Submitted:
08 August 2024
Posted:
09 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background on Head and Neck Cancer
- Heterogeneity: Oral cancer is not a single disease; it includes various subtypes with distinct genetic and clinical characteristics. This heterogeneity complicates diagnosis.
- Tissue sampling: Accurate diagnosis often requires a biopsy of the affected tissue. However, obtaining a representative sample can be challenging, and false negatives may occur if the biopsy misses the cancerous area.
- Recurrence prediction: Predicting the recurrence of oral cancer after treatment is challenging due to factors like tumor heterogeneity, incomplete removal of cancer cells during surgery, and resistance to therapy.
- Imaging limitations: While medical imaging, such as CT scans, is valuable for cancer diagnosis and staging, it may not always detect small or early-stage lesions accurately. This can lead to underdiagnosis.
- Patient variability: Patient-related factors, such as lifestyle choices, overall health, and genetic predisposition, can influence both the initial diagnosis and the likelihood of recurrence.
- Post-treatment changes: Treatments for oral cancer, such as surgery and radiation therapy, can result in changes to the oral cavity, affecting speech, swallowing, and quality of life. Distinguishing between post-treatment changes and cancer recurrence can be difficult.
- Limited biomarkers: Currently, there are limited specific biomarkers for oral cancer, making it challenging to identify individuals at high risk.
- Patient awareness and access: Some patients may lack awareness of oral cancer risk factors and symptoms, and others may face barriers to accessing healthcare, delaying diagnosis.
1.2. Review Objectives
2. AI Technologies and Methodologies
2.1. Machine Learning Techniques
2.2. Deep Learning Approaches
2.3. Natural Language Processing
3. Diagnostic Applications
3.1. Early Detection
3.2. Biomarker Discovery
4. Prognostic and Predictive Models
4.1. Risk Assessment Tools
4.2. Personalized Treatment Planning
4.3. Monitoring and Surveillance
5. Therapeutic Applications
5.1. Drug Discovery
5.2. Precision Medicine
5.3. Surgery
6. Challenges and Limitations
6.1. Data Quality and Accessibility
6.2. Algorithm Bias and Fairness
6.3. Integration into Clinical Practice
7. Future Directions
7.1. Innovations on the Horizon
7.1.1. Advanced Machine Learning Algorithms
7.1.2. Explainable Machine Intelligence
7.1.3. Integration of Multimodal Data
7.1.4. AI in Drug Discovery
7.1.5. Real-Time Monitoring and Decision Support
7.1.6. Telemedicine and Remote Care
7.2. Interdisciplinary Collaboration
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Head and neck cancer. NHS. https://www.nhs.uk/conditions/head-and-neck-cancer/. Date of access: 5 August 2024.
- Head and neck cancers. NIH. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Date of access: 5 August 2024.
- Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers 2020; 6(1):92. [CrossRef]
- Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015; 24(3):491-508. [CrossRef]
- Wenig, BM. Squamous cell carcinoma of the upper aerodigestive tract: dysplasia and select variants. Mod Pathol. 2017; 30(s1):S112-S118. [CrossRef]
- Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S, Nice EC, Tang J, Huang C. Oral squamous cell carcinomas: state of the field and emerging directions. Int J Oral Sci. 2023; 15(1):44. [CrossRef]
- Tarle M, Luksic I. Pathogenesis and therapy of oral carcinogenesis. Int J Mol Sci. 2024; 25(12):6343. [CrossRef]
- Young A, Okuyemi OT. Malignant Salivary Gland Tumors. Updated 2 Jan 12 2023. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563022/.
- Coletta RD, Yeudall WA, Salo T. Grand challenges in oral cancers. Front Oral Health. 2020; 1:3. [CrossRef]
- Oral health. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/oral-health. Date of access: 01 May 2024.
- Badwelan M, Muaddi H, Ahmed A, Lee KT, Tran SD. Oral squamous cell carcinoma and concomitant primary tumors, what do we know? A review of the literature. Curr Oncol. 2023; 30(4):3721-3734. [CrossRef]
- Tranby EP, Heaton LJ, Tomar SL, Kelly AL, Fager GL, Backley M, Frantsve-Hawley J. Oral cancer prevalence, mortality, and costs in medicaid and commercial insurance claims data. Cancer Epidemiol Biomarkers Prev. 2022; 31(9):1849-1857. [CrossRef]
- Sun R, Dou W, Liu W, Li J, Han X, Li S, Wu X, Wang F, Xu X, Li J. Global, regional, and national burden of oral cancer and its attributable risk factors from 1990 to 2019. Cancer Med. 2023 ; 12(12):13811-13820. [CrossRef]
- Sathish N, Wang X, Yuan Y. Human papillomavirus (HPV)-associated oral cancers and treatment strategies. J Dent Res. 2014; 93(7 Suppl):29S-36S. [CrossRef]
- Hernandez BY, Zhu X, Goodman MT, Gatewood R, Mendiola P, Quinata K, Paulino YC. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS One. 2017; 12(2):e0172196. [CrossRef]
- Warnakulasuriya S, Chen THH. Areca Nut and oral cancer: evidence from studies conducted in humans. J Dent Res. 2022; 101(10):1139-1146. [CrossRef]
- Rodriguez-Molinero J, Miguelanez-Medran BDC, Puente-Gutierrez C, Delgado-Somolinos E, Martin Carreras-Presas C, Fernandez-Farhall J, Lopez-Sanchez AF. Association between oral cancer and diet: an update. Nutrients. 2021; 13(4):1299. [CrossRef]
- Huang Y, Zhao J, Mao G, Lee GS, Zhang J, Bi L, Gu L, Chang Z, Valentino J, Li GM. Identification of novel genetic variants predisposing to familial oral squamous cell carcinomas. Cell Discov. 2019; 5:57. [CrossRef]
- Lima AM, Meira IA, Soares MS, Bonan PR, Mélo CB, Piagge CS. Delay in diagnosis of oral cancer: a systematic review. Med Oral Patol Oral Cir Bucal. 2021 Nov 1;26(6):e815-e824. [CrossRef]
- Watters C, Brar S, Pepper T. Cancer of the oral mucosa. Updated 15 Mar 2024. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK565867/.
- Daniel M, Rogers SN. Professional delays in referral of patients with mouth cancer: six case histories. Br Dent J. 2022; 233(12):1003-1008. [CrossRef]
- Homer JJ, Winter SC, Abbey EC, et al. Head and neck cancer: United Kingdom national multidisciplinary guidelines, sixth edition. The Journal of Laryngology & Otology. 2024;138(S1):S1-S224. [CrossRef]
- Sankaranarayanan R, Ramadas K, Amarasinghe H, Subramanian S, Johnson N. Oral cancer: prevention, early detection, and treatment. In: Gelband H, Jha P, Sankaranarayanan R, et al., editors. Cancer: Disease Control Priorities, Third Edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development. The World Bank, 2015. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343649/. [CrossRef]
- Ghantous Y, Nashef A, Sidransky D, Abdelraziq M, Alkeesh K, Araidy S, Koch W, Brait M, Abu El-Naaj I. Clinical and prognostic significance of the eighth edition oral cancer staging system. Cancers (Basel) 2022; 14(19):4632. [CrossRef]
- Huang SH, O’Sullivan B. Oral cancer: Current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013; 18(2):e233-40. [CrossRef]
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12(4):237-51. [CrossRef]
- Gupta SL, Basu S, Soni V, Jaiswal RK. Immunotherapy: an alternative promising therapeutic approach against cancers. Mol Biol Rep. 2022; 49(10):9903-9913. [CrossRef]
- Voss JO, Freund L, Neumann F, Mrosk F, Rubarth K, Kreutzer K, Doll C, Heiland M, Koerdt S. Prognostic value of lymph node involvement in oral squamous cell carcinoma. Clin Oral Investig. 2022; 26(11):6711-6720. [CrossRef]
- Gonzalez-Moles MA, Aguilar-Ruiz M, Ramos-Garcia P. Challenges in the early diagnosis of oral cancer, evidence gaps and strategies for improvement: a scoping review of systematic reviews. Cancers (Basel) 2022 Oct 10;14(19):4967. [CrossRef]
- Carter, N. Mouth cancer: the challenges ahead. BDJ In Pract 2021; 34:20–21. [CrossRef]
- Fuller C, Mohamed A, Elhalawani H. Predict from CT data the HPV phenotype of oropharynx tumors; compared to ground-truth results previously obtained by p16 or HPV testing. April 2017. Date of Access 07 October 2023. [CrossRef]
- Ribeiro-de-Assis MCF, Soares JP, de Lima LM, de Barros LAP, Grao-Velloso TR, Krohling RA, Camisasca DR. NDB-UFES: An oral cancer and leukoplakia dataset composed of histopathological images and patient data. Data Brief 2023; 48:109128. [CrossRef]
- Veeraraghavan VP, Daniel S, Dasari AK, Aileni KR, Patil C, Patil SR, Harnessing artificial intelligence for predictive modelling in oral oncology: opportunities, challenges, and clinical perspectives. Oral Oncology Reports 2024; 11:100591. [CrossRef]
- de Chauveron J, Unger M, Lescaille G, Wendling L, Kurtz C, Rochefort J. Artificial intelligence for oral squamous cell carcinoma detection based on oral photographs: a comprehensive literature review. Cancer Med. 2024; 13(1):e6822. [CrossRef]
- Sarker, IH. AI-based modeling: techniques, applications and research issues towards automation, intelligent and smart systems. SN Comput Sci. 2022;3(2):158. [CrossRef]
- Rawas, S. AI: the future of humanity. Discov Artif Intell 2014; 4:25. [CrossRef]
- Chen ZH, Lin L, Wu CF, Li CF, Xu RH, Sun Y. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun (Lond) 2021; 41(11):1100-1115. [CrossRef]
- Koh DM, Papanikolaou N, Bick U, Illing R, Kahn CE Jr, Kalpathi-Cramer J, Matos C, Marti-Bonmatí L, Miles A, Mun SK, Napel S, Rockall A, Sala E, Strickland N, Prior F. Artificial intelligence and machine learning in cancer imaging. Commun Med (Lond) 2022; 2:133. [CrossRef]
- Lipkova J, Chen RJ, Chen B, Lu MY, Barbieri M, Shao D, Vaidya AJ, Chen C, Zhuang L, Williamson DFK, Shaban M, Chen TY, Mahmood F. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 2022; 40(10):1095-1110. [CrossRef]
- Ke J, Shen Y, Lu Y, Guo Y, Shen D. Mine local homogeneous representation by interaction information clustering with unsupervised learning in histopathology images. Comput Methods Programs Biomed. 2023; 235:107520. [CrossRef]
- Yaqoob A, Aziz RM, Verma NK. Applications and techniques of machine learning in cancer classification: a systematic review. Hum-Cent Intell Syst 2013; 3:588–615. [CrossRef]
- Eckardt JN, Wendt K, Bornhäuser M, Middeke JM. Reinforcement learning for precision oncology. Cancers (Basel) 2021; 13(18):4624. [CrossRef]
- Liu M, Shen X, Pan W. Deep reinforcement learning for personalized treatment recommendation. Stat Med. 2022; 41(20):4034-4056. [CrossRef]
- Liu S, See KC, Ngiam KY, Celi LA, Sun X, Feng M. Reinforcement learning for clinical decision support in critical care: comprehensive review. J Med Internet Res. 2020; 22(7):e18477. [CrossRef]
- Datta S, Li Y, Ruppert MM, Ren Y, Shickel B, Ozrazgat-Baslanti T, Rashidi P, Bihorac A. Reinforcement learning in surgery. Surgery 2021; 170(1):329-332. [CrossRef]
- Lee H, Yoon HK, Kim J, Park JS, Koo CH, Won D, Lee HC. Development and validation of a reinforcement learning model for ventilation control during emergence from general anesthesia. NPJ Digit Med. 2023; 6(1):145. [CrossRef]
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015; 521:436-444. [CrossRef]
- Dixit S, Kumar A, Srinivasan K. A Current review of machine learning and deep learning models in oral cancer diagnosis: recent technologies, open challenges, and future research directions. Diagnostics 2023; 13(7):1353. [CrossRef]
- Albalawi E, Thakur A, Ramakrishna MT, Bhatia Khan S, SankaraNarayanan S, Almarri B, Hadi TH. Oral squamous cell carcinoma detection using EfficientNet on histopathological images. Front Med (Lausanne) 2024; 10:1349336. [CrossRef]
- Illimoottil M, Ginat D. Recent advances in deep learning and medical imaging for head and neck cancer treatment: MRI, CT, and PET scans. Cancers (Basel). 2023; 15(13):3267. [CrossRef]
- Warin K, Suebnukarn S. Deep learning in oral cancer–a systematic review. BMC Oral Health 2024; 24(1):212. [CrossRef]
- Varalakshmi D, Tharaheswari M, Anand T, Saravanan KM. Transforming oral cancer care: the promise of deep learning in diagnosis. Oral Oncology Reports 2024; 10: 100482. [CrossRef]
- Sheng JQ, Hu PJ, Liu X, Huang TS, Chen YH. Predictive analytics for care and management of patients with acute diseases: deep learning-based method to predict crucial complication phenotypes. J Med Internet Res. 2021; 23(2):e18372. [CrossRef]
- Maleki Varnosfaderani S, Forouzanfar M. The role of AI in hospitals and clinics: transforming healthcare in the 21st century. Bioengineering (Basel) 2024; 11(4):337. [CrossRef]
- Berahmand K, Daneshfar F, Salehi ES, Li Y, Xu Y. Autoencoders and their applications in machine learning: a survey. Artif Intell Rev 2024; 57:28. [CrossRef]
- Janoudi G, Uzun Rada M, Fell DB, Ray JG, Foster AM, Giffen R, Clifford T, Walker MC. Outlier analysis for accelerating clinical discovery: an augmented intelligence framework and a systematic review. PLOS Digit Health 2024; 3(5):e0000515. [CrossRef]
- Franco EF, Rana P, Cruz A, Calderon VV, Azevedo V, Ramos RTJ, Ghosh P. Performance comparison of deep learning autoencoders for cancer subtype detection using multi-omics data. Cancers (Basel) 2021; 13(9):2013. [CrossRef]
- Chen RJ, Lu MY, Chen TY, Williamson DKF, Mahmood F. Synthetic data in machine learning for medicine and healthcare. Nat Biomed Eng 2021; 5:493-497. [CrossRef]
- Song B, KC DR, Yang RY, Li S, Zhang C, Liang R. Classification of mobile-based oral cancer images using the vision transformer and the Swin transformer. Cancers (Basel) 2024; 16(5):987. [CrossRef]
- Friedman C, Rindflesch TC, Corn M. Natural language processing: state of the art and prospects for significant progress, a workshop sponsored by the National Library of Medicine. J Biomed Inform. 2013; 46(5):765-73. [CrossRef]
- Velupillai S, Suominen H, Liakata M, Roberts A, Shah AD, Morley K, Osborn D, Hayes J, Stewart R, Downs J, Chapman W, Dutta R. Using clinical natural language processing for health outcomes research: overview and actionable suggestions for future advances. J Biomed Inform. 2018; 88:11-19. [CrossRef]
- Wu H, Wang M, Wu J, Francis F, Chang YH, Shavick A, Dong H, Poon MTC, Fitzpatrick N, Levine AP, Slater LT, Handy A, Karwath A, Gkoutos GV, Chelala C, Shah AD, Stewart R, Collier N, Alex B, Whiteley W, Sudlow C, Roberts A, Dobson RJB. A survey on clinical natural language processing in the United Kingdom from 2007 to 2022. NPJ Digit Med. 2022; 5(1):186. [CrossRef]
- Clusmann J, Kolbinger FR, Muti HS, Carrero ZI, Eckardt JN, Laleh NG, Löffler CML, Schwarzkopf SC, Unger M, Veldhuizen GP, Wagner SJ, Kather JN. The future landscape of large language models in medicine. Commun Med (Lond). 2023; 3(1):141. [CrossRef]
- Aramaki E, Wakamiya S, Yada S, Nakamura Y. Natural language processing: from bedside to everywhere. Yearb Med Inform. 2022 ; 31(1):243-253. [CrossRef]
- Yang X, Chen A, PourNejatian N, Shin HC, Smith KE, Parisien C, Compas C, Martin C, Costa AB, Flores MG, Zhang Y, Magoc T, Harle CA, Lipori G, Mitchell DA, Hogan WR, Shenkman EA, Bian J, Wu Y. A large language model for electronic health records. NPJ Digit Med. 2022; 5(1):194. [CrossRef]
- Hossain E, Rana R, Higgins N, Soar J, Barua PD, Pisani AR, Turner K. Natural Language processing in electronic health records in relation to healthcare decision-making: a systematic review. Comput Biol Med. 2023; 155:106649. [CrossRef]
- Aggarwal, Dipanshu, Pallavi, Kriti. Advancements and challenges in natural language processing in oral cancer research: A narrative review. Cancer Research, Statistics and Treatment 2024; 7(2):228-233. [CrossRef]
- Demner-Fushman D, Chapman WW, McDonald CJ. What can natural language processing do for clinical decision support? J Biomed Inform. 2009; 42(5):760-72. [CrossRef]
- Wang L, Chen X, Zhang L, Li L, Huang Y, Sun Y, Yuan X. Artificial intelligence in clinical decision support systems for oncology. Int J Med Sci. 2023; 20(1):79-86. [CrossRef]
- Berge GT, Granmo OC, Tveit TO, Munkvold BE, Ruthjersen AL, Sharma J. Machine learning-driven clinical decision support system for concept-based searching: a field trial in a Norwegian hospital. BMC Med Inform Decis Mak. 2023; 23(1):5. [CrossRef]
- Elani HW, Giannobile WV. Harnessing Artificial intelligence to address oral health disparities. JAMA Health Forum. 2024; 5(4):e240642. [CrossRef]
- Tobias MAS, Nogueira BP, Santana MCS, Pires RG, Papa JP, Santos PSS. Artificial intelligence for oral cancer diagnosis: what are the possibilities? Oral Oncol. 2022; 134:106117. [CrossRef]
- Kavyashree C, Vimala HS, Shreyas J. A systematic review of artificial intelligence techniques for oral cancer detection. Healthcare Analytics 2024; 5:100304. [CrossRef]
- Pereira-Prado V, Martins-Silveira F, Sicco E, Hochmann J, Isiordia-Espinoza MA, Gonzalez RG, Pandiar D, Bologna-Molina R. Artificial intelligence for image analysis in oral squamous cell carcinoma: a review. Diagnostics (Basel) 2023; 13(14):2416. [CrossRef]
- Garcia-Pola M, Pons-Fuster E, Suarez-Fernandez C, Seoane-Romero J, Romero-Mendez A, Lopez-Jornet P. Role of Artificial intelligence in the early diagnosis of oral cancer. a scoping review. Cancers (Basel). 2021; 13(18):4600. [CrossRef]
- Talwar V, Singh P, Mukhia N, Shetty A, Birur P, Desai KM, Sunkavalli C, Varma KS, Sethuraman R, Jawahar CV, Vinod PK. AI-assisted screening of oral potentially malignant disorders using smartphone-based photographic images. Cancers (Basel) 2023; 15(16):4120. [CrossRef]
- Vollmer A, Hartmann S, Vollmer M, Shavlokhova V, Brands RC, Kübler A, Wollborn J, Hassel F, Couillard-Despres S, Lang G, Saravi B. Multimodal artificial intelligence-based pathogenomics improves survival prediction in oral squamous cell carcinoma. Sci Rep. 2024; 14(1):5687. [CrossRef]
- Mann M, Kumar C, Zeng WF, Strauss MT. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021; 12(8):759-770. [CrossRef]
- Ng S, Masarone S, Watson D, Barnes MR. The benefits and pitfalls of machine learning for biomarker discovery. Cell Tissue Res. 2023; 394(1):17-31. [CrossRef]
- Pham TD, Ravi V, Fan C, Luo B, Sun XF. Tensor decomposition of largest convolutional eigenvalues reveals pathologic predictive power of RhoB in rectal cancer biopsy. Am J Pathol. 2023; 193(5):579-590. [CrossRef]
- Pham TD, Sun XF. Wavelet scattering networks in deep learning for discovering protein markers in a cohort of Swedish rectal cancer patients. Cancer Med. 2023; 12(23):21502-21518. [CrossRef]
- Chen C, Wang J, Pan D, Wang X, Xu Y, Yan J, Wang L, Yang X, Yang M, Liu GP. Applications of multi-omics analysis in human diseases. MedComm 2023; 4(4):e315. [CrossRef]
- Adeoye J, Su YX. Artificial intelligence in salivary biomarker discovery and validation for oral diseases. Oral Dis. 2024; 30(1):23-37. [CrossRef]
- Viet CT, Asam KR, Yu G, Dyer EC, Kochanny S, Thomas CM, Callahan NF, Morlandt AB, Cheng AC, Patel AA, Roden DF, Young S, Melville J, Shum J, Walker PC, Nguyen KK, Kidd SN, Lee SC, Folk GS, Viet DT, Grandhi A, Deisch J, Ye Y, Momen-Heravi F, Pearson AT, Aouizerat BE. Artificial intelligence-based epigenomic, transcriptomic and histologic signatures of tobacco use in oral squamous cell carcinoma. NPJ Precis Oncol. 2024; 8(1):130. [CrossRef]
- Gu X, Salehi A, Wang L, Coates PJ, Sgaramella N, Nylander K. Early detection of squamous cell carcinoma of the oral tongue using multidimensional plasma protein analysis and interpretable machine learning. J Oral Pathol Med. 2023; 52(7):637-643. [CrossRef]
- Perumal MKK, Srinivasan GP, Thangavelu L, Renuka RR. Theragnostic applications of artificial intelligence (AI) in the field of oral cancer care. Oral Oncology Reports 2024; 10:100278. [CrossRef]
- Mahmood, H., Shaban, M., Rajpoot, N, Syed A. Khurram . Artificial Intelligence-based methods in head and neck cancer diagnosis: an overview. Br J Cancer 2021; 124:1934–1940. [CrossRef]
- Hegde S, Ajila V, Zhu W, Zeng C. Artificial intelligence in early diagnosis and prevention of oral cancer. Asia Pac J Oncol Nurs. 2022; 9(12):100133. [CrossRef]
- Uppal S, Kumar Shrivastava P, Khan A, Sharma A, Kumar Shrivastav A. Machine learning methods in predicting the risk of malignant transformation of oral potentially malignant disorders: a systematic review. Int J Med Inform. 2024; 186:105421. [CrossRef]
- Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019; 69(2):127-157. [CrossRef]
- Habibi M, Taheri G. A new machine learning method for cancer mutation analysis. PLoS Comput Biol. 2022; 18(10):e1010332. [CrossRef]
- Rajula HSR, Verlato G, Manchia M, Antonucci N, Fanos V. Comparison of conventional statistical methods with machine learning in medicine: diagnosis, drug development, and treatment. Medicina (Kaunas). 2020; 56(9):455. [CrossRef]
- Howard FM, Kochanny S, Koshy M, Spiotto M, Pearson AT. Machine Learning-Guided Adjuvant Treatment of Head and Neck Cancer. JAMA Netw Open. 2020; 3(11):e2025881. [CrossRef]
- Al-Rawi N, Sultan A, Rajai B, Shuaeeb H, Alnajjar M, Alketbi M, Mohammad Y, Shetty SR, Mashrah MA. The effectiveness of artificial intelligence in detection of oral cancer. Int Dent J. 2022; 72(4):436-447. [CrossRef]
- Li Z, Ding S, Zhong Q, Fang J, Huang J, Huang Z, Zhang Y. A machine learning model for predicting the three-year survival status of patients with hypopharyngeal squamous cell carcinoma using multiple parameters. J Laryngol Otol. 2023; 137(9):1041-1047. [CrossRef]
- Choi N, Kim J, Yi H, Kim H, Kim TH, Chung MJ, Ji M, Kim Z, Son YI. The use of artificial intelligence models to predict survival in patients with laryngeal squamous cell carcinoma. Sci Rep. 2023; 13(1):9734. [CrossRef]
- Zhang YF, Shen YJ, Huang Q, Wu CP, Zhou L, Ren HL. Predicting survival of advanced laryngeal squamous cell carcinoma: comparison of machine learning models and Cox regression models. Sci Rep. 2023; 13(1):18498. [CrossRef]
- Wang Y, Zhou C, Li T, Luo J. Prognostic value of CDKN2A in head and neck squamous cell carcinoma via pathomics and machine learning. J Cell Mol Med. 2024; 28(9):e18394. [CrossRef]
- Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022; 19(2):132-146. [CrossRef]
- Muthupandian S, Arockiaraj J, Belete MA. A commentary on `The use of multilayer perceptron and radial basis function: an artificial intelligence model to predict progression of oral cancer’: correspondence. Int J Surg. 2024; 110(4):2438-2439. [CrossRef]
- Chakrabarty N, Mahajan A. Imaging analytics using artificial intelligence in oncology: a comprehensive review. Clin Oncol (R Coll Radiol). 2024; 36(8):498-513. [CrossRef]
- Zhang B, Shi H, Wang H. Machine learning and ai in cancer prognosis, prediction, and treatment selection: a critical approach. J Multidiscip Healthc. 2023; 16:1779-1791. [CrossRef]
- Batra AM, Reche A. A new era of dental care: harnessing artificial intelligence for better diagnosis and treatment. Cureus 2023; 15(11):e49319. [CrossRef]
- Dhopte A, Bagde H. Smart smile: revolutionizing dentistry with artificial intelligence. Cureus. 2023 Jun 30;15(6):e41227. [CrossRef]
- Berger MF, Mardis ER. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018; 15(6):353-365. [CrossRef]
- Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, White C, Lowe C, Sherba JJ, Hartmanshenn C, O’Neill KM, Balter ML, Fritz ZR, Androulakis IP, Schloss RS, Yarmush ML. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018; 6(3-4):79-100. [CrossRef]
- Duan, XP., Qin, BD., Jiao, XD. Liu K, Wang Z, Zang YS.. New clinical trial design in precision medicine: discovery, development and direction. Sig Transduct Target Ther . 2024; 9: 57. [CrossRef]
- Xu Z, Wang X, Zeng S, Ren X, Yan Y, Gong Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm Sin B. 2021 Nov;11(11):3393-3405. [CrossRef]
- Chen ZH, Lin L, Wu CF, Li CF, Xu RH, Sun Y. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun (Lond). 2021; 41(11):1100-1115. [CrossRef]
- Liao J, Li X, Gan Y, Han S, Rong P, Wang W, Li W, Zhou L. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023; 12:998222. [CrossRef]
- Fionda B, Boldrini L, D’Aviero A, Lancellotta V, Gambacorta MA, Kovács G, Patarnello S, Valentini V, Tagliaferri L. Artificial intelligence (AI) and interventional radiotherapy (brachytherapy): state of art and future perspectives. J Contemp Brachytherapy. 2020; 12(5):497-500. [CrossRef]
- Ahervo H, Korhonen J, Lim Wei Ming S, Guan Yunqing F, Soini M, Lian Pei Ling C, Metsälä E. Artificial intelligence-supported applications in head and neck cancer radiotherapy treatment planning and dose optimisation. Radiography (Lond). 2023; 29(3):496-502. [CrossRef]
- Crowson MG, Ranisau J, Eskander A, Babier A, Xu B, Kahmke RR, Chen JM, Chan TCY. A contemporary review of machine learning in otolaryngology-head and neck surgery. Laryngoscope. 2020; 130(1):45-51. [CrossRef]
- Loperfido A, Celebrini A, Marzetti A, Bellocchi G. Current role of artificial intelligence in head and neck cancer surgery: a systematic review of literature. Explor Target Antitumor Ther. 2023;4(5):933-940. [CrossRef]
- Miragall MF, Knoedler S, Kauke-Navarro M, Saadoun R, Grabenhorst A, Grill FD, Ritschl LM, Fichter AM, Safi AF, Knoedler L. Face the future-artificial intelligence in oral and maxillofacial surgery. J Clin Med. 2023 Oct 30;12(21):6843. [CrossRef]
- Cai Y, Xie Y, Zhang S, Wang Y, Wang Y, Chen J, Huang Z. Prediction of postoperative recurrence of oral cancer by artificial intelligence model: multilayer perceptron. Head Neck 2023; 45(12):3053-3066. [CrossRef]
- Kar A, Wreesmann VB, Shwetha V, Thakur S, Rao VUS, Arakeri G, Brennan PA. Improvement of oral cancer screening quality and reach: The promise of artificial intelligence. J Oral Pathol Med. 2020; 49(8):727-730. [CrossRef]
- Talwar V, Singh P, Mukhia N, Shetty A, Birur P, Desai KM, Sunkavalli C, Varma KS, Sethuraman R, Jawahar CV, Vinod PK. AI-assisted screening of oral potentially malignant disorders using smartphone-based photographic images. Cancers (Basel). 2023; 15(16):4120. [CrossRef]
- Farina E, Nabhen JJ, Dacoregio MI, Batalini F, Moraes FY. An overview of artificial intelligence in oncology. Future Sci OA. 2022 Feb 10;8(4):FSO787. [CrossRef]
- Kurian M, Adashek JJ, West HJ. Cancer care in the era of artificial intelligence. JAMA Oncol. 2024; 10(5):683. [CrossRef]
- Lopez-Cortes XA, Matamala F, Venegas B, Rivera C. Machine-learning applications in oral cancer: a systematic review. Applied Sciences 2022; 12(11):5715. [CrossRef]
- Fatapour Y, Abiri A, Kuan EC, Brody JP. Development of a Machine Learning Model to Predict Recurrence of Oral Tongue Squamous Cell Carcinoma. Cancers (Basel). 2023; 15(10):2769. [CrossRef]
- OuYang PY, He Y, Guo JG, Liu JN, Wang ZL, Li A, Li J, Yang SS, Zhang X, Fan W, Wu YS, Liu ZQ, Zhang BY, Zhao YN, Gao MY, Zhang WJ, Xie CM, Xie FY. Artificial intelligence aided precise detection of local recurrence on MRI for nasopharyngeal carcinoma: a multicenter cohort study. EClinicalMedicine. 2023; 63:102202. [CrossRef]
- Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018; 243(3):213-221. [CrossRef]
- Albutt A, O’Hara J, Conner M, Lawton R. Involving patients in recognising clinical deterioration in hospital using the Patient Wellness Questionnaire: a mixed-methods study. J Res Nurs. 2020; 25(1):68-86. [CrossRef]
- Brands MR, Gouw SC, Beestrum M, Cronin RM, Fijnvandraat K, Badawy SM. Patient-centered digital health records and their effects on health outcomes: systematic review. J Med Internet Res 2022; 24(12):e43086. [CrossRef]
- Li Y, Tang H, Liu Y, Qiao Y, Xia H, Zhou J. Oral wearable sensors: health management based on the oral cavity. Biosensors and Bioelectronics: X 2022; 10:100135. [CrossRef]
- Dailah HG. Mobile health (mHealth) technology in early detection and diagnosis of oral cancer–a scoping review of the current scenario and feasibility. J Healthc Eng. 2022; 2022:4383303. [CrossRef]
- Babel A, Taneja R, Mondello Malvestiti F, Monaco A, Donde S. Artificial intelligence solutions to increase medication adherence in patients with non-communicable diseases. Front Digit Health. 2021; 3:669869. [CrossRef]
- Saber AF, Ahmed SK, Hussein S, Qurbani K. Artificial intelligence-assisted nursing interventions in psychiatry for oral cancer patients: a concise narrative review. Oral Oncology Reports 2024; 10:100343. doi.org/10.1016/j.oor.2024.100343.
- Derraz B, Breda G, Kaempf C, Baenke F, Cotte F, Reiche K, Köhl U, Kather JN, Eskenazy D, Gilbert S. New regulatory thinking is needed for AI-based personalised drug and cell therapies in precision oncology. NPJ Precis Oncol. 2024; 8(1):23. [CrossRef]
- Wu YP, Linder LA, Kanokvimankul P, Fowler B, Parsons BG, Macpherson CF, Johnson RH. Use of a smartphone application for prompting oral medication adherence among adolescents and young adults with cancer. Oncol Nurs Forum. 2018; 45(1):69-76. [CrossRef]
- Villanueva-Bueno C, Collado-Borrell R, Escudero-Vilaplana V, Revuelta-Herrero JL, Marzal-Alfaro MB, Gonzalez-Haba E, Arranz-Arija JÁ, Osorio S, Herranz-Alonso A, Sanjurjo-Saez M. A smartphone app to improve the safety of patients undergoing treatment with oral antineoplastic agents: 4 years of experience in a university hospital. Front Public Health. 2022; 10:978783. [CrossRef]
- Shaffer KM, Turner KL, Siwik C, Gonzalez BD, Upasani R, Glazer JV, Ferguson RJ, Joshua C, Low CA. Digital health and telehealth in cancer care: a scoping review of reviews. Lancet Digit Health. 2023; 5(5):e316-e327. [CrossRef]
- Batra P, Tagra H, Katyal S. Artificial intelligence in teledentistry. Discoveries (Craiova). 2022; 10(3):153. [CrossRef]
- You Y, Lai X, Pan Y, Zheng H, Vera J, Liu S, Deng S, Zhang L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct Target Ther. 2022; 7(1):156. [CrossRef]
- Wang L, Song Y, Wang H, Zhang X, Wang M, He J, Li S, Zhang L, Li K, Cao L. Advances of Artificial intelligence in anti-cancer drug design: a review of the past decade. Pharmaceuticals (Basel) 2023; 16(2):253. [CrossRef]
- Tran NL, Kim H, Shin CH, Ko E, Oh SJ. Artificial intelligence-driven new drug discovery targeting serine/threonine kinase 33 for cancer treatment. Cancer Cell Int. 2023; 23(1):321. [CrossRef]
- Sharma V, Singh A, Chauhan S, Sharma PK, Chaudhary S, Sharma A, Porwal O, Fuloria NK. Role of artificial intelligence in drug discovery and target identification in cancer. Curr Drug Deliv. 2024;21(6):870-886. [CrossRef]
- Issa NT, Stathias V, Schürer S, Dakshanamurthy S. Machine and deep learning approaches for cancer drug repurposing. Semin Cancer Biol. 2021; 68:132-142. [CrossRef]
- Rogers MF, Gaunt TR, Campbell C. Prediction of driver variants in the cancer genome via machine learning methodologies. Brief Bioinform. 2021; 22(4):bbaa250. [CrossRef]
- Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021; 26(1):80-93. [CrossRef]
- Dara S, Dhamercherla S, Jadav SS, Babu CM, Ahsan MJ. Machine learning in drug discovery: a review. Artif Intell Rev. 2022;55(3):1947-1999. [CrossRef]
- Visan AI, Negut I. Integrating artificial intelligence for drug discovery in the context of revolutionizing drug delivery. Life 2024; 14(2):233. [CrossRef]
- Tanoli Z, Vähä-Koskela M, Aittokallio T. Artificial intelligence, machine learning, and drug repurposing in cancer. Expert Opin Drug Discov. 2021; 16(9):977-989. [CrossRef]
- Vora LK, Gholap AD, Jetha K, Thakur RRS, Solanki HK, Chavda VP. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics. 2023; 15(7):1916. [CrossRef]
- Anand U, Dey A, Chandel AKS, Sanyal R, Mishra A, Pandey DK, De Falco V, Upadhyay A, Kandimalla R, Chaudhary A, Dhanjal JK, Dewanjee S, Vallamkondu J, Pérez de la Lastra JM. Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022; 10(4):1367-1401. [CrossRef]
- Xia Y, Sun M, Huang H, Jin WL. Drug repurposing for cancer therapy. Signal Transduct Target Ther. 2024; 9(1):92. [CrossRef]
- Qureshi R, Irfan M, Gondal TM, Khan S, Wu J, Hadi MU, Heymach J, Le X, Yan H, Alam T. AI in drug discovery and its clinical relevance. Heliyon. 2023 Jul;9(7):e17575. [CrossRef]
- Singh S, Kumar R, Payra S, Singh SK. Artificial intelligence and machine learning in pharmacological research: bridging the gap between data and drug discovery. Cureus 2023; 15(8):e44359. [CrossRef]
- Yadav S, Singh A, Singhal R, Yadav JP. Revolutionizing drug discovery: the impact of artificial intelligence on advancements in pharmacology and the pharmaceutical industry. Intelligent Pharmacy 2024; 2(3): 367-380. [CrossRef]
- Pawar AM, Desai R, Thakur B. From tedious to targeted: optimizing oral cancer research with Consensus AI. Oral Oncology Reports 2024; 10:100383. [CrossRef]
- Sobhani N, Tardiel-Cyril DR, Chai D, Generali D, Li JR, Vazquez-Perez J, Lim JM, Morris R, Bullock ZN, Davtyan A, Cheng C, Decker WK, Li Y. Artificial intelligence-powered discovery of small molecules inhibiting CTLA-4 in cancer. BJC Rep. 2024; 2:4. [CrossRef]
- Reardon, S. Precision-medicine plan raises hopes. Nature 2015; 517(7536):540. [CrossRef]
- Olson, MV. Precision medicine at the crossroads. Hum Genomics 2017; 11(1):23. [CrossRef]
- Vinks AA. Precision medicine–nobody is average. Clin Pharmacol Ther. 2017; 101(3):304-307. [CrossRef]
- Rosen A, Zeger SL. Precision medicine: discovering clinically relevant and mechanistically anchored disease subgroups at scale. J Clin Invest. 2019; 129(3):944-945. [CrossRef]
- Cutler DM. Early returns from the era of precision medicine. JAMA. 2020; 323(2):109–110. [CrossRef]
- Sahu M, Gupta R, Ambasta RK, Kumar P. Artificial intelligence and machine learning in precision medicine: A paradigm shift in big data analysis. Prog Mol Biol Transl Sci. 2022; 190(1):57-100. [CrossRef]
- Wang RC, Wang Z. Precision medicine: disease subtyping and tailored treatment. Cancers (Basel). 2023; 15(15):3837. [CrossRef]
- Alabi RO, Almangush A, Elmusrati M, Makitie AA. Deep machine learning for oral cancer: from precise diagnosis to precision medicine. Front Oral Health 2022; 2:794248. [CrossRef]
- Mesko B. The role of artificial intelligence in precision medicine. Expert Review of Precision Medicine and Drug Development 2017; 2(5), 239–241. [CrossRef]
- Johnson KB, Wei WQ, Weeraratne D, Frisse ME, Misulis K, Rhee K, Zhao J, Snowdon JL. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021; 14(1):86-93. [CrossRef]
- Babu M, Snyder M. Multi-omics profiling for health. Mol Cell Proteomics 2023; 22(6):100561. [CrossRef]
- Sharma A, Lysenko A, Jia S, Boroevich KA, Tsunoda T. Advances in AI and machine learning for predictive medicine. J Hum Genet. 2024 Feb 29. [CrossRef]
- Kwon YW, Jo HS, Bae S, Seo Y, Song P, Song M, Yoon JH. Application of proteomics in cancer: recent trends and approaches for biomarkers discovery. Front Med (Lausanne). 2021; 8:747333. [CrossRef]
- Sinha S, Vegesna R, Mukherjee S, Kammula AV, Dhruba SR, Wu W, Kerr DL, Nair NU, Jones MG, Yosef N, Stroganov OV, Grishagin I, Aldape KD, Blakely CM, Jiang P, Thomas CJ, Benes CH, Bivona TG, Schäffer AA, Ruppin E. PERCEPTION predicts patient response and resistance to treatment using single-cell transcriptomics of their tumors. Nat Cancer. 2024; 5(6):938-952. [CrossRef]
- Krishnan G, Singh S, Pathania M, Gosavi S, Abhishek S, Parchani A, Dhar M. Artificial intelligence in clinical medicine: catalyzing a sustainable global healthcare paradigm. Front Artif Intell. 2023; 6:1227091. [CrossRef]
- Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA, Al Yami MS, Al Harbi S, Albekairy AM. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. 2023; 23(1):689. [CrossRef]
- Knudsen JE, Ghaffar U, Ma R, Hung AJ. Clinical applications of artificial intelligence in robotic surgery. J Robot Surg. 2024 Mar 1;18(1):102. [CrossRef]
- Liu, Y., Wu, X., Sang, Y., Zhao, C., Wang, Y., Shi, B. and Fan, Y. Evolution of surgical robot systems enhanced by artificial intelligence: a review. Adv. Intell. Syst. 2024; 6: 2300268. [CrossRef]
- Marsden M, Weyers BW, Bec J, Sun T, Gandour-Edwards RF, Birkeland AC, Abouyared M, Bewley AF, Farwell DG, Marcu L. Intraoperative margin assessment in oral and oropharyngeal cancer using label-free fluorescence lifetime imaging and machine learning. IEEE Trans Biomed Eng. 2021; 68(3):857-868. [CrossRef]
- Zhou XY, Guo Y, Shen M, Yang GZ. Application of artificial intelligence in surgery. Front Med. 2020; 14(4):417-430. [CrossRef]
- Xu J, Zeng B, Egger J, Wang C, Smedby Ö, Jiang X, Chen X. A review on AI-based medical image computing in head and neck surgery. Phys Med Biol. 2022; 67(17). [CrossRef]
- Mithany RH, Aslam S, Abdallah S, Abdelmaseeh M, Gerges F, Mohamed MS, Manasseh M, Wanees A, Shahid MH, Khalil MS, Daniel N. Advancements and challenges in the application of artificial intelligence in surgical arena: a literature review. Cureus 2023; 15(10):e47924. [CrossRef]
- Reddy K, Gharde P, Tayade H, Patil M, Reddy LS, Surya D. Advancements in robotic surgery: a comprehensive overview of current utilizations and upcoming frontiers. Cureus 2023; 15(12):e50415. [CrossRef]
- Fairag M, Almahdi RH, Siddiqi AA, Alharthi FK, Alqurashi BS, Alzahrani NG, Alsulami A, Alshehri R. Robotic revolution in surgery: diverse applications across specialties and future prospects review article. Cureus 2024; 16(1):e52148. [CrossRef]
- Wu Y, Wang F, Fan S, Chow JK. Robotics in dental implantology. Oral Maxillofac Surg Clin North Am. 2019; 31(3):513-518. [CrossRef]
- Satapathy P, Hermis AH, Rustagi S, Pradhan KB, Padhi BK, Sah R. Artificial intelligence in surgical education and training: opportunities, challenges, and ethical considerations - correspondence. Int J Surg. 2023; 109(5):1543-1544. [CrossRef]
- Guerrero DT, Asaad M, Rajesh A, Hassan A, Butler CE. Advancing surgical education: the use of artificial intelligence in surgical training. Am Surg. 2023; 89(1):49-54. [CrossRef]
- Varas J, Coronel BV, Villagrán I, Escalona G, Hernandez R, Schuit G, Durán V, Lagos-Villaseca A, Jarry C, Neyem A, Achurra P. Innovations in surgical training: exploring the role of artificial intelligence and large language models (LLM). Rev Col Bras Cir. 2023; 50:e20233605. [CrossRef]
- Sinha A, West A, Vasdev N, Sooriakumaran P, Rane A, Dasgupta P, McKirdy M. Current practises and the future of robotic surgical training. Surgeon 2023; 21(5):314-322. [CrossRef]
- Brian R, Murillo A, Gomes C, Alseidi A. Artificial intelligence and robotic surgical education. Global Surg Educ. 2024; 3:60. [CrossRef]
- Chen G, Jin S, Xia Q, Wang Z, Shi Z, Chen G, Hong Y, Fan X, Lin H. Insight into the history and trends of surgical simulation training in education: a bibliometric analysis. Int J Surg. 2023; 109(8):2204-2213. [CrossRef]
- Khanagar SB, Alkadi L, Alghilan MA, Kalagi S, Awawdeh M, Bijai LK, Vishwanathaiah S, Aldhebaib A, Singh OG. Application and performance of artificial intelligence (AI) in oral cancer diagnosis and prediction using histopathological images: a systematic review. Biomedicines. 2023; 11(6):1612. [CrossRef]
- Behera A, Babu NA, Renuka RR, Jothinathan MKD, Harnessing artificial intelligence role in oral cancer diagnosis and prediction: a comprehensive exploration. Oral Oncology Reports 2024; 10:100314. [CrossRef]
- Edemekong PF, Annamaraju P, Haydel MJ. Health Insurance Portability and Accountability Act. Updated 2024 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500019/.
- Cabral BP, Braga LAM, Syed-Abdul S, Mota FB. Future of Artificial intelligence applications in cancer care: a global cross-sectional survey of researchers. Curr Oncol. 2023; 30(3):3432-3446. [CrossRef]
- S Alshuhri M, Al-Musawi SG, Al-Alwany AA, Uinarni H, Rasulova I, Rodrigues P, Alkhafaji AT, Alshanberi AM, Alawadi AH, Abbas AH. Artificial intelligence in cancer diagnosis: opportunities and challenges. Pathol Res Pract. 2024; 253:154996. [CrossRef]
- Adeoye J, Hui L, Su YX. Data-centric artificial intelligence in oncology: a systematic review assessing data quality in machine learning models for head and neck cancer. J Big Data 2023; 10: 28. [CrossRef]
- Istasy P, Lee WS, Iansavichene A, Upshur R, Gyawali B, Burkell J, Sadikovic B, Lazo-Langner A, Chin-Yee B. The impact of artificial intelligence on health equity in oncology: scoping review. J Med Internet Res. 2022; 24(11):e39748. [CrossRef]
- Khoury ZH, Ferguson A, Price JB, Sultan AS, Wang R (2024) Responsible artificial intelligence for addressing equity in oral healthcare. Front. Oral. Health 2024; 5: 1408867. [CrossRef]
- Makitie AA, Alabi RO, Ng SP, Takes RP, Robbins KT, Ronen O, Shaha AR, Bradley PJ, Saba NF, Nuyts S, Triantafyllou A, Piazza C, Rinaldo A, Ferlito A. Artificial Intelligence in head and neck cancer: a systematic review of systematic reviews. Adv Ther. 2023; 40(8):3360-3380. [CrossRef]
- Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019; 17(1):195. [CrossRef]
- Wachter RM, Brynjolfsson E. Will generative artificial intelligence deliver on its promise in health care? JAMA. 2024; 331(1):65-69. [CrossRef]
- Liu X, Faes L, Kale AU, Wagner SK, Fu DJ, Bruynseels A, Mahendiran T, Moraes G, Shamdas M, Kern C, Ledsam JR, Schmid MK, Balaskas K, Topol EJ, Bachmann LM, Keane PA, Denniston AK. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health 2019; 1(6):e271-e297. [CrossRef]
- Karalis VD. The integration of artificial intelligence into clinical practice. Applied Biosciences. 2024; 3(1):14-44. [CrossRef]
- Park SH, Choi J, Byeon JS. Key Principles of clinical validation, device approval, and insurance coverage decisions of artificial intelligence. Korean J Radiol. 2021 Mar;22(3):442-453. [CrossRef]
- Tsopra R, Fernandez X, Luchinat C, Alberghina L, Lehrach H, Vanoni M, Dreher F, Sezerman OU, Cuggia M, de Tayrac M, Miklasevics E, Itu LM, Geanta M, Ogilvie L, Godey F, Boldisor CN, Campillo-Gimenez B, Cioroboiu C, Ciusdel CF, Coman S, Hijano Cubelos O, Itu A, Lange B, Le Gallo M, Lespagnol A, Mauri G, Soykam HO, Rance B, Turano P, Tenori L, Vignoli A, Wierling C, Benhabiles N, Burgun A. A framework for validating AI in precision medicine: considerations from the European ITFoC consortium. BMC Med Inform Decis Mak. 2021; 21(1):274. [CrossRef]
- Mennella C, Maniscalco U, De Pietro G, Esposito M. Ethical and regulatory challenges of AI technologies in healthcare: a narrative review. Heliyon. 2024; 10(4):e26297. [CrossRef]
- Siontis GCM, Sweda R, Noseworthy PA, Friedman PA, Siontis KC, Patel CJ. Development and validation pathways of artificial intelligence tools evaluated in randomised clinical trials. BMJ Health Care Inform. 2021; 28(1):e100466. [CrossRef]
- McKee M, Wouters OJ. The challenges of regulating artificial intelligence in healthcare. Int J Health Policy Manag. 2023;12:7261. [CrossRef]
- Lambert SI, Madi M, Sopka S, Lenes A, Stange H, Buszello CP, Stephan A. An integrative review on the acceptance of artificial intelligence among healthcare professionals in hospitals. NPJ Digit Med. 2023; 6(1):111. [CrossRef]
- Mucci A, Green WM, Hill LH. Incorporation of artificial intelligence in healthcare professions and patient education for fostering effective patient care. New Directions for Adult and Continuing Education 2024; 2024: 51-62. [CrossRef]
- Shevtsova D, Ahmed A, Boot IWA, Sanges C, Hudecek M, Jacobs JJL, Hort S, Vrijhoef HJM trust in and acceptance of artificial intelligence applications in medicine: mixed methods study. JMIR Hum Factors 2024;11:e47031. [CrossRef]
- Richardson JP, Smith C, Curtis S, Watson S, Zhu X, Barry B, Sharp RR. Patient apprehensions about the use of artificial intelligence in healthcare. NPJ Digit Med. 2021; 4(1):140. [CrossRef]
- Wu C, Xu H, Bai D, Chen X, Gao J, Jiang X. Public perceptions on the application of artificial intelligence in healthcare: a qualitative meta-synthesis. BMJ Open 2023; 13(1):e066322. [CrossRef]
- Kotter E, Ranschaert E. Challenges and solutions for introducing artificial intelligence (AI) in daily clinical workflow. Eur Radiol. 2021; 31(1):5-7. [CrossRef]
- Bajwa J, Munir U, Nori A, Williams B. Artificial intelligence in healthcare: transforming the practice of medicine. Future Healthc J. 2021; 8(2):e188-e194. [CrossRef]
- Wenderott K, Krups J, Luetkens JA, Weigl M. Radiologists’ perspectives on the workflow integration of an artificial intelligence-based computer-aided detection system: A qualitative study. Appl Ergon. 2024 May;117:104243. [CrossRef]
- Brady AP, Allen B, Chong J, Kotter E, Kottler N, Mongan J, Oakden-Rayner L, Dos Santos DP, Tang A, Wald C, Slavotinek J. Developing, purchasing, implementing and monitoring ai tools in radiology: practical considerations. A multi-society statement from the ACR, CAR, ESR, RANZCR & RSNA. Can Assoc Radiol J. 2024; 75(2):226-244. [CrossRef]
- Waqas A , Tripathi A, Ramachandran R., Stewart PA, Rasool G. Multimodal data integration for oncology in the era of deep neural networks: a review. Frontiers in Artificial Intelligence 2024; 7:1408843. [CrossRef]
- Ilhan B, Guneri P, Wilder-Smith P. The contribution of artificial intelligence to reducing the diagnostic delay in oral cancer. Oral Oncol. 2021; 116:105254. [CrossRef]
- Khoury ZH, Sultan AS. Tele-oral oncology: reinvigorating telemedicine in oral cancer care. Journal of Cancer & Allied Specialties 2020; 6(1):e335. [CrossRef]
- Kutuk T, Atak E, Villa A, Kalman NS, Kaiser A. Interdisciplinary collaboration in head and neck cancer care: optimizing oral health management for patients undergoing radiation therapy. Current Oncology 2024; 31(4):2092-2108. [CrossRef]
- Giansanti D. Joint expedition: exploring the intersection of digital health and AI in precision medicine with team integration. J Pers Med. 2024; 14(4):388. [CrossRef]
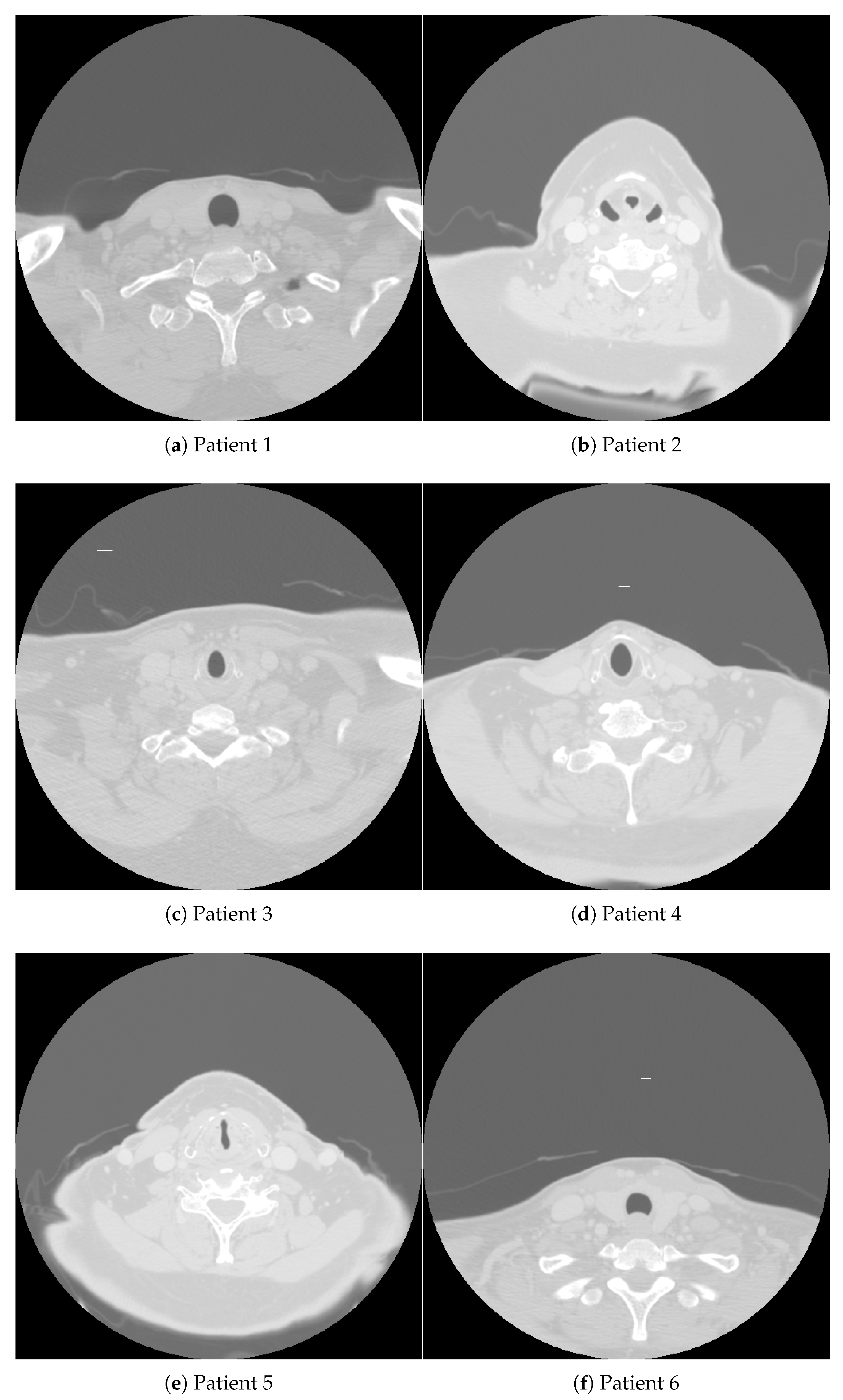
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| HPV/p16 status | positive | negative | positive | negative | positive | positive |
| Gender | male | female | female | male | male | female |
| Age | 58 | 78 | 56 | 59 | 55 | 47 |
| Race | white | white | white | black | hispanic | asian |
| Tumor side | left | right | right | right | right | left |
| Tumor subsite | tonsil | BOT | BOT | BOT | BOT | BOT |
| T category | 2 | 3 | 2 | 4 | 2 | 1 |
| N category | 0 | 0 | 2b | 1 | 2a | 2a |
| AJCC cancer stage | II | III | IV | III | IV | IV |
| Pathological grade | III | II | III | NA | III | II-III |
| Smoking status | former | former | never | current | never | never |
| Smoking pack-years | 5 | 70 | 0 | 66 | 0 | 0 |
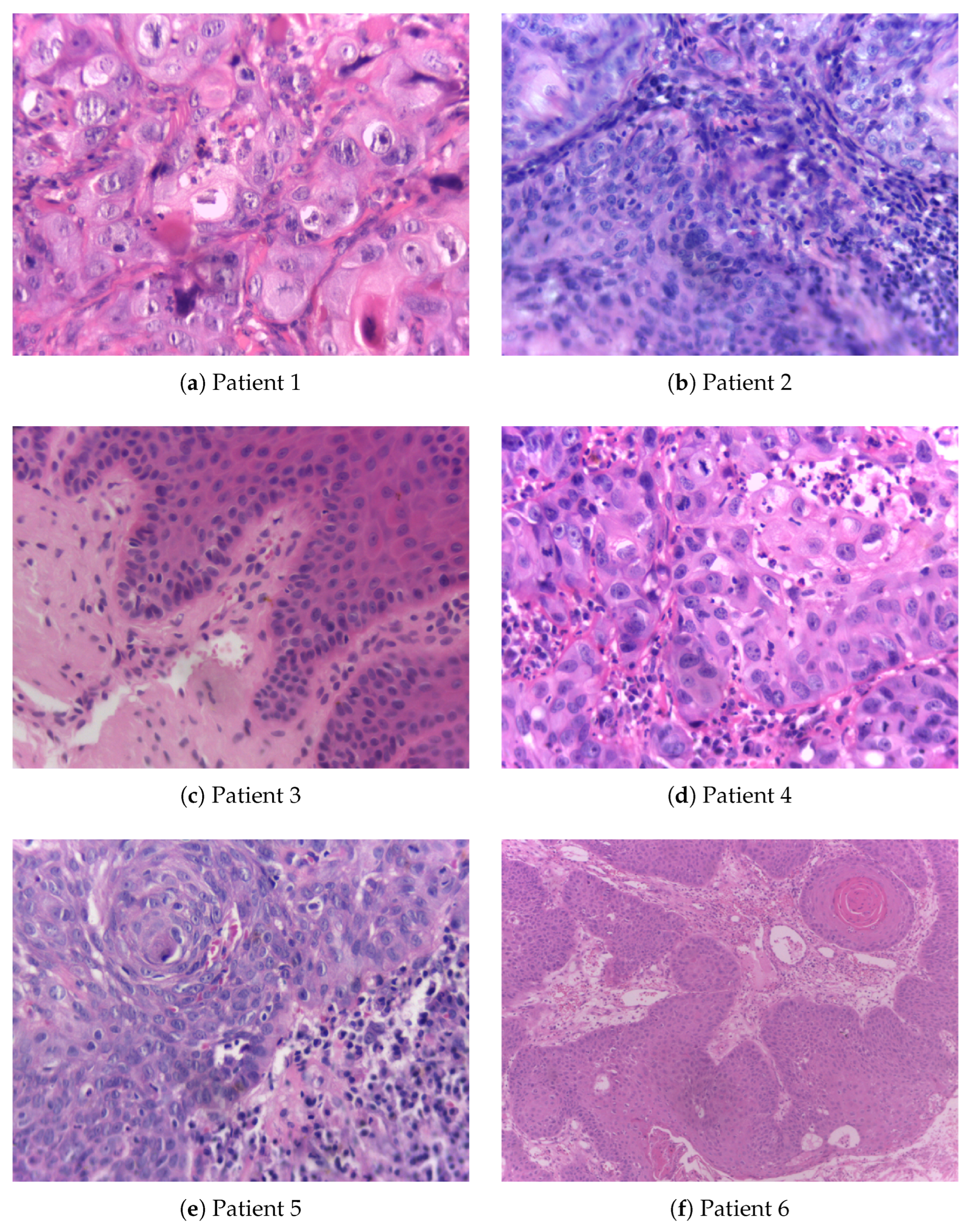
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| Gender | male | male | male | male | male | male |
| Age group | 1 | 1 | 2 | 2 | 1 | 1 |
| Race | white | white | black | white | NI | white |
| Tobacco use | Y | N | N | former | NI | Y |
| Alcohol consumption | former | Y | Y | former | NI | Y |
| Localization | tongue | FOM | BM | gingiva | palate | tongue |
| Diagnosis | OSCC | OSCC | LP (mild) | OSCC | LP (severe) | OSCC |
| Application | Examples | Benefits |
|---|---|---|
| Early Detection | Histopathological analysis, Imaging techniques (e.g., MRI, CT scans) | Improved accuracy in detecting early-stage lesions, Enhanced visualization of suspicious areas |
| Diagnosis | Automated lesion detection, Classification algorithms | Standardized diagnostic criteria, Reduction in diagnostic variability |
| Treatment Planning | Personalized medicine, Genomics integration | Tailored treatment regimens, Better patient outcomes based on individual profiles |
| Monitoring and Surveillance | Real-time monitoring, Recurrence detection, Patient compliance tracking | Early identification of recurrence, Improved patient follow-up, Enhanced compliance with treatment protocols |
| Drug Discovery | Identification of therapeutic targets, Drug screening and repurposing | Accelerated drug development, Identification of novel therapeutic compounds |
| Robotics and Surgery | AI-assisted surgery, Precision surgery techniques | Increased surgical precision, Reduced intraoperative risks, Faster recovery times |
| AI Technique | Applications | Description and Benefits |
|---|---|---|
| Supervised Learning | Image classification, Predictive modeling | Utilizes labeled data to train models for accurate diagnosis and prognosis prediction, improving early detection and personalized treatment plans |
| Unsupervised Learning | Biomarker discovery, Patient clustering | Analyzes unlabeled data to identify patterns and correlations, aiding in the discovery of novel biomarkers and understanding patient subgroups |
| Reinforcement Learning | Treatment optimization, Robotic surgery | Uses feedback loops to optimize treatment strategies and enhance precision in surgical procedures, resulting in better patient outcomes and reduced errors |
| Deep Learning | Histopathological image analysis, Radiographic image enhancement | Employs neural networks to analyze complex medical images, leading to improved detection and diagnosis accuracy |
| Natural Language Processing (NLP) | Literature mining, Patient data analysis | Extracts and analyzes information from medical literature and patient records, facilitating better decision-making and research insights |
| Explainable AI | Diagnostic decision support, Treatment planning transparency | Enhances the interpretability of AI models, providing clinicians with understandable insights and supporting transparent decision-making processes |
| Vision Transformer Networks | Medical image classification, Lesion detection | Utilizes advanced transformer models for image analysis, offering superior accuracy in detecting and classifying lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





