1. Introduction
Traditional (or conventional) farming is large-scale intensive production of crops based on mechanical tillage, the use of chemical fertilizers, herbicides, fungicides and other synthetic inputs. Although traditional methods are economically beneficial, they have the potential to be destructive [
1]. Compared to traditional farming methods, organic farming has the potential to reduce the harmful impact of economic growth on the environment [
2]. Reducing or eliminating tillage, implementing crop rotation, switching to biological products instead of synthetic ones, eliminating the use of synthetic fertilizers in favor of organic fertilizers, and utilizing cover crops as a form of green manure are among the most common practices employed in organic farming [
3,
4,
5].
However, the attitude of scientists towards organic farming is not entirely clear. In the study by Hammond et al. (2020), it was shown that organic fertilizers can improve grain quality but do not necessarily increase grain yield or farm profits compared to synthetic fertilizers [
6]. The main factors in choosing land use are crop yield and farm profit. How to evaluate the practice of organic farming from an environmental point of view and from the point of view of sustainable agriculture? What assessment criteria and soil indicators will be important?
First of all, we need to consider the concept of soil health and the parameters that are indicators of healthy soil. The concept of soil health emerged in the early 2000s and has since become linked to the emerging "One Health" concept, which recognizes the interconnectedness of human, animal, and environmental health. The concept of soil health includes soil fertility, quality, health and security [
7]. In terms of comparing crop production methods (organic versus conventional), the focus is on soil quality, which is understood as “the capacity of a soil to function within ecosystem and land-use boundaries to sustain biological productivity, maintain environmental quality, and promote plant and animal health” [
8,
9].
The most commonly proposed soil quality indicators include over 50 different parameters [
10]. These indicators can be grouped into three categories: physicochemical, physical, and biological. The physicochemical indicators include soil organic carbon content, pH, available phosphorus and potassium, total nitrogen, and other factors. Physical indicators include aggregation, texture, soil depth, and other characteristics. Biological indicators include soil respiration, microbial biomass, nitrogen mineralization, and other biological processes [
10].
Existing studies on organic and conventional farming have shown that organic methods can lead to an increase in the amount and quality of soil organic carbon (SOC) and nutrients [
11]. This, in turn, improves the physical properties of the soil and increases water availability [
12]. There are also studies that have shown that the transition from traditional agriculture to no-till farming, an increase in the number of crop species in crop rotation [
13]. Using cover crops not only did not result in SOC accumulation, but it also demonstrated SOC loss [
14,
15,
16].
In recent years, a number of new indicators for soil quality have been proposed, most of them being biological in nature. Particular attention to the biota is also due to its sensitivity to even the slightest changes in environmental conditions [
10]. In addition, living organisms are an important and still little-studied component of soil. The development of research methods such as metagenomics sheds light on the structural features and functional role of soil microbiota. Thus, we come to the conclusion that it is necessary to approach the comparison of agricultural farming practices with a focus on assessing their biological indicators.
Modern molecular research methods make it possible to identify the taxonomic structure of the soil microbiome. Subsequent bioinformatics processing enables us to analyze the qualitative and quantitative taxonomic composition of the microbiome and its functional genes, which aids in reconstructing the metabolic pathways within the community [
17,
18].
Metagenomic approach is actively used in studies of soil microbiomes [
19]. Shotgun sequencing method allow to profile not only the taxonomic composition of the microbiome, but also the genes responsible for biosynthesis and their abundance. However, additional omics technologies must be used to accurately assess the actual activity of the microbiome. A bridge between the predicted potential of the soil microbiome and its actual activity can be created by combining two approaches: profiling of functional genes, which is relatively accessible, and assessment of microbiological activity in soils, including activity of extracellular enzymes that play a crucial role in nutrient bioconversion.
In this study, we compared the indicators of soil quality in Chernozem soils located in organic and conventional cropping systems. Using metagenomics, we explored the functional potential of the microbiome in converting carbon, nitrogen, phosphorus, and sulfur. We used microplate fluorescence enzyme assay to assess the actual capacity of these microbial communities to convert these essential nutrients.
2. Materials and Methods
2.1. Soil Sampling and Preparation
Conventional cropping system (CCS). Crop rotation: winter wheat - winter wheat - peas - winter wheat. Crop rotation has been carried out since 2003 (five full rotation cycles). The field was treated with mineral fertilizers: diamophoska NPK(S) 10:26:26(2): N – 10%, P2O5 – 26%, K2O – 26%, MgO – 0.3-1%, S – 2% - 100 kg/ha, ammonium nitrate – 150 kg/ha. Seeds were treated with fungicides “Strike forte” (flutriafol + tebuconazole, 75 + 225 g L−1). The coordinates of the field: 47.335583, 38.305931. Organic cropping system (OCS). Crop rotation: peas – rye – lentils – spelt. Crop rotation has been carried out since 2011 (three full rotation cycles). No fertilizers were applied. The collection of soil samples occurred at the end of the rotation – spelt. The coordinates of the field: 47.348807, 38.312871. The soil type studied is Chernozem (Vorony-Calcic chernozems, according to the WRB classification).
From each field (organic and conventional), soil was collected for the experiment using the "checkerboard" method (four points at the corners and one in the center) from the surface of 0-5 cm and from a depth of 5-15 cm, in 4-fold repetition (16 samples in total), from both organic and conventional fields.
2.2. Physico-Chemical Soil Analysis
The particle size distribution (soil texture) was determined by the Integral Suspension Pressure method (ISP) [
20]. Soil texture, pH of soil samples, total carbon (TC) and total nitrogen (TN) contents, soil organic carbon (SOC), extractable organic carbon (EOC) and nitrogen (EON), phosphates were determined as described in our previous work [
21].
2.3. Soil Enzyme Activities
Enzyme activity of β-D-1,4-Cellobiosidase (CBH, EC 3.2.1.91), β-1,4-Glucosidase (BG, EC 3.2.1.21), β-1,4-Xylosidase (βX, EC 3.2.1.37), β-1,4-N-acetyl-glucosaminidase (NAG, EC 3.2.1.30), L-leucine aminopeptidase (LAP, EC 3.4.11.1), acid phosphatase (AP, EC 3.1.3.2) and arylsulfatase A (Sulf, EC 3.1.6.1) were determined using fluorimetric microplate enzyme assays using fluorogenically labeled substrates [
22].
One gram of soil was mixed with 50 ml of sterile water (V, total volume of homogenate). The soil suspension was homogenized by ultrasound and shaken on a horizontal shaker. Acetate buffer (0.5 M) with pH 5.5 was prepared for substrates labeled with 4-Methylumbelliferone (4-MUB). TRIZMA buffer (0.05 M) was prepared for substrates labeled with 7-Amino-4-Methyl Coumarin (AMC).
Preparation of 0.05 M TRIZMA buffer: 0.985 g of THAM (Tris-hydroxymethyl-aminomethane) and 2.66 g of Tris-HCl (Tris(hydroxymethyl)aminomethane–hydrochloric acid) were placed in one 500 ml volumetric flask and distilled water was added until the volume was reached. Solutions of standards (4-MUB and AMC) and substrates were prepared at specific concentrations. 50 μl of homogenate (
v, ml) was added to a 96-well black plate. We dispensed soil homogenate with wide orifice tips to permit the transfer of suspended soil particles. Then, 50 μl of buffer (acetate or TRIZMA buffer) and 100 μl of standard (4-MUB or AMC) (Quench Standard) or 100 μl of substrate (Sample assay) were added to all wells. The final reaction volume per well is 200 µl. Required controls: Soil control (150 µl buffer, 50 µl soil homogenate), Substrate Control Fluorescence (100 µl substrate, 100 µl buffer (acetate or TRIZMA buffer), Reference Standard Fluorescence (100 µl 4-MUB (or AMC), 100 µl of buffer (acetate or TRIZMA buffer)). The fluorescence of solutions in microplates was measured at the moment of adding the substrate, after 180 min (incubation time, T, h). The excitation wavelength is 360 nm, and the emission wavelength is 450 nm. For each soil sample, we calculated 9 analytical replicates (microplate wells). Activity is calculated as follow [
23]:
V ("ml") – total volume of homogenate, 50 ml for this experiment;
v("ml") – volume of homogenate in one incubation well, 0.05 ml for this experiment;
T("h") – incubation time, h;
DM("g") – mass of dry soil corresponding to 1 g of fresh soil.
2.4. Metagenomic Sequencing
Metagenomic sequencing was performed using HiSeq X Ten Illumina platform and a 2x150 bp paired-end sequencing reagent kit. Preparing reads for metagenome assembly: checking the quality of received reads using FastQC v.0.11.9 (
https://www.bioinformatics.babraham.ac.uk/projects/fastqc); clearing reads from artificial sequences and nucleotides with poor read quality using Trimmomatic v.0.39 [
24]; checking the quality of cleaned reads using FastQC. Sequencing of 16 DNA samples yielded a total of 700,829,024 paired reads. After purification, 407,386,189 paired readings were used for subsequent analysis. Reads filtered by quality were assembled de novo using metaSPAdes v. 3.15.0 using default options [
25].
The quality of the resulting metagenomic assemblies was assessed using QUAST v. 5.0 [
26]. The resulting assemblies had a length from 144.3 to 202.9 Mb, their GC composition varied from 62.7 to 65.05%. The protein functional annotation was performed using the domain-based annotation tool reCOGnizer (version 1.9.2) (
https://anaconda.org/bioconda/recognizer). The results derived from the clusters of orthologous groups of proteins (COGs) database [
27] and Pfam domens [
28] were used for functional categorization.
2.5. Statistical Processing
The obtained results were statistically manipulated using Origin 2021 (OriginLab Corporation, Northampton, MA, USA) software. The Shapiro–Wilk test was used to assess the normal distribution of values. In the presence of a normal distribution, the two-sample t-test was used, whereas if normality was rejected, the Mann–Whitney test was used. Differences were considered significant at p-values < 0.05.
To assess the contribution of factors (cropping system, soil depth) to enzyme activity, we implemented two-way PERMANOVA based on the Bray-Curtis similarity and 9999 permutations using Past v.4.06b [
29].
The coefficient of concordance (k) between predicted and measured enzyme activity was calculated based on the differences between the studied cropping systems in terms of soil enzyme activity or the functional potential of the soil microbiome. To do this, we expressed the difference between the compared groups through a fold change value (Log(FC)) using formula:
Log(FC) = (mean(log2(Group1))) /(mean(log2(Group2)))
Where,
Group 1 – this is enzyme activity (or gene relative abundance) in the OCS soils.
Group 1 - this is enzyme activity (or gene relative abundance) in the CCS soils.
Next, we calculated the k-coefficient of concordance using formula:
Where,
Log(FC) 1 – the difference in the measured enzyme activity between two cropping systems
Log(FC) 2 – the difference in the functional gene abundance between two cropping systems
3. Results
3.1. Soil Chemical Properties
During the transition to an organic cropping system, an increase in pH values is noted, but significant differences were observed only in the pHKCl variant for the 0–5 cm layer (
р - 0.02) (
Table S1). Soils of organic and traditional cropping system are neutral in pHKCl, and slightly alkaline in pHH
2O. The soil granulometric composition is Silty clay loam. The supply of soils with mobile forms of phosphorus varies. A higher content of available P was recorded in the top layer of soil compared to the samples from 5-15 cm. A significantly high content of available phosphorus was found in the OCS soils, which was 4.8 times higher than the average content (
p < 0.001). Additionally, a significant increase in the total carbon and nitrogen content was revealed for the subsoil (5-15 cm) layer of the CCS soils, with an increase of 1.6 and 3.5 times respectively (
p - 0.04 and
p - 0.01). There were no significant differences in the top (0-5 cm) soil layers between the two cropping systems in terms of TC and TN content (
p-values of 0.36 and 0.84, respectively). Inorganic carbon in organic soils accounts for a very small percentage of the total, averaging 3.5%. In contrast, SOC accounts for 97.8% in the top soil and 95.3% in the subsoil layers. In CCS, the SOC content was highest in the top layer, and in the subsoil layer its share of TC accounted for only 58.7%. There were no significant differences in the levels of SOC and EOC between the two cropping systems. However, the EON levels in the CCS soils were higher (on average, 4.5 times higher) than in the OCS soils, as shown in
Table S1 (
p < 0.01).
3.2. Predictable Functional Potential of Soils
A comparison of the functional potential of the two cropping systems showed that the main functional categories were significantly enriched in CCS compared to OCS (
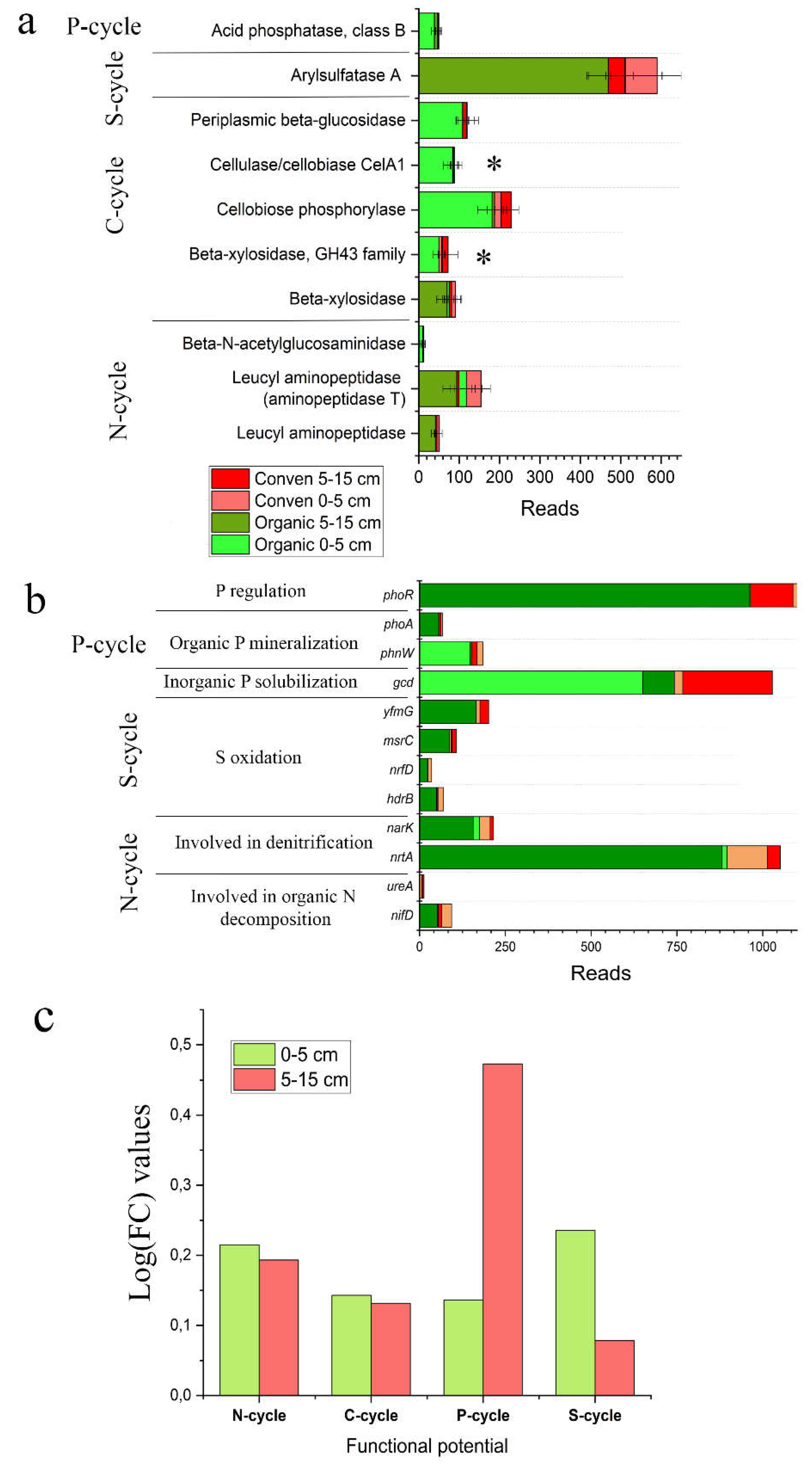
Figure S1). To better understand the distribution and abundance of functional genes involved in the cycling of carbon (C), phosphorus (P), nitrogen (N), and sulfur (S) within cropping systems, we analyzed the functional composition of microbial communities (
Figure 1). Specifically, we focused on the relative abundance of functional genes whose products are enzymes, the activity of which was measured in this study (
Figure 2).
C-cycling. We found that all soil samples contained the genes associated with the following enzymes: periplasmic beta-glucosidase (COG1472), beta-xylosidase (COGs 3507, 3664, 3940), cellobiose phosphorylase (COG 3459), сellulase/cellobiase CelA1 (COG 5297). Analysis of the representation of these genes in different comparison groups revealed that beta-xylosidase (COG 3664) и cellobiose phosphorylase (COG 3459) were significantly in greater abundance in the subsoil of CCS compared to OCS (
Figure 1 a). There were no differences between the two cropping systems for the remaining genes.
In addition to these genes, we also assessed differences between the cropping systems for the remaining genes included in category “Carbohydrate transport and metabolism”. We found significant differences between two studied cropping systems in the representation of 36 genes (out of 253 in total), with 94.45% of these genes being more prevalent in CCS soils (
Figure S2).
N-cycling. We have identified 23 genes whose products play a role in microbial nitrogen cycling processes (
Figure S3), and one gene whose product associated with beta-N-acetylglucosaminidase (COG 4193) (
Figure 1a). Among these genes, four of them showed a significant difference in representation between the comparison groups. The
nifD and
ureA genes determine the ability of the microbiome to decompose organic N. The abundance of the
nifD gene was greater in the top and subsoil layers of CCS soil compared to OCS. While in OCS, the abundance of these genes is greater in the subsoil layer compared to the top layer (
Figure 1b). The
ureA gene was identified in metagenomes in minor quantities with a predominance in CCS soils. Genes involved in denitrification were dominant in their abundance in the N-cycle gene pool. The abundance of the
nrtA and
narK genes was greater in CCS soils compared to OCS (
Figure 1 b). There was no significant difference in the abundance of the beta-N-acetylglucosaminidase gene between the two cropping systems (
Figure 1a).
S-cycling. A total of 12 genes were found whose products are associated with sulfur conversion (
Figure S2), among which 4 genes had significantly different representations in the microbiomes of the compared farming systems. The
hdrB and
nrfD genes were minor and their representation was different in the upper layers of the compared systems with a predominance in the CCS. The
msrC and
yfmG genes were dominant in the subsoil layer of the CCS compared to the OCS (
Figure 1b). The genes encoding arylsulfatase A (COG3119) was found in both cropping systems, but there was no difference in abundance between them (
Figure 1a).
P-cycling. In total were found 12 genes which are involved in the microbial turnover of soil P (
Figure S2). Among them, 4 genes were differently represented between cropping systems with predominance in CCS compared to OCS (
Figure 1b). These genes are associated with inorganic P solublization (
gcd), organic P mineralization (
phnW, phoA) and regulation (
phoR) [
30]. The gene encoded for acid phosphatase class B (COG3700) was found to be present in similar abundances in both cropping systems (
Figure 1a).
We expressed the difference between the two cropping systems in terms of log(FC) values, which is commonly used to quantify the differences between two groups being compared. It turned out that, based on the composition of functional genes involved in the cycling of elements, CCS soils had a greater potential for bioconversion of nutrients, but especially nitrogen and phosphorus (
Figure 1с).
Thus, a comparative analysis of functional genes showed that significant differences in their abundance were found for 25% of the total identified number. Regarding the differences between the CCS and OCS systems in terms of their functional potential for the bioconversion of phosphorus, nitrogen, and sulfur, the CCS system exhibited a higher potential.
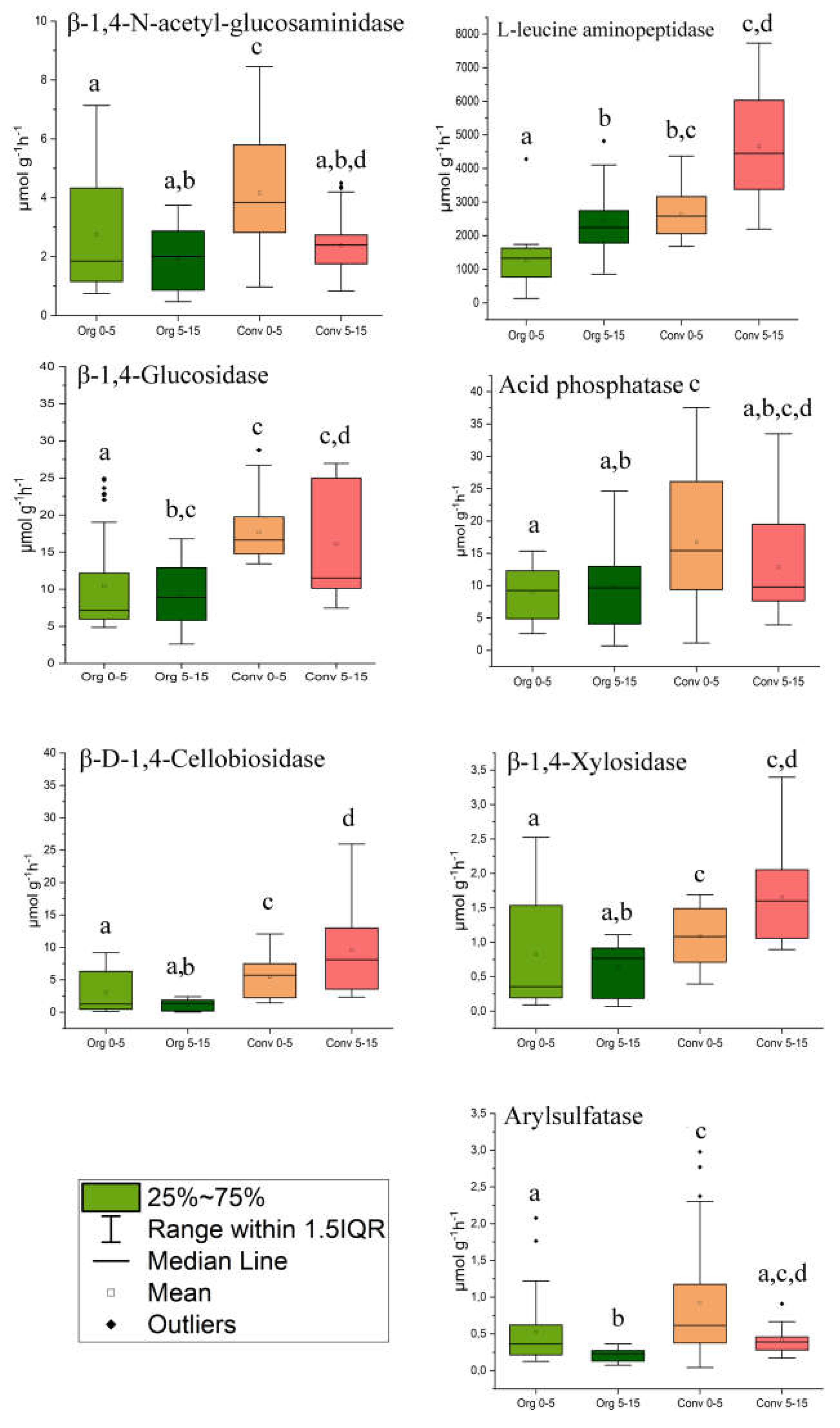
3.3. Measured Soil Enzyme Activities
Enzymes of the nitrogen cycle. NAG activity was significantly higher in CCS soils of the top layer compared to other comparison groups, where the activity of this enzyme was at the same level (
Figure 2). In the OCS soils, LAP activity was significantly higher in the subsoil layer (5-15 cm) compared to the top layer. In the CCS soils, enzyme activity did not differ between layers. When comparing two farming systems, LAP activity was higher in the CCS soils (
Figure 2).
Carbon cycle enzymes. The activity of BG differed to a lesser extent in the OCS samples from the topsoils, while in the remaining samples, the enzyme activity did not differ significantly. The activity of CBH between soils from different depths was not different in the OCS group. |In the CCS group, the activity of this enzyme increased with depth and was generally higher than the activity in the OCS soils. CBH activity in the OCS soils did not vary with depth and was lower compared to the CCS soils (
Figure 2).
Enzymes of the phosphorus and sulfur cycle. AP enzymatic activity in the OCS soils did not depend on the soil depth. In turn, the top layer of soil CCS differed in activity from OCS samples to a greater extent. Arylsulfatase A activity was found to be higher in the topsoil samples. When comparing two types of crop systems, the arylsulfatase A activity was observed to be greater in the topsoils of CCS compared to OCS (
Figure 2).
Performed two-way PERMANOVA showed that soil cropping system was the main (F=14.978,
p-0.009) variable determining enzymatic activity of soils followed by soil depth (F=9.6079,
p-0.006) (
Table S2).
3.4. Agreement and Discrepancy between Measured and Predicted Enzymatic Activity
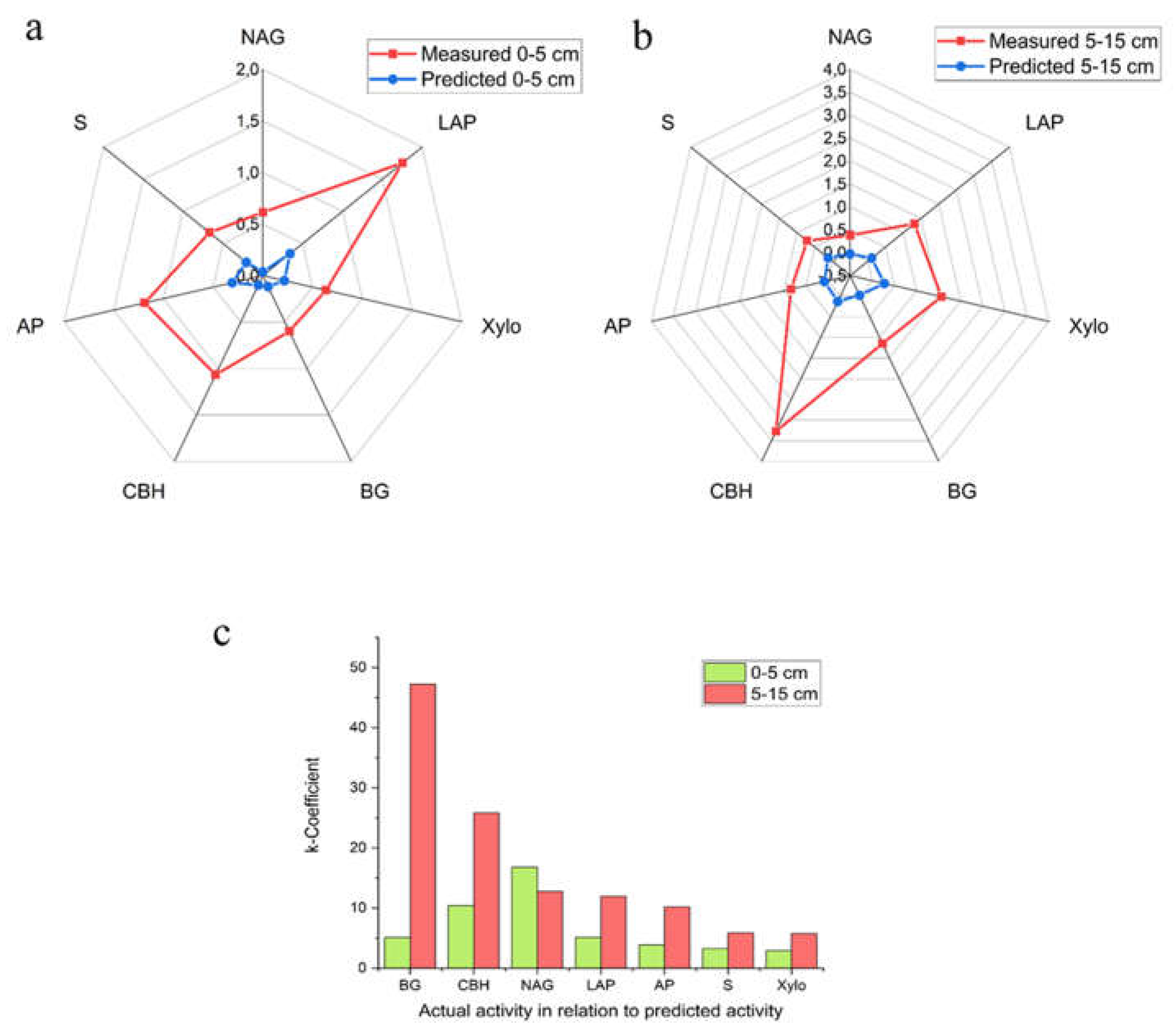
We compared two approaches to assessing the functional state of the soil environment, one of which is profiling functional genes, and the second is measuring the actual content of certain enzymes. The presence of two different cropping systems in this study allowed us to correlate and compare the predicted and measured approaches based on the severity of functional differences in the soils of these two fields.
It was found that the activity of all enzymes measured was higher in CCS soils compared to OCS soils. The following pattern is observed in the ranking of enzymes according to their log(FC) values. In the topsoils, enzyme activity measurements showed that the maximum differences between the cropping systems were for the LAP (log(FC) +1.75), and the minimum for BG (log(FC) +0.59). Profiling of functional genes resulted in following values: the maximum difference was in the LAP (log(FC) +0.34), and minimum difference was in NAG (log(FC) +0.036). For the subsoils, the following results from the comparison are: the maximum difference was observed in CBH (log(FC) +3.27). The minimum difference was found in NAG (+0.38). In terms of gene profiling, the maximum difference between systems was observed for Xylo (log(FC) +0.27), while the minimum difference was for NAG (log(FC) +0.029).
Thus, the predicted and measured enzyme activity have both concordance and discordance. In particular, if we rank the activity of enzymes based on their log(FC) values and build a series, we can see some qualitative similarities between the two assessment methods. In particular, in the case of LAP, AP and NAG these methods coincided. However, if we compare the two methods based on the absolute value of log(FC), the predicted method characterized the differences between the cropping systems as being minimal (log(FC) +0.34 to +0.036), whereas the measured activity method characterized the differences as being significant (log(FC) +1.75 to +0.59).
We quantified the similarities and differences between the predicted functional potential and measured enzymatic activity through the k-coefficient (
Figure 3c). We found that, firstly, there is no consensus between them, that is, each of the approaches characterizes the differences between cropping systems in terms of enzyme activity to varying degrees.
Second, it turned out that the similarity or difference between predicted activity and measured activity depended on the soil sampling depth (0-5 cm vs 5-15 cm). A high k-coefficient (discordance) was calculated for CBH and NAG in the topsoils, and BG and CBH in the subsoils (
Figure 3c).
4. Discussion
"Sustainable" organic farming, as opposed to traditional farming, aims to find alternative tools and techniques that are economically viable and have less potential for environmental harm [
31]. The cultivation of fields and the application of fertilizers, or their absence, significantly affect the transformation and movement of carbon, nitrogen, and phosphorus in soils.
Soil extracellular enzymes produced by plant roots and microorganisms act as biological catalysts. They are not only crucial for microbial nutrient uptake and soil element balance, but they are also essential indicators of soil nutrient limitations and microbial energy metabolism. Farming practices play a significant role in driving soil enzyme activity [
32,
33] and this activity significantly alters the structure and chemical composition of the soil [
34].
Numerous studies have been aimed at finding out how land cultivation and crop management practices affect such key indicators of soil quality as microbiota activity. It is not worth expecting to receive the final conclusion soon, since such studies were and are being conducted on different types of soils, with different textures, aggregations, mineralogy, in different climatic zones, with different initial conditions of the fields. This can explain the often-opposite results. For example, a comparison of two farming systems showed that enzyme activity in Haplic Chernozem soils was higher in the conventional farming system compared to the organic one [
34]. A similar result was obtained in the work Arcand et al. [
35] studied Dark Brown Chernozem and observed increased activity and production of enzymes in conventional farming soil compared to that in organic farming soil. Another study of Humic and Rhodic Nitisol soils revealed enhanced capacity for nitrification and nitrous oxide reduction in organic cropping system compared to conventional [
36]. There are also results that show no significant differences between conventional and organic soils (Eutric Cambisols) in terms of soil respiration, organic matter, nitrogen, and humidity [
37].
Key parameters that characterize the quality and health of soil include SOC, available nitrogen, phosphorus, potassium, and microbial activity. Microbial activity includes various indicators, such as enzymatic activity. In our study, we found significant differences between the two systems in terms of the content of nitrogen. The conventional system had a higher level of nitrogen, which we explained by the use of nitrogen-containing fertilizers. SOC did not differ significantly between organic and conventional cropping systems. Interestingly, the available phosphorus content in the soil of the organic system was 5 times higher than that of the conventional system.
Enzyme activity is a reflection of the qualitative and quantitative composition of the soil microbiome. In our previous work, we compared the soil bacterial communities of the two types of agriculture under study. Results from our previous study of the microbiome of these systems showed no significant differences in bacterial alpha biodiversity. However, the relative abundance of six (from nine identified) dominant phyla was significantly differ between the compared groups. Differences between the systems at the genera level affected 14 genera in the topsoils (0-5 cm) and 53 genera in the subsoils (5-15 cm) [
21].
It is difficult to say how differences in the representation of specific taxonomic groups are reflected in functional differences in microbiomes. To help with this, there are several bioinformatic tools available. For example, Tax4Fun [
38], PanFP [
39], and PicRUST [
40] can be used to predict functionality based on community structural data and reference genomes. These tools are in great demand in the study of soil microbial ecology [
41]. However, recent studies have shown the shortcomings of these tools. In the study, Toole et al (2020) compared the results of functional genome profiling using shotgun and prediction tools. The authors showed that PICRUSt2 and Tax4Fun2 significantly underestimated gene frequencies in select pathways [
42]. Recently, a study was conducted to verify the predictions of Tax4Fun and PanFP using instrumental measurements. The results showed that these prediction tools reflect functional potential qualitatively, but not quantitatively [
34].
In our study, we compared the results of functional gene profiling with instrumental measurement of soil enzyme activity. The functional gene profiling results showed that the conventional soil community as a whole had a greater potential for converting carbon, nitrogen, phosphorus, and sulfur. On the one hand, the soil samples collected from organic and conventional systems did not differ qualitatively, as they had the same set of functional genes. However, on the other hand, there were quantitative differences in the representation of these genes. In particular, the greatest difference was in the abundance of genes involved in all processes that determine P-cycling [
43], genes involved in organic N decomposition. Measurement of enzyme activity revealed that in the conventionally farmed soils, the activity of most enzymes was higher compared to the organically farmed soils. In particular, the activities of LAP, NAG, BG, Xylo, and CBH differed between the two cropping systems, while the activity of AP did not differ.
5. Conclusions
Thus, the results of the OCS vs CCS comparison suggest that functional gene profiling and enzymatic activity measurements both assess qualitative differences between these systems in a similar way, but assess quantitative differences differently. Indeed, the set of genes and their abundance is a potential activity that is conditional and depends on many variables. However, the measured enzyme activity also reflects the state of the soil system at a specific point in time, which is when the sample was taken. Apparently, a combination of these two methods for assessing soil condition is promising. In general, the study conducted showed that for the past 20 years, the practice of using chemical pesticides and mineral fertilizers in conventional farming has not only failed to lead to a decrease in activity of microbial community compared to organic farming, but it has also significantly increased the functional potential and microbiological activity of soil microbiomes for nutrient conversion. It is likely that the type of soil (Chernozem) contributes to a microbial community's greater resistance to agricultural practices used in conventional farming systems, compared to other types of soil. However, this is a hypothesis that requires further study.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Comparison of the functional potential of soil microbiomes in the organic and conventional cropping systems based on identified COG functional categories; Figure S2: Relative abundances of COG related with Carbohydrate transport and metabolism. Figure S3: The heatmap of abundance of the genes involved in P,N,S-cycles. Table S1. General soil chemical and physical properties of the organic and conventional cropping systems. Table S2. Two-way PERMANOVA based on Bray-Curtis similarity output of the effects of cropping systems, sampling depth and their interactions on enzymatic activity of soils.
Author Contributions
The following statements should be used “Conceptualization, A.S.V.; methodology, A.V.T.; formal analysis, A.S.V., A.V.T.; investigation, D.V.P., A.A.S., A.V.I.; resources, A.S.V.; data curation, A.V.T., A.S.V.; writing—original draft preparation, A.V.T., A.S.V.; writing—review and editing, A.S.V.; visualization, A.S.V.; supervision, A.S.V.; funding acquisition, A.S.V. All authors have read and agreed to the published version of the manuscript.”.
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (agreement № 075-15-2024-563).
Data Availability Statement
Data available upon request.
Conflicts of Interest
“The authors declare no conflicts of interest.”.
References
- Aytenew, M. Soil Biodiversity as a Key Sponsor of Regenerative Agriculture. Biodiversity of Ecosystems 2021. [CrossRef]
- Benbrook, C., Kegley, S., & Baker, B. Organic farming lessens reliance on pesticides and promotes public health by lowering dietary risks. Agronomy 2021, 11(7), 1266. [CrossRef]
- Pearsons, K.A., Omondi, E.C., Zinati, G., Smith, A., & Rui, Y. A tale of two systems: Does reducing tillage affect soil health differently in long-term, side-by-side conventional and organic agricultural systems? Soil and Tillage Research 2023, 226, 105562. [CrossRef]
- Rhodes, C.J. The imperative for regenerative agriculture. Science progress 2017, 100(1), 80–129. 10.3184/003685017X14876775256165.
- Soto, R.L., Martinez-Mena, M., Padilla, M.C., & de Vente, J. Restoring soil quality of woody agroecosystems in Mediterranean drylands through regenerative agriculture. Agriculture, Ecosystems & Environment 2021, 306, 107191. [CrossRef]
- Hammad, H.M., Khaliq, A., Abbas, F., Farhad, W., Fahad, S., Aslam, M., … Bakhat, H.F. Comparative Effects of Organic and Inorganic Fertilizers on Soil Organic Carbon and Wheat Productivity under Arid Region. Communications in Soil Science and Plant Analysis 2020, 1–17. [CrossRef]
- Lehmann, J., Bossio, D.A., Kögel-Knabner, I., & Rillig, M.C.The concept and future prospects of soil health. Nature Reviews Earth & Environment 2020, 1 (10), 544–553. [CrossRef]
- Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., & Haynes, R.J. Defining soil quality for a sustainable environment. Madison: Soil Science Society of America, 1994, pp. 3–21.
- Davidson, D.A. Soil quality assessment: recent advances and controversies. Progress in Environmental Science 2000, 2(4)(4), pp. 342–350.
- Bünemann, E.K., Bongiorno, G., Bai, Z., Creamer, R.E., De Deyn, G., De Goede, R., ... & Brussaard, L. Soil quality–A critical review. Soil biology and biochemistry 2018, 120, 105–125. [CrossRef]
- Jian, J., Du, X., Reiter, M.S., & Stewart, R.D. A meta-analysis of global cropland soil carbon changes due to cover cropping. Soil Biology and Biochemistry 2020, 143, 107735. [CrossRef]
- Lal, R. Soil quality impacts of residue removal for bioethanol production. Soil and Tillage Research 2009, 102(2), 233–241. [CrossRef]
- Luo, Z., Wang, E., and Sun, O.J. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agriculture, ecosystems & environment 2010, 139(1-2), 224–231. [CrossRef]
- Bandick, A.K., & Dick, R.P. Field management effects on soil enzyme activities. Soil biology and biochemistry 1999, 31(11), 1471–1479. [CrossRef]
- Idowu, O.J., Van Es, H.M., Abawi, G.S., Wolfe, D.W., Schindelbeck, R.R., Moebius-Clune, B.N., & Gugino, B.K. Use of an integrative soil health test for evaluation of soil management impacts. Renewable Agriculture and Food Systems 2009, 24(3), 214–224. [CrossRef]
- Ndiaye, E.L., Sandeno, J.M., McGrath, D., & Dick, R.P. Integrative biological indicators for detecting change in soil quality. American Journal of Alternative Agriculture 2000, 15(1), 26–36. [CrossRef]
- Lian, W.H., Mohamad, O.A.A., Dong, L., Zhang, L.Y., Wang, D., Liu, L., Han, M.X., Li, S., Wang, S., Antunes, A., Fang, B.Z., Jiao, J.Y., Li W.J. Culturomics-and metagenomics-based insights into the microbial community and function of rhizosphere soils in Sinai desert farming systems. Environ Microbiome 2023, 18(1), 4. [CrossRef]
- Garg, D., Patel, N., Rawat, A., & Rosado, A.S. Cutting edge tools in the field of soil microbiology. Current Research in Microbial Sciences 2024, 100226. [CrossRef]
- Semenov, M.V. Metabarcoding and metagenomics in soil ecology research: achievements, challenges, and prospects. Biology Bulletin Reviews 2021, 11(1), 40–53. [CrossRef]
- Durner, W., Iden, S.C., & von Unold, G. The integral suspension pressure method (ISP) for precise particle-size analysis by gravitational sedimentation. Water Resources Research 2017, 53(1), 33–48. [CrossRef]
- Vasilchenko A.S., Burlakov E.O., Poshvina D.V., Gruzdev D.S., Kravchenko S.V., Iashnikov A.V., Ning Ling, Vasilchenko A.V. The effect of long-term application of nitrogen-rich fertilizers on soil resistome: A study of conventional and organic cropping systems. Soil Ecology Letters 2024, 6(3), 230215 . [CrossRef]
- Razavi, B.S., Blagodatskaya, E., & Kuzyakov, Y. Nonlinear temperature sensitivity of enzyme kinetics explains canceling effect—a case study on loamy haplic Luvisol. Frontiers in Microbiology 2015, 6, 1126. [CrossRef]
- German, D.P., Weintraub, M.N., Grandy, A.S., Lauber, C.L., Rinkes, Z.L., & Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biology and Biochemistry 2011, 43(7), 1387–1397. [CrossRef]
- Bolger, A.M., Lohse, M., and Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30(15), 2114–2120. [CrossRef]
- Nurk, S., Meleshko, D., Korobeynikov, A., & Pevzner, P.A. metaSPAdes: a new versatile metagenomic assembler. Genome research 2017, 27(5), 824–834. [CrossRef]
- Gurevich, A., Saveliev, V., Vyahhi, N., and Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 2013, 29(8), 1072–1075. [CrossRef]
- Galperin, M.Y., Makarova, K.S., Wolf, Y.I., and Koonin, E.V., 2014. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Research43, D261–D269.
- Finn, R.D., Coggill, P., Eberhardt, R.Y., Eddy, S.R., Mistry, J., Mitchell, A.L., Potter, S.C., Punta, M., Qureshi, M., Sangrador-.
- Vegas, A., Salazar, G.A., Tate, J., Bateman, A., 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research 44, D279–D285.
- Hammer, Ø., Harper, D.A.T., Ryan, P.D. Past: paleontological statistics software package for educaton and data anlysis. Palaeontologia electronica 2001, 4(1), 1.
- Li, Y., Wang, J., He, L., Xu, X., Wang, J., Ren, C., ... & Zhao, F. Different mechanisms driving increasing abundance of microbial phosphorus cycling gene groups along an elevational gradient. Iscience 2022, 25(10), 105170. [CrossRef]
- Hue, N.V., & Silva, J.A. Organic soil amendments for sustainable agriculture: organic sources of nitrogen, phosphorus, and potassium. Plant nutrient management in Hawaii’s soils, approaches for tropical and subtropical agriculture. College of Tropical Agriculture and Human Resources, University of Hawaii, Manoa, 2000, 133–144.
- Kandeler, E., Tscherko, D., & Spiegel, H. Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biology and fertility of soils 1999, 28, 343–351doi: 10.1007/s003740050502.
- Mijangos, I., Pérez, R., Albizu, I., & Garbisu, C. Effects of fertilization and tillage on soil biological parameters. Enzyme and Microbial Technology 2006, 40(1), 100–106. [CrossRef]
- Breitkreuz C, Heintz-Buschart A, Buscot F, Wahdan SFM, Tarkka M, Reitz T. 2021. Can we estimate functionality of soil Breitkreuz, C., Heintz-Buschart, A., Buscot, F., Wahdan, S.F.M., Tarkka, M., & Reitz, T. Can we estimate functionality of soil microbial communities from structure-derived predictions? A reality test in agricultural soils. Microbiology Spectrum 2021, 9(1), 10-1128. [CrossRef]
- Arcand, M.M., Helgason, B.L., & Lemke, R.L. Microbial crop residue decomposition dynamics in organic and conventionally managed soils. Applied Soil Ecology 2016, 107, 347–359. [CrossRef]
- Krause, H.M., Ono-Raphel, J.G., Karanja, E., Matheri, F., Lori, M., Cifuentes, Y., ... & Mäder, P. Organic and conventional farming systems shape soil bacterial community composition in tropical arable farming. Applied Soil Ecology 2023, 191, 105054. [CrossRef]
- Mátyás, B., Andrade, M.E.C., Chida, N.C.Y., Velasco, C.M.T., Morales, D.E.G., Montero, G.N.M., ... & Acevedo, R.X.L.. Comparing organic versus conventional soil management on soil respiration. F1000Research 2018, 7. [CrossRef]
- Wemheuer, F., Taylor, J.A., Daniel, R., Johnston, E., Meinicke, P., Thomas, T., & Wemheuer, B. Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environmental Microbiome 2020, 15, 1–12. [CrossRef]
- Jun, S.R., Robeson, M.S., Hauser, L.J., Schadt, C.W., & Gorin, A.A. PanFP: pangenome-based functional profiles for microbial communities. BMC research notes 2015, 8, 1–7. [CrossRef]
- Douglas, G.M., Maffei, V.J., Zaneveld, J.R., Yurgel, S. N., Brown, J.R., Taylor, C.M., ... & Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nature biotechnology 2020, 38(6), 685–688. [CrossRef]
- Dubey, R.K., Tripathi, V., Prabha, R., Chaurasia, R., Singh, D.P., Rao, C.S., ... & Abhilash, P.C. Bioinformatics Tools for Soil Microbiome Analysis. Unravelling the Soil Microbiome: Perspectives for Environmental Sustainability 2020, 61–70. [CrossRef]
- Toole, D.R., Zhao, J., Martens-Habbena, W., Strauss, S.L. Bacterial functional prediction tools detect but underestimate metabolic diversity compared to shotgun metagenomics in southwest Florida soils. Applied Soil Ecology 2021, 168, 104129/ . [CrossRef]
- Li Y., Wang J., He L., Xu X., Wang J., Ren C., ... & Zhao, F. Different mechanisms driving increasing abundance of microbial phosphorus cycling gene groups along an elevational gradient. iScience 2022, 25(10). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).