Submitted:
10 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
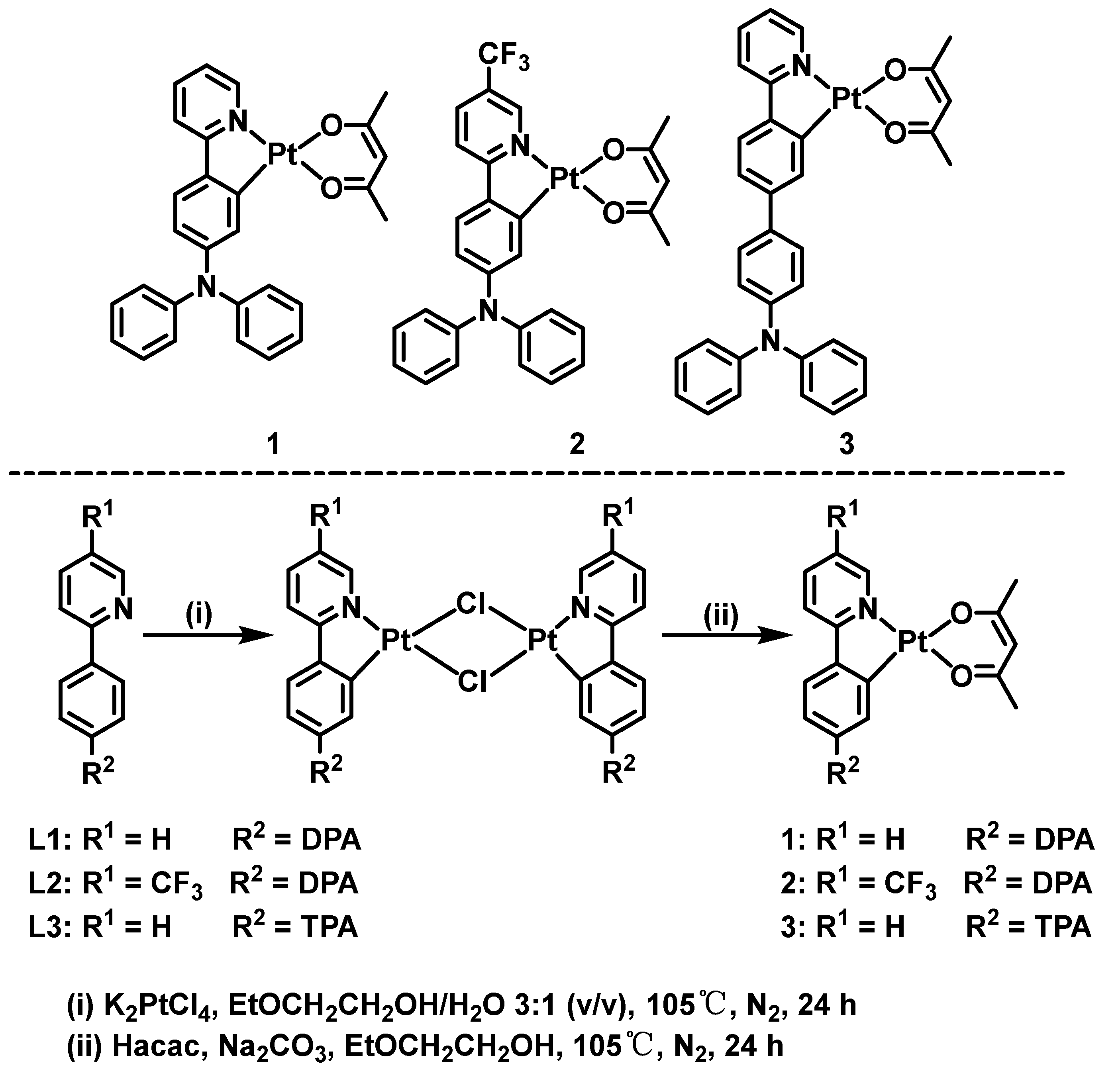
2.1. Materials and Instruments
2.2. Characterization of Complexes
2.3. Preparation of the Samples for Evaluating AIPE Properties and Detection of PA
3. Results and Discussion
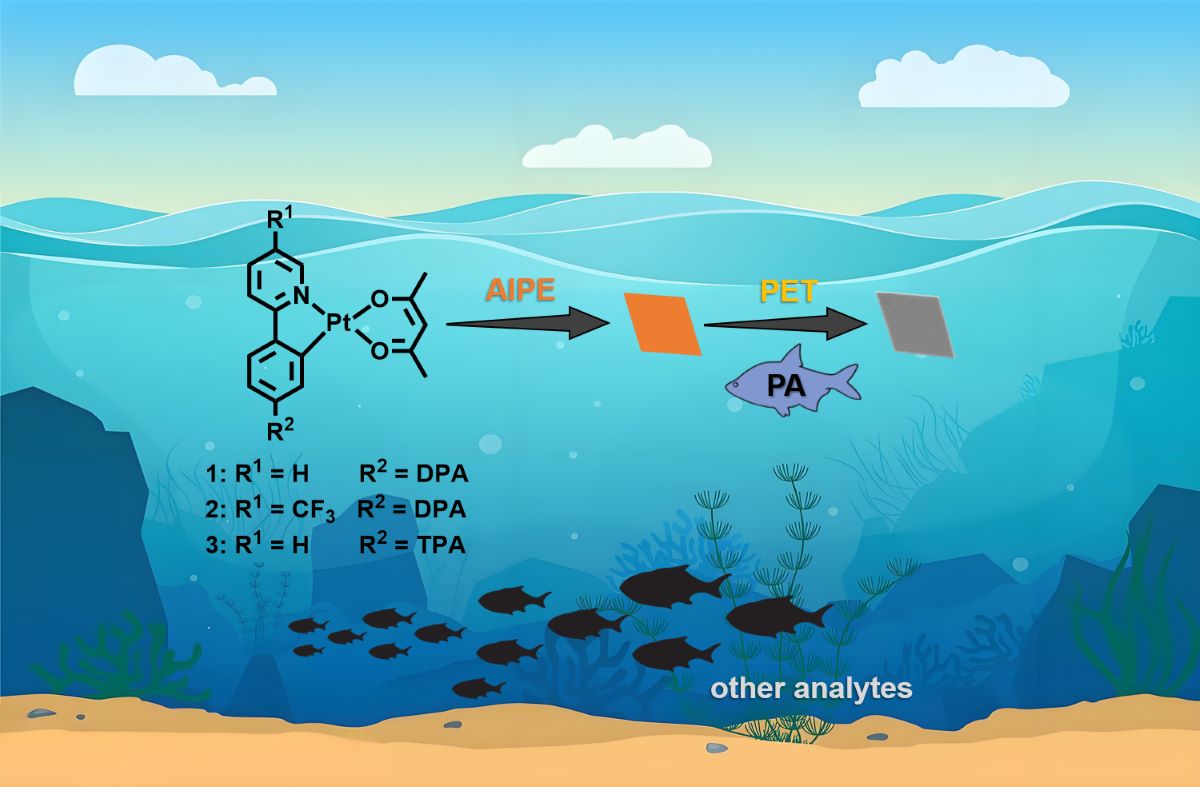
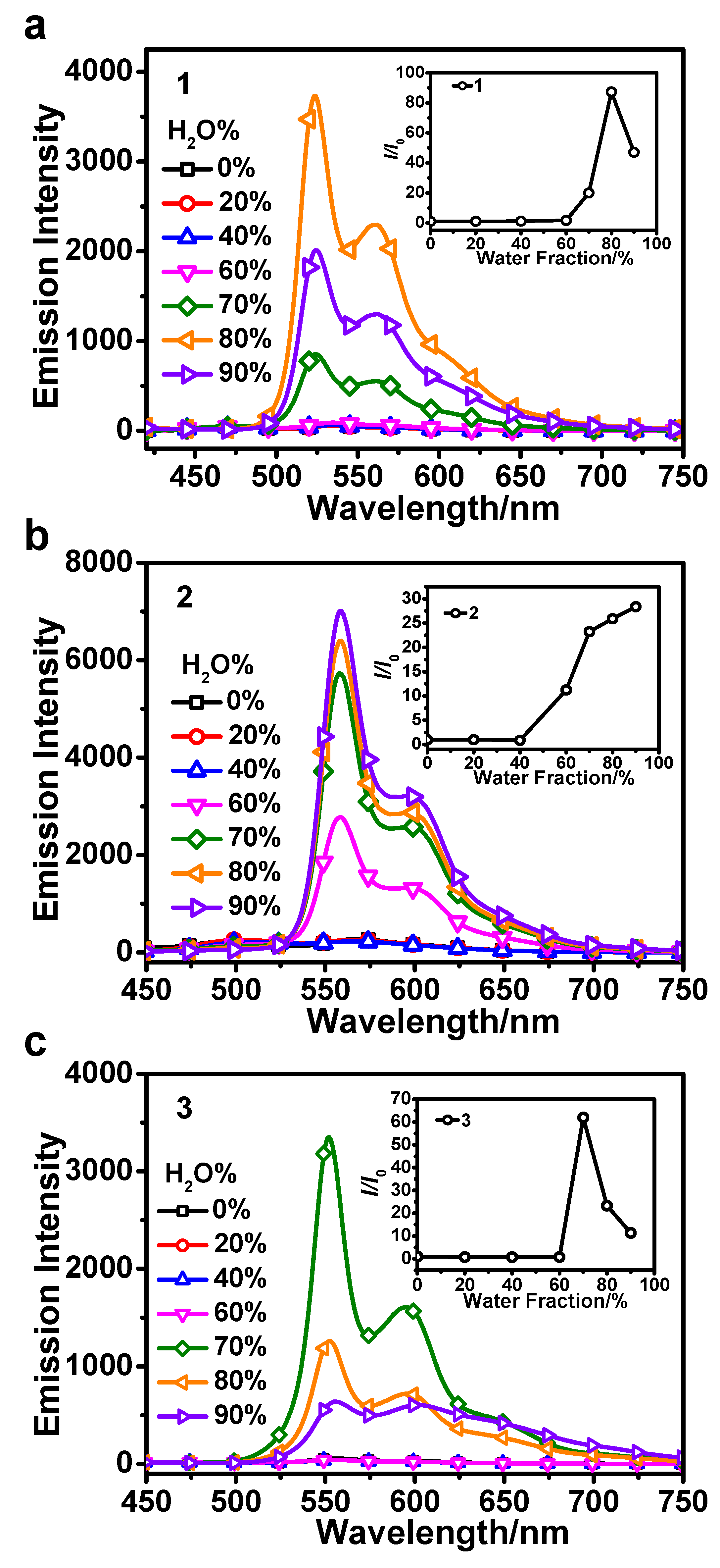
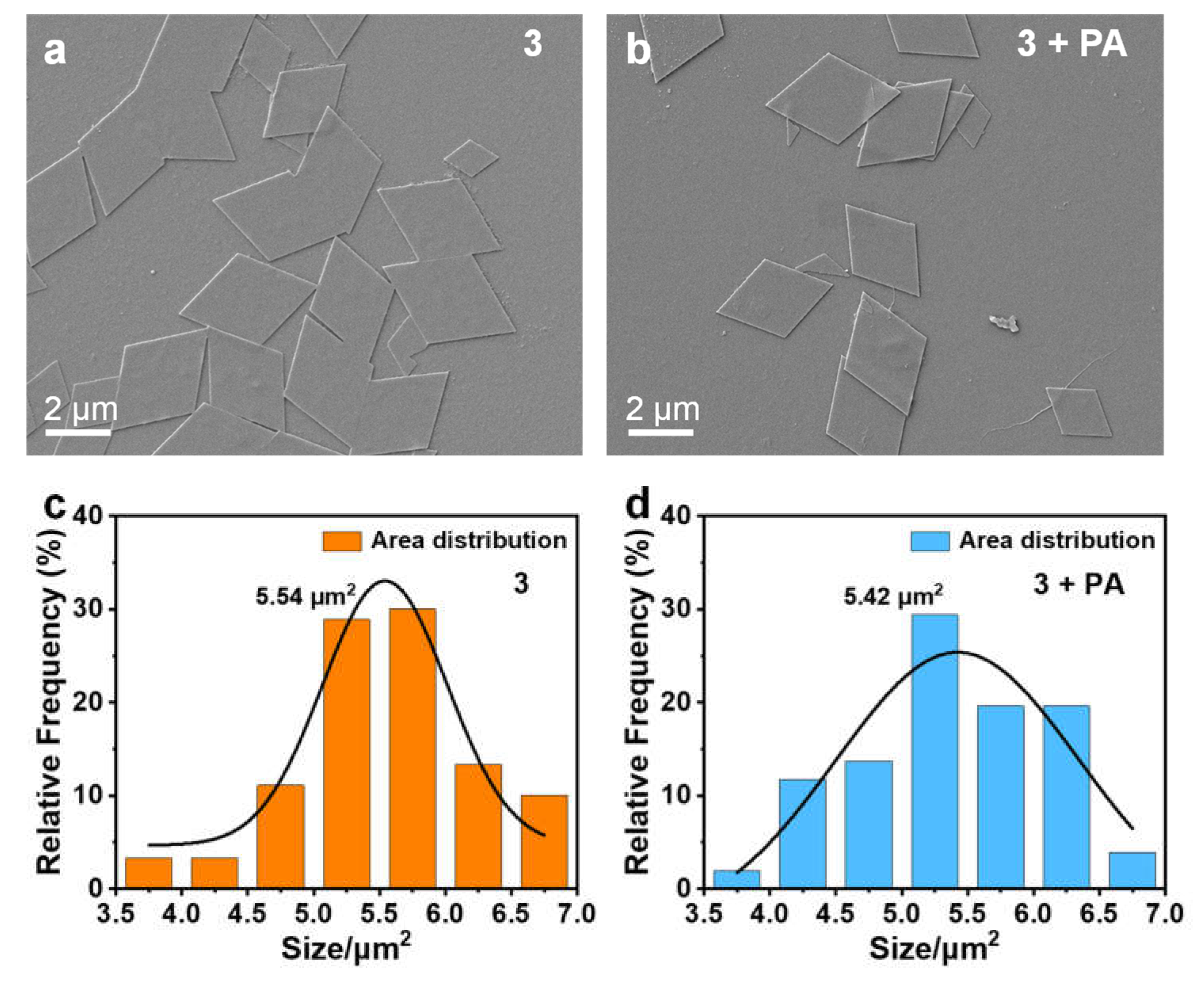
3.1. AIPE Properties
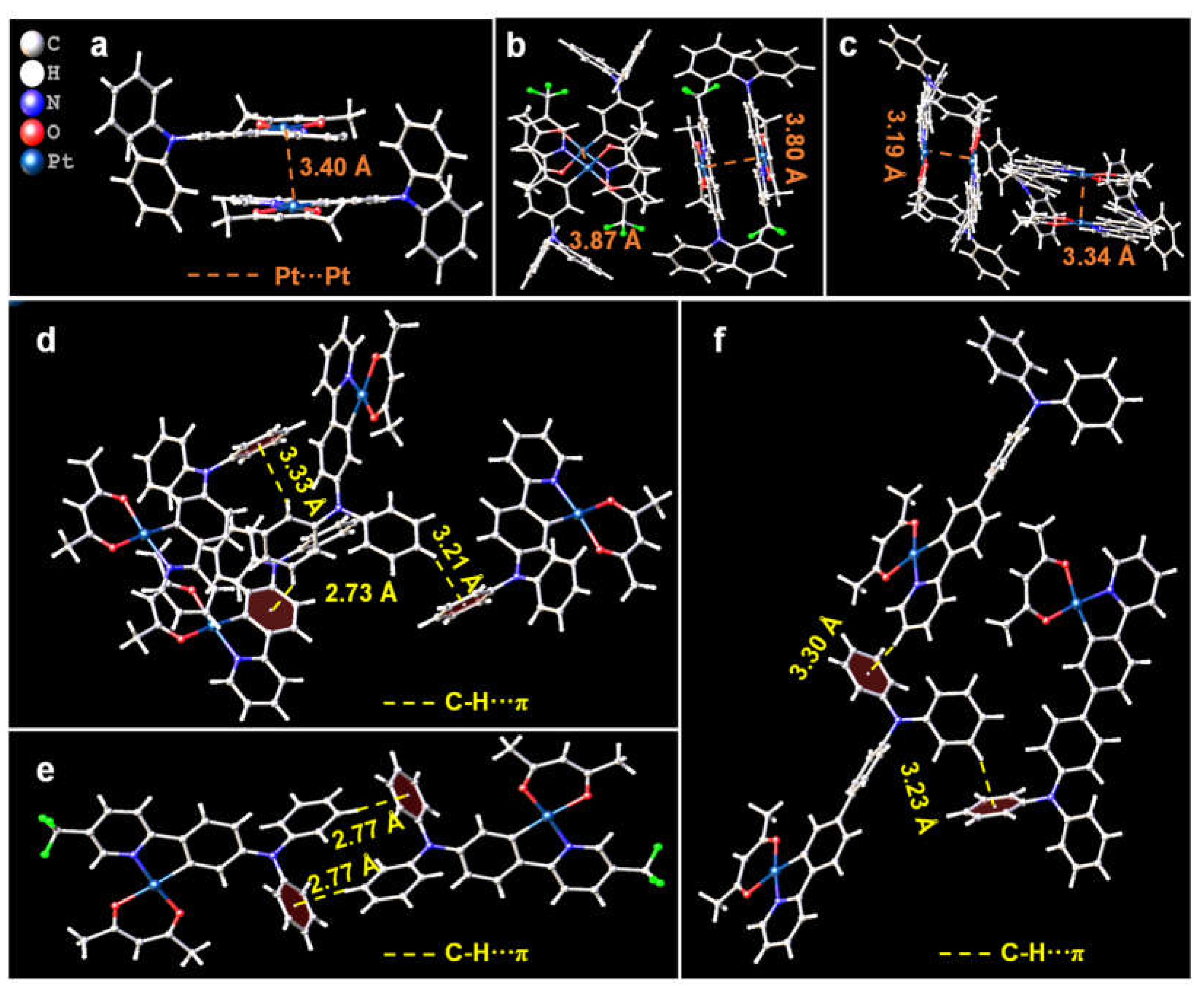
3.2. Molecular Packing Modes
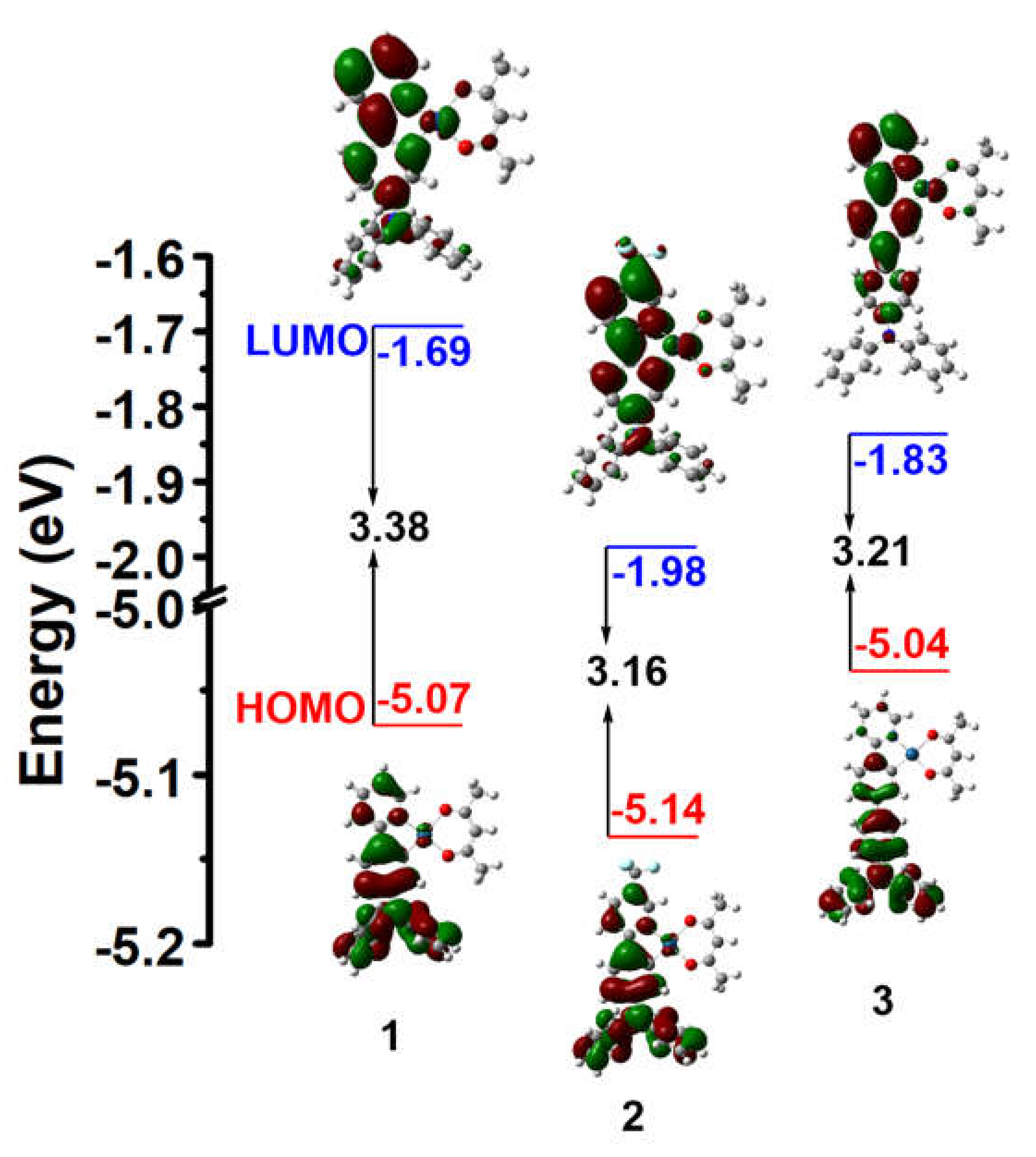
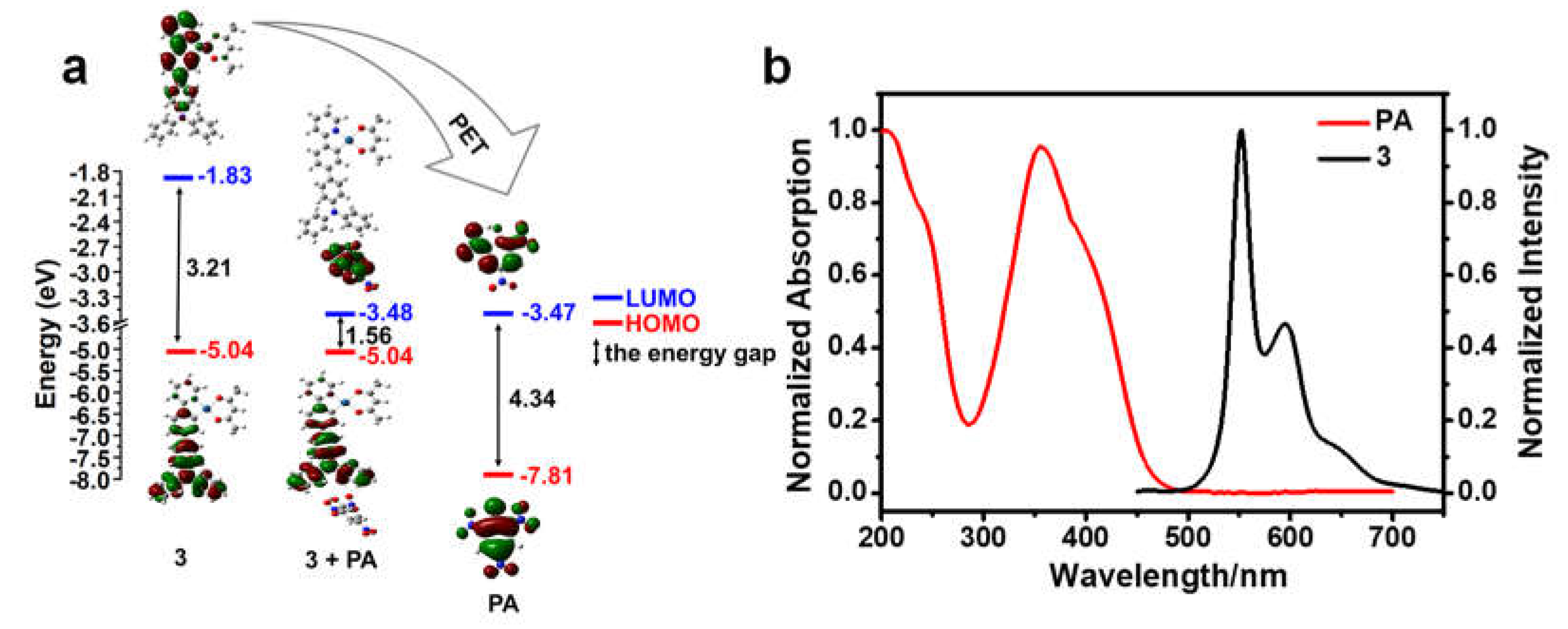
3.3. Theoretical Calculations
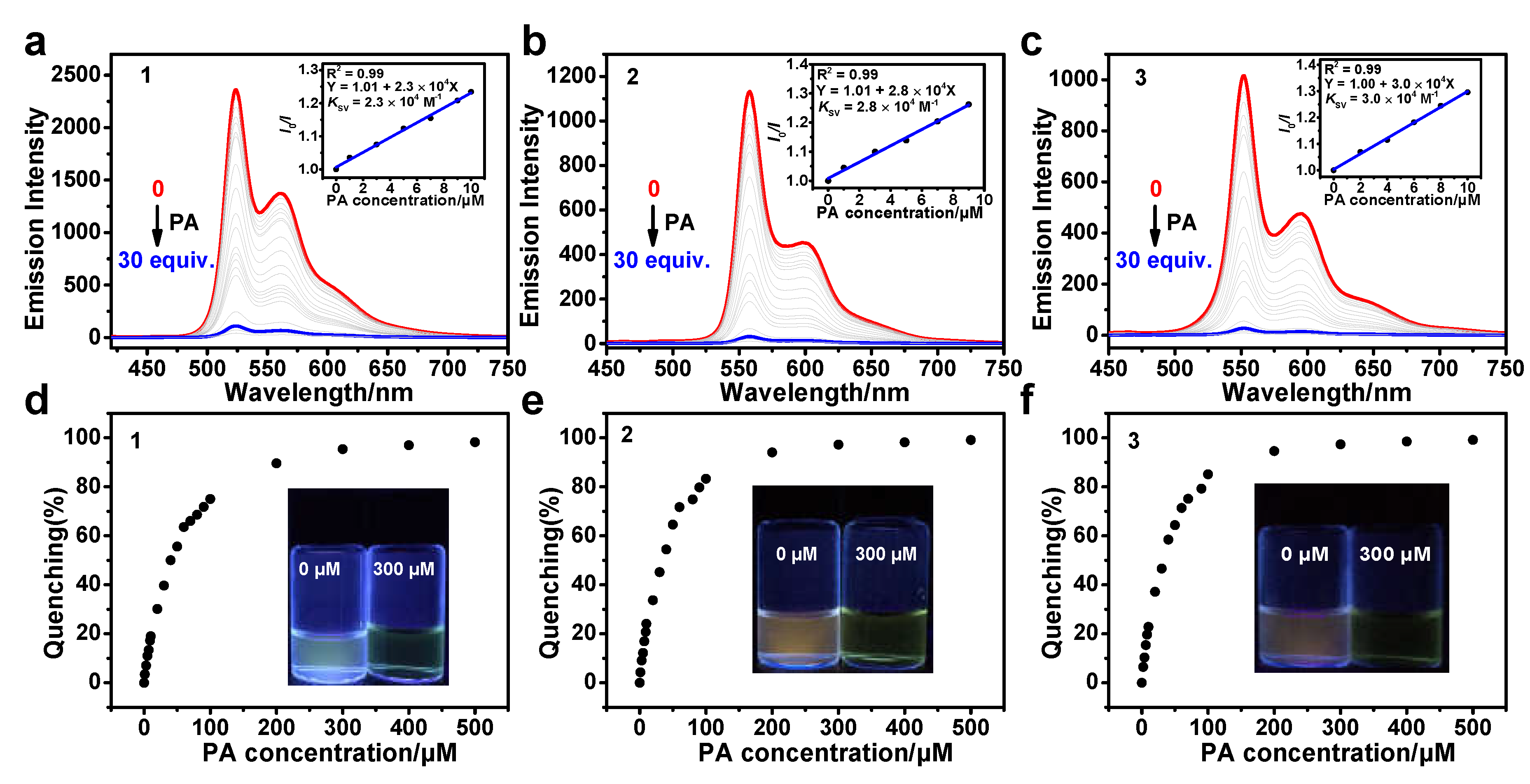
3.4. Sensing of PA
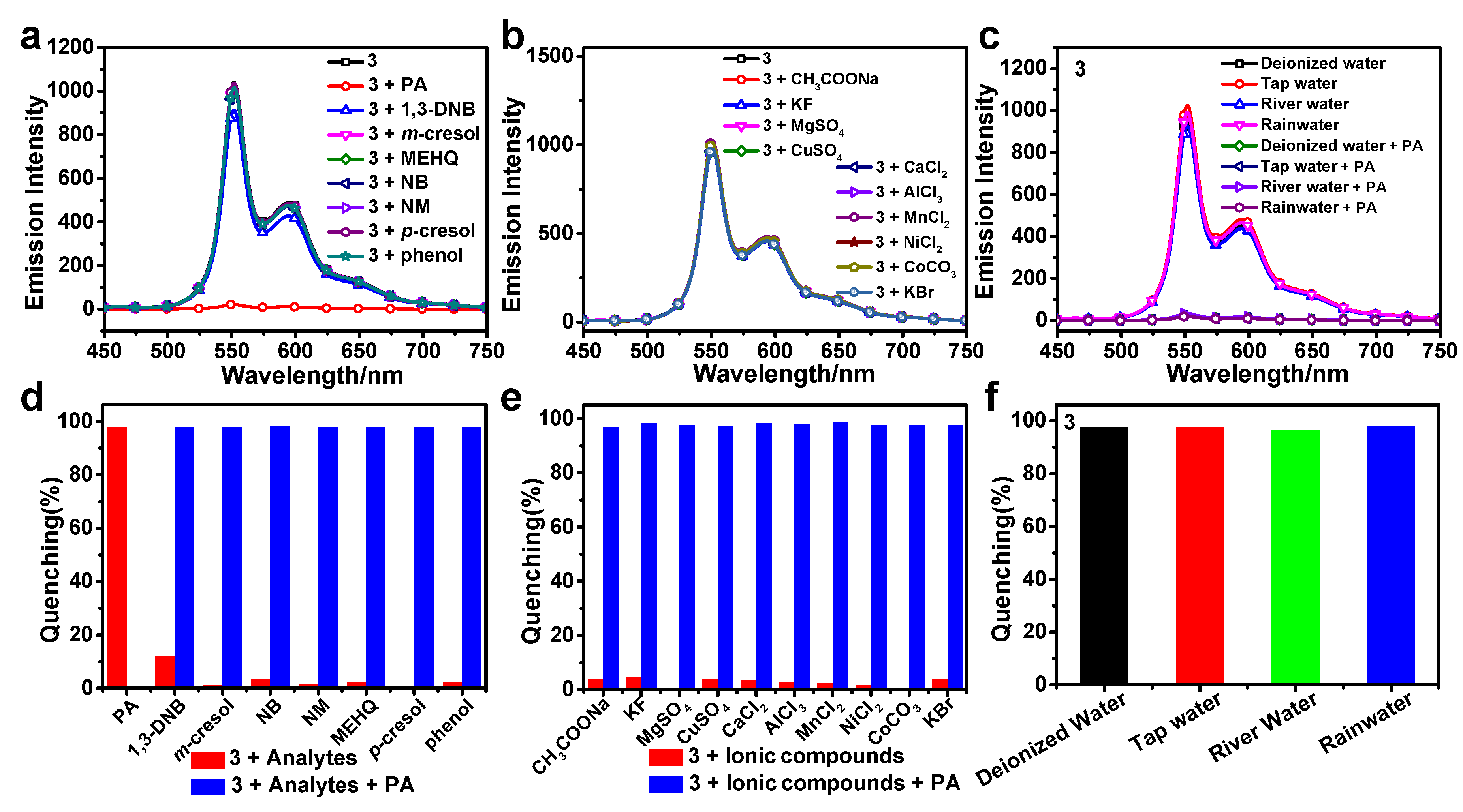
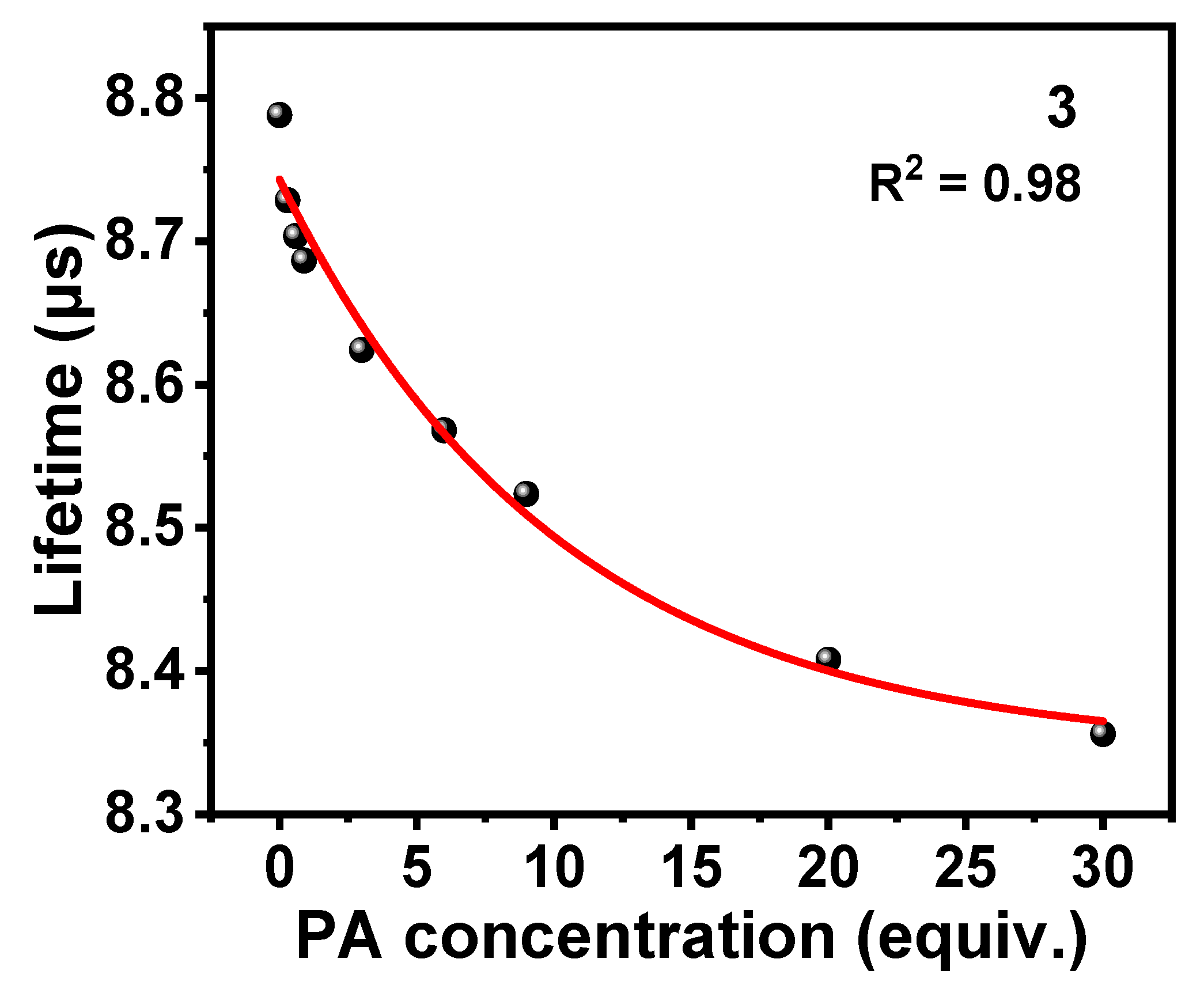
3.5. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang, S.; Hu, Z.; Zhang, H.; Zhang, X.; Liang, F.; Zhao, Z.; Liu, S. Enhancement of metal-metal interactions inside a large-cavity synthetic host in water. Chem. Commun. 2018, 54, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Chan, M.H.-Y.; Yam, V.W.-W. Dinuclear anthracene-containing alkynylplatinum(II) terpyridine complexes with photo-modulated self-assembly behaviors. Mater. Chem. Front. 2021, 5, 2409–2415. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Gao, Z.; Wang, F. Supramolecular engineering of discrete Pt(II)···Pt(II) interactions for visible-light photocatalysis. ACS Catal. 2017, 7, 4676–4681. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Z.; Wang, C.; Zhong, R.; Wang, F. Recent progress on supramolecular assembly of organoplatinum(II) complexes into long-range ordered nanostructures. Coord. Chem. Rev. 2020, 414, 213300. [Google Scholar] [CrossRef]
- Wan, Q.; Li, D.; Zou, J.; Yan, T.; Zhu, R.; Xiao, K.; Yue, S.; Cui, X.; Weng, Y.; Che, C.-M. Efficient long-range triplet exciton transport by metal-metal interaction at room temperature. Angew. Chem. Int. Ed. 2022, 61, e202114323. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Climent, C.; Alemany, P.; Laskar, I.R. “Aggregation-induced emission” of transition metal compounds: design, mechanistic insights, and applications. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100317. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Wong, K.M.-C.; Zhu, N. Solvent-induced aggregation through meta···metal/π···π interactions: large solvatochromism of luminescent organoplatinum(II) terpyridyl complexes. J. Am. Chem. Soc. 2002, 124, 6506–6507. [Google Scholar] [CrossRef]
- Cheng, H.-K.; Yeung, M.C.-L.; Yam, V.W.-W. Molecular engineering of platinum(II) terpyridine complexes with tetraphenylethylene-modified alkynyl ligands: supramolecular assembly via Pt···Pt and/or π-π stacking interactions and the formation of various superstructures. ACS Appl. Mater. Interfaces 2017, 9, 36220–36228. [Google Scholar] [CrossRef]
- Liu, C.; Song, X.; Rao, X.; Xing, Y.; Wang, Z.; Zhao, J.; Qiu, J. Novel triphenylamine-based cyclometalated platinum(II) complexes for efficient luminescent oxygen sensing. Dyes Pigm. 2014, 101, 85–92. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, R.; Ma, J.; Wang, S.; Wei, X.; Wang, H. Photoinduced DNA damage and cytotoxicity by a triphenylamine-modified platinum-diimine complex. J. Inorg. Biochem. 2015, 143, 64–68. [Google Scholar] [CrossRef]
- Wang, D.; Dong, H.; Wu, Y.; Yu, Y.; Zhou, G.; Li, L.; Wu, Z.; Gao, M.; Wang, G. Effect of diphenylamine substituent on charge-transfer absorption features of the iridium complexes and application in dye-sensitized solar cell. J. Organomet. Chem. 2015, 775, 55–59. [Google Scholar] [CrossRef]
- Chen, B.-L.; Liu, L.; Zhong, X.-X.; Asiri, A.M.; Alamry, K.A.; Li, F.-B.; Zhu, N.-Y.; Wong, W.-Y.; Qin, H.-M. Synthesis, characterization and luminescent properties of three-coordinate copper(I) halide complexes containing diphenylamino monodentate phosphine ligand. J. Coord. Chem. 2017, 70, 2916–2928. [Google Scholar] [CrossRef]
- Wang, D.; Shao, T.-F.; Li, S.-M.; Cao, W.; Yao, Q.; Ma, Y. A triphenylamine derivative with multistimuli responsive behavior and high-contrast vapochromism of its Zn(II) complex. Dyes Pigm. 2024, 226, 112114. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Wang, Y.; Wu, Z. The influence of numbers and ligation positions of the triphenylamine unit on the photophysical and electroluminescent properties of homoleptic iridium(III) complexes: a theoretical perspective. Dalton Trans. 2014, 43, 11915–11924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, Z.; Zhong, D.; Yang, X.; Wu, Y.; Zhou, G.; Wu, Z. Cyclometalated platinum complexes with aggregation-induced phosphorescence emission behavior and highly efficient electroluminescent ability. Chem. Mater. 2018, 30, 929–946. [Google Scholar] [CrossRef]
- Liu, X.; Hao, H.; Ge, X.; He, X.; Liu, Y.; Wang, Y.; Wang, H.; Shao, M.; Jing, Z.; Tian, L.; Liu, Z. Triphenylamine-appended cyclometallated iridium(III) complexes: Preparation, photophysical properties and application in biology/luminescence imaging. J. Inorg. Biochem. 2019, 199, 110757. [Google Scholar] [CrossRef]
- Guan, S.; Wei, Z.; Han, N.; Wei, Y.; Xu, B.; Wang, M.; Shi, J. Construction of metallo-complexes with 2,2′:6′,2"-terpyridine substituted triphenylamine in different modified positions and their photophysical properties. Chin. Chem. Lett. 2024, 35, 109348. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Liu, B.; Guo, H.; Zhou, G.; Ma, W.; Wu, Z. Aggregation-induced emission triggered by the radiative-transition-switch of a cyclometallated Pt(II) complex. J. Mater. Chem. C 2019, 7, 12552–12559. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Liu, C.; Jin, X. A diphenylamino-substituted cationic cyclometalated Ir(III) complex: its aggregation-induced phosphorescent emission and oxygen sensing properties. Mater. Chem. Front. 2019, 3, 1593–1600. [Google Scholar] [CrossRef]
- He, P.; Chen, Y.; Li, X.-N.; Yan, Y.-Y.; Liu, C. AIPE-active cationic Ir(III) complexes for efficient detection of 2,4,6-trinitrophenol and oxygen. Dalton Trans. 2023, 52, 128–135. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; Shu, Y.; Wang, J.; Qiu, H. Fabrication and application of 2,4,6-trinitrophenol sensors based on fluorescent functional materials. J. Hazard. Mater. 2022, 425, 127987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, L.; Tang, X.; Jiang, Z.; Guo, S.; Zou, L.; Xie, H.; Gong, Y.; Liu, Y. Preparation and characterization of carbazole-based luminogen with efficient emission in solid and solution states. Materials 2023, 16, 4193. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mukherjee, P.S. Self-Assembly of a nanoscopic prism via a new organometallic Pt3 acceptor and its fluorescent detection of nitroaromatics. Organometallics 2008, 27, 316–319. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Joshi, S.A.; Mukherjee, P.S. Self-assembly of metallamacrocycles using a dinuclear organometallic acceptor: synthesis, characterization, and sensing study. Inorg. Chem. 2011, 50, 11736–11745. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Mukherjee, P.S. PtII6 nanoscopic cages with an organometallic backbone as sensors for picric acid. Dalton Trans. 2013, 42, 16784–16795. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, S.; Zhang, Z.; Chen, L.; Zhang, M. A fluorescent platinum(II) metallacycle-cored supramolecular network formed by dynamic covalent bonds and its application in halogen ions and picric acid detection. Polym. Chem. 2020, 11, 254–258. [Google Scholar] [CrossRef]
- Hou, Y.; Shi, R.; Yuan, H.; Zhang, M. Highly emissive perylene diimide-based bowtie-shaped metallacycles. Chin. Chem. Lett. 2023, 34, 107688. [Google Scholar] [CrossRef]
- Tao, W.; Chen, Y.; Lu, L.; Liu, C. Luminescence properties of cyclometalated platinum(II) complexes in a dichloromethane/n-hexane system. Tetrahedron Lett. 2021, 66, 152802. [Google Scholar] [CrossRef]
- Jia, W.; Yan, Y.; Cai, R.; Yin, Q.; He, P.; Liu, C. Efficient detection of picric acid with aggregation-induced phosphorescent emission neutral cyclometalated platinum(II) complexes in aqueous media. Tetrahedron Lett. 2023, 126, 154663. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, W.; Cai, R.; Liu, C. An AIPE-active fluorinated cationic Pt(II) complex for efficient detection of picric acid in aqueous media. Chin. Chem. Lett. 2024, 35, 108819. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Gao, Z.; Wang, L.; Tang, Y.; Liu, J.; Liu, C. Supramolecular copolymers under kinetic, thermodynamic, or pathway-switching control. Angew. Chem. Int. Ed. 2023, 62, e202302581. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, Q.; Shi, Y.; Tang, B.; Che, C.-M.; Liu, C. Three-component multiblock 1D supramolecular copolymers of Ir(III) complexes with controllable sequences. Angew. Chem. Int. Ed. 2023, 62, e202312844. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Y.; Cai, R.; Zhang, H.; Liu, C. Controllable 1D, 2D and 3D supramolecular assemblies of Ir(III) complexes. Mater. Chem. Front. 2023, 7, 5915–5923. [Google Scholar] [CrossRef]
- Brooks, J.; Babayan, Y.; Lamansky, S.; Djurovich, P.I.; Tsyba, I.; Bau, R.; Thompson, M.E. Synthesis and characterization of phosphorescent cyclometalated platinum complexes. Inorg. Chem. 2002, 41, 3055–3066. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, C.; Wu, W.; Guo, H.; Ji, S.; Song, P.; Han, K.; Zhao, J.; Zhang, X.; Wu, Y.; Du, G. Tuning the emission colour of triphenylamine-capped cyclometallated platinum(II) complexes and their application in luminescent oxygen sensing and organic light-emitting diodes. Eur. J. Inorg. Chem. 2010, 2010, 4683–4696. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, B.; Thanasekaran, P.; Rajendran, T.; Lin, R.-J.; Chang, I.-J.; Lee, G.-H.; Peng, S.-M.; Rajagopal, S.; Lu, K.-L. Luminescence enhancement induced by aggregation of alkoxy-bridged rhenium(I) molecular rectangles. Inorg. Chem. 2002, 41, 5323–5325. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B. Aggregation-induced emission: fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Di, L.; Xia, Z.; Wang, H.; Xing, Y.; Yang, Z. Switchable and adjustable AIE activity of Pt(II) complexes achieving swift-responding and highly sensitive oxygen sensing. Sens. Actuators B Chem. 2021, 326, 128987. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, B.; Si, S.; Lin, Y. , Luo, C.S.; Pan, C.; Zhao, C.; Wang, L. A fluorescent chemosensor based on nonplanar donor-acceptor structure for highly sensitive and selective detection of picric acid in water. Dyes Pigm. 2017; 143, 463–469. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yao, Z.-Q.; Feng, R.; Sun, M.-H.; Shan, X.-T.; Su, Z.-H.; Li, W.; Bu, X.-H. A highly stable terbium metal-organic framework for efficient detection of picric acid in water. Chin. Chem. Lett. 2021, 32, 3095–3098. [Google Scholar] [CrossRef]
- Wang, Q.; Unno, M.; Liu, H. ; Silsesquioxane-based triphenylamine-linked fluorescent porous polymer for dyes a dsorption and nitro-aromatics detection. Materials 2021, 14, 3851. [Google Scholar] [CrossRef]
- Li, S.; Ouyang, T.; Guo, X.; Dong, W.; Ma, Z.; Fei, T. Tetraphenylethene-based cross-linked conjugated polymer nanoparticles for efficient detection of 2,4,6-trinitrophenol in aqueous phase. Materials 2023, 16, 6458. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Mahto, M.K.; Samanta, D.; Shaw, M.; Shaik, M.A.S.; Basu, R.; Mondal, I.; Bhattacharya, A.; Pathak, A. Blue-emissive nitrogen-doped carbon dots for picric acid detection: molecular fluorescence quenching mechanism. ACS Appl. Nano Mater. 2023, 6, 8059–8070. [Google Scholar] [CrossRef]
- Liu, X.; Lei, P.; Liu, X.; Li, Y.; Wang, Y.; Wang, L.; Zeng, Q.-D.; Liu, Y. From luminescent π-conjugated macrocycles to bridged multi-cyclic π-conjugated polymers: cyclic topology, aggregation-induced emission, and explosive sensing. Polym. Chem. 2023, 14, 2979–2986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




