1. Introduction
Mass spectrometry (MS) has emerged as a powerful analytical tool in clinical chemistry, offering unprecedented capabilities for qualitative and quantitative analysis of biomolecules [
1,
2]. This review examines the growing importance of MS, particularly when coupled with liquid chromatography (LC-MS), in identifying disease biomarkers and quantifying biomolecules and drugs for diagnostic and prognostic purposes [
1,
3]. The unique advantages of MS technology in accurately identifying and quantifying diverse endogenous and exogenous biomolecules have positioned it as a cornerstone in the advancement of personalized medicine [
1,
4]. As the field of clinical chemistry evolves, there is an increasing demand for technologies that can serve as reference methods and meet analytical needs with high accuracy and precision. Mass spectrometry, with its high specificity and sensitivity, is poised to fulfill this role, offering solutions to many of the limitations faced by traditional analytical techniques [
1,
2].
The integration of MS-based technologies into clinical practice has revolutionized the field of precision medicine, enabling a more comprehensive understanding of disease mechanisms and patient-specific responses to treatments [
4,
5]. LC-MS, in particular, has demonstrated exceptional utility in the analysis of complex biological matrices, allowing for the simultaneous detection and quantification of multiple analytes with high sensitivity and selectivity [
3,
6]. One of the key advantages of MS in clinical applications is its ability to provide detailed molecular information, which is crucial for the identification of novel biomarkers and the development of targeted therapies [
5]. This capability has been particularly valuable in oncology, where MS-based proteomics and metabolomics have facilitated the discovery of cancer-specific markers and potential therapeutic targets [
2,
5].
Furthermore, the application of MS in therapeutic drug monitoring has significantly improved patient care by enabling precise dosage adjustments based on individual pharmacokinetics and pharmacodynamics [
4]. This personalized approach to drug administration has led to enhanced treatment efficacy and reduced adverse effects, particularly in cases involving drugs with narrow therapeutic windows. The advent of high-resolution mass spectrometry (HRMS) has further expanded the analytical capabilities in clinical settings, allowing for the detection of low-abundance molecules and the elucidation of complex biological pathways. This technology has been instrumental in advancing our understanding of disease pathogenesis and in identifying novel therapeutic interventions.
In recent years, the integration of MS with other analytical techniques, such as ion mobility spectrometry, has opened new avenues for biomarker discovery and validation [
7]. These hyphenated techniques offer enhanced separation capabilities and structural information, further improving the specificity and sensitivity of clinical analyses [
8]. The role of MS in personalized medicine extends beyond biomarker discovery and drug monitoring [
4,
5]. It has also found applications in the field of pharmacogenomics, where it aids in the identification of genetic variants that influence drug metabolism and response. This integration of genomic and proteomic data has paved the way for more targeted and effective therapeutic strategies. As we move towards an era of precision medicine, the continued development and refinement of MS-based technologies will be crucial in addressing the complex challenges of individualized patient care. The ability of MS to provide comprehensive molecular profiles of biological samples positions it as an indispensable tool in the ongoing efforts to tailor medical treatments to individual patients.
In conclusion, mass spectrometry has established itself as a cornerstone technology in clinical chemistry and personalized medicine [
1,
5]. Its unparalleled analytical capabilities, coupled with ongoing technological advancements, promise to drive further innovations in disease diagnosis, prognosis, and treatment strategies. As we continue to unravel the complexities of human biology and disease, MS will undoubtedly play a pivotal role in shaping the future of healthcare and improving patient outcomes.
2. Advantages of Mass Spectrometry in Clinical Applications
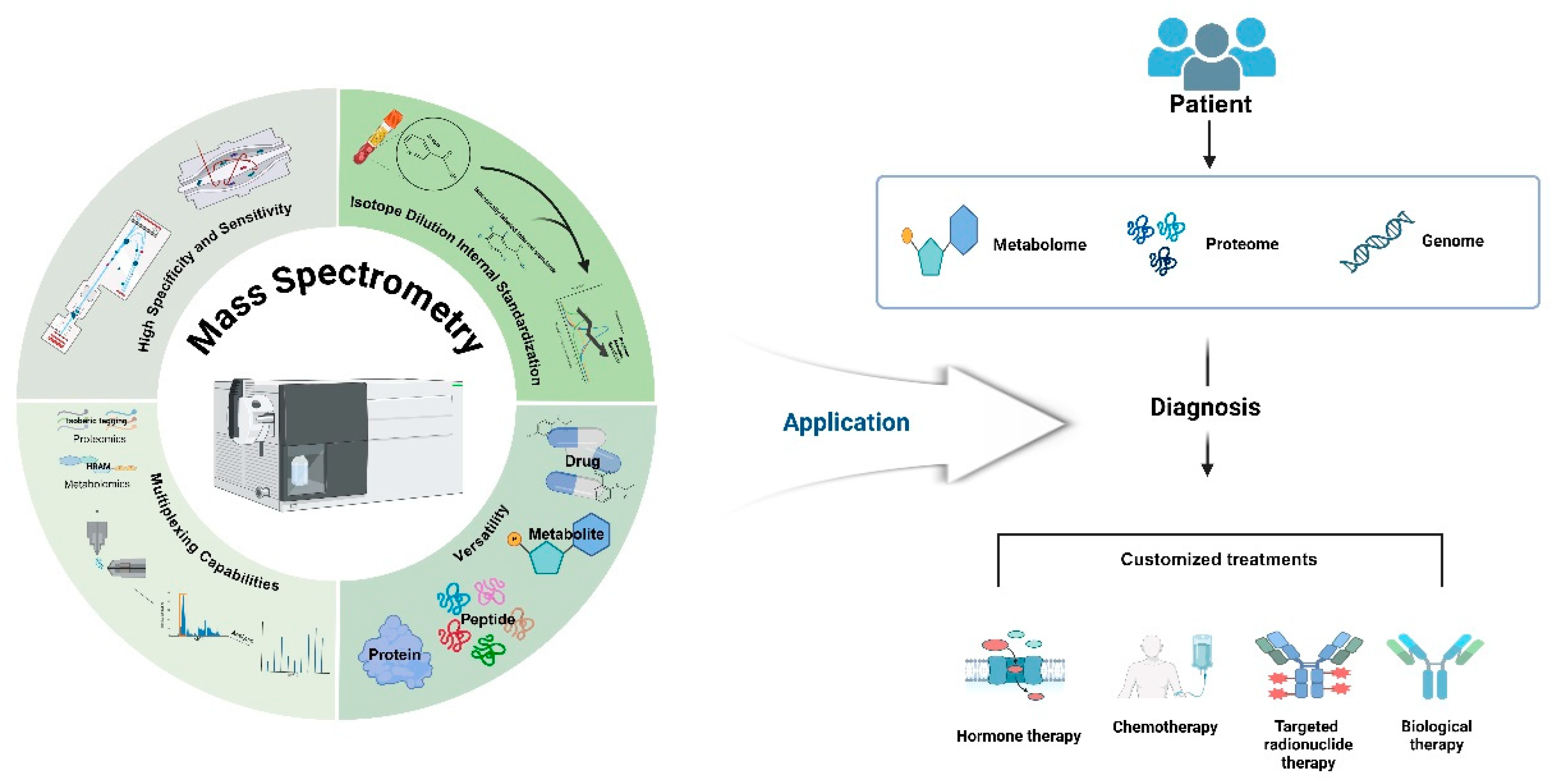
Figure 1.
Strengths of Mass Spectrometry in Diagnostics and Personalized Medicine.
Figure 1.
Strengths of Mass Spectrometry in Diagnostics and Personalized Medicine.
Mass spectrometry (MS) has significantly improved in sensitivity and specificity due to advancements in high-resolution accurate-mass (HRAM) analyzers, such as Time-of-Flight MS (TOF MS) and Orbitrap. These developments facilitate the accurate detection and quantification of low-abundance peptides. The integration of mass spectrometry with liquid chromatography (LC-MS) enhances separation efficiency and broadens its applicability, enabling precise analysis of a wide range of biomolecules. This approach not only facilitates detailed proteomic and metabolomic profiling using multiplexing technologies but also allows for drug analysis, demonstrating its versatility in molecular profiling. Isotope Dilution Internal Standardization (IDMS) further contributes to the accuracy and reproducibility of measurements by mitigating matrix effects. MS-based techniques are essential in advancing clinical diagnostics and personalized medicine, offering detailed molecular profiling that supports the understanding of disease mechanisms and the development of customized treatment strategies. This approach enables precise and reliable analysis of biomolecules across diverse concentrations in complex biological samples, ultimately enhancing the capability for personalized medicine.
2.1. High Specificity and Sensitivity
Mass spectrometry (MS) provides unparalleled specificity in molecular identification, allowing for the accurate detection and quantification of analytes even in complex biological matrices [
5]. Recent advancements in high-resolution accurate-mass (HRAM) spectrometers, such as time-of-flight MS (TOF MS) and Orbitrap, have significantly enhanced the sensitivity and resolution of MS, facilitating its transition from analytical chemistry laboratories to clinical settings [
9]. Techniques like ion mobility MS, which separates ionized molecules based on their mobility in a carrier gas, further improve the resolving power and sensitivity of MS, making it ideal for high-throughput proteomics [
10]. Additionally, liquid chromatography-mass spectrometry (LC-MS) has become a preferred analytical technique due to its high sensitivity and broad applicability, with strategies to optimize ionization efficiency and reduce contaminants enhancing its signal-to-noise ratio. Prioritized MS approaches, such as pSCoPE, have increased the depth and dynamic range of protein quantification in single-cell proteomics, demonstrating the ability to quantify low-abundance peptides that are often missed by traditional methods [
11]. These advancements underscore the critical role of MS in advancing both clinical diagnostics and research by providing highly specific and sensitive analytical capabilities.
2.2. Multiplexing Capabilities
Mass spectrometry (MS) techniques, especially when coupled with liquid chromatography (LC-MS), have emerged as powerful tools for simultaneously analyzing multiple analytes in a single run, enabling comprehensive metabolomic and proteomic profiling [
12]. The ability of MS to detect and quantify many thousands of metabolite features simultaneously has revolutionized the field of metabolomics, allowing for in-depth characterization of complex biological samples [
3]. In proteomics, MS-based approaches can routinely detect and quantify thousands of proteins, with recent advancements in instrumentation and methodology significantly enhancing sensitivity and resolution [
13]. Multiplexing strategies, such as isobaric tagging, have further improved the throughput and quantitative capabilities of MS-based proteomics, enabling the comparison of protein expression across multiple samples in a single experiment [
12]. These multiplexed approaches not only increase sample throughput but also improve measurement precision and reduce missing values in large-scale proteomic studies [
13]. In metabolomics, the combination of high-resolution accurate mass (HRAM) spectrometry with advanced separation techniques like ion mobility has greatly enhanced the ability to resolve and identify metabolites in complex mixtures. The integration of targeted and untargeted approaches in both metabolomics and proteomics has expanded the depth and breadth of biological insights that can be gained from MS-based analyses [
14]. Despite these advancements, challenges remain in fully characterizing the metabolome and proteome, particularly for low-abundance species and in distinguishing isomers [
15]. Ongoing developments in MS technology, including improvements in ionization efficiency, mass accuracy, and multiplexing capacity, continue to push the boundaries of what is possible in large-scale molecular profiling of biological systems [
5].
2.3. Versatility
Mass spectrometry (MS) can be applied to a wide range of biomolecules, including proteins, peptides, metabolites, and drugs, making it a versatile tool for various clinical applications [
1]. The ability of MS to analyze complex biological samples with high specificity and sensitivity has made it indispensable in clinical diagnostics and research [
16]. Recent advancements in MS technology, such as high-resolution accurate-mass (HRAM) spectrometers and tandem MS, have significantly enhanced the detection and quantification capabilities of this technique, allowing for the identification of disease biomarkers and therapeutic drug monitoring [
17]. MS-based proteomics, for instance, enables the comprehensive analysis of protein expression and post-translational modifications, which are crucial for understanding disease mechanisms and developing targeted therapies [
3]. Additionally, MS has been instrumental in metabolomics, providing detailed metabolic profiles that can reveal insights into metabolic disorders and potential therapeutic targets. The integration of liquid chromatography (LC) with MS further enhances its versatility by improving the separation and analysis of complex mixtures, thereby increasing the accuracy and reliability of the results [
18]. Techniques such as ion mobility spectrometry (IMS) coupled with MS have also been developed to separate ions based on their shape and size, adding another dimension to the analysis and improving the resolution of complex samples [
5]. Despite these advancements, challenges remain in fully characterizing low-abundance species and distinguishing isomers, but ongoing developments in MS technology continue to push the boundaries of what is possible in molecular profiling [
19]. The continuous evolution of MS, including improvements in ionization efficiency, mass accuracy, and data analysis, ensures its pivotal role in advancing clinical diagnostics and personalized medicine [
1].
2.4. Isotope Dilution Internal Standardization
Isotope dilution mass spectrometry (IDMS) is a unique feature of MS that allows for highly accurate quantification by compensating for matrix effects, a significant advantage over traditional immunoassays [
1]. Matrix effects, which can compromise the sensitivity and selectivity of MS, are mitigated in IDMS through the use of isotopically labeled internal standards that closely mimic the behavior of the analyte during the analytical process [
20]. This approach significantly enhances the precision and accuracy of quantitative measurements in complex biological matrices such as plasma, serum, and urine [
1]. The use of IDMS in clinical applications has been shown to provide superior specificity and expanded linear ranges compared to traditional immunoassays, making it an indispensable tool for therapeutic drug monitoring and biomarker quantification [
21]. Recent advancements in MS technology, including high-resolution accurate-mass (HRAM) spectrometers and tandem MS, have further improved the capabilities of IDMS, enabling more reliable and reproducible results [
17]. Calibration practices in clinical MS laboratories have also evolved, with the use of matrix-matched calibrators and stable isotope-labeled internal standards to mitigate the impact of matrix effects, ensuring the accuracy and precision of the regression models used for quantification [
22]. Despite the technical challenges associated with obtaining isotope-labeled internal standards, the benefits of IDMS in terms of reliability and quality control in targeted proteomic analysis are well-documented [
23]. The continuous evolution and optimization of IDMS methods continue to push the boundaries of what is possible in molecular profiling and clinical diagnostics, highlighting its pivotal role in advancing personalized medicine [
3].
3. Applications in Biomarker Discovery and Personalized Medicine
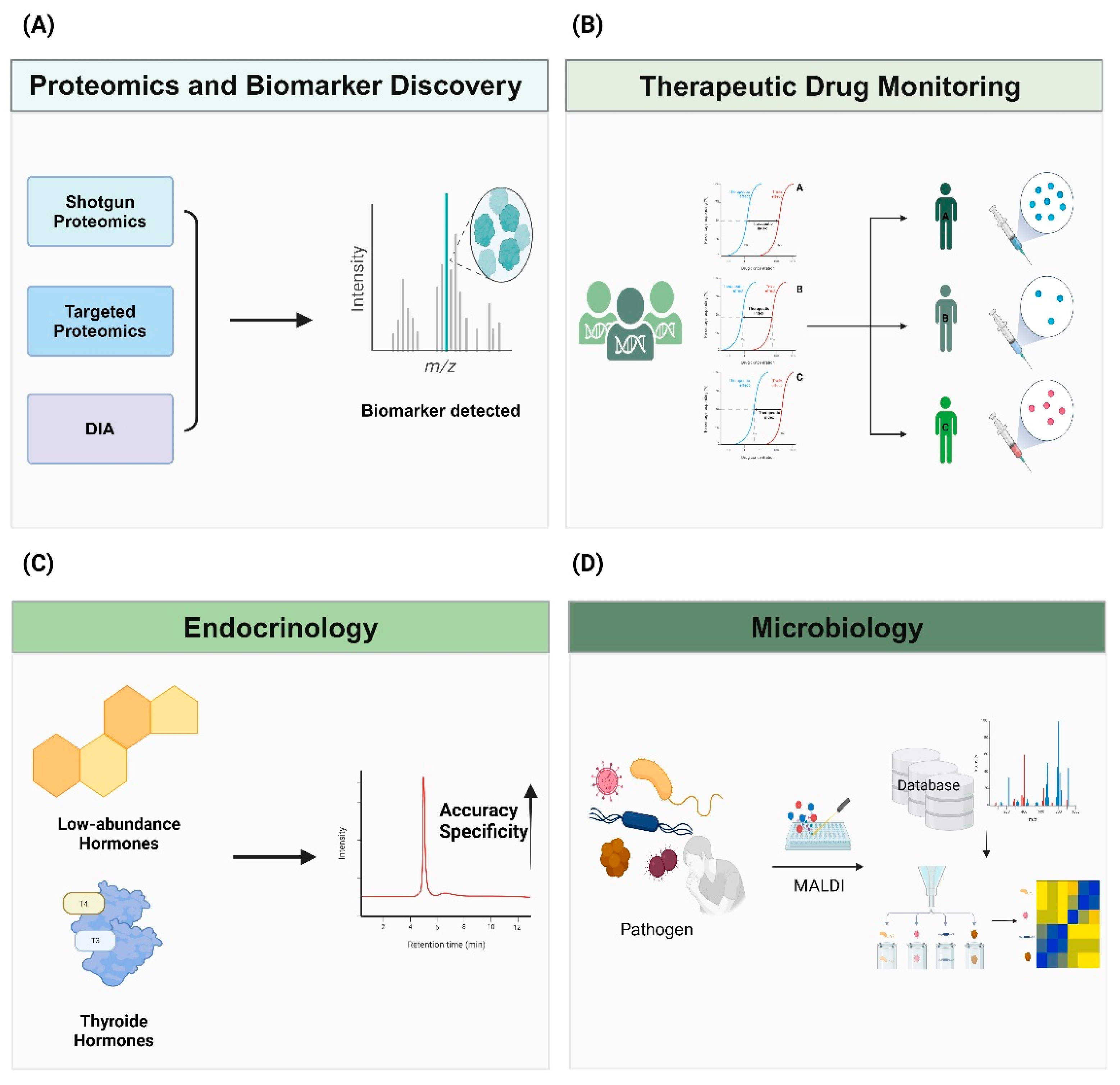
Figure 2.
Advancements in Personalized Medicine and Biomarker Discovery through Mass Spectrometry.
Figure 2.
Advancements in Personalized Medicine and Biomarker Discovery through Mass Spectrometry.
Mass spectrometry (MS) is a powerful tool for the discovery and validation of biomarkers in various diseases. (A) Techniques such as shotgun proteomics, targeted proteomics, and data-independent acquisition (DIA) enable comprehensive analysis of complex protein mixtures and precise quantification of proteins, facilitating biomarker identification and clinical application. (B) The integration of MS with liquid chromatography (LC-MS/MS) enhances the accurate detection and quantification of drugs. These strength of MS improving the efficiency and accuracy of therapeutic drug monitoring (TDM), and enabling personalized treatment strategies and accurate drug concentrations. (C) LC-MS/MS improves the precise detection and quantification of analytes in diverse and complex matrices, including low-abundance molecules. This capability extends to the simultaneous quantification of low-abundance hormones. These technologies enhance the reliability of clinical assays. (D) The integration of databases with mass spectrometry (MS) technology enhances its effectiveness. By comparing unique protein spectra obtained from MALDI-TOF MS with comprehensive microbial databases, it enables precise pathogen identification and aids in the prompt decision-making for treatment.
3.1. Proteomics and Biomarker Discovery
Mass spectrometry (MS)-based proteomics has emerged as a powerful tool for biomarker discovery and validation in various diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions [
5]. Techniques such as shotgun proteomics, targeted proteomics, and data-independent acquisition (DIA) have enabled the identification and quantification of disease-specific protein signatures [
24]. Shotgun proteomics allows for the comprehensive analysis of complex protein mixtures by identifying as many proteins as possible in a single run, which is crucial for discovering novel biomarkers [
25]. Targeted proteomics, on the other hand, focuses on the precise quantification of a predefined set of proteins, providing high specificity and sensitivity, which is essential for validating candidate biomarkers in clinical settings [
24]. DIA combines the strengths of both approaches by systematically acquiring data on all detectable peptides in a sample, allowing for both broad discovery and accurate quantification of proteins [
5]. The application of these MS-based techniques in biomarker discovery has led to significant advancements in understanding the molecular mechanisms underlying various diseases and has identified potential diagnostic and prognostic biomarkers [
26]. For instance, in cancer research, MS-based proteomics has been instrumental in identifying protein signatures associated with tumor progression and treatment response, thereby aiding in the development of targeted therapies [
24]. In neurodegenerative diseases, MS has facilitated the discovery of biomarkers that reflect pathological changes in the brain, providing insights into disease progression and potential therapeutic targets [
27]. Despite the challenges in translating these discoveries into clinical practice, ongoing advancements in MS technology and bioinformatics are continuously improving the accuracy, reproducibility, and clinical applicability of proteomics-based biomarker discovery [
25].
3.2. Therapeutic Drug Monitoring
Therapeutic drug monitoring (TDM) has become an indispensable tool in modern medicine, enabling clinicians to optimize drug dosages and improve patient outcomes across various therapeutic areas [
28]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has emerged as the gold standard for TDM due to its unparalleled specificity, sensitivity, and ability to simultaneously quantify multiple drugs and their metabolites in biological fluids [
29,
30]. This advanced analytical technique allows for precise measurement of drug concentrations, facilitating accurate dosage adjustments and personalized treatment strategies [
31]. Recent advancements in LC-MS/MS methodologies have further enhanced the efficiency and accuracy of TDM, with developments in sample preparation techniques, chromatographic separations, and mass spectrometric detection [
29,
30,
31]. The application of TDM has expanded beyond traditional narrow therapeutic index drugs to encompass a wide range of medications, including antiepileptics, immunosuppressants, and anticancer agents [
32]. In oncology, TDM of kinase inhibitors has gained particular attention, with exposure-response and exposure-toxicity relationships established for numerous compounds, potentially improving treatment efficacy and reducing adverse effects. The integration of TDM data with pharmacogenomic information and clinical parameters has paved the way for more comprehensive and individualized treatment approaches, ultimately enhancing patient care and reducing healthcare costs [
28,
32]. As mass spectrometers become more robust and user-friendly, the widespread adoption of MS-based TDM is expected to address additional clinical and analytical questions, further advancing the field of precision medicine [
31,
32].
3.3. Endocrinology
Mass spectrometry (MS) has significantly improved the accuracy and specificity of hormone measurements in clinical endocrinology, revolutionizing the field with its advanced analytical capabilities [
33,
34]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods have been developed for steroid hormone analysis, thyroid function tests, and vitamin D metabolite quantification, offering substantial advantages over traditional immunoassays in terms of selectivity and multiplexing capabilities [
33,
35]. LC-MS/MS allows for the precise quantification of low-abundance hormones such as dihydrotestosterone, estradiol, and aldosterone, which are often challenging to measure accurately with immunoassays [
34,
36]. The high specificity of MS has revealed inaccuracies in many automated immunoassays, particularly in complex physiological conditions, leading to improved diagnostic accuracy and patient outcomes [
35,
36]. Recent advancements in MS technology have facilitated the standardization and harmonization of hormone measurements, further enhancing the reliability of clinical assays [
34,
36]. The integration of MS-based hormone assays into routine clinical practice has enabled more accurate disease diagnosis and management, ultimately contributing to the advancement of precision medicine in endocrinology [
33,
34,
35].
3.4. Microbiology
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has revolutionized clinical microbiology by enabling rapid and accurate identification of microorganisms [
37]. This technique has significantly reduced the time required for pathogen identification, allowing for faster and more targeted treatment decisions [
38]. MALDI-TOF MS can identify a wide range of microorganisms, including bacteria, fungi, and viruses, by analyzing their unique protein spectra and comparing them to extensive databases [
39]. The high throughput and cost-effectiveness of MALDI-TOF MS make it an indispensable tool in modern clinical laboratories, facilitating the timely diagnosis of infectious diseases. Recent advancements in sample preparation and database enrichment have further improved the accuracy and reliability of this technology, addressing some of the challenges associated with the identification of closely related species [
37]. The ability of MALDI-TOF MS to detect antimicrobial resistance markers has also been demonstrated, providing critical information for the management of drug-resistant infections [
38]. Despite its limitations, such as the need for comprehensive and up-to-date spectral databases, MALDI-TOF MS continues to advance with ongoing research and technological improvements, promising even greater impact on clinical microbiology in the future [
40].
4. Enhancing Accessibility and Integration of Mass Spectrometry in Clinical Laboratories
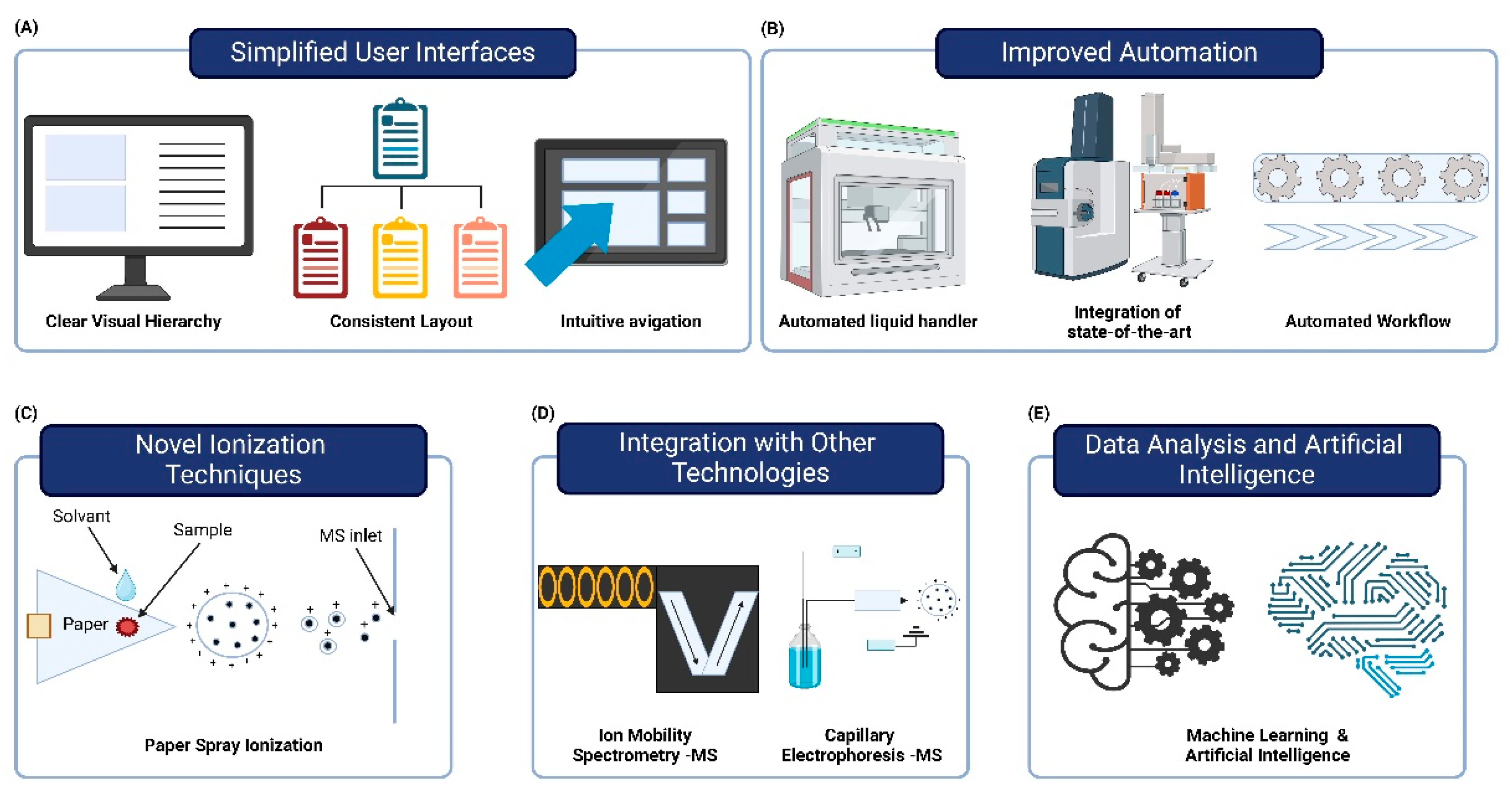
Figure 3.
Enhancing Accessibility and Integration of Mass Spectrometry in Clinical Laboratories. (A) The development of user-friendly mass spectrometry (MS) systems features simplified interfaces that require less specialized knowledge, improving usability. (B) The integration of multi-instrument automated systems and automated workflows enhances speed and consistency while reducing manual errors. (C) Novel ionization techniques like paper spray ionization enable rapid, low-cost analysis with minimal preparation. (D)Integration of MS with ion mobility spectrometry and capillary electrophoresis enhances biomolecule separation and characterization. (E) Advanced data analysis tools and AI improve interpretation of MS data, aiding in biomarker discovery and disease classification. These advancements make MS more accessible and efficient for routine clinical use, potentially revolutionizing diagnostic testing.
Figure 3.
Enhancing Accessibility and Integration of Mass Spectrometry in Clinical Laboratories. (A) The development of user-friendly mass spectrometry (MS) systems features simplified interfaces that require less specialized knowledge, improving usability. (B) The integration of multi-instrument automated systems and automated workflows enhances speed and consistency while reducing manual errors. (C) Novel ionization techniques like paper spray ionization enable rapid, low-cost analysis with minimal preparation. (D)Integration of MS with ion mobility spectrometry and capillary electrophoresis enhances biomolecule separation and characterization. (E) Advanced data analysis tools and AI improve interpretation of MS data, aiding in biomarker discovery and disease classification. These advancements make MS more accessible and efficient for routine clinical use, potentially revolutionizing diagnostic testing.
4.1. Simplified User Interfaces
Efforts to develop more user-friendly MS systems that require less specialized knowledge to operate are increasingly being prioritized in the field of user interface (UI) design. Recent studies emphasize the importance of creating digital solutions that are accessible to all users, regardless of their technical expertise, by adhering to well-established usability principles and design recommendations [
41]. The process often involves user-centered design, which places the user at the core of the design process, ensuring that the interface meets their needs and preferences through methods such as user interviews, surveys, and usability testing. Simplifying the interface by focusing on essential elements and eliminating unnecessary clutter has been shown to significantly improve user experience and engagement [
41]. Additionally, the use of clear visual hierarchy, consistent layout, and intuitive navigation is crucial in making interfaces more understandable and easier to use. Recent research also highlights the need for a critical analysis of existing design recommendations to standardize best practices and enhance the usability of digital solutions across different technologies and user profiles. By synthesizing and validating these recommendations, designers can create more effective and user-friendly interfaces that cater to a diverse audience [
41,
42].
4.2. Improved Automation
Recent advances in mass spectrometry-based proteomics have significantly improved automation, streamlining workflows and enhancing clinical applications [
43,
44]. Automated sample preparation has emerged as a crucial component for high-throughput and quantitative mass spectrometry analysis, addressing the tedious and time-consuming steps that can introduce analytical errors [
43]. The development and optimization of workflows utilizing automated liquid handling workstations have shown to improve speed and consistency in sample processing [
43]. Integration of state-of-the-art, multi-instrument automated systems has enabled the execution of complex methods involved in mass spectrometry analysis [
44]. These automated workflows facilitate increased throughput and reproducible quantitation of biomarker candidates, which is essential for processing large patient cohorts in clinical studies [
43]. Furthermore, advances in automation have extended to data acquisition and processing, with the introduction of automated mass spectrometry data acquisition systems and sophisticated data analysis tools. The implementation of fully automated FAIMS-DIA (high-field asymmetric waveform ion mobility spectrometry-data independent acquisition) mass spectrometry-based proteomic pipelines has demonstrated deep coverage of cellular proteomes with high throughput and reproducibility [
45]. These developments in automation are particularly beneficial for clinical laboratories, enabling technicians to focus on high-value tasks and improving operational efficiency. As the field progresses, the integration of automated sample preparation, data acquisition, and analysis is expected to further enhance the adoption of mass spectrometry in clinical settings, potentially revolutionizing diagnostic testing and biomarker discovery [
2].
4.3. Novel Ionization Techniques
Recent advancements in ambient ionization techniques, particularly paper spray ionization (PSI), have significantly enhanced the capabilities of mass spectrometry (MS) for rapid and direct analysis of complex mixtures [
46,
47]. PSI is a direct, fast, and low-cost sampling method that generates analyte ions by applying a high voltage to a small volume of spray solvent on a porous substrate [
46]. This technique has demonstrated the ability to provide both qualitative and quantitative MS analysis without the need for extensive sample preparation [
48]. Factors such as electric field, solvent supply rate, and paper thickness have been systematically evaluated to optimize ionization efficiency, revealing that the rim-jet mode offers the highest efficiency among different spray modes [
47]. The implementation of PSI in clinical settings has shown potential for point-of-care diagnostics, enabling rapid analysis of biofluids with minimal sample handling [
48]. Furthermore, recent studies have highlighted the versatility of PSI in analyzing a wide range of molecules, including illicit drugs, therapeutic drugs, metabolites, and proteins, making it a promising tool for clinical applications [
49]. The simplicity and efficacy of PSI, combined with its low cost and minimal biohazard and chemical waste, position it as a valuable technique for point-of-care MS analysis [
46,
48].
4.4. Integration with Other Technologies
The integration of mass spectrometry (MS) with ion mobility spectrometry (IMS) and capillary electrophoresis (CE) has significantly advanced clinical diagnostics by enhancing the separation and structural characterization of biomolecules. IMS-MS provides additional separation dimensions based on molecular shape and size, which is particularly beneficial for distinguishing isomers and improving the analysis of complex biological samples [
9,
10]. This hybrid technique has shown promise in clinical settings for high-throughput and high-confidence molecular characterization, aiding in the identification of biomarkers and improving diagnostic accuracy [
9]. Similarly, CE-MS combines the high-resolution separation capabilities of capillary electrophoresis with the sensitive detection of mass spectrometry, allowing for the analysis of a wide range of analytes, from small ions to large protein complexes [
50,
51]. This combination reduces sample complexity and ion suppression, leading to more straightforward data interpretation and enhanced detection of clinically relevant compounds [
50]. The use of CE-MS in clinical diagnostics has been demonstrated in various applications, including the analysis of urinary biomarkers for disease diagnosis and prognosis [
50,
51]. Overall, the integration of MS with IMS and CE represents a powerful approach to improving the analytical performance and clinical utility of mass spectrometry in the diagnosis and monitoring of diseases [
9,
10,
51].
4.5. Data Analysis and Artificial Intelligence
Recent advancements in data analysis algorithms and artificial intelligence have greatly enhanced the interpretation of complex mass spectrometry (MS) data. These developments are largely driven by recent software innovations, with new data processing tools such as MZmine 3, MS-DIAL, and XCMS providing improved capabilities for exploring and processing raw MS data [
52]. In conjunction with these tools, machine learning (ML) techniques offer novel approaches for interpreting clinical data, making it essential for improving biomarker discovery, disease classification, and treatment response prediction [
53,
54]. For instance, ML methods have demonstrated significant potential in identifying differentially expressed proteins in cancers, thus enhancing biomarker discovery [
55]. Furthermore, AI applications have shown promise in clinical settings, particularly by classifying patient samples with high accuracy based on MS data, ultimately aiding in more effective disease diagnosis [
56,
57]. In drug response prediction, sophisticated ML algorithms are increasingly used to tailor therapies to individual patients' profiles, marking a shift towards personalized medicine [
58]. As these analytical frameworks continue to evolve, they hold the potential to redefine diagnostic processes and therapeutic strategies within clinical diagnostics [
59,
60,
61].
5. Challenges and Opportunities in Implementing Mass Spectrometry in Clinical Laboratories
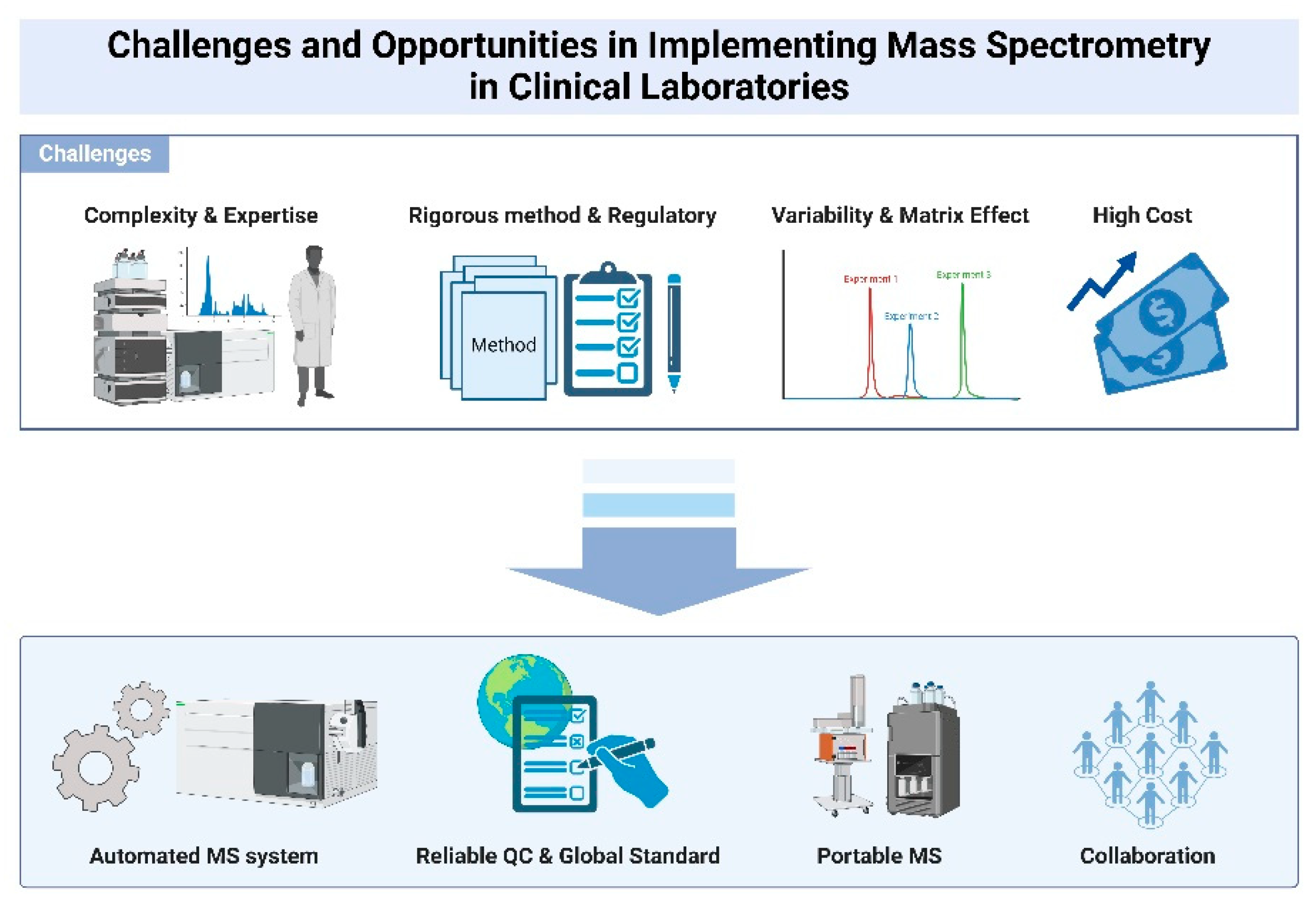
Figure 4.
Challenges and Opportunities in Implementing Mass Spectrometry in Clinical Laboratories.
Figure 4.
Challenges and Opportunities in Implementing Mass Spectrometry in Clinical Laboratories.
High technical expertise required for MS operation and data interpretation, alongside rigorous method validation and high equipment costs, limits adoption in standard laboratories. Lack of standardized protocols and reference materials creates variability in MS results, necessitating uniform procedures and quality control measures for reliable diagnostics. Advances in automated MS systems improve efficiency and reduce human error, with developments like the Cascadion SM Clinical Analyzer moving towards fully automated workflows. High acquisition and operating costs of MS equipment restrict access for smaller labs, though miniaturization and shared facilities offer potential cost-mitigation strategies.
5.1. Complexity and Expertise
Despite its potential, the implementation of mass spectrometry (MS) in routine clinical laboratories faces several challenges, particularly in terms of complexity and the need for specialized expertise [
62]. MS techniques require a high level of technical expertise for operation and data interpretation, which may limit their adoption in standard clinical laboratories. The intricate nature of MS, involving sophisticated instrumentation and complex data analysis, necessitates highly trained personnel, which can be a significant barrier for many laboratories [
63]. Additionally, the integration of MS into clinical workflows is complicated by the need for rigorous method validation and adherence to regulatory standards, which can be resource-intensive and time-consuming [
64]. The high costs associated with MS equipment and maintenance further exacerbate these challenges, making it difficult for smaller laboratories to adopt this technology [
65]. Despite advancements in automation and user-friendly interfaces, MS systems still require significant manual intervention and expertise to ensure accurate and reliable results [
5]. Moreover, the variability in sample preparation and the potential for matrix effects necessitate meticulous optimization and standardization, adding another layer of complexity to the implementation process [
64]. The development of comprehensive spectral databases and robust quality control measures is essential to overcome these hurdles and facilitate the broader adoption of MS in clinical settings [
62]. Ongoing efforts to streamline MS workflows and enhance automation hold promise for reducing the technical barriers and making this powerful analytical tool more accessible to clinical laboratories [
65].
5.2. Standardization
The implementation of mass spectrometry (MS) in routine clinical laboratories faces several challenges, particularly in terms of standardization and quality control measures needed to ensure reproducibility and comparability of results across different laboratories [
66]. The lack of standardized protocols for sample preparation, data acquisition, and analysis can result in significant variability in MS results between laboratories, undermining the reliability of diagnostic tests. Comprehensive guidelines, such as those provided by the Clinical and Laboratory Standards Institute (CLSI), are essential to establish uniform procedures and quality assurance practices that can be adopted universally [
67]. The development of standardized reference materials and calibration standards is crucial to achieving consistency in MS measurements, as these materials provide benchmarks against which laboratories can validate their methods and instruments (Annotation 4). Quality control (QC) in MS-based proteomics has seen significant advances, yet the rate of adoption of these QC measures remains inconsistent, highlighting the need for more robust and accessible QC tools and protocols [
68]. The establishment of metrological traceability, ensuring that measurement procedures are linked to internationally recognized standards, is another critical aspect of standardization that enhances the comparability of results across different settings [
66]. Efforts by organizations such as the Joint Committee for Traceability in Laboratory Medicine (JCTLM) to promote global standardization of clinical test results are pivotal in addressing these challenges. Despite these efforts, the high level of technical expertise required for MS operation and data interpretation continues to be a barrier, emphasizing the need for ongoing training and education of laboratory personnel [
69]. As MS technology continues to advance, the integration of automated systems and user-friendly interfaces holds promise for reducing the complexity of MS workflows and facilitating its broader adoption in clinical laboratories [
3].
5.3. Automation
The development of automated MS-based analyzer systems is crucial for shifting from specialized laboratories to standard clinical laboratories [
3]. The transition to automated MS systems offers numerous benefits, including improved efficiency, reduced human error, and the ability to bring previously outsourced testing in-house [
70]. Automated liquid handling systems and data analysis solutions have been introduced by various manufacturers to streamline pre- and post-analytical processes [
71]. These advancements have led to significant reductions in manual manipulations and processing times, enhancing overall laboratory workflow [
5]. Recent developments, such as the Cascadion SM Clinical Analyzer, represent significant progress towards fully automated "black box" LC-MS/MS systems that can operate without specialized technical staff [
72]. These systems aim to integrate sample preparation, analysis, and result generation into a single automated workflow, making MS more accessible to standard clinical laboratories. However, challenges remain, including the need for a wider range of available tests and regulatory approvals. As automation in clinical MS continues to advance, it is expected to drive wider adoption of this technology in routine laboratory settings, potentially improving patient care through faster turnaround times and more comprehensive diagnostic capabilities [
3,
59]. Future developments in instrumentation, software, and machine learning algorithms are likely to further enhance the clinical applications of MS and facilitate its integration into standard laboratory practices [
5].
5.4. Cost
Mass spectrometry (MS) is a powerful analytical technique that provides both qualitative and quantitative information based on the mass-to-charge ratios of analytes. However, the cost of acquiring such sophisticated equipment is significant [
73]. This high acquisition cost hinders the potential of MS analysis for a broader audience, particularly smaller laboratories that may not have the financial resources to invest in such expensive equipment [
74]. Driven by the goal of expanding the accessibility and applicability of MS analysis, efforts to miniaturize MS instrumentation have been ongoing since the 1970s [
73]. The past two decades have seen rapid advancements in the development of portable mass spectrometers, facilitated by improvements in microfabrication techniques, precise machining, integrated circuits, and computational modeling tools. Despite these advancements, the adoption of portable MS is still at the earliest stages, and further expansion is needed to fully realize their potential. Beyond the initial purchase, operating costs, including maintenance, calibration, and consumables, can also be significant and must be factored into the overall cost of ownership [
74]. Smaller laboratories can mitigate some of these costs by forming collaborations or utilizing shared facilities that provide access to MS instrumentation on a fee-for-service basis. Recent literature has highlighted the cost considerations in proteomics workflows, emphasizing the importance of efficient use of instrument time and resources to ensure cost-effectiveness.
6. Conclusions
Mass spectrometry has demonstrated immense potential in advancing clinical chemistry, particularly in the realms of biomarker discovery and personalized medicine. Its ability to provide accurate, sensitive, and comprehensive molecular profiles makes it an invaluable tool for modern healthcare. As technological advancements continue to address current limitations, mass spectrometry is poised to play an increasingly central role in clinical laboratories, ultimately contributing to improved patient care through more precise diagnostics and tailored therapeutic strategies. The integration of mass spectrometry into routine clinical practice represents a paradigm shift in laboratory medicine, offering new possibilities for understanding disease mechanisms, identifying novel biomarkers, and personalizing treatment approaches. As the field continues to evolve, ongoing research and development efforts will be crucial in overcoming existing challenges and fully realizing the potential of mass spectrometry in clinical chemistry.
Author Contributions
Conceptualization, investigation, writing, and original draft preparation, A.S. (Ahrum Son); H.K. (Hyunsoo Kim) – Visualization, and proofreading, W.K. (Woojin Kim); J.P. (Jongham Park); Y.P (Yongho Park); W.L. (Wonseok Lee); S.L. (Sangwoon Lee) – Supervision, Project Administration, Funding Acquisition, Review and Editing, H.K. (Hyunsoo Kim). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00209456). This work was supported by the Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Korea government (MSIT) (RS-2024-00402298). This work was partly supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (RS-2022-00155857, Artificial Intelligence Convergence Innovation Human Resources Development (Chungnam National University).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, X.; Zhang, W.; Ouyang, Z. Recent advances in on-site mass spectrometry analysis for clinical applications. Trends Analyt Chem 2022, 149, 116548. [Google Scholar] [CrossRef]
- Wenk, D.; Zuo, C.; Kislinger, T.; Sepiashvili, L. Recent developments in mass-spectrometry-based targeted proteomics of clinical cancer biomarkers. Clin Proteomics 2024, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid chromatography-tandem mass spectrometry for clinical diagnostics. Nat Rev Methods Primers 2022, 2, 96. [Google Scholar] [CrossRef]
- Cui, J.J.; Wang, L.Y.; Tan, Z.R.; Zhou, H.H.; Zhan, X.; Yin, J.Y. Mass Spectrometry-Based Personalized Drug Therapy. Mass Spectrom Rev 2020, 39, 523–552. [Google Scholar] [CrossRef]
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin Proteomics 2023, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Rischke, S.; Hahnefeld, L.; Burla, B.; Behrens, F.; Gurke, R.; Garrett, T.J. Small molecule biomarker discovery: Proposed workflow for LC-MS-based clinical research projects. J Mass Spectrom Adv Clin Lab 2023, 28, 47–55. [Google Scholar] [CrossRef]
- Grebe, S.K.; Singh, R.J. LC-MS/MS in the Clinical Laboratory - Where to From Here? Clin Biochem Rev 2011, 32, 5–31. [Google Scholar] [PubMed]
- Beck, O.; Olin, A.C.; Mirgorodskaya, E. Potential of Mass Spectrometry in Developing Clinical Laboratory Biomarkers of Nonvolatiles in Exhaled Breath. Clin Chem 2016, 62, 84–91. [Google Scholar] [CrossRef]
- Koomen, D.C.; May, J.C.; McLean, J.A. Insights and prospects for ion mobility-mass spectrometry in clinical chemistry. Expert Rev Proteomics 2022, 19, 17–31. [Google Scholar] [CrossRef]
- Chouinard, C.D.; Wei, M.S.; Beekman, C.R.; Kemperman, R.H.; Yost, R.A. Ion Mobility in Clinical Analysis: Current Progress and Future Perspectives. Clin Chem 2016, 62, 124–133. [Google Scholar] [CrossRef]
- Huffman, R.G.; Leduc, A.; Wichmann, C.; Di Gioia, M.; Borriello, F.; Specht, H.; Derks, J.; Khan, S.; Khoury, L.; Emmott, E.; et al. Prioritized mass spectrometry increases the depth, sensitivity and data completeness of single-cell proteomics. Nat Methods 2023, 20, 714–722. [Google Scholar] [CrossRef]
- Arul, A.B.; Robinson, R.A.S. Sample Multiplexing Strategies in Quantitative Proteomics. Anal Chem 2019, 91, 178–189. [Google Scholar] [CrossRef]
- Recchia, M.J.J.; Baumeister, T.U.H.; Liu, D.Y.; Linington, R.G. MultiplexMS: A Mass Spectrometry-Based Multiplexing Strategy for Ultra-High-Throughput Analysis of Complex Mixtures. Anal Chem 2023, 95, 11908–11917. [Google Scholar] [CrossRef]
- Tsumagari, K.; Isobe, Y.; Imami, K.; Arita, M. Exploring protein lipidation by mass spectrometry-based proteomics. J Biochem 2024, 175, 225–233. [Google Scholar] [CrossRef]
- Egertson, J.D.; Kuehn, A.; Merrihew, G.E.; Bateman, N.W.; MacLean, B.X.; Ting, Y.S.; Canterbury, J.D.; Marsh, D.M.; Kellmann, M.; Zabrouskov, V.; et al. Multiplexed MS/MS for improved data-independent acquisition. Nat Methods 2013, 10, 744–746. [Google Scholar] [CrossRef]
- Swiner, D.J.; Jackson, S.; Burris, B.J.; Badu-Tawiah, A.K. Applications of Mass Spectrometry for Clinical Diagnostics: The Influence of Turnaround Time. Anal Chem 2020, 92, 183–202. [Google Scholar] [CrossRef]
- Holbrook, J.H.; Kemper, G.E.; Hummon, A.B. Quantitative mass spectrometry imaging: therapeutics & biomolecules. Chem Commun (Camb) 2024, 60, 2137–2151. [Google Scholar] [CrossRef]
- Banerjee, S. Empowering Clinical Diagnostics with Mass Spectrometry. ACS Omega 2020, 5, 2041–2048. [Google Scholar] [CrossRef]
- Zhang, G.; Annan, R.S.; Carr, S.A.; Neubert, T.A. Overview of peptide and protein analysis by mass spectrometry. Curr Protoc Mol Biol 2014, 108, 10 21 11–10 21 30. [Google Scholar] [CrossRef]
- Lanekoff, I.; Stevens, S.L.; Stenzel-Poore, M.P.; Laskin, J. Matrix effects in biological mass spectrometry imaging: identification and compensation. Analyst 2014, 139, 3528–3532. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, X.; Liu, T.; Jiang, J.; Cui, X.; Zhao, Q.; Hu, P. Liquid chromatography-tandem mass spectrometry methods for quantification of roxadustat (FG-4592) in human plasma and urine and the applications in two clinical pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci 2022, 1203, 123274. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Markus, C.; Lim, C.Y.; Tan, R.Z.; Sethi, S.K.; Loh, T.P.; Protocols, I.W.G.o.M.E. Calibration Practices in Clinical Mass Spectrometry: Review and Recommendations. Ann Lab Med 2023, 43, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Carrascal, M.; Abian, J. Isotope dilution mass spectrometry for absolute quantification in proteomics: concepts and strategies. J Proteomics 2014, 96, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Morris, C.F.; Silva, A.R.; Fonseca, M.P.; Forget, P.; Castro, M.S.; Fontes, W. A Timely Shift from Shotgun to Targeted Proteomics and How It Can Be Groundbreaking for Cancer Research. Front Oncol 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, E.S.; Gritsenko, M.; Piehowski, P.D.; Gao, Y.; Orton, D.J.; Schepmoes, A.A.; Fillmore, T.L.; Frohnert, B.I.; Rewers, M.; Krischer, J.P.; et al. Tutorial: best practices and considerations for mass-spectrometry-based protein biomarker discovery and validation. Nat Protoc 2021, 16, 3737–3760. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Dashatan, N.; Koushki, M.; Abbaszadeh, H.A.; Rostami-Nejad, M.; Rezaei-Tavirani, M. Proteomics Applications in Health: Biomarker and Drug Discovery and Food Industry. Iran J Pharm Res 2018, 17, 1523–1536. [Google Scholar] [PubMed]
- Azevedo, R.; Jacquemin, C.; Villain, N.; Fenaille, F.; Lamari, F.; Becher, F. Mass Spectrometry for Neurobiomarker Discovery: The Relevance of Post-Translational Modifications. Cells 2022, 11. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J Intern Med 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zheng, X.; Liu, X.; Liu, W.; Wu, J. LC-MS/MS-based multiplex antibacterial platform for therapeutic drug monitoring in intensive care unit patients. Front Pharmacol 2023, 14, 1116071. [Google Scholar] [CrossRef]
- Barco, S.; Mesini, A.; Barbagallo, L.; Maffia, A.; Tripodi, G.; Pea, F.; Saffioti, C.; Castagnola, E.; Cangemi, G. A liquid chromatography-tandem mass spectrometry platform for the routine therapeutic drug monitoring of 14 antibiotics: Application to critically ill pediatric patients. J Pharm Biomed Anal 2020, 186, 113273. [Google Scholar] [CrossRef]
- Gaspar, V.P.; Ibrahim, S.; Zahedi, R.P.; Borchers, C.H. Utility, promise, and limitations of liquid chromatography-mass spectrometry-based therapeutic drug monitoring in precision medicine. J Mass Spectrom 2021, 56, e4788. [Google Scholar] [CrossRef]
- van der Kleij, M.B.A.; Guchelaar, N.A.D.; Mathijssen, R.H.J.; Versluis, J.; Huitema, A.D.R.; Koolen, S.L.W.; Steeghs, N. Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin Pharmacokinet 2023, 62, 1333–1364. [Google Scholar] [CrossRef]
- Vogeser, M.; Parhofer, K.G. Liquid chromatography tandem-mass spectrometry (LC-MS/MS)--technique and applications in endocrinology. Exp Clin Endocrinol Diabetes 2007, 115, 559–570. [Google Scholar] [CrossRef]
- Holmes, D.T. A brief update on mass spectrometry applications to routine clinical endocrinology. Clin Mass Spectrom 2019, 13, 18–20. [Google Scholar] [CrossRef]
- French, D. Clinical utility of laboratory developed mass spectrometry assays for steroid hormone testing. J Mass Spectrom Adv Clin Lab 2023, 28, 13–19. [Google Scholar] [CrossRef]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol 2015, 173, D1–D12. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 2015, 6, 791. [Google Scholar] [CrossRef]
- Grenga, L.; Pible, O.; Armengaud, J. Pathogen proteotyping: A rapidly developing application of mass spectrometry to address clinical concerns. Clin Mass Spectrom 2019, 14 Pt A, 9–17. [Google Scholar] [CrossRef]
- Calderaro, A.; Chezzi, C. MALDI-TOF MS: A Reliable Tool in the Real Life of the Clinical Microbiology Laboratory. Microorganisms 2024, 12. [Google Scholar] [CrossRef]
- Al-Manei, K.; Ghorbani, M.; Naud, S.; Al-Manei, K.K.; Sobkowiak, M.J.; Lund, B.; Hazirolan, G.; Sallberg Chen, M.; Ozenci, V. Clinical Microbial Identification of Severe Oral Infections by MALDI-TOF Mass Spectrometry in Stockholm County: an 11-Year (2010 to 2020) Epidemiological Investigation. Microbiol Spectr 2022, 10, e0248722. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.; Martins, A.; Almeida, A.; Silva, T.; Ribeiro, O.; Santinha, G.; Rocha, N.; Silva, A.G. Defining Recommendations to Guide User Interface Design: Multimethod Approach. JMIR Hum Factors 2022, 9, e37894. [Google Scholar] [CrossRef]
- Silva, A.G.; Caravau, H.; Martins, A.; Almeida, A.M.P.; Silva, T.; Ribeiro, O.; Santinha, G.; Rocha, N.P. Procedures of User-Centered Usability Assessment for Digital Solutions: Scoping Review of Reviews Reporting on Digital Solutions Relevant for Older Adults. JMIR Hum Factors 2021, 8, e22774. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Murray, C.I.; Karpov, O.A.; Van Eyk, J.E. Automated proteomic sample preparation: The key component for high throughput and quantitative mass spectrometry analysis. Mass Spectrom Rev 2023, 42, 873–886. [Google Scholar] [CrossRef]
- Waldenmaier, H.E.; Gorre, E.; Poltash, M.L.; Gunawardena, H.P.; Zhai, X.A.; Li, J.; Zhai, B.; Beil, E.J.; Terzo, J.C.; Lawler, R.; et al. "Lab of the Future" horizontal line Today: Fully Automated System for High-Throughput Mass Spectrometry Analysis of Biotherapeutics. J Am Soc Mass Spectrom 2023, 34, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.; Lara, E.; Ramos, D.; Li, Z.; Pantazis, C.B.; Stadler, J.; Santiana, M.; Roberts, J.; Faghri, F.; Hao, Y.; et al. A fully automated FAIMS-DIA mass spectrometry-based proteomic pipeline. Cell Rep Methods 2023, 3, 100593. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Maas, J.D.; Chappell, W.J.; Manicke, N.E.; Cooks, R.G.; Ouyang, Z. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int J Mass Spectrom 2012, 312, 201–207. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Song, W.Y.; Kim, T.Y. Characterization of Spray Modes and Factors Affecting the Ionization Efficiency of Paper Spray Ionization. Front Chem 2022, 10, 864184. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Zhang, W.; Ouyang, Z. Paper spray ionization mass spectrometry: recent advances and clinical applications. Expert Rev Proteomics 2018, 15, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal Chem 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Helena, H.; Ivona, V.; Roman, R.; Frantisek, F. Current applications of capillary electrophoresis-mass spectrometry for the analysis of biologically important analytes in urine (2017 to mid-2021): A review. J Sep Sci 2022, 45, 305–324. [Google Scholar] [CrossRef]
- Mischak, H.; Coon, J.J.; Novak, J.; Weissinger, E.M.; Schanstra, J.P.; Dominiczak, A.F. Capillary electrophoresis-mass spectrometry as a powerful tool in biomarker discovery and clinical diagnosis: an update of recent developments. Mass Spectrom Rev 2009, 28, 703–724. [Google Scholar] [CrossRef]
- Ebbels, T.M.D.; van der Hooft, J.J.J.; Chatelaine, H.; Broeckling, C.; Zamboni, N.; Hassoun, S.; Mathe, E.A. Recent advances in mass spectrometry-based computational metabolomics. Curr Opin Chem Biol 2023, 74, 102288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jonassen, I.; Goksoyr, A. Machine Learning Approaches for Biomarker Discovery Using Gene Expression Data. In Bioinformatics, Helder, I.N., Ed.; Brisbane (AU), 2021.
- Beck, A.G.; Muhoberac, M.; Randolph, C.E.; Beveridge, C.H.; Wijewardhane, P.R.; Kenttamaa, H.I.; Chopra, G. Recent Developments in Machine Learning for Mass Spectrometry. ACS Meas Sci Au 2024, 4, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Swan, A.L.; Mobasheri, A.; Allaway, D.; Liddell, S.; Bacardit, J. Application of machine learning to proteomics data: classification and biomarker identification in postgenomics biology. OMICS 2013, 17, 595–610. [Google Scholar] [CrossRef]
- Naji, Y.; Mahdaoui, M.; Klevor, R.; Kissani, N. Artificial Intelligence and Multiple Sclerosis: Up-to-Date Review. Cureus 2023, 15, e45412. [Google Scholar] [CrossRef]
- Bonacchi, R.; Filippi, M.; Rocca, M.A. Role of artificial intelligence in MS clinical practice. Neuroimage Clin 2022, 35, 103065. [Google Scholar] [CrossRef]
- Adam, G.; Rampasek, L.; Safikhani, Z.; Smirnov, P.; Haibe-Kains, B.; Goldenberg, A. Machine learning approaches to drug response prediction: challenges and recent progress. NPJ Precis Oncol 2020, 4, 19. [Google Scholar] [CrossRef]
- Boiko, D.A.; Kozlov, K.S.; Burykina, J.V.; Ilyushenkova, V.V.; Ananikov, V.P. Fully Automated Unconstrained Analysis of High-Resolution Mass Spectrometry Data with Machine Learning. J Am Chem Soc 2022, 144, 14590–14606. [Google Scholar] [CrossRef] [PubMed]
- Torun, F.M.; Virreira Winter, S.; Doll, S.; Riese, F.M.; Vorobyev, A.; Mueller-Reif, J.B.; Geyer, P.E.; Strauss, M.T. Transparent Exploration of Machine Learning for Biomarker Discovery from Proteomics and Omics Data. J Proteome Res 2023, 22, 359–367. [Google Scholar] [CrossRef]
- Abdelmoula, W.M.; Lopez, B.G.; Randall, E.C.; Kapur, T.; Sarkaria, J.N.; White, F.M.; Agar, J.N.; Wells, W.M.; Agar, N.Y.R. Peak learning of mass spectrometry imaging data using artificial neural networks. Nat Commun 2021, 12, 5544. [Google Scholar] [CrossRef]
- Chace, D.H. Mass spectrometry in the clinical laboratory. Chem Rev 2001, 101, 445–477. [Google Scholar] [CrossRef]
- Annesley, T.; Majzoub, J.; Hsing, A.; Wu, A.; Rockwood, A.; Mason, D. Mass spectrometry in the clinical laboratory: how have we done, and where do we need to be? Clin Chem 2009, 55, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.; Rhea, J.M.; Molinaro, R. Challenges in implementing clinical liquid chromatography-tandem mass spectrometry methods--the light at the end of the tunnel. J Mass Spectrom 2013, 48, 755–767. [Google Scholar] [CrossRef]
- Mugueta, C.; Gonzalez, A.; Deza, S.; Roca, C.A.; Contreras, T.; Puig, N.; Nerea, V. Mass spectrometry in clinical protein laboratories. Adv Lab Med 2024, 5, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.W.; Myers, G.L.; Miller, W.G. Current practices and challenges in the standardization and harmonization of clinical laboratory tests. Am J Clin Nutr 2016, 104 Suppl 3, 907S–912S. [Google Scholar] [CrossRef]
- Annesley, T.M.; Cooks, R.G.; Herold, D.A.; Hoofnagle, A.N. Clinical Mass Spectrometry-Achieving Prominence in Laboratory Medicine. Clin Chem 2016, 62, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bittremieux, W.; Tabb, D.L.; Impens, F.; Staes, A.; Timmerman, E.; Martens, L.; Laukens, K. Quality control in mass spectrometry-based proteomics. Mass Spectrom Rev 2018, 37, 697–711. [Google Scholar] [CrossRef]
- Rankin-Turner, S.; Heaney, L.M. Mass spectrometry in the clinical laboratory. A short journey through the contribution to the scientific literature by CCLM. Clin Chem Lab Med 2023, 61, 873–879. [Google Scholar] [CrossRef]
- Vicente, F.B.; Lin, D.C.; Haymond, S. Automation of chromatographic peak review and order to result data transfer in a clinical mass spectrometry laboratory. Clin Chim Acta 2019, 498, 84–89. [Google Scholar] [CrossRef]
- Lunt, A.M.; Fakhruldeen, H.; Pizzuto, G.; Longley, L.; White, A.; Rankin, N.; Clowes, R.; Alston, B.; Gigli, L.; Day, G.M.; et al. Modular, multi-robot integration of laboratories: an autonomous workflow for solid-state chemistry. Chem Sci 2024, 15, 2456–2463. [Google Scholar] [CrossRef]
- Junger, S.; Hoene, M.; Shipkova, M.; Danzl, G.; Schoberl, C.; Peter, A.; Lehmann, R.; Wieland, E.; Braitmaier, H. Automated LC-MS/MS: Ready for the clinical routine Laboratory? J Mass Spectrom Adv Clin Lab 2023, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pursell, M.E.; DeVor, A.; Awoyemi, O.; Valentine, S.J.; Li, P. Portable mass spectrometry system: instrumentation, applications, and path to 'omics analysis. Proteomics 2022, 22, e2200112. [Google Scholar] [CrossRef] [PubMed]
- Houfani, A.A.; Foster, L.J. Review of the Real and Sometimes Hidden Costs in Proteomics Experimental Workflows. Methods Mol Biol 2022, 2456, 1–14. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).