Submitted:
22 July 2024
Posted:
15 August 2024
You are already at the latest version
Abstract
Keywords:
- Psoriasis, extensively researched and prevalent as a recurrent chronic dermatological condition, presents formidable challenges in its management due to its profound impact on patients’ quality of life (QOL).
- Lifestyle factors play a crucial role in managing psoriasis effectively. For example, incorporating regular exercise and maintaining a balanced diet can help reduce the inflammatory symptoms associated with the condition.
- Dietary fats, particularly polyunsaturated fatty acids (PUFAs), play a crucial role in regulating epidermal homeostasis and inflammation.
- An adequate intake of PUFAs alongside conventional medical treatment contributes to the improvement of inflammatory symptoms associated with psoriasis.
- The abundance of saturated fatty acids is associated with obesity. Obesity worsens the psoriatic symptoms. Hence, it is suggested to restrict the intake of these fatty acids.
- This review encompasses the intricate disease mechanism of cutaneous inflammation in psoriasis along with clinical trials designed to assess the impact of dietary fats on psoriasis disease state.
1. Introduction
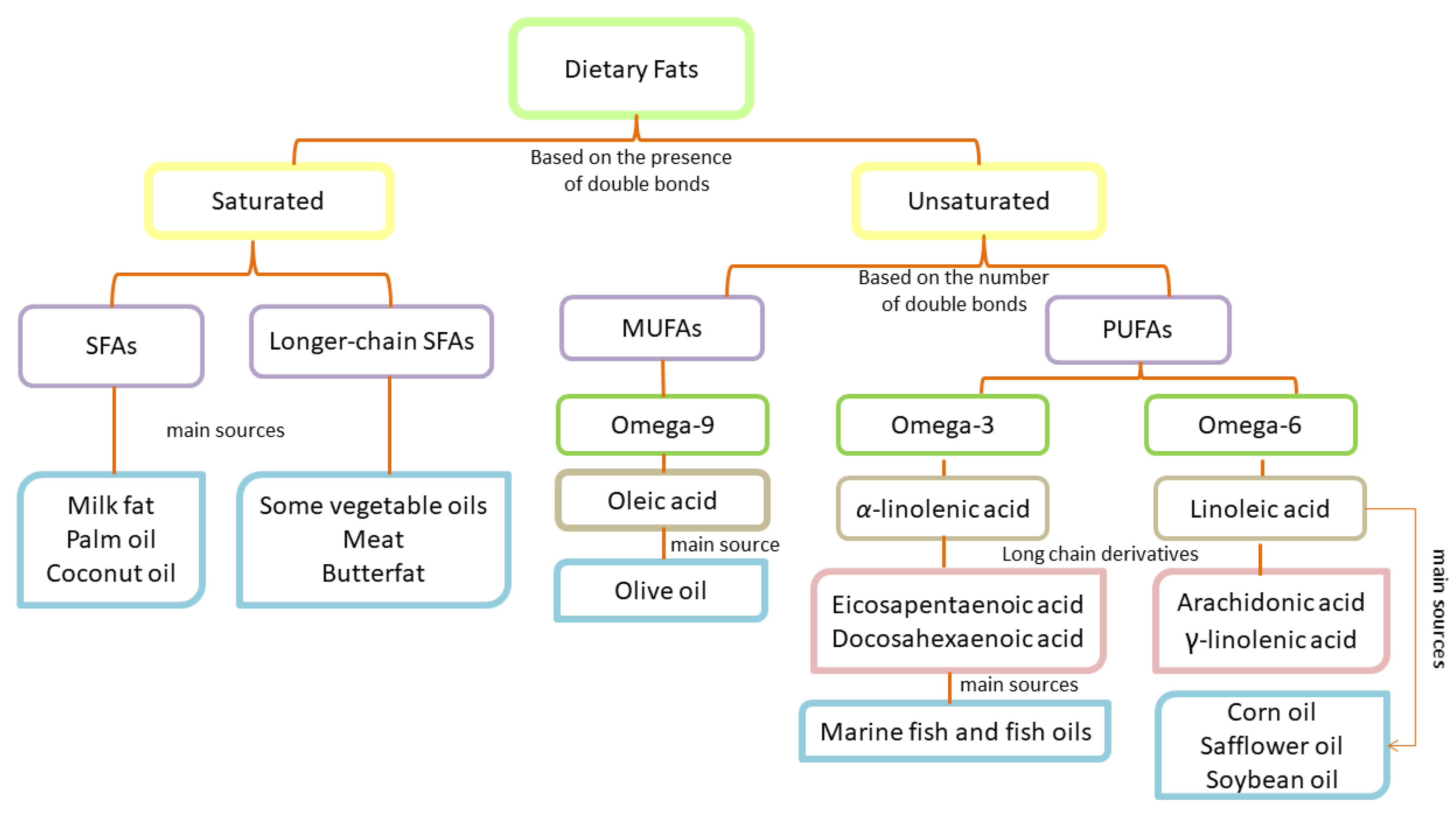
2. Dietary Fats: Essential Components and Classification
2.1. Classification of Saturated Fatty Acids
2.2. Classification of Unsaturated Fatty Acids
2.3. Fatty Acids and Their Significance in Dermatological Physiology
3. The Interplay of Obesity, Insulin Resistance, and Inflammation in Psoriasis Pathogenesis
4. How Dietary Fats Might Impact Inflammation in Psoriasis
4.1. Immunological Mechanisms Underlying Psoriasis Pathogenesis
4.2. Omega-3 and Omega-6 Polyunsaturated Fatty Acids in Inflammation
4.3. Impact of Fatty Acid Levels on Psoriasis Severity and Comorbidities
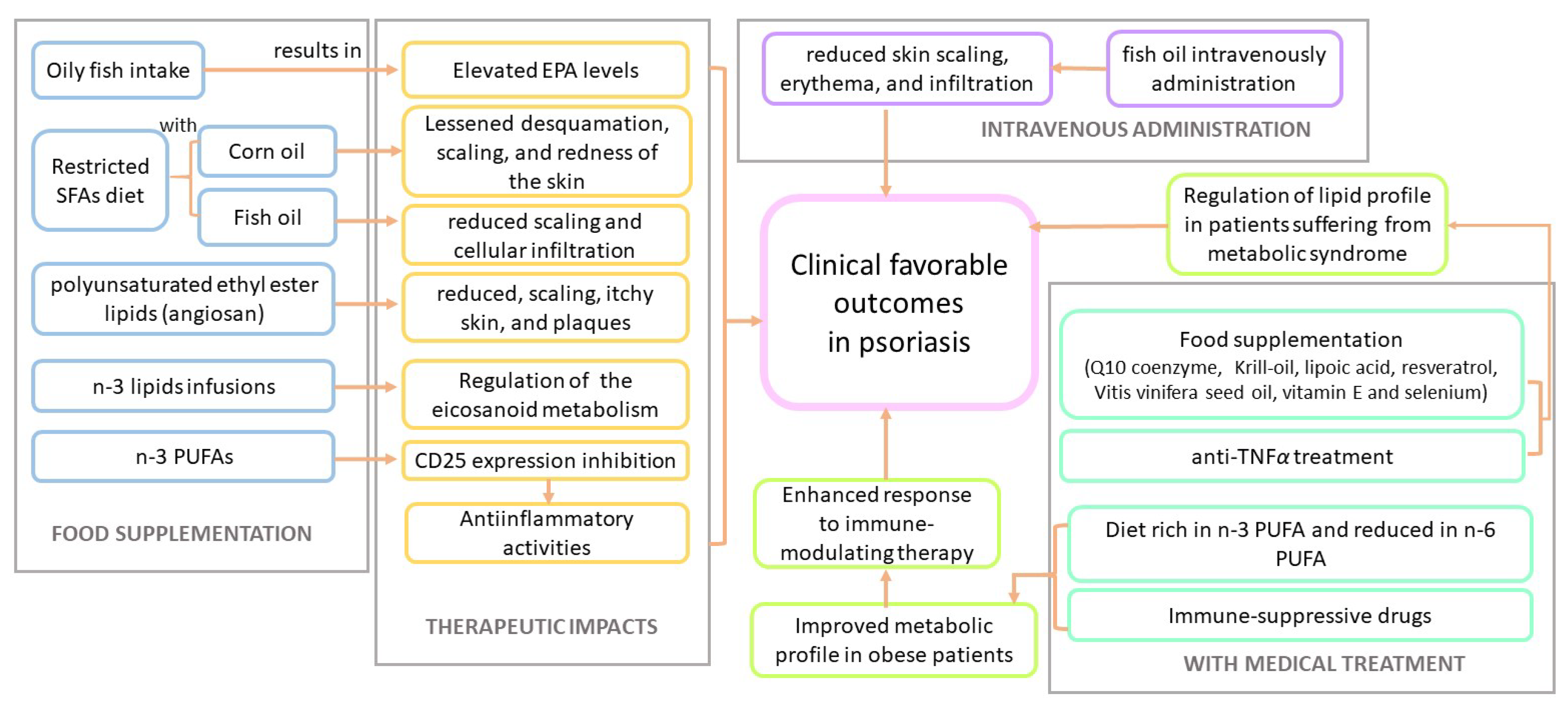
4.4. The Therapeutic Role of Polyunsaturated Fatty Acids in Psoriasis
4.4.1. Essential Fatty Acids: Implications for Skin Health
5. Clinical Trials That Assessed the Impact of Fatty Acids in Psoriasis and Psoriatic Arthritis
5.1. Psoriasis
5.2. Psoriatic Arthritis
| Sr. no. | Fatty acid | No. of patients | Patient info | Year | Region | Combined with |
Intake type | PMID & Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | polyunsaturated ethyl ester lipids (Angiosan) |
80 | psoriasis and psoriatic arthritis |
1990 | Finland | - | food supplementation |
PMID: 2139859 [62] |
| 2 | oily fish consumption | 18 | plaque psoriasis | 1993 | UK | - | food supplementation |
PMID: 8491161 [58] |
| 3 | fish and corn oils | 145 | moderate to severe psoriasis |
1993 | Norway | - | food supplementation |
PMID: 8502270 [59] |
| 4 | lipid infusion (-3 PUFAs) | 20 | guttate psoriasis | 1993 | Germany | - | infusions | PMID: 8219661 [63] |
| 5 | Highly purified -3 PUFAs | 52 | moderate plaque psoriasis |
1993 | Germany | - | topical | PMID: 8286257 ZEPELIN et al. |
| 6 | -3 PUFAs (long-chain) | 19 psoriasis and 21 atopic dermatitis |
moderate to severe psoriasis and atopic dermatitis |
1994 | Norway | - | food supplementation |
PMID: 8050452 [65] |
| 7 | lipid emulsion (fish oil based) | 83 | plaque psoriasis | 1998 | Europe | - | intravenously administrated |
PMID: 9555791 [60] |
| 8 | natural honey, beeswax and olive oil mixture |
18 psoriasis, 21 dermatitis |
psoriasis and dermatitis |
2003 | UAE | - | topical | PMID: 15022655 [72] |
| 9 | nutraceutical comprising Q10 coenzyme, Krill-oil, lipoic acid, resveratrol, Vitis vinifera seed oil, vitamin E and selenium |
40 | moderate to severe psoriasis |
2013 | Italy | anti-TNF treatment |
food supplementation |
PMID: 24442048 [68] |
| 10 | -3 PUFAs rich diet | 44 | obese patients with plaque psoriasis |
2013 | Italy | mmuno-suppressive drugs |
food supplementation |
PMID: 24120032 [69] |
| Sr. no. | Fatty acid | No. of patients | Patient info | Year | Region | Combined with |
Intake type | PMID & Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | seal oil | 43 | Psoriatic arthritis | 2006 | Norway | - | oral treatment | PMID: 16465662 [74] |
| 2 |
-3 PUFA and -linolenic acid |
60 | 6 Psoriatic arthritis and 54 rheumatoid arthritis |
2011 | Germany | - | food supplementation |
PMID: 21816071 [75] |
| 3 | marine -3 PUFA | 145 | Psoriatic arthritis | 2016 | Denmark | - | food supplementation |
PMID: 27955663 [76] |
| 4 | marine -3 PUFA | 145 | Psoriatic arthritis | 2018 | Denmark | - | food supplementation |
PMID: 28303758 [78] |
| 5 | Dietary fish oils (-3 PUFA) |
142 | Psoriatic arthritis | 2021 | Denmark | - | food supplementation |
PMID: 33885930 [80] |
6. Discussion
7. Conclusion
Author Contributions
Conflicts of Interest
References
- Globe, D.; Bayliss, M.S.; Harrison, D.J. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health and quality of life outcomes 2009, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parrish, L. Psoriasis: symptoms, treatments and its impact on quality of life. British journal of community nursing 2012, 17, 524–528. [Google Scholar] [CrossRef]
- Böhm, D.; Stock Gissendanner, S.; Bangemann, K.; Snitjer, I.; Werfel, T.; Weyergraf, A.; Schulz, W.; Jäger, B.; Schmid-Ott, G. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. Journal of the European Academy of Dermatology and Venereology 2013, 27, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Ainali, C.; Valeyev, N.; Perera, G.; Williams, A.; Gudjonsson, J.E.; Ouzounis, C.A.; Nestle, F.O.; Tsoka, S. Transcriptome classification reveals molecular subtypes in psoriasis. BMC genomics 2012, 13, 1–15. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. bmj 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, K.; Seemanthini, C.; Rashmi, R. Diet in dermatology: present perspectives. Indian journal of dermatology 2010, 55, 205. [Google Scholar] [CrossRef] [PubMed]
- Ada, L.S.; Anna, A.; Vincenzo, G. Foods, diet, and skin diseases. SKINmed: Dermatology for the Clinician 2004, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Katta, R.; Desai, S.P. Diet and dermatology: the role of dietary intervention in skin disease. The Journal of clinical and aesthetic dermatology 2014, 7, 46. [Google Scholar]
- Afifi, L.; Danesh, M.J.; Lee, K.M.; Beroukhim, K.; Farahnik, B.; Ahn, R.S.; Yan, D.; Singh, R.K.; Nakamura, M.; Koo, J.; et al. Dietary behaviors in psoriasis: patient-reported outcomes from a US national survey. Dermatology and therapy 2017, 7, 227–242. [Google Scholar] [CrossRef]
- Roe, D.A. Nutrition and the skin; Liss, 1986.
- Boelsma, E.; Hendriks, H.F.; Roza, L. Nutritional skin care: health effects of micronutrients and fatty acids. The American journal of clinical nutrition 2001, 73, 853–864. [Google Scholar] [CrossRef]
- Boelsma, E.; Van de Vijver, L.P.; Goldbohm, R.A.; Klöpping-Ketelaars, I.A.; Hendriks, H.F.; Roza, L. Human skin condition and its associations with nutrient concentrations in serum and diet. The American journal of clinical nutrition 2003, 77, 348–355. [Google Scholar] [CrossRef]
- Ahmed, S.; Shah, P.; Ahmed, O. Biochemistry, lipids. StatPearls [Internet] 2021.
- Council, N.R.; others. Diet and health: implications for reducing chronic disease risk. - 1989.
- Nogoy, K.M.C.; Kim, H.J.; Lee, Y.; Zhang, Y.; Yu, J.; Lee, D.H.; Li, X.Z.; Smith, S.B.; Seong, H.A.; Choi, S.H. High dietary oleic acid in olive oil-supplemented diet enhanced omega-3 fatty acid in blood plasma of rats. Food Science & Nutrition 2020, 8, 3617–3625. [Google Scholar]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: the structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clinics in Dermatology 2010, 28, 440–451. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Advances in food and nutrition research 2012, 65, 211–222. [Google Scholar] [PubMed]
- Linder, K.E. Structure and Function of the Skin. Feline Dermatology 2020, pp. 3–21.
- Monteiro-Riviere, N.A. Structure and function of skin. In Toxicology of the Skin; CRC Press, 2010; pp. 15–32.
- Wang, Z.; Man, M.Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging (Albany NY) 2020, 12, 5551. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, A.; Kendall, A.C. Current insights into skin lipids and their roles in cutaneous health and disease. Current Opinion in Clinical Nutrition & Metabolic Care 2023, 26, 83–90. [Google Scholar]
- Nicolaou, A.; Harwood, J.L. Skin lipids in health and disease. Lipid Technology 2016, 28, 36–39. [Google Scholar] [CrossRef]
- Vietri Rudan, M.; Watt, F.M. Mammalian epidermis: a compendium of lipid functionality. Frontiers in physiology 2022, 12, 804824. [Google Scholar] [CrossRef]
- Pappas, A.; others. Lipids and skin health. Technical report, Springer, 2015.
- Feng, F.; Ma, L.; Qu, Z.; Dong, Y.; Yi, F.; Feng, F.; Ma, L.; Qu, Z.; Dong, Y.; Yi, F. Effects of skin surface lipids on skin health. Asian Journal of Beauty and Cosmetology 2019, 17, 149–155. [Google Scholar] [CrossRef]
- Nicolaou, A. Polyunsaturated Fatty Acid Oxygenated Metabolites in Skin. In Lipids and Skin Health; Springer, 2014; pp. 43–63.
- Greb, J.; Goldminz, A.; Elder, J.; Lebwohl, M.; Gladman, D.; Wu, J.; Mehta, N.; Finlay, A.; Gottlieb, A. Psoriasis. Nature Reviews Disease Primers 2016, 2. [Google Scholar] [CrossRef]
- Ziboh, V.A. Arachidonic acid metabolism in the skin. In Arachidonic Acid Metabolism and Tumor Promotion; Springer, 1985; pp. 5–20.
- Ziboh, V.A. The significance of polyunsaturated fatty acids in cutaneous biology. Lipids 1996, 31, S249–S253. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. The role of fatty acid desaturases in epidermal metabolism. Dermato-endocrinology 2011, 3, 62–64. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and vascular biology 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Gross, E.; Ruzicka, T.; Restorff, B.v.; Stolz, W.; Klotz, K.N. High-affinity binding and lack of growth-promoting activity of 12 (S)-hydroxyeicosatetraenoic acid (12 (S)-HETE) in a human epidermal cell line. Journal of investigative dermatology 1990, 94, 446–451. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khazaâai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Archives of medical science: AMS 2017, 13, 851. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatric gastroenterology, hepatology & nutrition 2013, 16, 143. [Google Scholar]
- Jensen, P.; Skov, L. Psoriasis and obesity. Dermatology 2016, 232, 633–639. [Google Scholar] [CrossRef]
- Napolitano, M.; Megna, M.; Monfrecola, G. Insulin resistance and skin diseases. The Scientific World Journal 2015, 2015. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Cordeiro-Rodríguez, M.; Carnero-Gregorio, M.; López-Barcenas, A.; Martínez-Herrera, E.; Fabbrocini, G.; Sinani, A.; Arenas-Guzmán, R.; González-Cespón, J.L. Biomarkers of inflammation in obesity-psoriatic patients. Mediators of inflammation 2019, 2019. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S.; et al. Obesity and insulin resistance. The Journal of clinical investigation 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Buerger, C.; Richter, B.; Woth, K.; Salgo, R.; Malisiewicz, B.; Diehl, S.; Hardt, K.; Boehncke, S.; Boehncke, W.H. Interleukin-1β interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. Journal of Investigative Dermatology 2012, 132, 2206–2214. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. International journal of molecular sciences 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Batycka-Baran, A.; Maj, J.; Wolf, R.; Szepietowski, J. The new insight into the role of antimicrobial proteins-alarmins in the immunopathogenesis of psoriasis. Journal of immunology research 2014, 2014. [Google Scholar] [CrossRef]
- Grine, L.; Dejager, L.; Libert, C.; Vandenbroucke, R.E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine & growth factor reviews 2015, 26, 25–33. [Google Scholar]
- Zhang, L.j. Type1 interferons potential initiating factors linking skin wounds with psoriasis pathogenesis. Frontiers in Immunology 2019, 10, 1440. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Lv, P.; Li, X.; Liu, G.; Chen, Y.; Wang, Z.; Qian, X.; Shen, Y.; Li, Y.; et al. The role of Th17 cells in psoriasis. Immunologic Research 2020, 68, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins, leukotrienes and essential fatty acids 2006, 75, 197–202. [Google Scholar] [CrossRef]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. Journal of Investigative Dermatology 2018, 138, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Myśliwiec, H.; Baran, A.; Harasim-Symbor, E.; Myśliwiec, P.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Serum fatty acid profile in psoriasis and its comorbidity. Archives of Dermatological Research 2017, 309, 371–380. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. Journal of the American College of Cardiology 2015, 66, 1538–1548. [Google Scholar] [CrossRef]
- Ring, J.; Kunz, B. Unsaturated fatty acids in the treatment of atopic eczema. In Handbook of atopic eczema; Springer, 1991; pp. 429–434.
- Murphrey, M.B.; Miao, J.H.; Zito, P.M. Histology, stratum corneum. Europe PMC 2018.
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods-a review. Journal of food science and technology 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Horrobin, D.F. Essential fatty acids in clinical dermatology. Journal of the American Academy of Dermatology 1989, 20, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Berbis, P.; Hesse, S.; Privat, Y. Essential fatty acids and the skin. Allergie et immunologie 1990, 22, 225–231. [Google Scholar]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and therapeutic applications of fish oilâs fatty acids on the skin. Marine drugs 2018, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.; Ursell, A.; Zaremba, K.; Payne, C.; Staughton, R.; Sanders, T. Effect of regular consumption of oily fish compared with white fish on chronic plaque psoriasis. European journal of clinical nutrition 1993, 47, 251–254. [Google Scholar]
- Soyland, E.; Funk, J.; Rajka, G.; Sandberg, M.; Thune, P.; Rustad, L.; Helland, S.; Middelfart, K.; Odu, S.; Falk, E.S.; et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. New England Journal of Medicine 1993, 328, 1812–1816. [Google Scholar] [CrossRef]
- Mayser, P.; Mrowietz, U.; Arenberger, P.; Bartak, P.; Buchvald, J.; Christophers, E.; Jablonska, S.; Salmhofer, W.; Schill, W.B.; Krämer, H.J.; et al. ω-3 Fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. Journal of the American Academy of Dermatology 1998, 38, 539–547. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. International journal of molecular sciences 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- Lassus, A.; Dahlgren, A.L.; Halpern, M.; Santalahti, J.; Happonen, H.P. Effects of dietary supplementation with polyunsaturated ethyl ester lipids (Angiosan®) in patients with psoriasis and psoriatic arthritis. Journal of international medical research 1990, 18, 68–73. [Google Scholar] [CrossRef]
- Grimminger, F.; Mayser, P.; Papavassilis, C.; Thomas, M.; Schlotzer, E.; Heuer, K.U.; Führer, D.; Hinsch, K.D.; Walmrath, D.; Schill, W.B.; et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis. The clinical investigator 1993, 71, 634–643. [Google Scholar] [CrossRef]
- Calder, P.C. n- 3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American journal of clinical nutrition 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Søyland, E.; Lea, T.; Sandstad, B.; Drevon, A. Dietary supplementation with very long-chain n-3 fatty acids in man decreases expression of the interleukin-2 receptor (CD25) on mitogen-stimulated lymphocytes from patients with inflammatory skin diseases. European Journal of Clinical Investigation 1994, 24, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Fostini, A.C.; Fossà, I.; Girolomoni, G.; Targher, G. Psoriasis and the metabolic syndrome. Clinics in dermatology 2018, 36, 21–28. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Yeung, H. Metabolic syndrome in patients with psoriatic disease. The Journal of Rheumatology Supplement 2012, 89, 24–28. [Google Scholar] [CrossRef]
- Skroza, N.; Proietti, I.; Bernardini, N.; La Viola, G.; Nicolucci, F.; Pampena, R.; Tolino, E.; Zuber, S.; Mancini, M.; Soccodato, V.; et al. Efficacy of food supplement to improve metabolic syndrome parameters in patients affected by moderate to severe psoriasis during anti-TNFα treatment. Giornale Italiano di Dermatologia e Venereologia: Organo Ufficiale, Societa Italiana di Dermatologia e Sifilografia 2013, 148, 661–665. [Google Scholar]
- Guida, B.; Napoleone, A.; Trio, R.; Nastasi, A.; Balato, N.; Laccetti, R.; Cataldi, M. Energy-restricted, n-3 polyunsaturated fatty acids-rich diet improves the clinical response to immuno-modulating drugs in obese patients with plaque-type psoriasis: a randomized control clinical trial. Clinical nutrition 2014, 33, 399–405. [Google Scholar] [CrossRef]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. International journal of endocrinology 2012, 2012. [Google Scholar] [CrossRef]
- Castela, E.; Archier, E.; Devaux, S.; Gallini, A.; Aractingi, S.; Cribier, B.; Jullien, D.; Aubin, F.; Bachelez, H.; Joly, P.; et al. Topical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalities. Journal of the European Academy of Dermatology and Venereology 2012, 26, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: partially controlled, single-blinded study. Complementary therapies in medicine 2003, 11, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Brent, L.H. Psoriatic arthritis. In StatPearls [Internet]; StatPearls Publishing, 2021.
- Madland, T.M.; Björkkjaer, T.; Brunborg, L.A.; Fröyland, L.; Berstad, A.; Brun, J.G. Subjective improvement in patients with psoriatic arthritis after short-term oral treatment with seal oil. A pilot study with double blind comparison to soy oil. The Journal of Rheumatology 2006, 33, 307–310. [Google Scholar]
- Dawczynski, C.; Hackermeier, U.; Viehweger, M.; Stange, R.; Springer, M.; Jahreis, G. Incorporation of n-3 PUFA and γ-linolenic acid in blood lipids and red blood cell lipids together with their influence on disease activity in patients with chronic inflammatory arthritis-a randomized controlled human intervention trial. Lipids in Health and Disease 2011, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.; Schmidt, E.B.; Schlemmer, A.; Rasmussen, C.; Lindgreen, E.; Johansen, M.B.; Christensen, J.H. The effect of marine n-3 polyunsaturated fatty acids on cardiac autonomic and hemodynamic function in patients with psoriatic arthritis: a randomised, double-blind, placebo-controlled trial. Lipids in health and disease 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Loft, N.; Nguyen, T.T.; Kristensen, L.E.; Thyssen, J.P.; Egeberg, A. Disease burden, symptoms, and use of analgesics in patients with psoriasis with or without psoriatic arthritis: A cross-sectional study. Journal of the American Academy of Dermatology 2022, 86, 590–597. [Google Scholar] [CrossRef]
- Kristensen, S.; Schmidt, E.; Schlemmer, A.; Rasmussen, C.; Johansen, M.B.; Christensen, J. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scandinavian journal of rheumatology 2018, 47, 27–36. [Google Scholar] [CrossRef]
- Wagner, M.F.M.G.; Theodoro, T.R.; Oyafuso, L.K.M.; Pinhal, M.A.S.; et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC molecular and cell biology 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Holm Nielsen, S.; Sardar, S.; Siebuhr, A.S.; Schlemmer, A.; Schmidt, E.B.; Bay-Jensen, A.C.; Karsdal, M.A.; Christensen, J.H.; Kristensen, S. Effect of n-3 PUFA on extracellular matrix protein turnover in patients with psoriatic arthritis: a randomized, double-blind, placebo-controlled trial. Rheumatology International 2021, 41, 1065–1077. [Google Scholar] [CrossRef]
- ZEPELIN, H.H.; Mrowietz, U.; Farber, L.; Bruck-Borchers, K.; Schober, C.; Huber, J.; LETZ, G.; KHNEN, R.; HRISTOPHERS, E.; Welzel, D. Highly purified omega-3-polyunsaturated fatty acids for topical treatment of psoriasis. Results of a double-blind, placebo-controlled multicentre study. British Journal of Dermatology 1993, 129, 713–717. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites. The American journal of clinical nutrition 2000, 71, 361s–366s. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedicine & pharmacotherapy 2006, 60, 502–507. [Google Scholar]
- Calder, P.; Grimble, R. Polyunsaturated fatty acids, inflammation and immunity. European journal of clinical nutrition 2002, 56, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; OâKeefe, J.H. Good fats versus bad fats: a comparison of fatty acids in the promotion of insulin resistance, inflammation, and obesity. Missouri medicine 2017, 114, 303. [Google Scholar]
- Phillips, C.M.; Kesse-Guyot, E.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity–associated gene in adults. The Journal of nutrition 2012, 142, 824–831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




