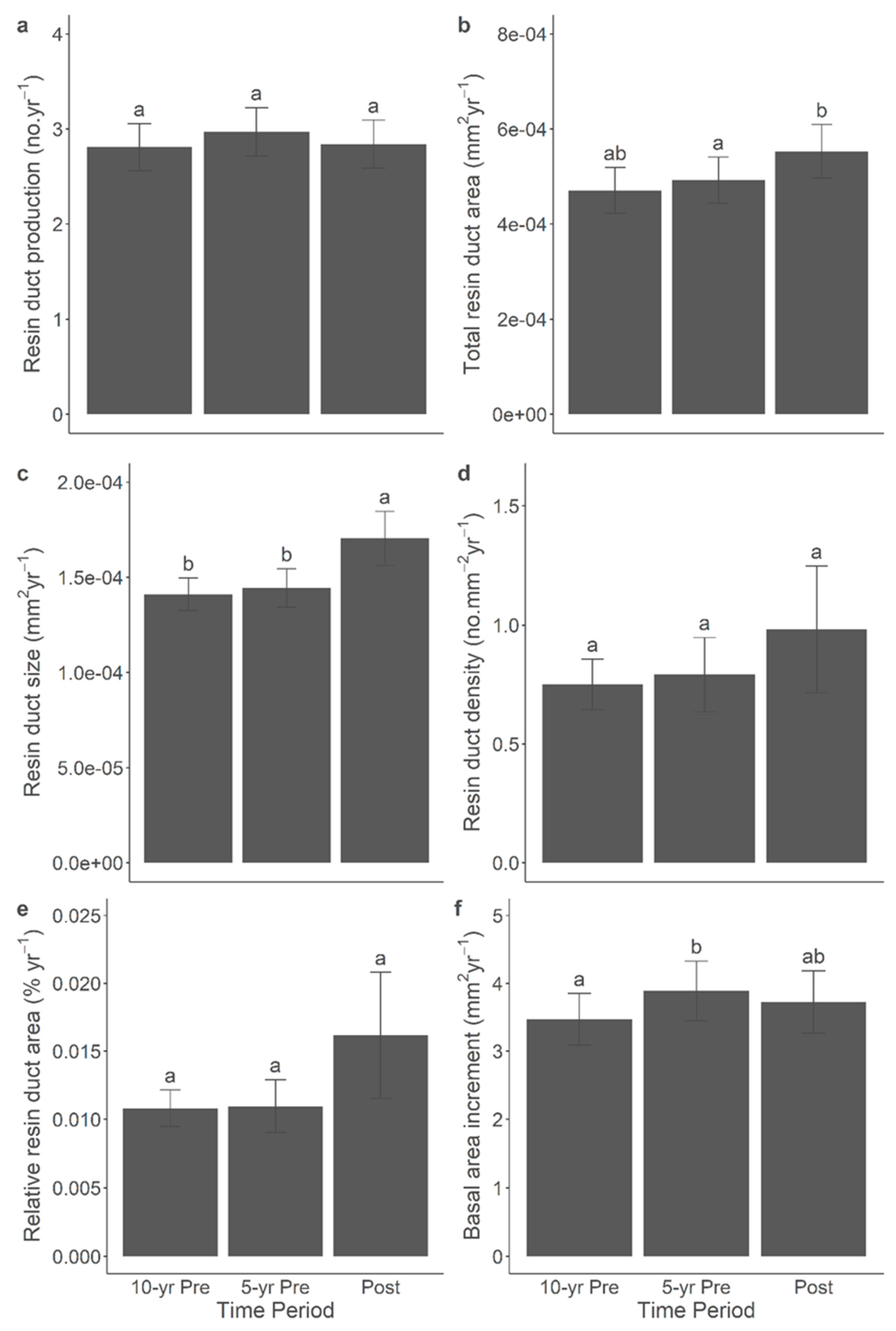
| Outbreak Years | Period | Variables* | Total monoterpenes | ||
| Coefficient | t-value | P-value | |||
| 2014 | Post-outbreak | Resin duct production | 1.1e3 | 4.3 | <0.001 |
| Total resin duct area | 4.8e6 | 4.8 | <0.001 | ||
| Individual resin duct size | 4.2e7 | 2.4 | 0.029 | ||
| Resin duct density | 1.4e3 | 0.5 | 0.626 | ||
| Relative resin duct area (%) | 2.2e5 | 1.4 | 0.187 | ||
| 5-yr pre-outbreak | Resin duct production | 1.1e3 | 3.1 | 0.005 | |
| Total resin duct area | 4.8e6 | 3.2 | 0.005 | ||
| Individual resin duct size | 3.0e7 | 1.7 | 0.107 | ||
| Resin duct density | 2.7e3 | 0.5 | 0.637 | ||
| Relative resin duct area (%) | 4.2e5 | 1.4 | 0.189 | ||
| 10-yr pre-outbreak | Resin duct production | 1.4e3 | 4.2 | <0.001 | |
| Total resin duct area | 6.2e6 | 4.1 | <0.001 | ||
| Individual resin duct size | 6.0e7 | 2.4 | 0.025 | ||
| Resin duct density | 1.5e3 | 0.3 | 0.761 | ||
| Relative resin duct area (%) | 4.2e5 | 1.3 | 0.219 | ||
| 2015 | Post-outbreak | Resin duct production | 78.7 | 0.2 | 0.831 |
| Total resin duct area | 3.4e6 | 2.1 | 0.045 | ||
| Individual resin duct size | 2.3e7 | 4.2 | <0.001 | ||
| Resin duct density | -100.8 | -0.3 | 0.767 | ||
| Relative resin duct area (%) | -1.3e3 | -0.1 | 0.946 | ||
| 5-yr pre-outbreak | Resin duct production | 584.0 | 1.6 | 0.127 | |
| Total resin duct area | 5.8e6 | 3.3 | 0.002 | ||
| Individual resin duct size | 3.0e7 | 3.7 | 0.002 | ||
| Resin duct density | -127.4 | -0.2 | 0.830 | ||
| Relative resin duct area (%) | -8.0e3 | -0.2 | 0.868 | ||
| 10-yr pre-outbreak | Resin duct production | 741.6 | 1.8 | 0.081 | |
| Total resin duct area | 6.2e6 | 3.3 | 0.002 | ||
| Individual resin duct size | 4.0e7 | 4.1 | <0.001 | ||
| Resin duct density | -280.3 | -0.3 | 0.762 | ||
| Relative resin duct area (%) | -6.8e3 | -0.1 | 0.926 | ||
| Monoterpenes | 2014-Outbreak | 2015-Outbreak | ||||||||||||
| Post-outbreak | 5-yr pre-outbreak | 10-yr pre-outbreak | Post-outbreak | 5-yr pre-outbreak | 10-yr pre-outbreak | |||||||||
| RDP | RDA | RDS | RDP | RDA | RDP | RDA | RDS | RDA | RDS | RDA | RDS | RDA | RDS | |
| 4-Allylanisole | ||||||||||||||
| (-)-Borneol | ++ | ++ | ++ | ++ | + | + | + | + | ++ | + | + | + | ++ | |
| Bornyl acetate | + | + | + | + | + | + | ||||||||
| (+)-Camphene | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | + | +++ | +++ | |||
| 3-Carene | + | + | + | |||||||||||
| (+)-Limonene | +++ | +++ | + | ++ | ++ | +++ | +++ | + | +++ | ++ | ++ | ++ | +++ | |
| (-)-Limonene | ||||||||||||||
| Linalool | + | ++ | ++ | +++ | +++ | ++ | ++ | + | ++ | + | ++ | ++ | ++ | |
| Myrcene | ++ | +++ | ++ | ++ | +++ | +++ | + | +++ | ++ | +++ | ++ | ++ | ||
| Ocimene | ++ | ++ | +++ | +++ | +++ | +++ | ||||||||
| β-Phellandrene | ++ | ++ | + | + | ++ | +++ | +++ | + | +++ | + | ++ | + | +++ | |
| (+)-α-Pinene | + | ++ | + | + | + | |||||||||
| (-)-α-Pinene | +++ | +++ | + | +++ | +++ | +++ | +++ | + | + | +++ | +++ | ++ | +++ | +++ |
| (+)-β-Pinene | + | ++ | + | + | + | + | + | ++ | + | + | + | + | + | ++ |
| (-)-β-Pinene | ++ | +++ | ++ | + | ++ | ++ | ++ | + | ++ | +++ | ||||
| β-Phellandrene | ++ | ++ | + | + | ++ | +++ | +++ | + | +++ | + | ++ | + | +++ | |
| (R)-(+)-Pulegone | + | + | ++ | ++ | + | ++ | ++ | |||||||
| α-Terpinene | + | + | + | |||||||||||
| γ-Terpinene | + | + | + | ++ | + | + | ++ | ++ | + | ++ | ||||
| α-Terpineol | +++ | ++ | + | +++ | ||||||||||
| Terpinolene | ++ | +++ | + | + | + | |||||||||
| Terpene classes | 2014-Outbreak | 2015-Outbreak | ||||
| Post-outbreak BAI |
5-yr pre-outbreak BAI |
10-yr pre-outbreak BAI |
Post-outbreak BAI |
5-yr pre-outbreak BAI |
10-yr pre-outbreak BAI |
|
| Monoterpenes | ||||||
| 4-Allylanisole | ||||||
| (-)-Borneol | +++ | + | ++ | |||
| Bornyl acetate | ++ | + | + | |||
| (+)-Camphene | ++ | ++ | ++ | + | + | + |
| 3-Carene | ||||||
| (+)-Limonene | + | + | ++ | ++ | +++ | +++ |
| (-)-Limonene | ||||||
| Linalool | +++ | +++ | +++ | +++ | ++ | +++ |
| Myrcene | ++ | ++ | +++ | ++ | +++ | +++ |
| Ocimene | + | + | ++ | |||
| β-Phellandrene | + | ++ | ++ | ++ | ++ | +++ |
| (+)-α-Pinene | ++ | + | ||||
| (-)-α-Pinene | +++ | ++ | +++ | ++ | +++ | +++ |
| (+)-β-Pinene | + | + | + | + | + | + |
| (-)-β-Pinene | ++ | + | ++ | ++ | ++ | |
| (R)-(+)-Pulegone | ++ | +++ | +++ | |||
| α-Terpinene | ||||||
| γ-Terpinene | + | ++ | ++ | |||
| α-Terpineol | + | +++ | +++ | +++ | ||
| Terpinolene | + | + | + | |||
| Diterpenes | ||||||
| Abietic | + | + | + | |||
| Dehydroabietic | ++ | ++ | +++ | |||
| Levopiramic | +++ | +++ | +++ | |||
| Neoabietic | +++ | +++ | +++ | |||
| Palustric | ++ | ++ | +++ | |||
| Sandaracopiramic | ||||||
| Tree age | ||
| Monoterpenes | 2014-Outbreak | 2015-Outbreak |
| 4-Allylanisole | ||
| (-)-Borneol | ||
| Bornyl acetate | ||
| (+)-Camphene | - | |
| 3-Carene | ||
| (+)-Limonene | - | -- |
| (-)-Limonene | ||
| Linalool | --- | -- |
| Myrcene | - | |
| Ocimene | ||
| β-Phellandrene | - | |
| (+)-α-Pinene | ||
| (-)-α-Pinene | - | -- |
| (+)-β-Pinene | - | |
| (-)-β-Pinene | - | |
| (R)-(+)-Pulegone | - | |
| α-Terpinene | - | |
| γ-Terpinene | -- | -- |
| α-Terpineol | -- | |
| Terpinolene | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





