Submitted:
09 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
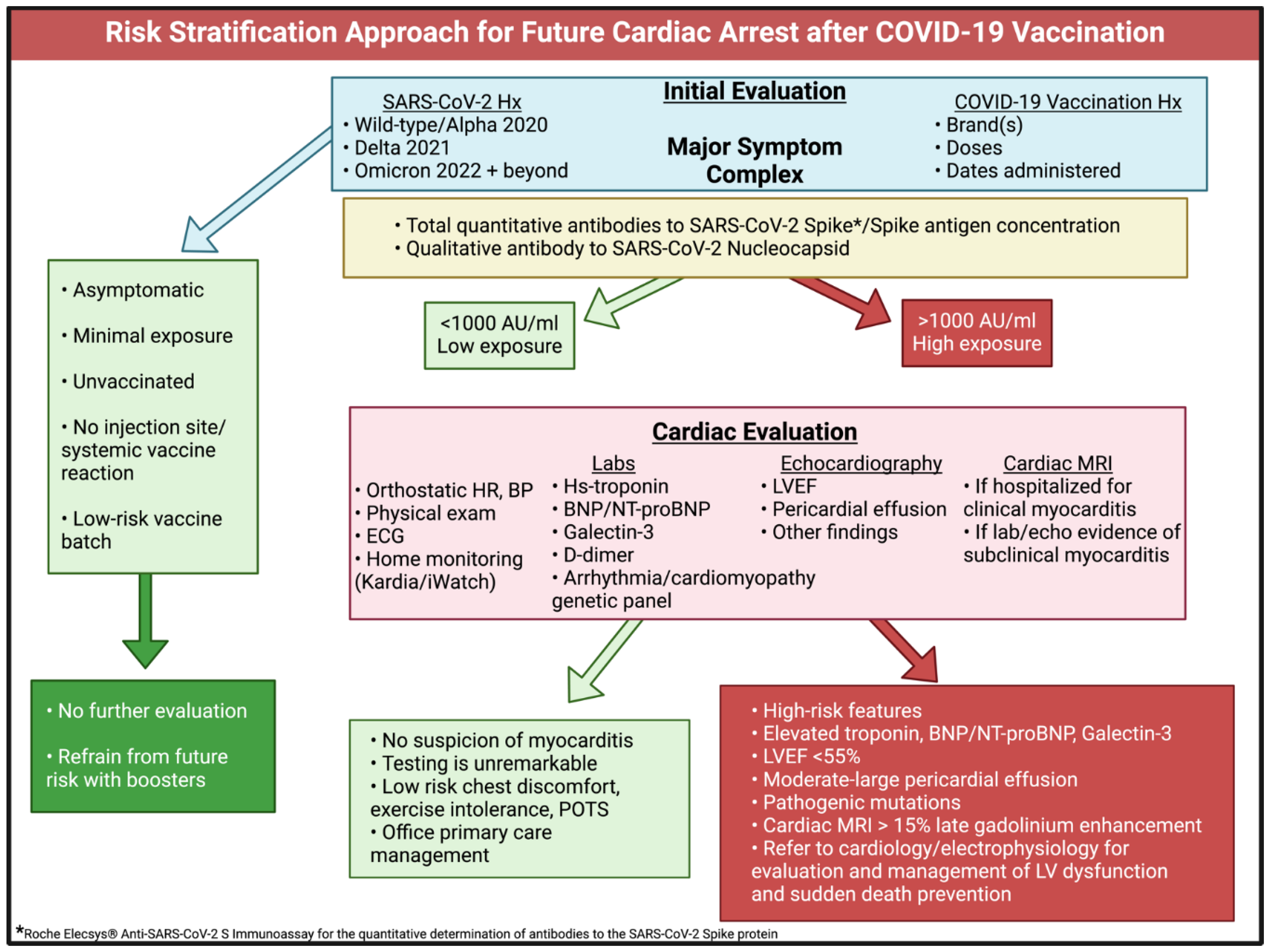
Rationale for Risk Stratification
Risk Stratification Approach
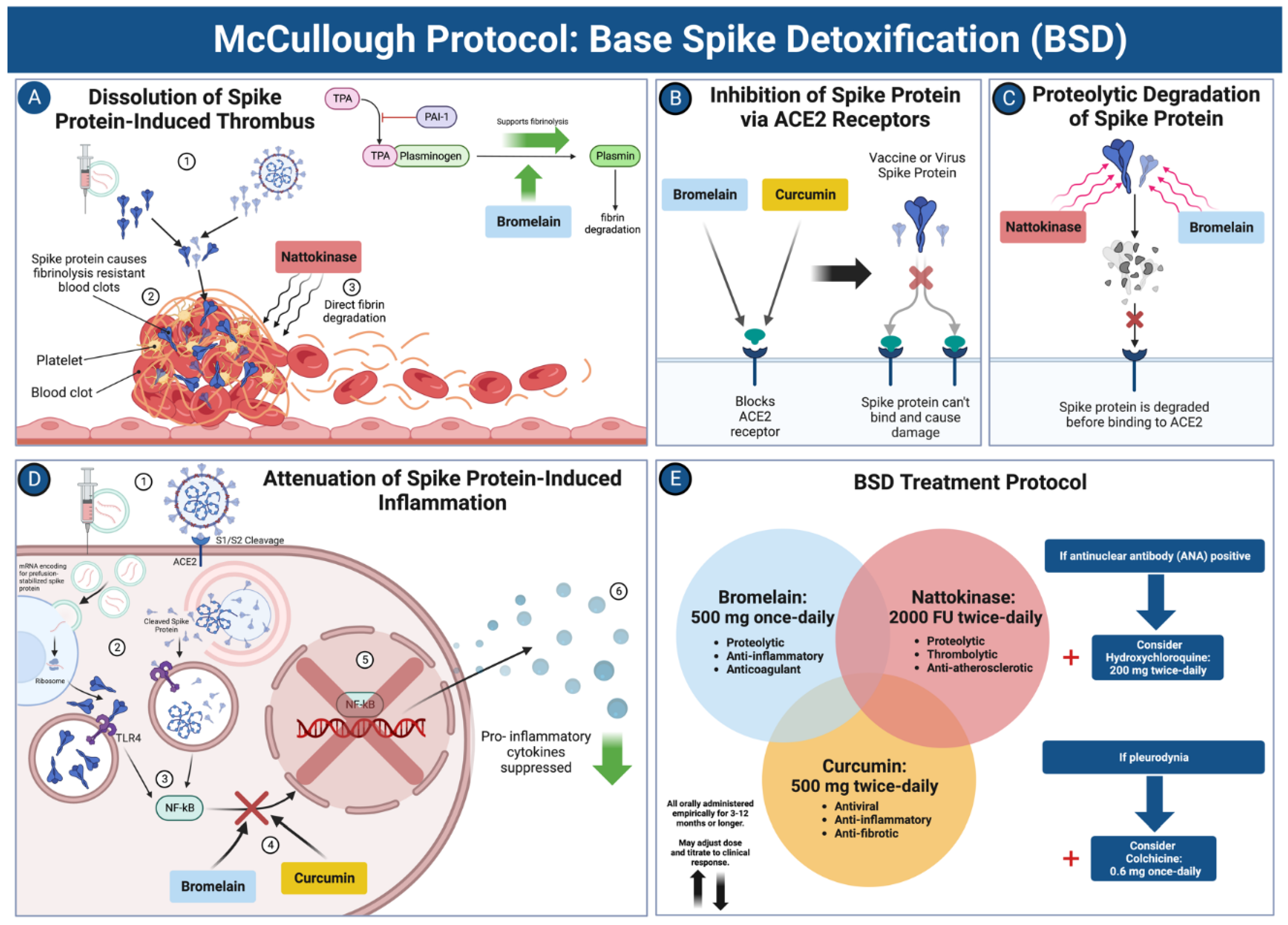
Conclusions
Acknowledgments
Conflicts of Interest
Data Availability Statement
Ethics statement
Author Contributions
Funding
References
- Hulscher, N.; Cook, M.; Stricker, R.; McCullough, P. A. Excess Cardiopulmonary Arrest and Mortality after COVID-19 Vaccination in King County, Washington. Preprints 2024, 2024051665. [CrossRef]
- Li YE, Wang S, Reiter RJ, Ren J. Clinical cardiovascular emergencies and the cellular basis of COVID-19 vaccination: from dream to reality?. Int J Infect Dis. 2022;124:1-10. [CrossRef]
- Sun CLF, Jaffe E, Levi R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave [published correction appears in Sci Rep. 2023 Aug 15;13(1):13276. doi: 10.1038/s41598-023-40234-1]. Sci Rep. 2022;12(1):6978. Published 2022 Apr 28. [CrossRef]
- Sadiq W, Waleed MS, Suen P, Chalhoub MN. Cardiopulmonary Arrest After COVID-19 Vaccination: A Case Report. Cureus. 2022;14(1):e21141. Published 2022 Jan 12. [CrossRef]
- Maruyama T, Uesako H. Lessons Learnt from Case Series of Out-of-hospital Cardiac Arrest and Unexpected Death after COVID-19 Vaccination. Intern Med. 2023;62(22):3267-3275. [CrossRef]
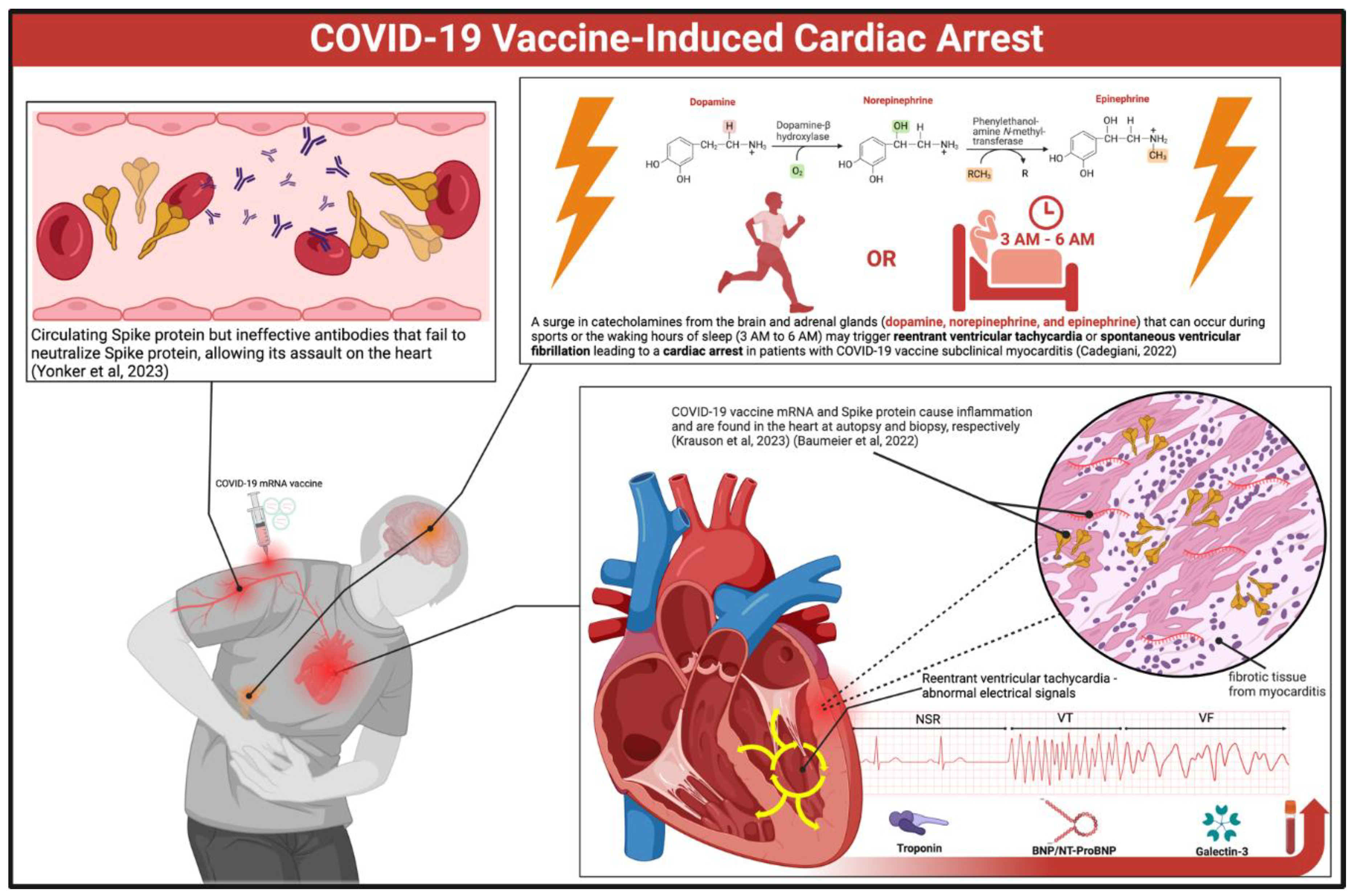
- Krauson AJ, Casimero FVC, Siddiquee Z, Stone JR. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines. 2023;8(1):141. Published 2023 Sep 27. [CrossRef]
- Baumeier C, Aleshcheva G, Harms D, et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int J Mol Sci. 2022;23(13):6940. Published 2022 Jun 22. [CrossRef]
- Yonker LM, Swank Z, Bartsch YC, et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147(11):867-876. [CrossRef]
- Nakahara T, Iwabuchi Y, Miyazawa R, et al. Assessment of Myocardial 18F-FDG Uptake at PET/CT in Asymptomatic SARS-CoV-2-vaccinated and Nonvaccinated Patients. Radiology. 2023;308(3):e230743. [CrossRef]
- Scheim DE, Vottero P, Santin AD, Hirsh AG. Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19. Int J Mol Sci. 2023;24(23):17039. Published 2023 Dec 1. [CrossRef]
- Vähätalo JH, Huikuri HV, Holmström LTA, et al. Association of Silent Myocardial Infarction and Sudden Cardiac Death. JAMA Cardiol. 2019;4(8):796-802. [CrossRef]
- Cadegiani FA. Catecholamines Are the Key Trigger of COVID-19 mRNA Vaccine-Induced Myocarditis: A Compelling Hypothesis Supported by Epidemiological, Anatomopathological, Molecular, and Physiological Findings. Cureus. 2022;14(8):e27883. Published 2022 Aug 11. [CrossRef]
- Polykretis P, McCullough PA. Rational harm-benefit assessments by age group are required for continued COVID-19 vaccination. Scand J Immunol. 2023;98(1):e13242. [CrossRef]
- Alessandria M, Malatesta GM, Berrino F, Donzelli A. A Critical Analysis of All-Cause Deaths during COVID-19 Vaccination in an Italian Province. Microorganisms. 2024; 12(7):1343. [CrossRef]
- Faksova K, Walsh D, Jiang Y, et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine. 2024;42(9):2200-2211. [CrossRef]
- Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. Published online January 14, 2024. [CrossRef]
- Rose J, Hulscher N, McCullough PA. Determinants of COVID-19 vaccine-induced myocarditis. Ther Adv Drug Saf. 2024;15:20420986241226566. Published 2024 Jan 27. [CrossRef]
- Long Term Follow-Up After Administration of Human Gene Therapy Products [Internet]. FDA; 2020 [cited 2024 Aug 5]. Available from: https://www.fda.gov/media/113768/download.
- Krumholz HM, Wu Y, Sawano M, et al. Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization. Preprint. medRxiv. 2023;2023.11.09.23298266. Published 2023 Nov 10. [CrossRef]
- Shrestha Y, Venkataraman R: The prevalence of post-COVID-19 vaccination syndrome and quality of life among COVID-19-vaccinated individuals [IN PRESS]. Vacunas. 2023. [CrossRef]
- Said KB, Al-Otaibi A, Aljaloud L, et al. The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines (Basel). 2022;10(7):1015. Published 2022 Jun 24. [CrossRef]
- Brogna C, Cristoni S, Marino G, et al. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteomics Clin Appl. 2023;17(6):e2300048. [CrossRef]
- Yu CK, Tsao S, Ng CW, et al. Cardiovascular Assessment up to One Year After COVID-19 Vaccine-Associated Myocarditis. Circulation. 2023;148(5):436-439. [CrossRef]
- Parry PI, Lefringhausen A, Turni C, et al. 'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023;11(8):2287. Published 2023 Aug 17. [CrossRef]
- Hulscher N, Procter BC, Wynn C, McCullough PA. Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination. Cureus. 2023;15(11):e49204. Published 2023 Nov 21. [CrossRef]
- Elecsys® Anti-SARS-CoV-2 S. Roche Diagnostics. Accessed August 5, 2024. https://diagnostics.roche.com/us/en/products/lab/elecsys-anti-sars-cov-2-s-cps-000616.html.
- Komici K, Verderosa S, D'Amico F, Guerra G. Self-reported side effects following COVID-19 vaccination in athletes: A retrospective study. Hum Vaccin Immunother. 2023;19(2):2234788. [CrossRef]
- Schwab C, Domke LM, Hartmann L, Stenzinger A, Longerich T, Schirmacher P. Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin Res Cardiol. 2023;112(3):431-440. [CrossRef]
- Schmeling M, Manniche V, Hansen PR. Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine. Eur J Clin Invest. 2023;53(8):e13998. [CrossRef]
- Fürst T, Šourek P, Krátká Z, Janošek J. Batch-dependent safety of COVID-19 vaccines in the Czech Republic and comparison with data from Denmark. Eur J Clin Invest. Published online June 27, 2024. [CrossRef]
- Knoll F. How Bad is My Batch? [Online]. GitHub; 2024 [cited 2024 Aug 5]. Available at: https://knollfrank.github.io/HowBadIsMyBatch/HowBadIsMyBatch.html.
- Sen G, Scully P, Gordon P, Sado D. Advances in the diagnosis of myocarditis in idiopathic inflammatory myopathies: an overview of diagnostic tests. Rheumatology (Oxford). 2024;63(7):1825-1836. [CrossRef]
- Silver MA, Maisel A, Yancy CW, et al. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases [published correction appears in Congest Heart Fail. 2005 Mar-Apr;11(2):102]. Congest Heart Fail. 2004;10(5 Suppl 3):1-30. [CrossRef]
- McCullough PA, Olobatoke A, Vanhecke TE. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure [published correction appears in Rev Cardiovasc Med. 2012;13(1):e52]. Rev Cardiovasc Med. 2011;12(4):200-210. [CrossRef]
- Bounds EJ, Kok SJ. D Dimer. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 31, 2023.
- Patel, F., Roux, J.L., Sawry, S., Kieser, R., Dhar, M., Gill, K., Lazarus, E., Nana, A., Garrett, N., Moore, P.L., Sigal, A., Gray, G., Rees, H., Jacobson, B.F., & Fairlie, L. Clot Twist - D-dimer analysis of healthy adults receiving heterologous or homologous booster COVID-19 vaccine after a single prime dose of Ad26.COV2.S in a phase II randomised open-label trial, BaSiS. 2024. medRxiv. [CrossRef]
- Ittiwut C, Mahasirimongkol S, Srisont S, et al. Genetic basis of sudden death after COVID-19 vaccination in Thailand. Heart Rhythm. 2022;19(11):1874-1879. [CrossRef]
- Maheshwari S, Dagor H. Evolving the Scope of Cardiac Point-of-Care Ultrasound in the Current Era. Cureus. 2024;16(2):e53985. Published 2024 Feb 10. [CrossRef]
- Money DB, Mehio M, Scoma C, Gupta S. Cardiac Point-of-Care Ultrasound (P.O.C.U.S.) Utilization for Hospitalists in the Assessment of Patients with Cardiac Complaints: An Educational Overview. J Community Hosp Intern Med Perspect. 2023;13(4):1-8. Published 2023 Jun 29. [CrossRef]
- Rajiah PS, François CJ, Leiner T. Cardiac MRI: State of the Art. Radiology. 2023;307(3):e223008. [CrossRef]
- Sen G, Scully P, Gordon P, Sado D. Advances in the diagnosis of myocarditis in idiopathic inflammatory myopathies: an overview of diagnostic tests. Rheumatology (Oxford). 2024;63(7):1825-1836. [CrossRef]
- Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130(6):484-495. [CrossRef]
- Karur GR, Aneja A, Stojanovska J, et al. Imaging of Cardiac Fibrosis: An Update, From the AJR Special Series on Imaging of Fibrosis. AJR Am J Roentgenol. 2024;222(6):e2329870. [CrossRef]
- Zhu L, Wang Y, Zhao S, Lu M. Detection of myocardial fibrosis: Where we stand. Front Cardiovasc Med. 2022;9:926378. Published 2022 Sep 29. [CrossRef]
- Valore L, Junker T, Heilmann E, et al. Case report: mRNA-1273 COVID-19 vaccine-associated myopericarditis: Successful treatment and re-exposure with colchicine. Front Cardiovasc Med. 2023;10:1135848. Published 2023 Apr 17. [CrossRef]
- Sanada Y, Azuma J, Hirano Y, Hasegawa Y, Yamamoto T. Overlapping Myocarditis and Postural Orthostatic Tachycardia Syndrome After COVID-19 Messenger RNA Vaccination: A Case Report. Cureus. 2022;14(11):e31006. Published 2022 Nov 2. [CrossRef]
- Iqbal AM, Butt N, Jamal SF. Automatic Internal Cardiac Defibrillator. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 22, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




