Submitted:
08 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
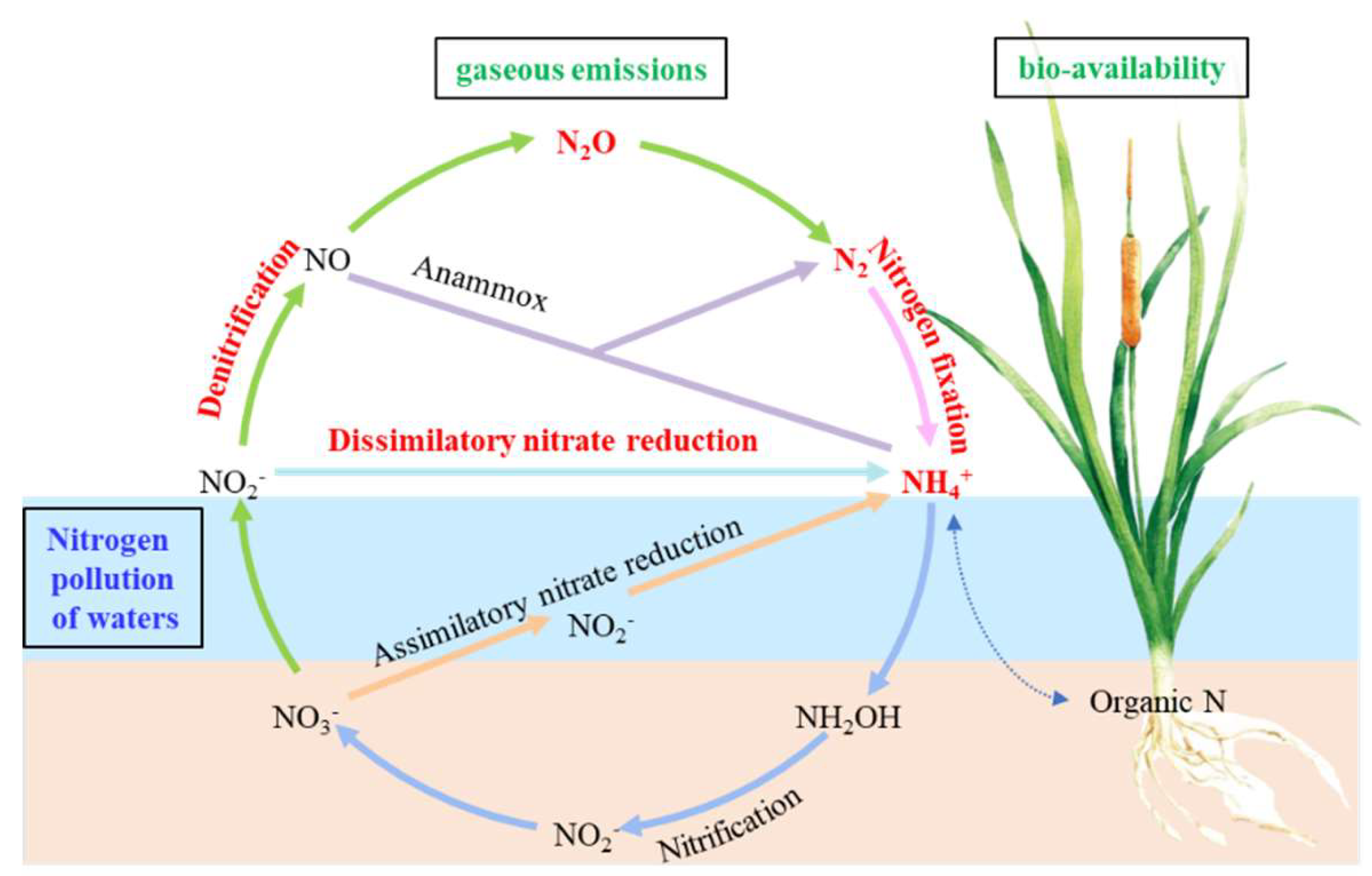
1. Introduction
2. Materials and Methods
2.1. Research Site and Sediment Sampling
2.2. Nitrogen Transformation Rate in Sediment
- In the slurry preculture experiment, fresh sediment (20 g) was mixed thoroughly with in situ overlying water (1:7 w/v). The mixture was homogenized by rinsing with high-purity helium (purity >99.999%) for 30 minutes and transferred to a 200 mL hermetic vial (Exetainer, Labco, UK). The vial was sealed with a butyl rubber septum and incubated for 24 hours in the dark.
- For the 15N isotope tracing experiment, after the precultivation experiment, the vials were divided into two groups, and 15N-labeled sodium nitrate solution (Na15NO3, 99% 15N, Cambridge Isotope Laboratories, Inc., Tewksbury, USA) was added to achieve a final 15N-labeled solution concentration of 100 μM. One group of vials was injected with 0.2 mL of ZnCl2 solution at a 50% w/v concentration, which was the initial sample. Another group of vials was placed in a constant-temperature incubator for further cultivation for 8 hours. After the cultivation was completed, the groups of vials were injected with ZnCl2 solution as the final samples.
- For the analysis of potential DNRA rates (DNRARs), after the slurry precultivation experiment and 15N isotope tracing experiment were completed, 200 μL of hypobromic acid oxidant was added to the initial and final sample vials. Subsequently, the difference in the concentration of 15NH4+-N in the initial and final sample vials was analyzed using MIMS to calculate the potential DNRARs [48,49].
- For the analysis of potential nitrogen fixation rates (NFRs, after the slurry precultivation experiment was completed, the vials were divided into two groups. One group of vials was injected with 0.2 mL of ZnCl2 solution at a 50% w v-1 concentration, which was the initial sample. Another group of vials was injected with 0.5 mL of 99 atom% 15N-N2 (Campro Scientific, Germany), sealed and cultured at in situ temperature for 24 h. After cultivation was completed, the group of vials was injected with ZnCl2 solution, which was the final sample. Subsequently, all the vials were washed with He for 30 minutes to remove N2, and iodine hypobromide solution was injected to oxidize the 15N label produced by fixing N into N2. Subsequently, the difference in the 15N concentration between the initial and final sample vials was analyzed via MIMS to calculate the potential NFRs [50].
2.3. Analysis of Sediment and Water Physical and Chemical Properties
2.4. High-Throughput Sequencing and Metagenomic Sequencing
2.5. Statistical Analysis
3. Results
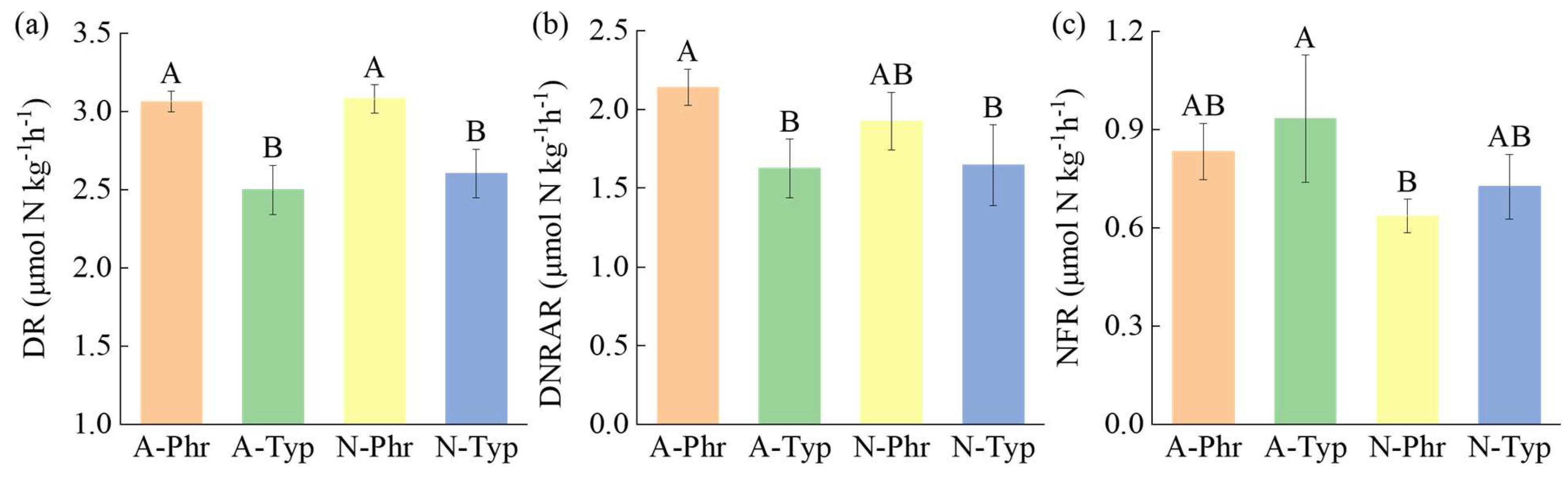
3.1. Nitrogen Transformation Rates in Rhizosphere Sediments
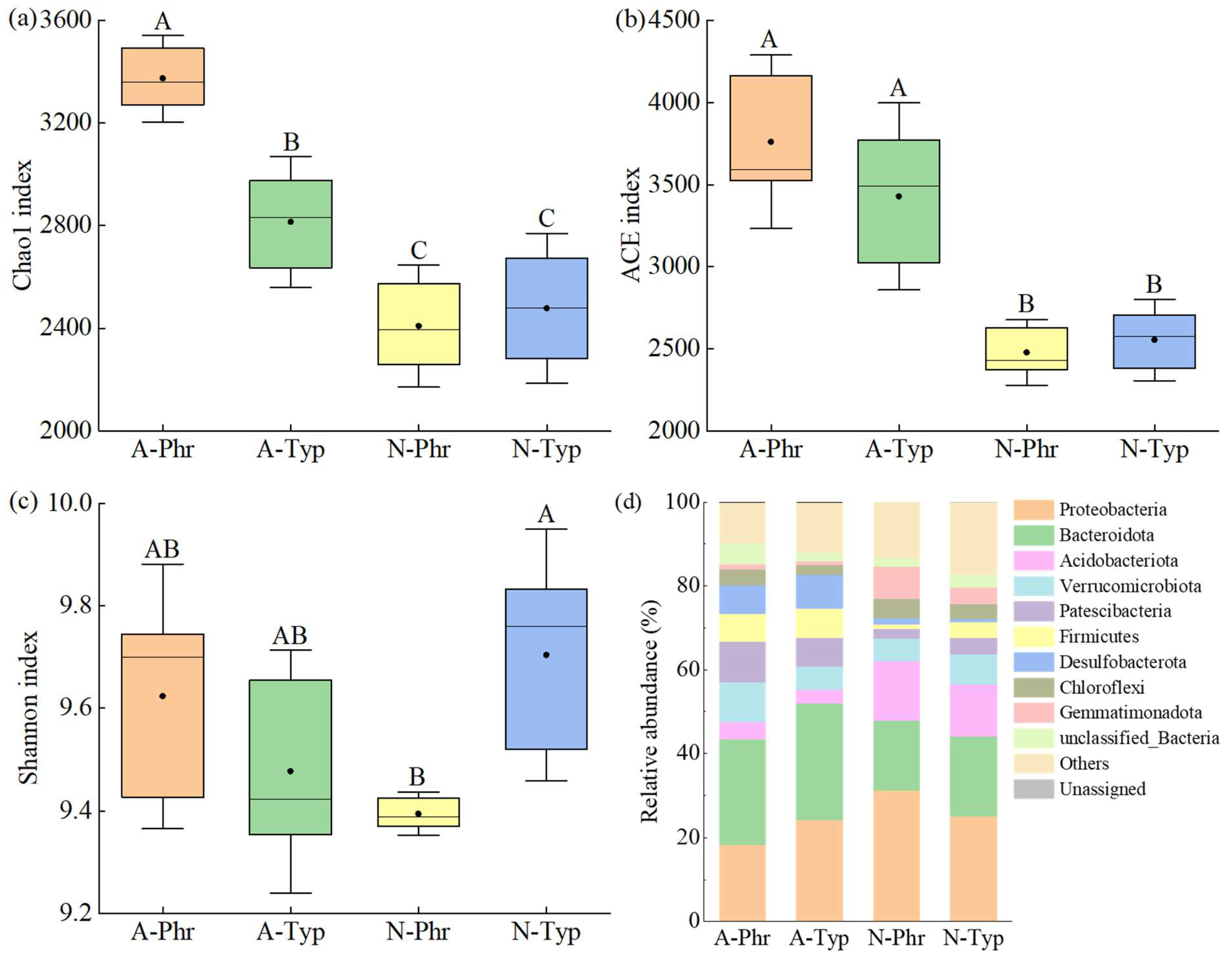
3.2. Diversity and Composition of the Bacterial Community in Rhizosphere Sediments and Their Driving Factors
3.3. Relative Abundance of Nitrogen Transformation Functional Genes in Rhizosphere Sediments and Their Driving Factors
3.4. Influence of Environmental and Microbial Factors on the Nitrogen Conversion Rate of Sediment
4. Discussion
4.1. Differences in Nitrogen Transformation Rates in Rhizosphere Sediments between Artificially Cultivated Ditches and Natural Ditches
4.2. Diversity and Composition of the Bacterial Community in Rhizosphere Sediments and Their Driving Factors
4.3. Relative Abundance of Nitrogen Transformation Functional Genes in Rhizosphere Sediments and Their Driving Factors
4.4. Influence of Environmental and Microbial Factors on the Nitrogen Conversion Rate of Sediment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Zhang, Q.; Wang, G.; Singh, V.P.; Zhao, J.; Sun, S.; Wang, D.; Liu, T.; Duan, L. Ecological Degradation in the Inner Mongolia Reach of the Yellow River Basin, China: Spatiotemporal Patterns and Driving Factors. Ecological Indicators 2023, 154, 110498. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Qi, J.; Wang, Q. Evaluation of the Stability and Suitable Scale of an Oasis Irrigation District in Northwest China. Water 2020, 12, 2837. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y. Associations among ecosystem services from local perspectives. Science of The Total Environment 2019, 690, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lei, Q.; Liang, X.; Lindsey, S.; Luo, J.; Pei, W.; Du, X.; Wu, S.; An, M.; Qiu, W.; et al. Optimization of the N Footprint Model and Analysis of Nitrogen Pollution in Irrigation Areas: A Case Study of Ningxia Hui Autonomous Region, China. Journal of Environmental Management 2023, 340, 118002. [Google Scholar] [CrossRef]
- Tian, L. Analysis and Investigation of the Third Drainage Ditch Pollution Situation in Ningxia Based on Multivariate Statistics. E3S Web Conf. 2020, 145, 02082. [Google Scholar] [CrossRef]
- Pei, W.; Yan, T.; Lei, Q.; Zhang, T.; Fan, B.; Du, X.; Luo, J.; Lindsey, S.; Liu, H. Spatio-Temporal Variation of Net Anthropogenic Nitrogen Inputs (NANI) from 1991 to 2019 and Its Impacts Analysis from Parameters in Northwest China. Journal of Environmental Management 2022, 321, 115996. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, M.V.; Hashemi, F.; Hoffmann, C.C.; Zak, D.; Audet, J.; Kronvang, B. Efficiency of Mitigation Measures Targeting Nutrient Losses from Agricultural Drainage Systems: A Review. Ambio 2020, 49, 1820–1837. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, J.; Liu, L.; Li, Y.; Li, X. A Source-Sink Landscape Approach to Mitigation of Agricultural Non-Point Source Pollution: Validation and Application. Environmental Pollution 2022, 314, 120287. [Google Scholar] [CrossRef]
- Li, S.; Wu, M.; Jia, Z.; Luo, W.; Fei, L.; Li, J. Influence of Different Controlled Drainage Strategies on the Water and Salt Environment of Ditch Wetland: A Model-Based Study. Soil and Tillage Research 2021, 208, 104894. [Google Scholar] [CrossRef]
- Clifford, C.C.; Heffernan, J.B. B. North Carolina Coastal Plain Ditch Types Support Distinct Hydrophytic Communities. Wetlands 2023, 43, 1–27. [Google Scholar] [CrossRef]
- Hou, L.; Gao, D.; Zheng, Y.; Li, X.; Yin, G.; Dong, H.; Liang, X.; Liu, M. Nitrogen Cycling in Aquatic Environments of China: Progress and Future Challenges. Progress in Physical Geography: Earth and Environment 2022, 46, 846–868. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat Rev Microbiol 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Y.; Yang, W.; Huang, J.; Hou, K.; Zhang, L.; Song, H.; Yang, L.; Tian, C.; Rong, X.; et al. A Comparison of the Mechanisms and Performances of Acorus Calamus, Pontederia Cordata and Alisma Plantagoaquatica in Removing Nitrogen from Farmland Wastewater. Bioresource Technology 2021, 332, 125105. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Zhao, S.; Wang, X.; Hefting, M.M.; Schwark, L.; Zhu, G. Anammox and Denitrification Separately Dominate Microbial N-Loss in Water Saturated and Unsaturated Soils Horizons of Riparian Zones. Water Research 2019, 162, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Sanford, R.A.; Chee-Sanford, J.; Ooi, S.K.; Löffler, F.E.; Konstantinidis, K.T.; Yang, W.H. Beyond Denitrification: The Role of Microbial Diversity in Controlling Nitrous Oxide Reduction and Soil Nitrous Oxide Emissions. Global Change Biology 2021, 27, 2669–2683. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-W.; Chen, D.; He, J.-Z. Microbial Regulation of Terrestrial Nitrous Oxide Formation: Understanding the Biological Pathways for Prediction of Emission Rates. FEMS Microbiology Reviews 2015, 39, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barros, C.M.; Jia, M.; Van Loosdrecht, M.C.M.; Volcke, E.I.P.; Winkler, M.K.H. Evaluating the Potential for Dissimilatory Nitrate Reduction by Anammox Bacteria for Municipal Wastewater Treatment. Bioresource Technology 2017, 233, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.; Sanford, R.A.; Connor, L.; Yang, W.H.; Chee-Sanford, J. Optimization of PCR Primers to Detect Phylogenetically Diverse NrfA Genes Associated with Nitrite Ammonification. Journal of Microbiological Methods 2019, 160, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Hester, E.R.; Harpenslager, S.F.; Lüke, C.; Lücker, S.; Welte, C.U. Linking Nitrogen Load to the Structure and Function of Wetland Soil and Rhizosphere Microbial Communities. 2018, 3, e00214–17. [Google Scholar] [CrossRef]
- Uesaka, K.; Banba, M.; Chiba, S.; Fujita, Y. Restoration of the Functional Nif Gene Cluster by Complex Recombination Events during Heterocyst Development in the Nitrogen-Fixing Cyanobacterium Calothrix Sp. NIES-4101. Plant And Cell Physiology 2024, 65, 1050–1064. [Google Scholar] [CrossRef]
- He, R.; Zeng, J.; Zhao, D.; Wang, S.; Wu, Q.L. Decreased Spatial Variation and Deterministic Processes of Bacterial Community Assembly in the Rhizosphere of Phragmites Australis across the Middle–Lower Yangtze Plain. Molecular Ecology 2022, 31, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jia, M.; Xun, M.; Wang, X.; Chen, K.; Yang, H. Nitrogen Transformation and Microbial Community Structure Varied in Apple Rhizosphere and Rhizoplane Soils under Biochar Amendment. J Soils Sediments 2021, 21, 853–868. [Google Scholar] [CrossRef]
- Sun, L.; Tsujii, Y.; Xu, T.; Han, M.; Li, R.; Han, Y.; Gan, D.; Zhu, B. Species of Fast Bulk-soil Nutrient Cycling Have Lower Rhizosphere Effects: A Nutrient Spectrum of Rhizosphere Effects. Ecology 2023, 104, e3981. [Google Scholar] [CrossRef]
- Supreeth, M. Enhanced remediation of pollutants by microorganisms-plant combination. International Journal of Environmental Science and Technology 2021, (5), 1–12. [Google Scholar] [CrossRef]
- Cong, S.; Yuelu, J. Physiological and Ecological Responses and Changes of Phaeodactylum Tricornutum under Long-Term Stress of Naphthalene. IOP Conf. Ser.: Earth Environ. Sci. 2019, 310, 052021. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, R.; Cao, Q.; Quan, Q.; Sun, R.; Liu, J. Effects of Emergent Aquatic Plants on Nitrogen Transformation Processes and Related Microorganisms in a Constructed Wetland in Northern China. Plant Soil 2019, 443, 473–492. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.; Zhang, Q.; Bao, Y.; Yang, N.; Huo, Y.; He, P. Epiphytic Bacterial Community Composition on the Surface of the Submerged Macrophyte Myriophyllum Spicatum in a Low-Salinity Sea Area of Hangzhou Bay. Oceanological and Hydrobiological Studies 2019, 48, 43–55. [Google Scholar] [CrossRef]
- Yang, J.; Pei, H.; Lü, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S.; Feng, J. Effects of Phytoplankton Community and Interaction between Environmental Variables on Nitrogen Uptake and Transformations in an Urban River. J. Ocean. Limnol. 2022, 40, 1012–1026. [Google Scholar] [CrossRef]
- Wei, W.; Tong, J.; Hu, B.X. Study on Ecological Dynamic Model for Phytoremediation of Farmland Drainage Water. Journal of Hydrology 2019, 578, 124026. [Google Scholar] [CrossRef]
- Bai, S.; Chen, J.; Guo, M.; Ren, N.; Zhao, X. Vertical-Scale Spatial Influence of Radial Oxygen Loss on Rhizosphere Microbial Community in Constructed Wetland. Environment International 2023, 171, 107690. [Google Scholar] [CrossRef]
- Duan, Z.; Xie, S.; Chen, Z.; Wang, J.; Li, Z. Effects of Aquatic Plants in Constructed Wetlands to Removal of Water Pollutants. In Proceedings of the World Environmental and Water Resources Congress 2018; American Society of Civil Engineers: Minneapolis, Minnesota,, 31 May 2018; pp. 239–245. [Google Scholar] [CrossRef]
- Riva, V.; Mapelli, F.; Syranidou, E.; Crotti, E.; Choukrallah, R.; Kalogerakis, N.; Borin, S. Root Bacteria Recruited by Phragmites Australis in Constructed Wetlands Have the Potential to Enhance Azo-Dye Phytodepuration. Microorganisms 2019, 7, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mao, W.; Pang, L.; Li, R.; Li, S. Influence of Phragmites Communis and Zizania Aquatica on Rhizosphere Soil Enzyme Activity and Bacterial Community Structure in a Surface Flow Constructed Wetland Treating Secondary Domestic Effluent in China. Environ Sci Pollut Res 2020, 27, 26141–26152. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Zhang, X.; Cai, M.; Zhou, L.; Chen, G.; Zou, G. Roles of Vegetation in Nutrient Removal and Structuring Microbial Communities in Different Types of Agricultural Drainage Ditches for Treating Farmland Runoff. Ecological Engineering 2020, 155, 105941. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Xiao, R.; Li, Y.; He, Y.; Wu, J. Effects of Vegetation on Ammonium Removal and Nitrous Oxide Emissions from Pilot-Scale Drainage Ditches. Aquatic Botany 2016, 130, 37–44. [Google Scholar] [CrossRef]
- Veraart, A.J.; Dimitrov, M.R.; Schrier-Uijl, A.P.; Smidt, H.; De Klein, J.J.M. Abundance, Activity and Community Structure of Denitrifiers in Drainage Ditches in Relation to Sediment Characteristics, Vegetation and Land-Use. Ecosystems 2017, 20, 928–943. [Google Scholar] [CrossRef]
- Meuleman, A.F.M.; Beekman, J.Ph.; Verhoeven, J.T.A. Nutrient Retention and Nutrient-Use Efficiency in Phragmites Australis Stands after Wasterwater Application. Wetlands 2002, 22, 712–721. [Google Scholar] [CrossRef]
- Fang, J.; Yang, R.; Cao, Q.; Dong, J.; Li, C.; Quan, Q.; Huang, M.; Liu, J. Differences of the Microbial Community Structures and Predicted Metabolic Potentials in the Lake, River, and Wetland Sediments in Dongping Lake Basin. Environ Sci Pollut Res 2020, 27, 19661–19677. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; He, R.; Zeng, J.; Zhao, D.; Wang, S.; He, F.; Yu, Z.; Wu, Q.L. Lower Compositional Variation and Higher Network Complexity of Rhizosphere Bacterial Community in Constructed Wetland Compared to Natural Wetland. Microb Ecol 2023, 85, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, Q.; Zhu, Y.; Christakos, G.; Wu, J. Changes to the Structure and Function of Microbial Communities in Spartina Alterniflora and Kandelia Obovata Sediments as a Factor of Stand Age. Applied Soil Ecology 2022, 177, 104544. [Google Scholar] [CrossRef]
- Ding, J.; Jia, Y.; Zhao, C.; Bo, W.; Xu, X.; Lv, R.; Zhou, G.; Kong, Q.; Du, Y.; Xu, F.; et al. Microbial Abundance and Community i46n Constructed Wetlands Planted with Phragmites Australis and Typha Orientalis in Winter. International Journal of Phytoremediation 2021, 23, 1476–1485. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Li, X.; Li, C.; Zhao, Y.; Gao, Y.; Liu, J. Effects of Plants and Soil Microorganisms on Organic Carbon and the Relationship between Carbon and Nitrogen in Constructed Wetlands. Environ Sci Pollut Res 2023, 30, 62249–62261. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.R.; Zak, D.; Audet, J.; Petersen, R.J.; Lange, J.; Oehmke, C.; Wichtmann, W.; Kreyling, J.; Grygoruk, M.; Jabłońska, E.; et al. Wetland Buffer Zones for Nitrogen and Phosphorus Retention: Impacts of Soil Type, Hydrology and Vegetation. Science of The Total Environment 2020, 727, 138709. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.P. Denitrification in Sediment Determined from Nitrogen Isotope Pairing. FEMS Microbiology Letters 1992, 86, 357–362. [Google Scholar] [CrossRef]
- Hou, L.; Liu, M.; Carini, S.A.; Gardner, W.S. Transformation and Fate of Nitrate near the Sediment–Water Interface of Copano Bay. Continental Shelf Research 2012, 35, 86–94. [Google Scholar] [CrossRef]
- Montoya, J.P.; Voss, M.; Kahler, P.; Capone, D.G. A Simple, High-Precision, High-Sensitivity Tracer Assay for N(Inf2) Fixation. Appl Environ Microbiol 1996, 62, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Rysgaard, S.; Glud, R.N.; Risgaard-Petersen, N.; Dalsgaard, T. Denitrification and Anammox Activity in Arctic Marine Sediments. Limnology & Oceanography 2004, 49, 1493–1502. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, L.; Cheng, Z.; Xian, J.; Yang, Y.; Liu, L.; Xu, X. Dissimilatory Nitrate Reduction in Urban Lake Ecosystems: A Comparison Study between Closed and Open Lakes in Chengdu, China. Water Research 2022, 214, 118218. [Google Scholar] [CrossRef]
- Deng, F.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Li, X.; Lin, X.; Chen, F.; Gao, J.; Jiang, X. Dissimilatory Nitrate Reduction Processes and Associated Contribution to Nitrogen Removal in Sediments of the Yangtze Estuary. JGR Biogeosciences 2015, 120, 1521–1531. [Google Scholar] [CrossRef]
- Hou, L.; Wang, R.; Yin, G.; Liu, M.; Zheng, Y. Nitrogen Fixation in the Intertidal Sediments of the Yangtze Estuary: Occurrence and Environmental Implications. JGR Biogeosciences 2018, 123, 936–944. [Google Scholar] [CrossRef]
- Li, D.; Gao, G.; Lü, Y.; Fu, B. Multi-Scale Variability of Soil Carbon and Nitrogen in the Middle Reaches of the Heihe River Basin, Northwestern China. CATENA 2016, 137, 328–339. [Google Scholar] [CrossRef]
- Guo, L.; Wang, X.; Lin, Y.; Yang, X.; Ni, K.; Yang, F. Microorganisms That Are Critical for the Fermentation Quality of Paper Mulberry Silage. Food and Energy Security 2021, 10, e304. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat Biotechnol 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Research 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. The KEGG Resource for Deciphering the Genome. Nucleic Acids Research 2004, 32, 277D–280. [Google Scholar] [CrossRef]
- Tu, Q.; Lin, L.; Cheng, L.; Deng, Y.; He, Z. NCycDB: A Curated Integrative Database for Fast and Accurate Metagenomic Profiling of Nitrogen Cycling Genes. Bioinformatics 2019, 35, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- You, A.; Hua, L.; Hu, J.; Tian, J.; Ding, T.; Cheng, N.; Hu, L. Patters of Reactive Nitrogen Removal at the Waters in the Semi-Constructed Wetland. Journal of Environmental Management 2023, 344, 118733. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, W.; Gao, J.; Hu, X.; Zhang, C.; He, Q.; Yang, F.; Wang, H.; Wang, X.; Zhan, X. A pilot-scale study on the treatment of landfill leachate by a composite biological system under low dissolved oxygen conditions: Performance and microbial community. Bioresource Technology 2020, 296, 122344. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Poursat, B.A.J.; De Ridder, D.; Smidt, H.; Van Der Wal, A.; Sutton, N.B. Unravelling the Contribution of Nitrifying and Methanotrophic Bacteria to Micropollutant Co-Metabolism in Rapid Sand Filters. Journal of Hazardous Materials 2022, 424, 127760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bu, C.; Yang, H.; Qiao, Z.; Ding, S.; Ni, S.-Q. Survey of Dissimilatory Nitrate Reduction to Ammonium Microbial Community at National Wetland of Shanghai, China. Chemosphere 2020, 250, 126195. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Luo, H.; Zeng, D.; Sima, M.; Huang, S. Linking Microbial Community and Biological Functions to Redox Potential during Black-Odor River Sediment Remediation. Environ Sci Pollut Res 2020, 27, 40392–40404. [Google Scholar] [CrossRef]
- Deng, M.; Li, L.; Dai, Z.; Senbati, Y.; Song, K.; He, X. Aerobic Denitrification Affects Gaseous Nitrogen Loss in Biofloc-Based Recirculating Aquaculture System. Aquaculture 2020, 529, 735686. [Google Scholar] [CrossRef]
- Xu, T.; Tao, Y.; Song, L.; Wang, H.; Ren, B. A Unique Microbiome in a Highly Polluted and Alkalic Lake in a Seasonally Frozen Area. Environmental Research 2022, 204, 112056. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, L.; Yang, L.; Liu, C.; Li, J.; Li, N. Combined Impact of Organic Matter, Phosphorus, Nitrate, and Ammonia Nitrogen on the Process of Blackwater. Environ Sci Pollut Res 2021, 28, 32831–32843. [Google Scholar] [CrossRef]
- Sarkodie, E.K.; Jiang, L.; Li, K.; Guo, Z.; Yang, J.; Shi, J.; Peng, Y.; Wu, X.; Huang, S.; Deng, Y.; et al. The Influence of Cysteine in Transformation of Cd Fractionation and Microbial Community Structure and Functional Profile in Contaminated Paddy Soil. Science of The Total Environment 2024, 906, 167535. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, Q.; She, Z.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Nitrogen Removal Pathway and Dynamics of Microbial Community with the Increase of Salinity in Simultaneous Nitrification and Denitrification Process. Science of The Total Environment 2019, 697, 134047. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.R.L.; De Alcantara Neto, F.; Dutra, A.F.; Mendes, L.W.; Antunes, J.E.L.; Melo, V.M.M.; Oliveira, F.A.S.; Rocha, S.M.B.; Pereira, A.P.D.A.; Prado, R.D.M.; et al. Silicon Application Influences the Prokaryotic Communities in the Rhizosphere of Sugarcane Genotypes. Applied Soil Ecology 2023, 187, 104818. [Google Scholar] [CrossRef]
- Yun, L.; Yu, Z.; Li, Y.; Luo, P.; Jiang, X.; Tian, Y.; Ding, X. Ammonia Nitrogen and Nitrite Removal by a Heterotrophic Sphingomonas Sp. Strain LPN080 and Its Potential Application in Aquaculture. Aquaculture 2019, 500, 477–484. [Google Scholar] [CrossRef]
- Ma, H.; Gao, X.; Chen, Y.; Zhu, J.; Liu, T. Fe(II) Enhances Simultaneous Phosphorus Removal and Denitrification in Heterotrophic Denitrification by Chemical Precipitation and Stimulating Denitrifiers Activity. Environmental Pollution 2021, 287, 117668. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Luo, P.; Xiao, R.; Chen, J.; Chen, L.; Wu, J. Does rice straw application reduce N2O emissions from surface ffow CWs for swine wastewater treatment? Chemosphere 2019, 226, 273–281. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.; Yu, X.; Gu, H.; Zheng, X.; Wang, C.; Xiao, F.; Shu, L.; et al. Co-Symbiosis of Arbuscular Mycorrhizal Fungi (AMF) and Diazotrophs Promote Biological Nitrogen Fixation in Mangrove Ecosystems. Soil Biology and Biochemistry 2021, 161, 108382. [Google Scholar] [CrossRef]
- Cheng, J.Z.; White, J.R. Dredge-Material Created Coastal Marshes Are More Effective at Improving Water Quality than Natural Marshes in Early-Stage Development. Ecological Engineering 2022, 185, 106814. [Google Scholar] [CrossRef]
- Ma, L.; Xiong, Z.; Yao, L.; Liu, G.; Zhang, Q.; Liu, W. Soil Properties Alter Plant and Microbial Communities to Modulate Denitrification Rates in Subtropical Riparian Wetlands. Land Degrad Dev 2020, 31, 1792–1802. [Google Scholar] [CrossRef]
- Wang, W.; Pan, X.; Shu, X.; Tan, X.; Zhao, B.; Zhang, Q. Direct Evidence Indicates That Revegetation Improves Organic Carbon Limitation in Sediment Denitrification in a Eutrophic Headwater River. Ecological Engineering 2024, 198, 107132. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Z.; Li, X.; Long, Z.; Pei, Y. Comprehensive Analysis of the Migration and Transformation of Nutrients between Sediment and Overlying Water in Complex Habitat Systems. Science of The Total Environment 2022, 852, 158433. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, C.J.; Brock, M.T.; Van Diepen, L.T.A.; Maignien, L.; Ewers, B.E.; Weinig, C. The Plant Circadian Clock Influences Rhizosphere Community Structure and Function. The ISME Journal 2018, 12, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, J.; Liu, H.; Li, B.; Yu, X.; Cao, X.; Liu, D.; Wen, L.; Zhuo, Y.; Wang, L. Characteristics of Bacterial Biodiversity and Community Structure in Non-Rhizosphere Soils along Zonal Distribution of Plants within Littoral Wetlands in Inner Mongolia, China. Global Ecology and Conservation 2020, 24, e01310. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, S.; Qin, J.; Dai, J.; Zhao, F.; Gao, L.; Lian, X.; Shang, W.; Xu, X.; Hu, X. Changes in the Microbiome in the Soil of an American Ginseng Continuous Plantation. Front. Plant Sci 2020, 11, 572199. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Q.; Feng, J.; Yang, Z.; Yu, C.; Zhang, J.; Ling, J.; Dong, J. Introduction of Exotic Species Sonneratia Apetala Alters Diazotrophic Community and Stimulates Nitrogen Fixation in Mangrove Sediments. Ecological Indicators 2022, 142, 109179. [Google Scholar] [CrossRef]
- Zhang, M.; Zha, J.; Dong, Y.; Zhang, Q.; Pang, S.; Tian, S.; Sun, Q. Regulation of Potential Denitrification Rates in Sediments by Microbial-Driven Elemental Coupled Metabolisms. Journal of Environmental Management 2023, 348, 119320. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, Z.; Tobias, C.R.; Wang, Y.; Xiao, K.; Yu, Q.; Lin, J.; Huang, G.; Chen, N. Salt Marsh Expansion into Estuarine Mangrove Mudflats Reduces Nitrogen Removal Capacity. CATENA 2023, 232, 107459. [Google Scholar] [CrossRef]
- Wang, S.; Pi, Y.; Song, Y.; Jiang, Y.; Zhou, L.; Liu, W.; Zhu, G. Hotspot of Dissimilatory Nitrate Reduction to Ammonium (DNRA) Process in Freshwater Sediments of Riparian Zones. Water Research 2020, 173, 115539. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat Commun 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zeng, J.; Zhao, D.; Huang, R.; Yu, Z.; Wu, Q.L. Contrasting Patterns in Diversity and Community Assembly of Phragmites Australis Root-Associated Bacterial Communities from Different Seasons. Appl Environ Microbiol 2020, 86, e00379–20. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, S.; Yan, P.; Huo, J.; Aurangzeib, M. Microbial Community and Their Potential Functions after Natural Vegetation Restoration in Gullies of Farmland in Mollisols of Northeast China. Land 2022, 11, 2231. [Google Scholar] [CrossRef]
- Ye, F.; Duan, L.; Sun, Y.; Yang, F.; Liu, R.; Gao, F.; Wang, Y.; Xu, Y. Nitrogen Removal in Freshwater Sediments of Riparian Zone: N-Loss Pathways and Environmental Controls. Front. Microbiol. 2023, 14, 1239055. [Google Scholar] [CrossRef]
- Rankovi’c, V.; Radulovi’c, J.; Radojevi’c, I.; Ostoji’c, A.; Čomić, L. Neural network modeling of dissolved oxygen in the Gruža reservoir, Serbia. Ecological Modelling 2010, 221, 1239–1244. [Google Scholar] [CrossRef]
- Lu, B.; Xu, Z.; Li, J.; Chai, X. Removal of Water Nutrients by Different Aquatic Plant Species: An Alternative Way to Remediate Polluted Rural Rivers. Ecological Engineering 2018, 110, 18–26. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of Root Exudates on Denitrifier Gene Abundance, Community Structure and Activity in a Micro-Polluted Constructed Wetland. Science of The Total Environment 2017, 598, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Husson, O. Redox Potential (Eh) and PH as Drivers of Soil/Plant/Microorganism Systems: A Transdisciplinary Overview Pointing to Integrative Opportunities for Agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, J.; Liu, J.; Yu, L. Shifts of Sediment Bacterial Community and Respiration along a Successional Gradient in a Typical Karst Plateau Lake Wetland (China). J. Ocean. Limnol. 2021, 39, 880–891. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Cao, X.; Qi, W.; Peng, J.; Liu, H.; Qu, J. The Influence of Wet-to-Dry Season Shifts on the Microbial Community Stability and Nitrogen Cycle in the Poyang Lake Sediment. Science of The Total Environment 2023, 903, 166036. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.; Babalola, O. The Influence of Soil Fertilization on the Distribution and Diversity of Phosphorus Cycling Genes and Microbes Community of Maize Rhizosphere Using Shotgun Metagenomics. Genes 2021, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.D.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.A.; et al. Stochasticity, Succession, and Environmental Perturbations in a Fluidic Ecosystem. Proc. Natl. Acad. Sci. U.S.A. 2014, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Yuan, S.-W.; Che, F.-F.; Wan, X.; Wang, Y.-F.; Yang, D.-H.; Yang, H.-J.; Zhu, D.; Chen, P. Strong Bacterial Stochasticity and Fast Fungal Turnover in Taihu Lake Sediments, China. Environmental Research 2023, 237, 116954. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ping, Y.; Cui, L.; Zhang, X.; Li, W.; Hu, Y.; Cornelissen, J.H.C. Nutrient Resorption from Leaves of Wetland Plants in a Constructed Wetland Depends on Green Leaf Nutrient Content and Life Form. Wetlands 2020, 40, 983–991. [Google Scholar] [CrossRef]
- Hu, J.; Yu, H.; Li, Y.; Wang, J.; Lv, T.; Liu, C.; Yu, D. Variation in resource allocation strategies and environmental driving factors for different life-forms of aquatic plants in cold temperate zones. Journal of Ecology 2021, 109, 3046. [Google Scholar] [CrossRef]
- Bao, Y.; Huo, Y.; Duan, Y.; He, P.; Wu, M.; Yang, N.; Sun, B. Growth and Nutrient Uptake of Myriophyllum Spicatum under Different Nutrient Conditions and Its Potential Ecosystem Services in an Enclosed Sea Area in the East China Sea. Marine Pollution Bulletin 2020, 151, 110801. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, J.; Ma, X.; Lv, T.; Wang, L.; Li, J.; Liu, C. Community Structure and Function of Epiphytic Bacteria Attached to Three Submerged Macrophytes. Science of The Total Environment 2022, 835, 155546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, J.; Liu, X.; Zhong, H.; Wang, B.; Kong, Z.; Wu, L. Tracking the Changes of Wetland Soil Bacterial Community and Metabolic Potentials under Drought and Flooding Conditions in Experimental Microcosms. J Soils Sediments 2021, 21, 2404–2417. [Google Scholar] [CrossRef]
- Wang, X.; Jain, A.; Chen, B.; Wang, Y.; Jin, Q.; Yugandhar, P.; Xu, Y.; Sun, S.; Hu, F. Differential Efficacy of Water Lily Cultivars in Phytoremediation of Eutrophic Water Contaminated with Phosphorus and Nitrogen. Plant Physiology and Biochemistry 2022, 171, 139–146. [Google Scholar] [CrossRef]
- Xu, C.; Chen, L.; Chen, S.; Chu, G.; Wang, D.; Zhang, X. Effects of Rhizosphere Oxygen Concentration on Root Physiological Characteristics and Anatomical Structure at the Tillering Stage of Rice. Annals of Applied Biology 2020, 177, 61–73. [Google Scholar] [CrossRef]
- Pietrangelo, L.; Bucci, A.; Maiuro, L.; Bulgarelli, D.; Naclerio, G. Unraveling the Composition of the Root-Associated Bacterial Microbiota of Phragmites Australis and Typha Latifolia. Front. Microbiol. 2018, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Isobe, K.; Nishizawa, T.; Zhu, L.; Shiratori, Y.; Ohte, N.; Koba, K.; Otsuka, S.; Senoo, K. Higher Diversity and Abundance of Denitrifying Microorganisms in Environments than Considered Previously. The ISME Journal 2015, 9, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, G.; Li, X.; Li, C.; Li, J.; Zhao, C.; Cui, J.; Du, C.; Tian, Z.; Shi, Y.; et al. Effect of Microbial Network Complexity and Stability on Nitrogen and Sulfur Pollutant Removal during Sediment Remediation in Rivers Affected by Combined Sewer Overflows. Chemosphere 2023, 331, 138832. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, F.; Chen, Y.; Zou, W.; Zhu, Z. Diazotrophic Community in the Sediments of Poyang Lake in Response to Water Level Fluctuations. Front. Microbiol. 2024, 15, 1324313. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Z.; Li, J.; Wang, H.; Guo, G.; Wu, W. Effects of Muddy Water Irrigation with Different Sediment Particle Sizes and Sediment Concentrations on Soil Microbial Communities in the Yellow River Basin of China. Agricultural Water Management 2022, 270, 107750. [Google Scholar] [CrossRef]
- Hu, J.; Yu, H.; Li, Y.; Wang, J.; Lv, T.; Liu, C.; Yu, D. Variation in resource allocation strategies and environmental driving factors for different life-forms of aquatic plants in cold temperate zones. Journal of Ecology 2021, 109, 3046. [Google Scholar] [CrossRef]
- Wang, L.; Xing, P.; Li, H.; Zhou, L.; Wu, Q.L. Distinct Intra-Lake Heterogeneity of Diazotrophs in a Deep Oligotrophic Mountain Lake. Microb Ecol 2020, 79, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; McIlroy, S.J.; Tyson, G.W.; Guo, J. Phylogenetic and Metabolic Diversity of Microbial Communities Performing Anaerobic Ammonium and Methane Oxidations under Different Nitrogen Loadings. ISME Communications 2023, 3, 39. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, R.-Z.; Wang, Y.; Chen, G.-L.; Fu, Y.-Y.; Yu, H.-Q. Carbon Source Shaped Microbial Ecology, Metabolism and Performance in Denitrification Systems. Water Research 2023, 243, 120330. [Google Scholar] [CrossRef]
- Shao, Y.; He, Q.; Fu, Y.; Zhang, G.; Liu, Y. Environmental impact and variation analysis of different CaO2 and Ca (NO3)2 dosing modes on microbial community in black-odorous sediment. Process Safety and Environmental Protection 2022, 167, 641–650. [Google Scholar] [CrossRef]
- Tian, L.Q.; Jiang, H.L.; Bai, L.L.; Wang, C.L.; Xu, S.Q. Q. Biological Nitrogen Fixation in Sediments of a Cyanobacterial Bloom-Occurring Bay in One Eutrophic Shallow Lake: Occurrence and Related Environmental Factors. Journal of Geophysical Research: Biogeosciences 2021, 126, 1–15. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Guo, Y.; Zhao, J.; Bao, Z. Rhizosphere-Associated Anammox Bacterial Diversity and Abundance of Nitrogen Cycle-Related Functional Genes of Emergent Macrophytes in Eutrophic Wetlands. Curr Microbiol 2024, 81, 107. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Peng, L.; Liu, G.; Wan, X.; Hua, Y.; Zhu, D.; Hamilton, D.P. Diversity of Anammox Bacteria and Abundance of Functional Genes for Nitrogen Cycling in the Rhizosphere of Submerged Macrophytes in a Freshwater Lake in Summer. J Soils Sediments 2019, 19, 3648–3656. [Google Scholar] [CrossRef]
- Fang, J.; Lü, T.; Liu, J.; He, S.; Yang, X.; Dou, H.; Zhang, H. Responses of Nitrogen Cycling and Related Microorganisms to Brackish Wetlands Formed by Evapotranspiration. Pedosphere 2024, 34, 252–266. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, W.; Xia, X.; Xia, L.; Li, S.; Zhang, L.; Bai, Y.; Wang, G. Ammonia Oxidizers in River Sediments of the Qinghai-Tibet Plateau and Their Adaptations to High-Elevation Conditions. Water Research 2020, 173, 115589. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; Myrold, D.D.; Firestone, M.; Voytek, M. Environmental Controls on Denitrifying Communities And Denitrification Rates: Insights From Molecular Methods. Ecological Applications 2006, 16, 2143–2152. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 1997, 61, 533–616. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Wang, F.; Ren, L.; Ma, S.; Li, S.; Liu, L.; Wang, J. Ratio of Nitrate to Ammonium Mainly Drives Soil Bacterial Dynamics Involved in Nitrate Reduction Processes. Applied Soil Ecology 2022, 169, 104164. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Nelson, W.C.; Shi, L.; Xu, F.; Liu, Y.; Yan, A.; Zhong, L.; Thompson, C.; Fredrickson, J.K.; et al. Effect of Water Chemistry and Hydrodynamics on Nitrogen Transformation Activity and Microbial Community Functional Potential in Hyporheic Zone Sediment Columns. Environ. Sci. Technol. 2017, 51, 4877–4886. [Google Scholar] [CrossRef]
- Kang, P.; Chen, S.; Huang, T.; Zhang, H.; Shang, P.; Zhao, Z.; Tan, X. Denitrification Characteristics and Functional Genes of Denitrifying Bacteria Under Aerobic or Anaerobic Conditions. Huan Jing Ke Xue 2018, 8, 3789–3796. [Google Scholar] [CrossRef]
- Wang, F.; Cui, Q.; Liu, W.; Jiang, W.; Ai, S.; Liu, W.; Bian, D. Synergistic Denitrification Mechanism of Domesticated Aerobic Denitrifying Bacteria in Low-Temperature Municipal Wastewater Treatment. npj Clean Water 2024, 7, 6. [Google Scholar] [CrossRef]
- Maruyama, R.; Yasumoto, K.; Mizusawa, N.; Iijima, M.; Yasumoto-Hirose, M.; Iguchi, A.; Hermawan, O.R.; Hosono, T.; Takada, R.; Song, K.-H.; et al. Metagenomic Analysis of the Microbial Communities and Associated Network of Nitrogen Metabolism Genes in the Ryukyu Limestone Aquifer. Sci Rep 2024, 14, 4356. [Google Scholar] [CrossRef] [PubMed]
- Saarenheimo, J.; Rissanen, A.J.; Arvola, L.; Nykänen, H.; Lehmann, M.F.; Tiirola, M. Genetic and Environmental Controls on Nitrous Oxide Accumulation in Lakes. PLoS ONE 2015, 10, e0121201. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Lv, J.; He, X.; Wang, J.; Teng, D.; Jiang, L.; Wang, H.; Lv, G. Rhizosphere Effect Alters the Soil Microbiome Composition and C, N Transformation in an Arid Ecosystem. Applied Soil Ecology 2022, 170, 104296. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 1997, 61, 533–616. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Van Meter, K.J.; Byrnes, D.K.; Basu, N.B. Maximizing US Nitrate Removal through Wetland Protection and Restoration. Nature 2020, 588, 625–630. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Wang, X.; Yuan, D.; Zhu, G. Dissimilatory Nitrate Reduction to Ammonium (DNRA) in Traditional Municipal Wastewater Treatment Plants in China: Widespread but Low Contribution. Water Research 2020, 179, 115877. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhang, M.; Zheng, C.; Yang, L.; Yang, L. Review of the Mechanisms Involved in Dissimilatory Nitrate Reduction to Ammonium and the Efficacies of These Mechanisms in the Environment. Environmental Pollution 2024, 345, 123480. [Google Scholar] [CrossRef]
- Huang, X.; Luoluo; Xie, D.; Li, Z. Dissimilatory Nitrate Reduction to Ammonium in Four Pseudomonas Spp. under Aerobic Conditions. Heliyon 2023, 9, e14983. [Google Scholar] [CrossRef]
- Wei, Z.; Jin, K.; Li, C.; Wu, M.; Shan, J.; Yan, X. Environmental Factors Controlling Dissimilatory Nitrate Reduction to Ammonium in Paddy Soil. J Soil Sci Plant Nutr 2022, 22, 4241–4248. [Google Scholar] [CrossRef]
- Pang, Y.; Ji, G. Biotic Factors Drive Distinct DNRA Potential Rates and Contributions in Typical Chinese Shallow Lake Sediments. Environmental Pollution 2019, 254, 112903. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Md.M.; Roberts, K.L.; Grace, M.R.; Kessler, A.J.; Cook, P.L.M. Role of Organic Carbon, Nitrate and Ferrous Iron on the Partitioning between Denitrification and DNRA in Constructed Stormwater Urban Wetlands. Science of The Total Environment 2019, 666, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kasak, K.; Espenberg, M.; Anthony, T.L.; Tringe, S.G.; Valach, A.C.; Hemes, K.S.; Silver, W.L.; Mander, Ü.; Kill, K.; McNicol, G.; et al. Restoring Wetlands on Intensive Agricultural Lands Modifies Nitrogen Cycling Microbial Communities and Reduces N2O Production Potential. Journal of Environmental Management 2021, 299, 113562. [Google Scholar] [CrossRef] [PubMed]
- Broman, E.; Zilius, M.; Samuiloviene, A.; Vybernaite-Lubiene, I.; Politi, T.; Klawonn, I.; Voss, M.; Nascimento, F.J.A.; Bonaglia, S. Active DNRA and Denitrification in Oxic Hypereutrophic Waters. Water Research 2021, 194, 116954. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bai, Y.; Qu, J. The Phragmites Root-Inhabiting Microbiome: A Critical Review on Its Composition and Environmental Application. Engineering 2022, 9, 42–50. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Lu, J.; Duan, R.; Chen, H.; Ma, Y.; Si, T.; Luo, M. Reed Restoration Decreased Nutrients in Wetlands with Dredged Sediments: Microbial Community Assembly and Function in Rhizosphere. Journal of Environmental Management 2023, 344, 118700. [Google Scholar] [CrossRef]
- Singh, R.P.; Reddy, C.R.K. Seaweed–microbial interactions: key functions of seaweed-associated bacteria. FEMS Microbiology Ecology 2014, 88, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Zehr, J.P.; Braun, S.; Chen, Y.; Mellon, M. Nitrogen Fixation in the Marine Environment: Relating Genetic Potential to Nitrogenase Activity. Journal of Experimental Marine Biology and Ecology 1996, 203, 61–73. [Google Scholar] [CrossRef]
- Barraquio, W.L.; Watanabe, I. Occurrence of Aerobic Nitrogen Fixing Bacteria in Wetland and Dryland Plants. Soil Science and Plant Nutrition 1981, 27, 121–125. [Google Scholar] [CrossRef]
- Ketelboeter, L.M.; Mitra, S.; Gyaneshwar, P. A Thiamine Transporter Is Required for Biofilm Formation by Rhizobium Sp. IRBG74. FEMS Microbiology Letters 2023, 370, fnad046. [Google Scholar] [CrossRef]
- Luo, Z.; Qiu, Z.; Wei, Q.; Du Laing, G.; Zhao, Y.; Yan, C. Dynamics of Ammonia-Oxidizing Archaea and Bacteria in Relation to Nitrification along Simulated Dissolved Oxygen Gradient in Sediment–Water Interface of the Jiulong River Estuarine Wetland, China. Environ Earth Sci 2014, 72, 2225–2237. [Google Scholar] [CrossRef]
- Deng, D.; Pan, Y.; Liu, G.; Liu, W.; Ma, L. Seeking the Hotspots of Nitrogen Removal: A Comparison of Sediment Denitrification Rate and Denitrifier Abundance among Wetland Types with Different Hydrological Conditions. Science of The Total Environment 2020, 737, 140253. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Chen, J.; Wang, D.; Yang, Z.; Wang, B.; Zhuang, Y.; Wang, R. Nitrogen Removal through Sediment Denitrification in the Yangtze Estuary and Its Adjacent East China Sea: A Nitrate Limited Process during Summertime. Science of The Total Environment 2021, 795, 148616. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, Y.; Yang, F.; Guan, Q.; Li, Q.; Liang, H.; Zhao, J. Impact of Environmental Factors on the Diversity of Nitrogen-Removal Bacteria in Wetlands in the Sanmenxia Reservoir of the Yellow River. J Soils Sediments 2023, 23, 512–525. [Google Scholar] [CrossRef]
- Zhang, M.; Daraz, U.; Sun, Q.; Chen, P.; Wei, X. Denitrifier Abundance and Community Composition Linked to Denitrification Potential in River Sediments. Environ Sci Pollut Res 2021, 28, 51928–51939. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; She, D.; Pan, Y.; Abulaiti, A.; Huang, Y.; Liu, R.; Wang, F.; Xia, Y.; Shan, J. Ditch Level-Dependent N Removal Capacity of Denitrification and Anammox in the Drainage System of the Ningxia Yellow River Irrigation District. Science of The Total Environment 2024, 916, 170314. [Google Scholar] [CrossRef]
- Nifong, R.L.; Taylor, J.M. Vegetation and Residence Time Interact to Influence Metabolism and Net Nutrient Uptake in Experimental Agricultural Drainage Systems. Water 2021, 13, 1416. [Google Scholar] [CrossRef]
- Rachel, L.N.; Jason, M.T. Vegetation and Residence Time Interact to Influence Metabolism and Net Nutrient Uptake in Experimental Agricultural Drainage Systems. Water 2021, 13, 1416. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, H.; Tian, L.; Yuan, J.; Chen, Y.; Li, Y.; Chen, J.; Chang, S.X.; Fang, Y.; Tavakkoli, E.; et al. Relationships between Denitrification Rates and Functional Gene Abundance in a Wetland: The Roles of Single- and Multiple-Species Plant Communities. Science of The Total Environment 2023, 863, 160913. [Google Scholar] [CrossRef]
- Surey, R.; Kaiser, K.; Schimpf, C.M.; Mueller, C.W.; Böttcher, J.; Mikutta, R. Contribution of Particulate and Mineral-Associated Organic Matter to Potential Denitrification of Agricultural Soils. Front. Environ. Sci. 2021, 9, 640534. [Google Scholar] [CrossRef]
- Davidsson, T.E.; Ståhl, M. The Influence of Organic Carbon on Nitrogen Transformations in Five Wetland Soils. Soil Science Soc of Amer J 2000, 64, 1129–1136. [Google Scholar] [CrossRef]
- Sigleo, A.C. Denitrification Rates Across a Temperate North Pacific Estuary, Yaquina Bay, Oregon. Estuaries and Coasts 2019, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Xie, C.; Qin, Y.; Che, S. Effects of Revetments on Nitrification and Denitrification Potentials in the Urban River–Riparian Interface. Land 2024, 13, 333. [Google Scholar] [CrossRef]
- Li, S.; Twilley, R.R.; Hou, A. Heterotrophic Nitrogen Fixation in Response to Nitrate Loading and Sediment Organic Matter in an Emerging Coastal Deltaic Floodplain within the Mississippi River Delta Plain. Limnology & Oceanography 2021, 66, 1961–1978. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Gao, D.; Liu, M.; Cheng, L. Ammonium Production and Removal in the Sediments of Shanghai River Networks: Spatiotemporal Variations, Controlling Factors, and Environmental Implications. JGR Biogeosciences 2017, 122, 2461–2478. [Google Scholar] [CrossRef]
- Semedo, M.; Song, B. From Genes to Nitrogen Removal: Determining the Impacts of Poultry Industry Wastewater on Tidal Creek Denitrification. Environ. Sci. Technol. 2020, 54, 146–157. [Google Scholar] [CrossRef]
- Tsiknia, M.; Paranychianakis, N.V.; Varouchakis, E.A.; Nikolaidis, N.P. Environmental Drivers of the Distribution of Nitrogen Functional Genes at a Watershed Scale. FEMS Microbiology Ecology 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Gao, H. Enhanced Long-Term Advanced Denitrogenation from Nitrate Wastewater by Anammox Consortia: Dissimilatory Nitrate Reduction to Ammonium (DNRA) Coupling with Anammox in an Upflow Biofilter Reactor Equipped with EDTA-2Na/Fe(II) Ratio and PH Control. Bioresource Technology 2020, 305, 123083. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yin, G.; Hou, L.; Liu, M.; Zheng, Y.; Han, P.; Dong, H.; Liang, X.; Gao, D.; Liu, C. Nitrogen Removal Processes Coupled with Nitrification in Coastal Sediments off the North East China Sea. J Soils Sediments 2021, 21, 3289–3299. [Google Scholar] [CrossRef]
- Hu, Y.; Hong, Y.; Ye, J.; Wu, J.; Wang, Y.; Ye, F.; Chang, X.; Long, A. Shift of DNRA Bacterial Community Composition in Sediment Cores of the Pearl River Estuary and the Impact of Environmental Factors. Ecotoxicology 2021, 30, 1689–1703. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Wang, Y.; Tan, X.; Qi, W.; Li, H.; Wei, J.; You, Y.; Shi, W.; Zhang, Q. Dissimilatory Nitrate Reduction and Functional Genes in Two Subtropical Rivers, China. Environ Sci Pollut Res 2021, 28, 68155–68173. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, N.; Shao, X.; Gao, D.; Xia, J.; Cui, Q.; Zhang, D. Effects of Coastal Marsh Conversion to Shrimp Aquaculture Ponds on Sediment Nitrogen Fixation. Front. Mar. Sci. 2022, 9, 1034145. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Yang, Y. Nitrogen Fixation in Surface Sediments of the East China Sea: Occurrence and Environmental Implications. Marine Pollution Bulletin 2018, 137, 542–548. [Google Scholar] [CrossRef]
- Huang, X.; Feng, J.; Yang, Q.; Chen, L.; Zhang, J.; Yang, B.; Tang, X.; Yu, C.; Ling, J.; Dong, J. High Site Elevation Enhanced Nitrogen Fixation and the Stability of Diazotrophic Community in Planted Sonneratia Apetala Mangrove Sediments. Applied Soil Ecology 2023, 191, 105059. [Google Scholar] [CrossRef]
- Spinette, R.; Brown, S.; Ehrlich, A.; Puggioni, G.; Deacutis, C.; Jenkins, B. Diazotroph Activity in Surface Narragansett Bay Sediments in Summer Is Stimulated by Hypoxia and Organic Matter Delivery. Mar. Ecol. Prog. Ser. 2019, 614, 35–50. [Google Scholar] [CrossRef]
- Luo, Z.; Zhong, Q.; Han, X.; Hu, R.; Liu, X.; Xu, W.; Wu, Y.; Huang, W.; Zhou, Z.; Zhuang, W.; et al. Depth-Dependent Variability of Biological Nitrogen Fixation and Diazotrophic Communities in Mangrove Sediments. Microbiome 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hou, L.; Zhang, Z.; Ge, J.; Li, M.; Yin, G.; Han, P.; Dong, H.; Liang, X.; Gao, J.; et al. Overlooked Contribution of Water Column to Nitrogen Removal in Estuarine Turbidity Maximum Zone (TMZ). Science of The Total Environment 2021, 788, 147736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, W.; Bai, Y.; Zhang, Z.; Wang, J.; Gao, H.; Gu, J. Linkages between anammox and denitrifying bacterial communities and nitrogen loss rates in highelevation rivers. Limnology and Oceanography 2020, 9999, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yang, A.; Hou, L.; Zheng, Y.; Zhai, W.; Gong, J. Incorporation of Microbial Functional Traits in Biogeochemistry Models Provides Better Estimations of Benthic Denitrification and Anammox Rates in Coastal Oceans. JGR Biogeosciences 2018, 123, 3331–3352. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmad, H.A.; Teng, Z.-J.; Sun, M.; Ismail, S.; Qiao, Z.; Ni, S.-Q. Widespread but Overlooked DNRA Process in a Full-Scale Simultaneous Partial Nitrification, Anammox, and Denitrification Plant. ACS EST Water 2022, 2, 1360–1369. [Google Scholar] [CrossRef]
- Taylor, J.M.; Moore, M.T.; Scott, J.T. Contrasting Nutrient Mitigation and Denitrification Potential of Agricultural Drainage Environments with Different Emergent Aquatic Macrophytes. J. Environ. Qual. 2015, 44, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Speir, S.L.; Taylor, J.M.; Thad, S.J. Seasonal differences in relationships between nitrate concentration and denitrification rates in ditch sediments vegetated with rice cutgrass. Journal of Environmental Quality 2017, 46, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.B.; Kumar, U.; Kaviraj, M.; Minick, K.J.; Mishra, A.K.; Singh, J.S. DNRA: A Short-Circuit in Biological N-Cycling to Conserve Nitrogen in Terrestrial Ecosystems. Science of The Total Environment 2020, 738, 139710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dai, H.; Yuan, S. The Competition between Heterotrophic Denitrification and DNRA Pathways in Hyporheic Zone and Its Impact on the Fate of Nitrate. Journal of Hydrology 2023, 626, 130175. [Google Scholar] [CrossRef]
- Quick, A.M.; Reeder, W.J.; Farrell, T.B.; Tonina, D.; Feris, K.P.; Benner, S.G. Nitrous Oxide from Streams and Rivers: A Review of Primary Biogeochemical Pathways and Environmental Variables. Earth-Science Reviews 2019, 191, 224–262. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Huang, Z.; Xiao, R.; Zhu, H.; Wu, J. Are vegetated drainage ditches effective for nitrogen removal under cold temperatures? Bioresource Technology 2020, 301, 122744. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yan, Z.; Wang, C.; Xu, S.; Jiang, H. Habitat Heterogeneity Induces Regional Differences in Sediment Nitrogen Fixation in Eutrophic Freshwater Lake. Science of The Total Environment 2021, 772, 145594. [Google Scholar] [CrossRef] [PubMed]
- Aoki, L.; McGlathery, K. Restoration Enhances Denitrification and DNRA in Subsurface Sediments of Zostera Marina Seagrass Meadows. Mar. Ecol. Prog. Ser. 2018, 602, 87–102. [Google Scholar] [CrossRef]
- Hansen, J.; Reidenbach, M. Wave and Tidally Driven Flows in Eelgrass Beds and Their Effect on Sediment Suspension. Mar. Ecol. Prog. Ser. 2012, 448, 271–287. [Google Scholar] [CrossRef]
- Aoki, L.R.; McGlathery, K.J.; Oreska, M.P.J. Seagrass Restoration Reestablishes the Coastal Nitrogen Filter through Enhanced Burial. Limnology & Oceanography 2020, 65, 1–12. [Google Scholar] [CrossRef]
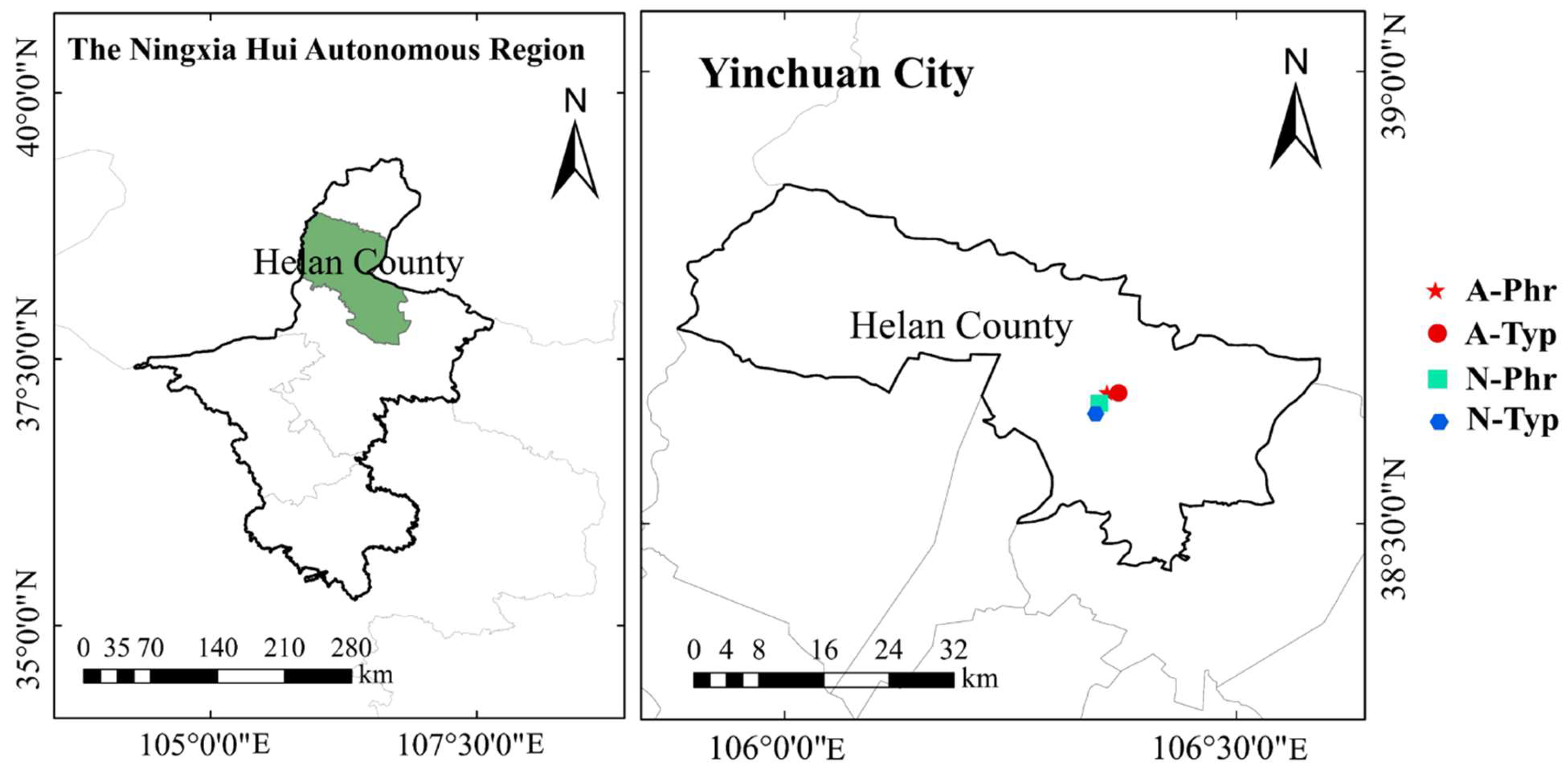
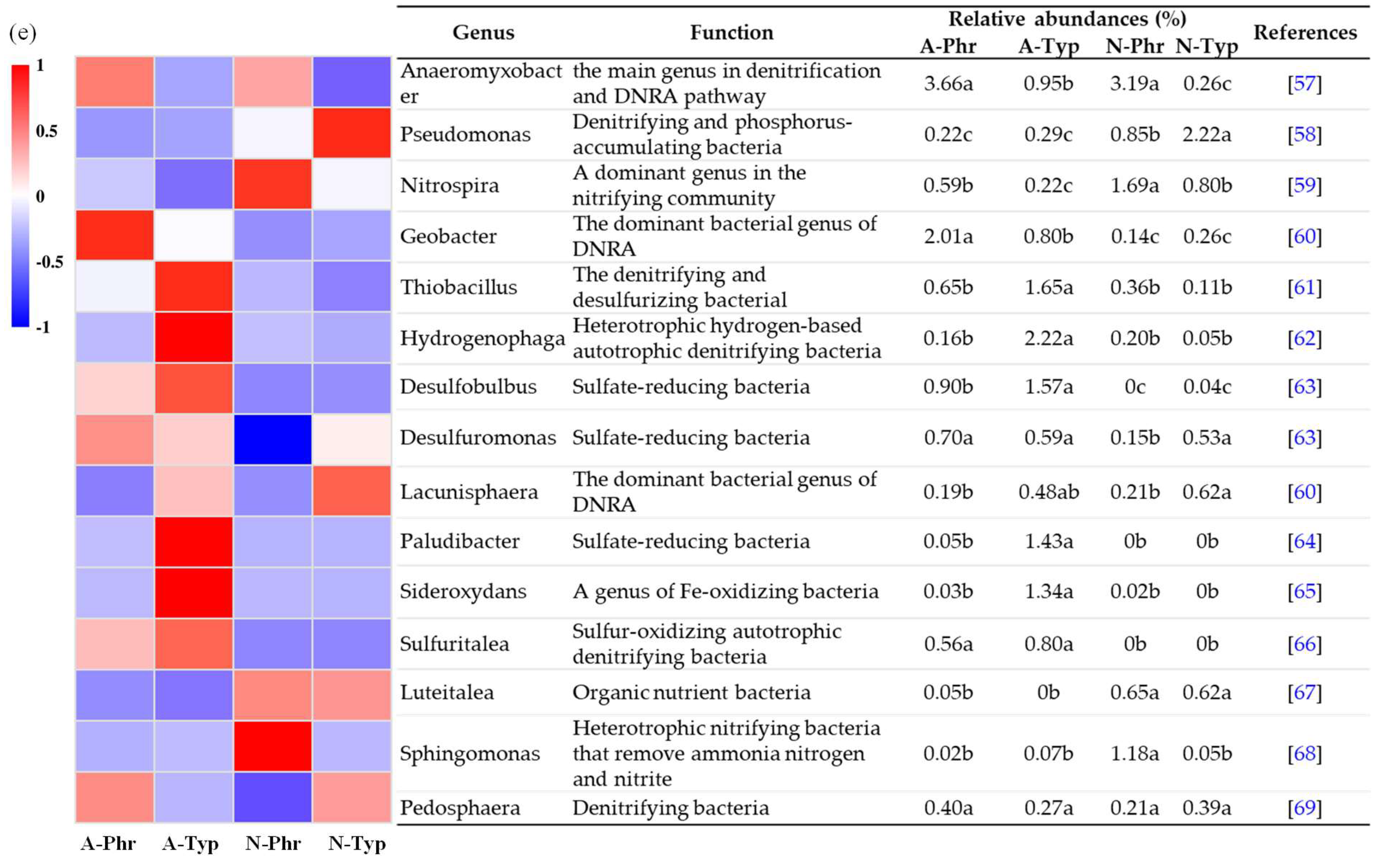
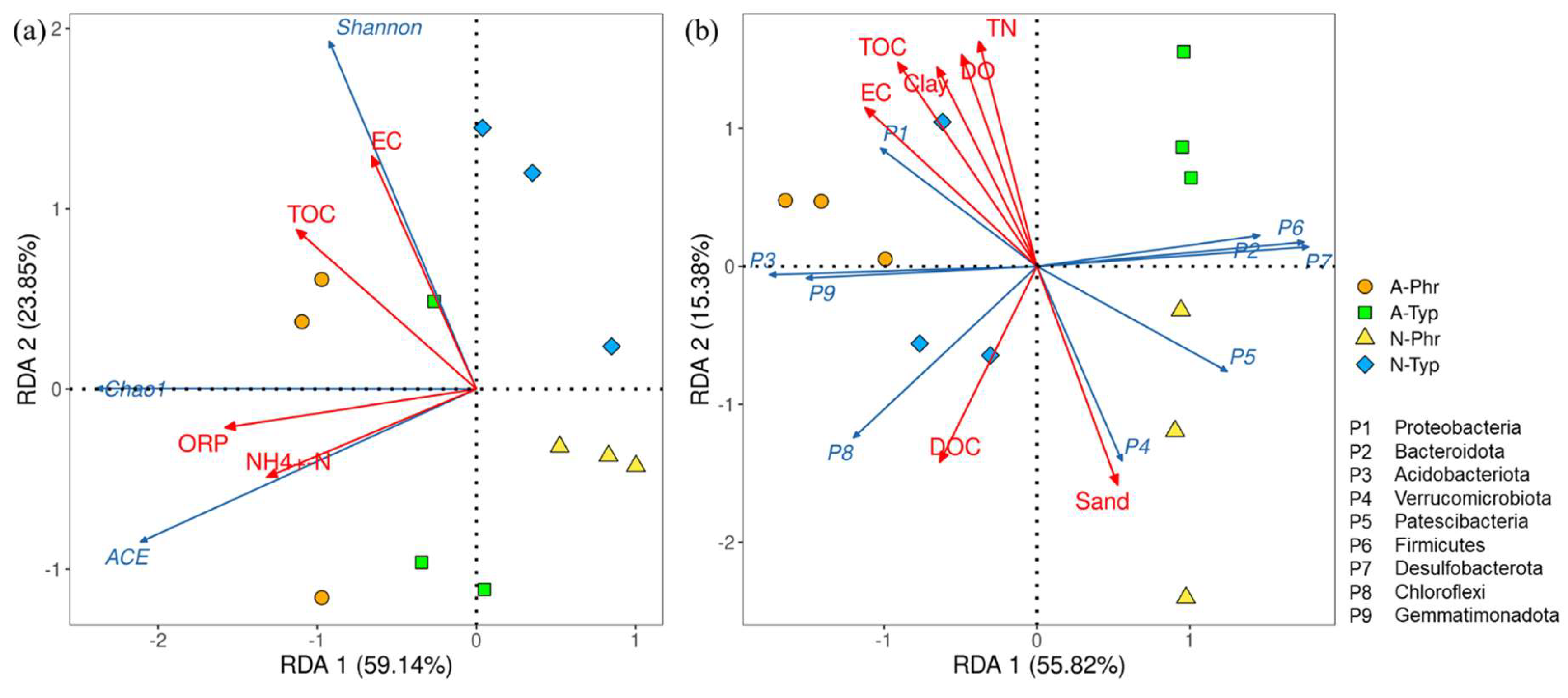


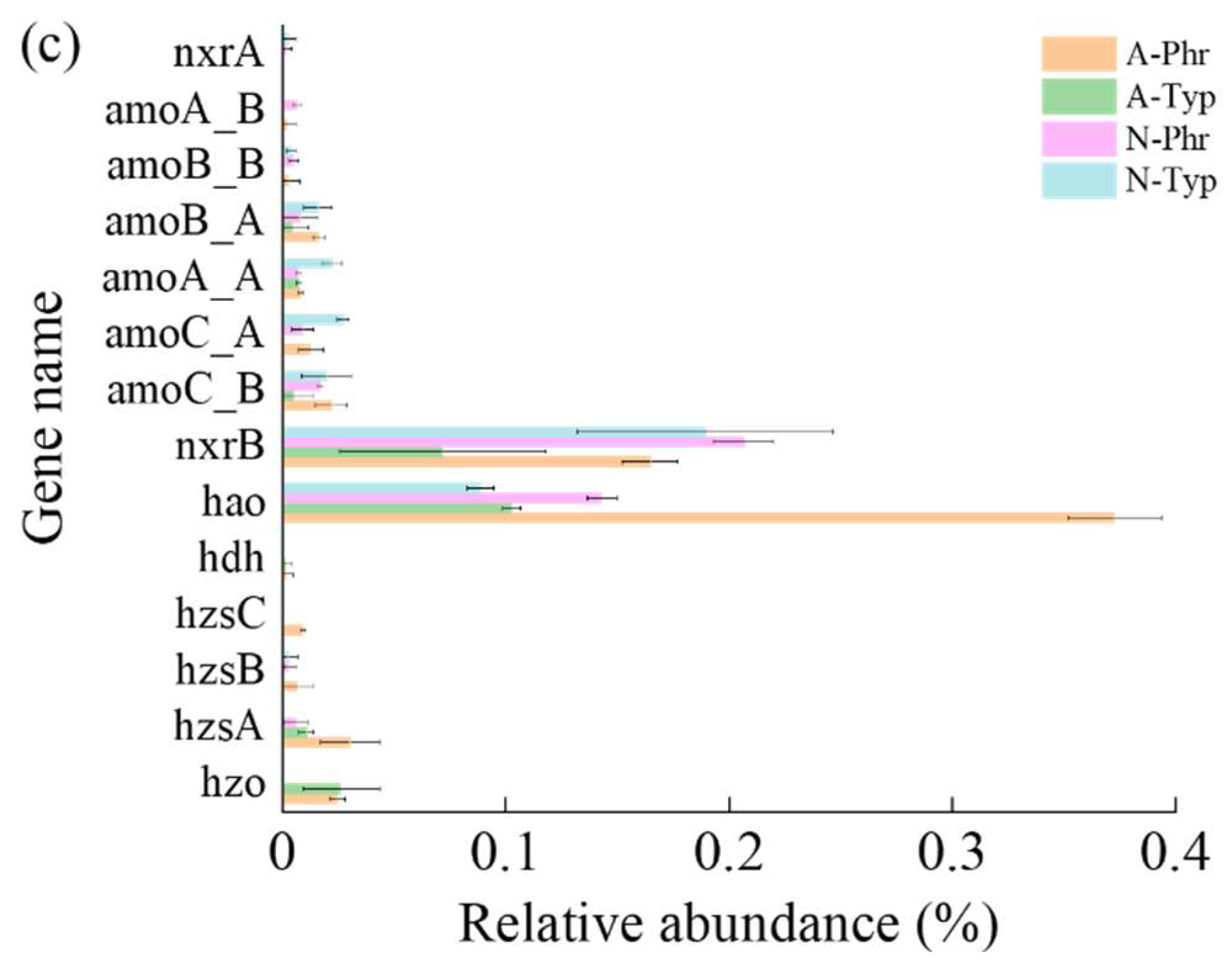
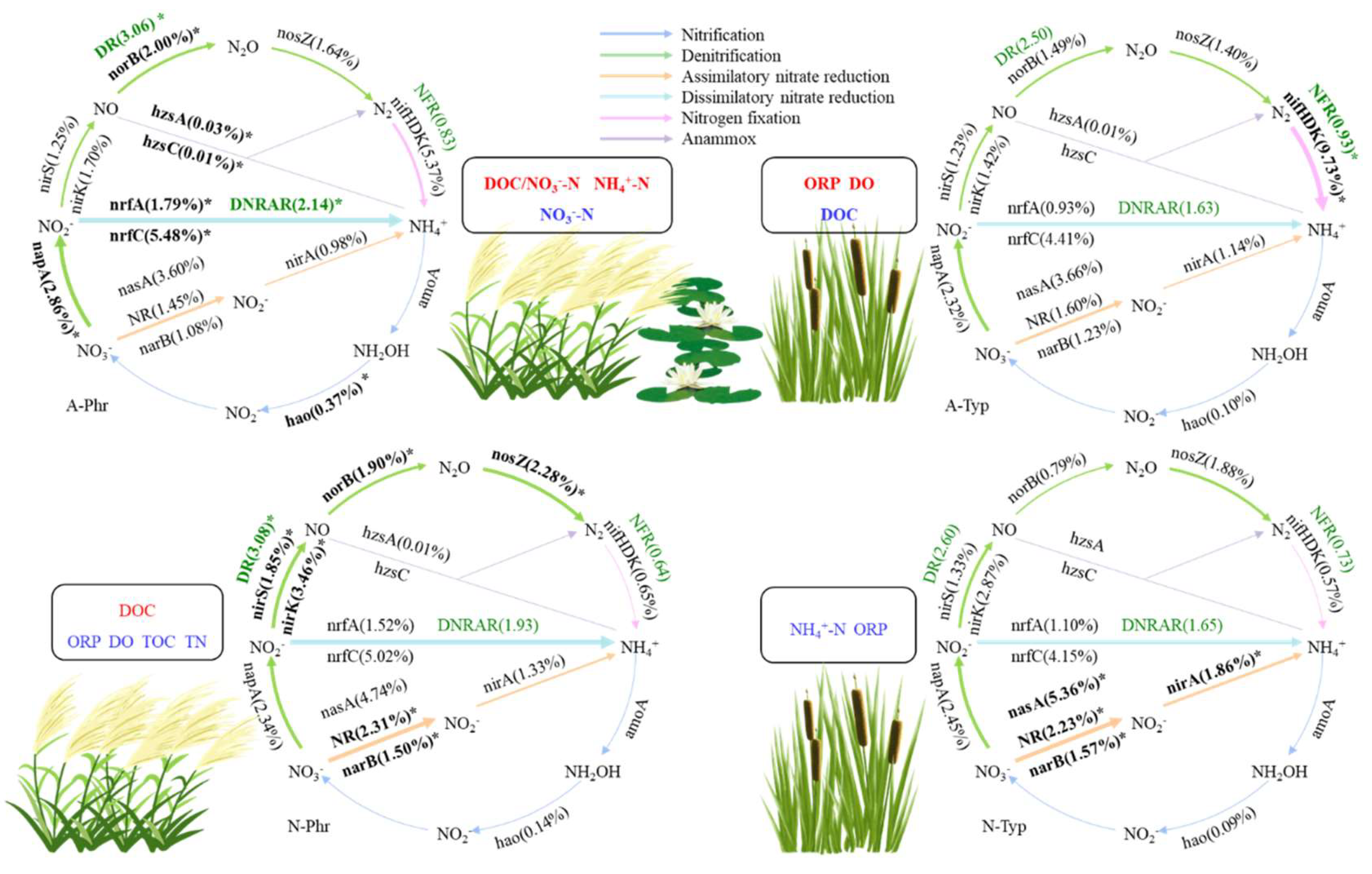
| Type of ditch | Principal species | Associated species | Relative coverage | Sample location | Abbreviation |
|---|---|---|---|---|---|
| Artificial cultivated ditch | Phragmites australis, Typha orientalis, Nymphaea tetragona | - | 57% | Phragmites australis- Rhizosphere | A-Phr |
| Typha orientalis - Rhizosphere | A-Typ | ||||
| Natural ditch1 | Phragmites australis | Scirpus triqueter, Echinochloa crusgali | 37.5% | Phragmites australis- Rhizosphere | N-Phr |
| Natural ditch2 | Typha orientalis | Phragmites australis, Scirpus triqueter | 30% | Typha orientalis - Rhizosphere | N-Typ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





