1. Introduction
Diabetes is a chronic condition that leads to premature death and an economic burden on the health care system, with 46.6% of deaths in adults aged <60 years [
1]. Per the Health Promotion Administration, Ministry of Health and Welfare in Taiwan, Taiwan has >2 million diabetic patients, increasing by 25,000 per year. Of individuals with prediabetes (i.e., having glycemic variables higher than normal but lower than diabetes thresholds), approximately 5%–10% develop diabetes per year [
2]. Fortunately, restoring normoglycemia during prediabetes or very early type 2 diabetes can prevent diabetes progression [
3].
Research suggests that chronic pain [
4] and body weight [
4] are associated with diabetes/prediabetes. For instance, individuals with diabetes/prediabetes have a high prevalence of chronic pain symptoms (e.g., lower limb, back, and neck pain) [
4]. Obesity was associated with a greater risk of type 2 diabetes [
5]. The prevalence of prediabetes was significantly greater in adolescents and young adults with obesity than in those with normal weight [
6]. A study of men and women aged ≥18 years in South Korea suggested that long working hours increased the risk of developing diabetes in prediabetes patients [
7]. For mental health and diabetes, a systematic review and meta-analysis suggested that burnout is associated with an increased risk of type 2 diabetes [
8]. For workstyle, night shifts and rotating shifts were associated with an increased risk of type 2 diabetes [
9].
Research has also suggested that lifestyle interventions such as diet or exercise effectively reduce the progression from prediabetes to diabetes [
10,
11]. One study showed poor diet as a main risk factor for diabetes [
12]. Becoming married (or living as married) can change eating habits [
13], possibly due to women’s dinner choices being often limited by their husbands’ preferences for meat, vegetables, and a smaller variety of foods than they prefer [
14]. Another study supported the aforementioned view that compared with women who were married or single, women eat more meat and snacks and drink higher fat milk and more alcohol when married or cohabitating with a man. Conversely, men tended to eat less meat, drink lower-fat milk, and use less alcohol after marriage than before marriage [
15]. Eating together after marriage might also affect the development of diabetes in spouses. For instance, a large prospective biracial cohort and meta-analysis showed a positive association between spousal diabetes status and diabetes development [
16]. Research has suggested that spouses are less likely to have diabetes than those who are unmarried, widowed, or divorced [
17]. However, another study showed less risk of type 2 diabetes in widowed women than in married women [
18]. An exploratory cross-sectional survey of females showed that being married significantly increased the risk for prediabetes and diabetes [
19]. These results are inconsistent. Thus, to provide insights into prediabetes development, we explored whether marriage is a protective or risk factor for prediabetes.
Triglycerides (TG) are a risk factor for type 2 diabetes [
20] and are the main lipid component of dietary fat, animal fat [
21], and milk lipids (>98%) [
22]. Elevated TG levels might be closely associated with changes in diet habits [
23]. Thus, we posed two hypotheses (H1 and H2) to verify whether TG change is a primary cause of increased prediabetes risk during marriage.
H1: Marrying is an independent risk factor for prediabetes.
H2: Increased TG is a mediating factor that increases prediabetes risk during marriage.
2. Materials and Methods
To conduct this cross-sectional observational study, we distributed QR code-linked questionnaires by email March 2023 to 2,369 staff members from a hospital affiliated with a medical university in Taichung, Taiwan, who were participating in an employee health check. We received 1,630 responses; 1,039 were deemed valid [exclusion criteria were missing data, a history of diabetes, or fasting blood sugar (FBG) over 125 mg/dl]. Participants’ demographic/living/work data (e.g., family structure, living habits, occupation, physical health) were collected using questionnaires. Because burnout [
8] and musculoskeletal pain [
4] are closely related to prediabetes and diabetes, the Copenhagen Burnout Inventory and the Nordic Musculoskeletal Questionnaire (NMQ) were used to measure those of the participants. The Copenhagen Burnout Inventory has very high internal reliability; it includes a personal burnout scale, a work-related burnout scale, and a client burnout scale, measuring the dimensions of burnout separately [
24].
To ensure suitability for all participants and the close relationship between burnout and diabetes risk, we adopted personal burnout scales to measure burnout for health care workers. The first six questions pertained to personal burnout (PB): how often do you feel tired, how often are you physically exhausted, how often are you emotionally exhausted, how often do you think “I can’t take it anymore?”, how often do you feel worn out, and how often do you feel weak and susceptible to illness. Response options were always, often, sometimes, seldom, and never/almost never, with scores of 100, 75, 50, 25, and 0, respectively. The mean score of the six items represented each participant’s burnout level.
We also used the NMQ to measure the presence of pain attributable to work-related factors in the preceding year [
25,
26,
27]. The options for the frequency of each pain site were every day, once per week, once per month, once per half a year, and at least once every half a year (relatively scored as 100, 80, 60, 40, and 20 points). The options of serious degree were even life is affected, so take leave to recuperate, work ability is significantly reduced, slightly reduce work capacity, and does not affect life and work at all (relatively scored as 100, 75, 50, 25, and 5 points). According to the frequency score multiplied by the serious degree score, we used factor analysis [
28] to determine new underlying variables to effectively explain the questionnaire; the new variables identified by the new factor loadings are listed and marked in Supplementary Information
Table S1.
The response options for sex on the questionnaire were female or male. Age was filled out by participants. Married and others were the response options for marital status. Yes and No were the response options for whether the participant raises children and whether he or she lives with his or her parents, respectively. Never, occasionally, one cup every day, two cups per day, and at least two cups per day were the response options for drinking coffee. The response options for alcohol use habits in the past month were never, occasionally, and drinking every day. In addition, daily sleeping time was surveyed, and the response options were < 5 h, 5–6 h, 6–7 h, 7–8 h, and > 8 h. We also surveyed participants’ exercise habits. The response options were never, less than one time monthly, at least than one time monthly, at least than one time weekly, and at least one time daily, with scores of 100, 75, 50, 25, and 0 points, respectively.
Height and weight were recorded and classified per the definitions of underweight [body mass index (BMI) < 18.5], healthy weight (18.5 ≤ BMI < 24.0), overweight (24.0 ≤ BMI < 27.0), and obese (BMI ≥ 27.0) by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Chronic diseases were prelisted in the questionnaire and ticked by the participants. One or more diseases indicated the category "suffering from chronic disease.” The response options for professional field were nurses, administration staff, physicians, including attending physicians, residents and nurse practitioners, and technical staff. The daily work time (hours) was self-reported. For shift work, response options were irregular, regular, night, and day shift work. Response options for education degree were PhD, Master, Bachelor, and others.
FBG and TG levels were checked and confirmed by the laboratory of a hospital affiliated with a medical university. In 2003, the American Diabetes Association issued new impaired fasting glycemia diagnostic criteria, widening the FBG range from 110–125 to 100–125 mg/dl [
29]. Thus, we used the new criteria (100–125 mg/dl) to define prediabetes. Because the criteria fit FBG, prediabetes is called impaired fasting glucose (IFG) hereafter.
We used three steps to confirm H1 and H2:
Step 1: The chi-square test or Fisher’s exact test was used to determine confounders of IFG for every stratified age group, owing to the close relationship between age and diabetes.
Step 2: A logistic regression model was established for the correlation between marital status and the IFG risk in a stratified analysis of age, confirming marital status as an independent risk factor for increased risk of IFG.
Step 3: Mediation models were used to confirm whether increased TG is a mediating factor between marriage status and IFG. The mediating effect was assessed using the strategy of Baron and Kenny [
30], in which (1) the independent variable significantly affects the mediating factor (the first-stage effect), (2) the independent variable significantly affects the dependent variable in the absence of the mediating factor, (3) the mediating factor has a significant unique effect on the dependent variable (the second-stage effect), and (4) the effect of the independent variable on the dependent variable (the direct effect) weakens upon the addition of a mediating factor to the model. If the mediation factor or dependent variable is a categorical variable, the original formula of the Sobel test is rederived into a new formula per Iacobucci (2012) [
31]:
Z_(mediation ) (Z_m )=(a/s_a ×b/s_b )/√(〖 (a/s_a )〗^2+ (〖b/s_b )〗^2+1)
First-stage effect: a is the linear or logistic regression coefficient of the independent variable against the mediating factor.
Second-stage effect: b is the regression coefficient of the mediating factor against the dependent variable in the linear or logistic regression model.
where s_a and s_b are the standard deviations of a and b, respectively. Z_m values exceeding |1.96|, |2.57|, and |3.90| (for a two-tailed test) are significant at α = 0.05, 0.01, and 0.0001, respectively.
SAS Enterprise Guide 7.1 software (SAS Institute Inc., Cary, NC, USA) was used for analysis; significance was set at P < 0.05.
3. Results
Table 1 describes the survey and demographic variables for 1,039 female-dominated health workers (85.18%): average age, 37.50 ± 9.95 years; 43.60% married, 38.02% raising children; 59.29%, 18.48% and 13.76% were healthy weight, overweight and obese, respectively; 45.72% nurses; 11.65% physicians; average daily work time, 8.52 ± 0.88 h per participant; and 27.91% reported recent shift work (i.e., irregular and regular). Notably, 220 participants (21.17%) fulfilled the prediabetes criteria, per FBG. For all participants, the mean FBG and TG levels were 93.49 ± 8.60 and 74.03 ± 53.63 mg/dl, respectively.
Regarding prediabetes, males had a greater prevalence of IFG than females did (23.46%–38.36%, P < .05). IFG prevalence was greater for married than not married, with no age stratification (28.26% vs. 15.70%, P < .001). Only married participants aged 20–37 years had a greater risk of IFG than their unmarried counterparts (24.11% vs. 12.59%, P = 0.004). Participants raising children had a greater risk of developing IFG than those not raising children, with no age stratification (27.34 vs. 17.39%, < 0.001); however, only participants aged 20–37 years and raising children had a greater risk of developing IFG than did those not raising children in age stratification (25.61 vs. 13.08%, P = 0.007). Despite no association between coffee drinking frequency and IFG (P = 0.111), we detected a dose‒response relationship between increased frequency and increased risk (never-at least two cups per day, 18.34%–35.71%). For body weight, obese or overweight participants had a greater risk of IFG than healthy or underweight participants, regardless of age (for all P < 0.001). The proportion of obese participants with IFG in the group aged >38 years was >50% (52.94%). Prediabetes risk in participants with chronic diseases (excluding diabetes) was greater than that for all participants without chronic diseases (25.45 vs. 18.62%, P = 0.009). IFG risk varied significantly among professions with no age stratification (P = 0.005): nurses were the lowest (17.05%), and administrative staff and others were the highest (26.69%). IFG risk only differed among professions in the >38 years group (P = 0.043) and was highest for administrative staff and others (34.57%). With no age stratification, IFG risk differed among the shift work types (P = 0.008), with the highest risk for the day shift (24.68%).
Education level was associated with IFG risk with no age stratification and for the group aged 20–37 years (P = 0.013; 0.004). The proportion of participants with IFG who had a PhD was the highest (44.44%), followed by those with a master’s degree (24.09%) and bachelor’s degree (19.74%). Among the group aged 20–37 years, for those with IFG, 18.37% and 14.05% had a master’s degree and a bachelor’s degree, respectively.
The mean frequency coefficients of pain in the neck and shoulder and the ankle were determined using factor analysis with no age stratification for those aged 20–37 years and aged >38 years (
Table 1).
Mean FBG was 93.49 ± 8.60 mg/dl for no age stratification and 91.89 ± 7.62 mg/dl and 95.18 ± 9.25 mg/dl for the groups aged 20–37 and 38 years, respectively. The mean TG concentration was 74.03 ± 53.63 mg/dl for no age stratification and 65.82 ± 48.36 mg/dl and 82.68 ± 57.47 mg/dl for the groups aged 20–37 and years, respectively.
Table 2 shows the simple (M0) and multiple (M1) logistic regression models for prediabetes by age. For the M0 model with no age stratification, female (OR = 0.55, 95% CI: 0.38, 0.81), aged >38 years (OR = 2.17, 95% CI: 1.59, 2.95), married (OR = 2.12, 95% CI: 1.56, 2.86), raising children (OR = 1.79, 95% CI: 1.32, 2.42), drinking coffee every day (OR = 1.47, 95% CI: 1.09, 1.99), overweight (OR = 2.15, 95% CI: 1.47, 3.14), obese (OR = 3.98, 95% CI: 2.68, 5.91), chronic diseases (excluding diabetes) (OR = 1.49, 95% CI: 1.10, 2.02), nurses (OR = 0.57, 95% CI: 0.40, 0.79), neck and shoulder pain (OR = 1.23, 95% CI: 1.07, 1.43), and higher TG (OR = 1.01, 95% CI: 1.006, 1.011) were confounders of IFG risk. Because of possible collinearity concerns between being married and raising children, we excluded the dummy variable of raising children from the regression models. The confounders of IFG were adjusted variables in the M1 models. For all participants, the M1 model showed that marital status (OR = 1.65 (1.14, 2.38), overweight (OR = 1.66, 95% CI: 1.10, 2.50), obesity (OR = 3.45, 95% CI: 2.22, 5.37), neck and shoulder pain (OR = 1.19, OR = 1.02, 1.40), and higher TG (OR = 1.004 (1.00, 1.01) were independent risk factors for IFG.
The M0 model for the group aged 20–37 years showed females (OR = 0.51, 95% CI: 0.29, 0.91), married (OR = 2.21, 95% CI: 1.31, 3.71), raising children (OR = 2.29, 95% CI: 1.30, 4.03), sleeping for less than 6 h (OR = 1.68, 95% CI: 1.04, 2.71), obesity (OR = 3.70, 95% CI: 2.06, 6.65), neck and shoulder pain (OR = 1.30, 95% CI: 1.03, 1.65), and TG (OR = 1.01, 95% CI: 1.005, 1.015) were confounders of IFG risk. The M1 model for the group aged 20–37 years showed that marital status (OR = 1.89, 95% CI: 1.08, 3.33), obesity (OR = 2.95, 95% CI: 1.49, 5.83), neck and shoulder pain (OR = 1.31, 95% CI: 1.01, 1.69), and TG (OR = 1.01, 95% CI: 1.00, 1.01) were independent risk factors for IFG.
The M0 model for the group >38 years of age showed that female (OR = 0.56, 95% CI: 0.33, 0.94), exercising at least once weekly (OR = 1.51, 95), overweight (OR = 2.28, 95% CI: 1.43, 3.63), obese (OR = 4.54, 95% CI: 2.61, 7.91), nurse (OR = 0.57, 95% CI: 0.37, 0.89), technical staff member (OR = 0.47, 95% CI: 0.23, 0.98), and higher TG (OR = 1.01, 95% CI: 1.003, 1.01) were confounders of IFG. The M1 model for the group >38 years of age showed that overweight (OR = 2.08, 95% CI: 1.27, 3.43), obese (OR = 4.30, 95% CI: 2.38, 7.79), and higher TG (OR = 1.003, 95% CI: 1.00–1.01) were the only independent risk factors for IFG.
In summary, we confirmed that being married in H1 is an independent risk factor for IFG. Nevertheless, the effect of marriage status on IFG in the group >38 years of age was not significant (OR = 1.43, P > 0.05)
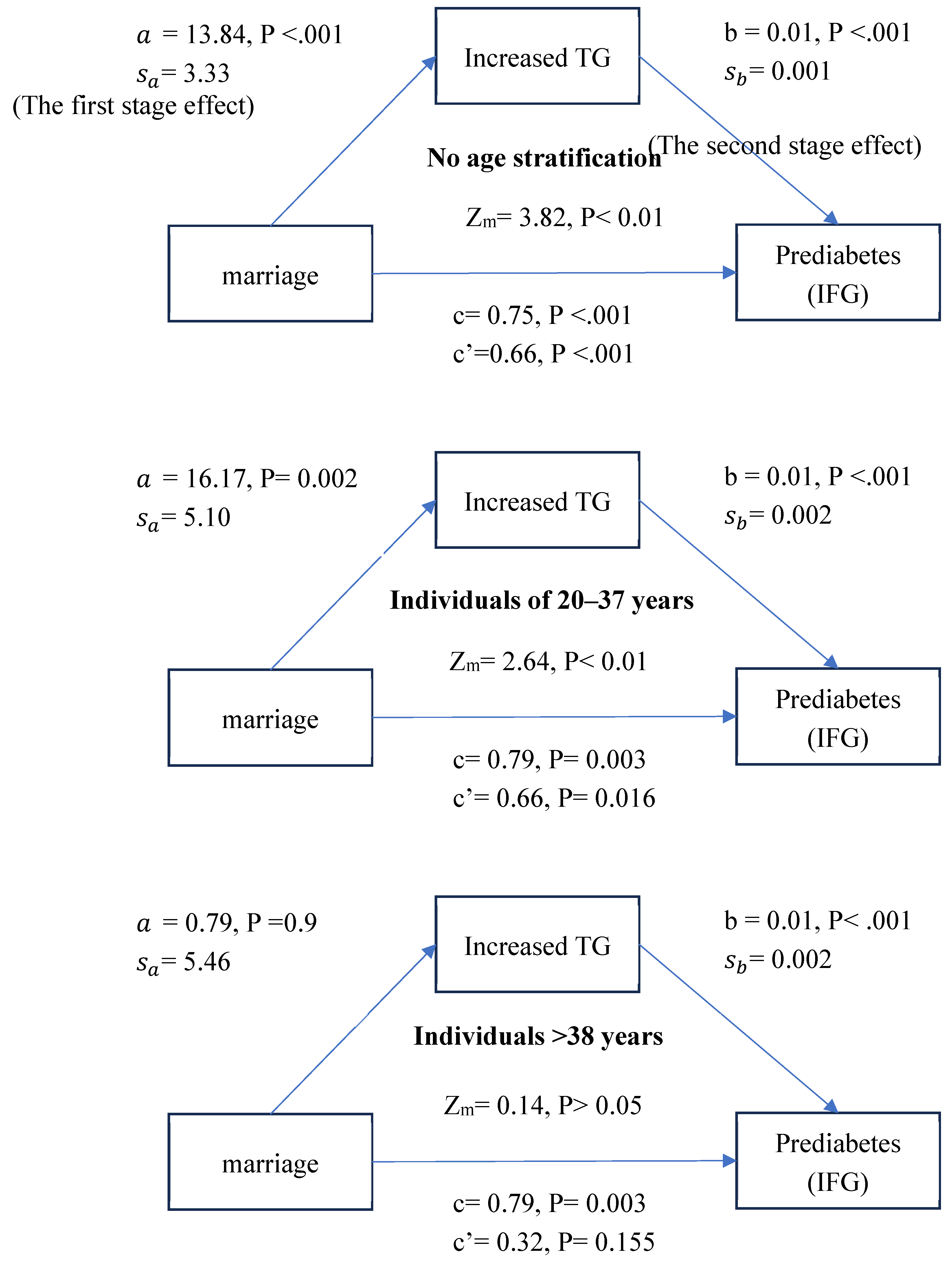
Figure 1 shows that the first- and second-stage effects were significant for no age stratification and the group aged 20–37 years (P<.001; P = 0.002/P<.001 for both) but not the group aged >38 years. Further statistical tests for the presence of a mediating effect used a formula in Iacobucci (2012) [
31] and showed that elevated TG is a mediating factor for the married group and increases the risk of prediabetes with no age stratification and for the group aged 20–37 years (Zm= 3.82; Zm= 2.64, P < 0.05 for both).
The results confirmed H2 that increased TG is a mediating factor by which being married increases the IFG risk. The effect was not significant for the group aged >38 years.
4. Discussion
Prediabetes prevalence in adults aged ≥20 years in the United States (National Health and Nutrition Examination Survey 2015–2016) was 43.5% [
32]. Prediabetes prevalence in adults in Taiwan aged ≥18 years (National Nutrition and Health Status Change Survey Results Report 2017–2020) was 25.5% [
33]. Compared with that of health care workers in our study, the proportion of individuals with IFG was 21.17%, close to Taiwan’s general prevalence. Next, we deeply examine confounders of prediabetes, including sex, age, marital status, overweight or obesity status, chronic diseases, and TG levels.
The M1 model of
Table 2 with no age stratification showed no sex difference in IFG risk (female vs. male, OR = 0.81, P < .05). A cross-sectional study in England from 2003 to 2011 showed that the odds ratios of prediabetes for males vs. females were 0.96 (95% CI: 0.83, 1.12) and 1.07 (95% CI: 0.91, 1.25), respectively [
34]. Despite differences in race and adjusted variables, the findings across studies remain consistent.
A study in Texas, 2004–2017, showed that the odds ratio for prediabetes was 1.7 for individuals aged 40–64 years and 3.1 for those aged >65 years, compared with those aged 18–39 years (P< 0.05 for both) [
35]. We found that the IFG risk for the group >38 years was 2.17 times greater than that for those aged 20–37 years (
Table 2, M0, OR = 2.17, 95% CI: 1.59, 2.95). In summary, middle-aged and older participants had a greater risk of developing prediabetes than the younger participants.
Obesity is associated with a greater risk for incident type 2 diabetes [
5] and may be linked to prediabetes incidence or risk. For instance, a study of prediabetes and diabetes in adults aged 25–64 years in the Czech Republic showed that the prevalence of prediabetes for overweight and obese was 1.419 (95% CI: 1.007, 1.200) and 2.401 times (95% CI: 1.666, 3.461) greater, respectively, than that for healthy individuals [
36]. We showed that in the M0 and M1 models for all age stratification, prediabetes risk for obese was 2.95–4.54 times than for healthy weight (
Table 2, OR = 2.95~4.54, P < 0.05 for all). Therefore, maintaining a healthy weight can effectively prevent the occurrence of prediabetes.
Higher glucose levels and diabetes are associated with daily chronic pain in adults [
37,
38]. Individuals with diabetes/prediabetes often experience chronic pain symptoms in the lower limbs, back, and neck [
4]. We also found a close relationship between prediabetes and chronic pain. For example, the participants with IFG more commonly reported neck and shoulder pain than those without IFG with no age stratification, regardless of the M0 or M1 model (
Table 2, OR = 1.23, 95% CI: 1.07, 1.43; OR = 1.19, 95% CI: 1.02–1.40), particularly in the group aged 20–37 years (OR = 1.30, 95% CI: 1.03, 1.65; 1.31, 95% CI: 1.01, 1.69). This finding supports the literature. Notably, chronic pain can pose a significant health risk during physical activity and in dietary habits. These lifestyle changes may increase the risk of developing type 2 diabetes [
39].
A study of adults aged 20–39 years showed a greater proportion of married than single males and females had prediabetes [
40]. A study among females in Saudi Arabia showed that the prediabetes risk in those who were married was 2.51 times greater (OR = 2.51, 95% CI: 1.61, 3.93) than for their single counterparts [
19]. As shown in
Table 2, in this study, IFG risk for married was 2.12 (M0, OR = 2.12, 95% CI: 1.56, 2.86) and 1.65 (M1, OR = 1.65, 95% CI: 1.14, 2.38) times greater than for not married. Nevertheless, another study showed that diabetes was less prevalent among married than unmarried, widowed, or divorced adults [
17]. The effect of marriage on diabetes development in adults with prediabetes might be complex and staged by age. For instance, diabetes incidence in males increased with their income, and the opposite was observed for females [
17]. We showed that the effect of marriage on IFG was significant for the group aged 20–37 years (M1 in
Table 2, OR = 1.89, 95% CI: 1.08, 3.33) but not for those aged >38 years. (M1 in
Table 2, OR = 1.43, 95% CI: 0.91, 2.26). Therefore, whether being married is a protective factor against prediabetes or diabetes in middle-aged and older adults requires further research.
Studies have shown that dieting habits can change after marriage because spouses eat together [
13,
14,
15], which can increase the risk of diabetes [
16] and prediabetes. TG levels in men and women were associated with the percentage of calories from fats [
23]. In overnutrition, hepatic fatty acid levels are increased due to enhanced lipolysis within adipocytes, leading to increased circulating levels of fatty acids in the bloodstream and increased hepatic de novo lipogenesis. Excess fatty acids cannot be consumed by oxidative pathways; fatty acids are instead directed toward the synthesis of TG, leading to increased hepatic TG storage and very low-density lipoprotein overproduction [
41]. Excess TG is packaged into chylomicrons, secreted into the lymphatic system, ultimately reaches the plasma, and is taken up by muscle and adipose tissue [
42]. Therefore, triglycerides are closely related to fat intake. Notably, in the group aged 20-37 years, TG levels were higher for the married than the unmarried group (
Figure 1, a = 13.84, P < 0.001; a = 16.17, P = 0.002). One possible explanation is that the dietary habits of married and unmarried adults differ, particularly in higher fat consumption.
Similar to eating habits, TG levels are associated with the risk of type 2 diabetes [
43,
44,
45]. TG might also be associated with an increased risk of prediabetes. For instance, TG may directly contribute to disorders of glucose metabolism [
46]. A study of prediabetes in Mexican Americans showed that high TG levels were associated with the risk of prediabetes, particularly in males aged 18-39 years (OR = 1.003, 95% CI: 1.001, 1.005) [
35]. We found that increased TG levels increased the IFG risk in all age stratifications (
Table 2, OR = 1.003–1.01, P< 0.05), indicating the close relationship between TG and IFG regardless of physiology or epidemiology.
The mediation models in
Figure 1 showed higher TG levels for married than unmarried adults, which further increased the risk of prediabetes for the former, especially those aged 20-37 years. A study of eating habits in Australia showed that younger people eat more carbohydrates and meat than older adults do [
47]. Other studies have shown that the greater TG level in older than younger people [
48,
49] can be attributed to delayed postprandial TG clearance [
50]. Middle-aged and older adults sustain a higher TG level than young adults, regardless of marital status, weakening the effect of marriage on TG levels increasing with age. We also found no significant difference in TG levels between married and unmarried adults >38 years of age (
Figure 1, a = 0.79, P = 0.9). TG levels were greater for married adults aged 20-37 years than for unmarried adults (
Figure 1, a = 16.17, P = 0.002).
Age, overweight/obesity status, pain in the neck and shoulders, and increased TG levels are independent risk factors for prediabetes and are highly consistent with the risk factors for diabetes, indicating that prediabetes is an important period for diabetes prevention. Because prediabetes can be reversed [
3], increased investment in resources to help individuals with prediabetes improve their living habits, maintain healthy weight, relieve pain, and provide healthy eating information should increase. Moreover, increased attention to the eating habits of young couples and additional healthy eating education to reduce fat intake are necessary.
Some studies have suggested that marriage is not an independent risk factor for prediabetes [
19,
40]. This finding differs from that of our study and might result from different sample sources (our focus was health care workers) and adjustment variables (e.g., we ignored physical activity and family history of diabetes). Specifically, our participants were mainly health care workers from a single medical institution, excluding the effects of other occupations on prediabetes (e.g., working time, rotation shifts, work environment conditions). In addition, we did not use an accurate physical activity questionnaire (e.g., Global Physical Activity Questionnaire) to measure the amount of exercise. According to a study of prediabetes and diabetes in South Korea [
40], low physical activity in adults was a predictor of prediabetes, which might explain the partial effect of marriage on prediabetes.
A study of 4,190 young adults aged 20-39 years in South Korea showed that the prevalence of prediabetes according to fasting blood glucose and hemoglobin A1c standards was 21.2% [
40]. We used fasting blood glucose levels between 100 and 125 mg/dl to indicate prediabetes, and the prevalence of prediabetes in the group aged 20-37 years was 15.01% (=80/533). This might be because individuals whose hemoglobin A1c <5.7% were excluded from prediabetes, leading us to underestimate the proportion of those with prediabetes. A study showed that solely using fasting glucose levels may result in an underestimation of diabetes and prediabetes [
51]. However, there was a significant positive correlation between HbA1c and FBG (r2 = 0.713, p < 0.05) [
52], and we used the FBG level because the determination of the association between prediabetes and marriage-related prediabetes is suitable.
5. Conclusions
Increasing age, marital status, being overweight or obese, neck and shoulder pain, and high TG levels are independent risk factors for prediabetes among health care workers. Additionally, married adults had higher TG levels were higher in married than in unmarried adults, elevating prediabetes risk, particularly in those aged 20-37 years. Consequently, the dietary habits of young couples warrant increased attention. Our results link marital status to diabetes and will support advances in diabetes prevention.
Author Contributions
Conceptualization, Y.-H.C., G.-P.J. and Y.S.Y.; Data curation, Y.-H.C., J.J.L., H.-M.T., C.-w.Y. and G.-P.J.; Formal analysis, Y.-H.C., G.-P.J. and Y.S.Y.; Funding acquisition, Y.-H.C.; Methodology, Y.-H.C., G.-P.J. and Y.S.Y.; Supervision, G.-P.J. and Y.S.Y.; Writing – original draft, Y.-H.C.; Writing – review & editing, G.-P.J.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in Chung Shan Medical University Hospital (grant CSH-2024-A-017).
Institutional Review Board Statement
All procedures performed in this study were approved by the ethical committee of Chung Shan Medical University Hospital. Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the staff and participants of Chung Shan Medical University Hospital for their important contributions.
Conflicts of Interest
The authors declare no competing interests.
References
- Atlas, I. IDF diabetes atlas, 9th ed. International Diabetes Federation. 2019.
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.S.; Ratner, R.E.; Buse, J.B.; Kahn, S.E. We can change the natural history of type 2 diabetes. Diabetes Care 2014, 37, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Aldossari, K.K.; Shubair, M.M.; Al-Zahrani, J.; Alduraywish, A.A.; AlAhmary, K.; Bahkali, S.; et al. Association between chronic pain and diabetes/prediabetes: A population-based cross-sectional survey in Saudi Arabia. Pain Res. Manage. 2020, 2020, 8239474. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatrics 2020, 174, e194498. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Lee, Y.; Mun, E.; Kim, D.H.; Jeong, Y.; Lee, J.; et al. The effect of long working hours on developing type 2 diabetes in adults with prediabetes: The Kangbuk Samsung cohort study. Ann Occupa. Environ. Med. 2022, 34, e4. [Google Scholar] [CrossRef] [PubMed]
- Strikwerda, M.; Beulens, J.W.; Remmelzwaal, S.; Schoonmade, L.J.; van Straten, A.; Schram, M.T.; et al. The association of burnout and vital exhaustion with type 2 diabetes: A systematic review and meta-analysis. Psycho Med. 2021, 83, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gan, T.; Jiang, L.; Yu, L.; Tang, D.; Wang, Y.; et al. Association between shift work and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of observational studies. Chronobiology Int. 2020, 37, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Krishnappa, M.; Patil, K.; Parmar, K.; Trivedi, P.; Mody, N.; Shah, C.; et al. Effect of saroglitazar 2 mg and 4 mg on glycemic control, lipid profile and cardiovascular disease risk in patients with type 2 diabetes mellitus: A 56-week, randomized, double blind, phase 3 study (PRESS XII study). Cardiovas. Diabetol. 2020, 19, 93. [Google Scholar] [CrossRef]
- Sobal, J.; Bove, C.F.; Rauschenbach, B.S. Commensal careers at entry into marriage: Establishing commensal units and managing commensal circles. Sociological Rev. 2002, 50, 378–397. [Google Scholar] [CrossRef]
- Brown, J.L.; Miller, D. Couples’ gender role preferences and management of family food preferences. J Nutri. Edu. Behavior. 2002, 34, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bove, C.F.; Sobal, J.; Rauschenbach, B.S. Food choices among newly married couples: Convergence, conflict, individualism, and projects. Appetite 2003, 40, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Schreiner, P.J.; Selvin, E.; Demerath, E.W.; Pankow, J.S. Spousal diabetes status as a risk factor for incident type 2 diabetes: A prospective cohort study and meta-analysis. Acta Diabetologica 2019, 56, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, L.A. Are there associations between socio-economic status and known diabetes in an elderly Finnish population? Central Euro. J. Public Health. 2005, 13, 187–190. [Google Scholar]
- Ramezankhani, A.; Azizi, F.; Hadaegh, F. Associations of marital status with diabetes, hypertension, cardiovascular disease and all-cause mortality: A long term follow-up study. PLOS ONE 2019, 14, e0215593. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, J.M.; Aldiab, A.; Aldossari, K.K.; Al-Ghamdi, S.; Batais, M.A.; Javad, S.; et al. Prevalence of prediabetes, diabetes and its predictors among females in AlKharj, Saudi Arabia: A cross-sectional study. Ann Global Health. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Wei, F.; Song, J.; Cao, Z.; Chen, C.; et al. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: A prospective study with 8-year follow-ups in two cohorts. J. Translation Med. 2019, 17, 403. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.A.; García-Palmieri, M.R. Cholesterol, triglycerides, and associated lipoproteins. 1990.
- Liu, Z.; Rochfort, S.; Cocks, B. Milk lipidomics: What we know and what we don’t. J Translation Med. 2018, 71, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Fisher, M.; Ernst, N.; Rifkind, B.M. Relation of diet to LDL cholesterol, VLDL cholesterol, and plasma total cholesterol and triglycerides in white adults. The Lipid Research Clinics prevalence study. Arteriosclerosis. 1982, 2, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.S.; Borritz, M.; Villadsen, E.; Christensen, K.B. The Copenhagen burnout inventory: A new tool for the assessment of burnout. Work Stress 2005, 19, 192–207. [Google Scholar] [CrossRef]
- Aulia, C. Validity and reliability test of the Nordic musculoskeletal questionnaire with formal and informal sector workers. In Proceedings of the 7th International Conference on Public Health. Indonesia; 2020; pp. 100–106. [Google Scholar]
- Palmer, K.; Smith, G.; Kellingray, S.; Cooper, C. Repeatability and validity of an upper limb and neck discomfort questionnaire: The utility of the standardized Nordic questionnaire. Occupa. Med. 1999, 49, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.G.; Havang, S.; Hagen, K. Reliability of a self-administrated musculoskeletal questionnaire: The fourth Trøndelag health study. Musculoskelet. Sci. Pract. 2022, 57, 102496. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate data analysis with readings, 4th ed. Prentice Hall, Inc. 1995.
- Genuth, S.; Alberti, K.G.; Bennett, P.; Buse, J.; DeFronzo, R.; Kahn, R.; et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003, 26, 3160–3167. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal Social Psycho. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Iacobucci, D. Mediation analysis and categorical variables: The final frontier. J. Consum. Psycho. 2012, 22, 582–594. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Selvin, E. Prediabetes and what it means: The epidemiological evidence. Ann. Rev. Public Health 2021, 42, 59–77. [Google Scholar] [CrossRef] [PubMed]
- National Nutrition and Health Status Change Survey Results Report 2017-2020. 2019.
- Mainous, A.G., 3rd; Tanner, R.J.; Baker, R.; Zayas, C.E.; Harle, C.A. Prevalence of prediabetes in England from 2003 to 2011: Population-based, cross-sectional study. BMJ. Open 2014, 4, e005002. [Google Scholar] [CrossRef] [PubMed]
- Vatcheva, K.P.; Fisher-Hoch, S.P.; Reininger, B.M.; McCormick, J.B. Sex and age differences in prevalence and risk factors for prediabetes in Mexican-Americans. Diabetes Res. Clin. Pract. 2020, 159, 107950. [Google Scholar] [CrossRef] [PubMed]
- Brož, J.; Malinovská, J.; Nunes, M.A.; Kučera, K.; Rožeková, K.; Žejglicová, K.; et al. Prevalence of diabetes and prediabetes and its risk factors in adults aged 25-64 in the Czech Republic: A cross-sectional study. Diabetes Res. Clin. Pract. 2020, 170, 108470. [Google Scholar] [CrossRef] [PubMed]
- Mäntyselkä, P.; Miettola, J.; Niskanen, L.; Kumpusalo, E. Chronic pain, impaired glucose tolerance and diabetes: A community-based study. Pain 2008, 137, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mäntyselkä, P.; Miettola, J.; Niskanen, L.; Kumpusalo, E. Persistent pain at multiple sites—Connection to glucose derangement. Diabetes Res. Clin. Pract. 2009, 84, e30–e32. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.L.; Fleischer, J.; Hoeyem, P.; Laugesen, E.; Poulsen, P.L.; Christiansen, J.S.; et al. Objective measurements of activity patterns in people with newly diagnosed type 2 diabetes demonstrate a sedentary lifestyle. Diabetic Med. 2013, 30, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Hwang, S.Y. Lifestyle-related predictors affecting prediabetes and diabetes in 20-30-year-old young Korean adults. Epidemi. Health. 2020, 42, e2020014. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Comprehen Physio. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physio. Endocri. Meta. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed]
- Mc Donald Posso, A.J.; Bradshaw Meza, R.A.; Mendoza Morales, E.A.; Jaen, Y.; Cumbrera Ortega, A.; Mendoza Posada, E.J. Diabetes in panama: Epidemiology, risk factors, and clinical management. Ann. Global Health. 2015, 81, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Suvitaival, T.; Bondia-Pons, I.; Yetukuri, L.; Pöhö, P.; Nolan, J.J.; Hyötyläinen, T.; et al. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Koh, W.-P.; Talaei, M.; Yuan, J.-M.; Pan, A. Association between the ratio of triglyceride to high-density lipoprotein cholesterol and incident type 2 diabetes in Singapore Chinese men and women. J. Diabetes. 2017, 9, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, M.; Tricò, D.; Natali, A. The impact of triglycerides on glucose tolerance: Lipotoxicity revisited. Diabetes Metab. 2017, 43, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, T.M.; Egloff, B. Personality and eating habits revisited: Associations between the big five, food choices, and body mass index in a representative Australian sample. Appetite 2020, 149, 104607. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.S.; McNamara, J.R.; Cohn, S.D.; Ordovas, J.M.; Schaefer, E.J. Postprandial plasma lipoprotein changes in human subjects of different ages. J. Lipid Res. 1988, 29, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Cassader, M.; Gambino, R.; Ruiu, G.; Marena, S.; Bodoni, P.; Pagano, G. Postprandial triglyceride-rich lipoprotein changes in elderly and young subjects. Aging 1996, 8, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.G.; Freitas, F.R.; de Mesquita, C.H.; Vinagre, J.C.; Mariani, A.C.; Kalil-Filho, R.; Maranhão, R.C. Removal of chylomicron remnants from the bloodstream is delayed in aged subjects. Aging Dis. 2018, 9, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Ko, S.-H.; Kwon, H.-S.; Kim, N.H.; Kim, J.H.; Kim, C.S.; et al. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab. J. 2013, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kam-On Chung, J.; Xue, H.; Wing-Hang Pang, E.; Chuen-Chu Tam, D. Accuracy of fasting plasma glucose and hemoglobin A1c testing for the early detection of diabetes: A pilot study. Front. Lab. Med. 2017, 1, 76–81. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).