1. Introduction
Out-of-hospital cardiac arrest (OHCA) remains a leading cause of death in the US and worldwide. While global survival rates from OHCA continue to show modest improvements over recent decades, the disease is still characterized by a high mortality rate; the latest data from the US national OHCA registry, the Cardiac Arrest Registry to Enhance Survival (CARES), indicate a 24% survival rate to hospital admission and 9% survival rate to discharge [
1]. The high incidence of OHCA, accompanied by this high mortality rate, emphasizes the enormous need for improved care approaches to OHCA. Increasing prompt OHCA recognition, encouraging bystander cardiopulmonary resuscitation (CPR), and improving emergency medical services (EMS) response time and preparedness are factors that would increase survival. However, given the marked drop in survival between survival to admission and survival to discharge, the management of patients after resuscitation represents an important area for research and care improvements.
Caring for resuscitated OHCA patients encompasses a complex range of diagnostic tools and multidisciplinary interventions. Increasing efforts have been employed to study interventions aimed at improving outcomes for patients suffering from OHCA, such as targeted temperature management (TTM) and coronary angiography. Several key challenges are inherent in these efforts; the patient population presenting with OHCA to the emergency department (ED) is a highly heterogeneous population of patients with regards to medical and surgical history, age, hereditary disorders, and other factors. Additionally, interventions to reverse treatable causes are likely highly time sensitive, yet in many cases, no attributable cause for the arrest is found, and interventions for those patients are thus limited. This underscores the importance of focusing on post-resuscitation diagnostic modalities that enable the detection of reversible causes or sequelae of the arrest to boost survival rates after return of spontaneous circulation (ROSC) is achieved.
In this review, we will discuss available and evolving diagnostic modalities helpful in post-resuscitation assessment of OHCA patients, while linking these modalities to interventions aimed at reversing the respective cause or sequelae of arrest.
2. Electrocardiography
Electrocardiography (ECG) is one of the first tools used to assess patients during and after OHCA. During resuscitation efforts, patients are routinely monitored through continuous acquisition of cardiac electrical activity. Recent studies have attempted to use ECG features during pulseless electrical activity (PEA) to predict ROSC. It has been shown that heart rate, ECG when combined in a logistic regression model with specific electrical waveform characteristics, are potentially useful prognostic markers of ROSC during PEA [
2]. In addition, the role of artificial intelligence algorithms in assessing for ECG-based markers of ROSC has been explored. Two deep neural networks, trained to differentiate between PEA and pulse generating rhythms using defibrillator lead-pads only, were able to do so with high sensitivity, specificity, and balanced accuracy [
3].
After successful resuscitation from OHCA, the acquisition of 12-lead ECGs remains a crucial diagnostic approach. Guidelines from the American Heart Association (AHA) in 2020 recommend routinely obtaining a 12-lead ECG immediately after ROSC and performing prompt coronary angiography on those patients with evidence of ST-elevation on ECG [
4].
Early post-ROSC ECG is important to identify coronary artery disease (CAD) as a reversible cause in OHCA. While this strategy is important for early intervention in cases of myocardial infarction, recent observational data from several studies suggest that early ECG should be interpreted with caution. Cohort data from Norway suggest that early ECG post-ROSC is not reliable in identifying patients with indication for immediate coronary angiography, and it is evident that a considerable subset of patients may suffer from a coronary cause for the OHCA without concerning ischemic changes on ECG [
5]. In the setting of mechanical trauma to the heart during resuscitation, defibrillation, and severe metabolic derangements may complicate interpretation of immediate ECG data. Consequently, some investigations have suggested to either delay ECG acquisition for several minutes post-ROSC allowing transient injury patterns to dissipate or to repeat an ECG after some time had passed from an early post-ROSC ECG exhibiting changes consistent with myocardial ischemia [
6,
7]. However, recent observational data suggest that even a delayed repeat ECG acquisition post-ROSC has similar accuracy in predicting acute thrombotic coronary occlusion compared to the first early post-ROSC ECG [
8]. Interestingly, a study from South Korea that combined ECG, echocardiographic, and biomarker criteria for diagnosing obstructive CAD as a cause for the OHCA, indicate that ECG criteria alone had superior diagnostic accuracy than combined criteria [
9].
An important opportunity to improve post-arrest ECG interpretation could lie in the use of artificial intelligence. A trained model was able to successfully diagnose total or near-total coronary occlusion on post-ROSC ECG and was non-inferior to the consensus interpretation of emergency physicians and cardiologists [
10].
Finally, the potential role of ECG in prognosticating patients resuscitated from OHCA has been studied. Post-ROSC ECG has exhibited utility in predicting 30-day mortality: in a multivariate analysis of an international multicenter OHCA cohort, 30-day mortality was found to be significantly higher OHCA patients >62 years old, with post-ROSC ECG findings of QRS>120ms and ST-segment elevation in >1 segment [
11]. In addition, heart rate variability as measured on ECG during the first day post-ROSC during TTM measures was able to successfully predict long-term patient outcomes in comatose patients after cardiac arrest [
12].
3. Coronary Angiography
The role of immediate cardiac catheterization after OHCA remains an ongoing focus of research and controversy. Currently, AHA guidelines recommend emergent coronary angiography in OHCA patients suspected to have myocardial infarction and accompanying ST-segment elevations on ECG [
4,
13]. However, for patients with post-arrest ECG findings that suggest potential ischemia but do not exhibit ST-elevations, selecting patients that would benefit from coronary angiography remains a key challenge in post-resuscitation care.
Obstructive CAD may trigger OHCA without exhibiting ischemic changes on ECG, complicating decision-making and patient selection for angiography. Earlier observational data examining the use of early cardiac catheterization in comatose patients resuscitated from OHCA without evidence of STEMI on ECG found significantly decreased mortality [
14,
15]. A subsequent meta-analysis also supported the use of early coronary angiography but highlighted the need for randomized trial evidence [
16]. This prompted the development of prospective clinical investigations such as the Coronary Angiography after Cardiac Arrest (COACT) trial. In this work, patients were randomized to immediate versus delayed coronary angiography in OHCA if they presented with initial shockable rhythms, without evidence of ST-elevation myocardial infarction (STEMI) on ECG. The trial demonstrated no significant difference in survival at 90 days, suggesting that patients without ECG evidence of STEMI findings may not uniformly require immediate angiography [
17]. Similar results emerged from additional randomized trials, further highlighting no difference in clinical outcome between immediate and delayed angiography [
18,
19,
20,
21,
22].
Whether a subset of patients can be prospectively identified, via either ECG or clinical factors, who might benefit from immediate angiography despite lack of ECG evidence of STEMI, remains an unresolved question [
23].
4. Computed Tomography
The use of computed tomography (CT) imaging immediately following OHCA resuscitation has varied widely in clinical practice due to the absence of clear guidelines for its use. The European Resuscitation Council and European Society of Intensive Care Medicine Guidelines in 2021 recommended the use of head CT and/or CT pulmonary angiography in patients without an identifiable cardiac cause for the arrest and in those presenting with pre-arrest symptoms suggestive of pulmonary or neurological etiology [
24]. This is predicated on the notion that CT evaluation may identify treatable underlying causes of arrest, potentially improving outcomes for patients with OHCA.
Observational studies have evaluated the diagnostic yield and utility of CT imaging following OHCA. These studies highlight the ability of CT to detect a wide range of potential causes and sequelae of OHCA or resuscitation, such as subarachnoid hemorrhage, cerebral edema, pulmonary embolism, pneumothorax, sternal or rib fractures, lacerations findings, among others [
24,
25,
26,
27,
28,
29]. Recent prospective data employing a standardized protocol for head-to-pelvis CT imaging in patients following OHCA resuscitation concluded that such a protocol is safe, expedites time-sensitive diagnoses, and provides meaningful clinical information when compared to standard of care in which clinicians opted for CT imaging at their discretion. These findings were recently confirmed by work from our team in a multihospital cohort [
30,
31,
32].
The impact of CT evaluation on the timing of other interventions remains a potential issue, however; one retrospective study suggested that head CT imaging for OHCA patients failed to identify any cases of intracranial hemorrhage, but also resulted in delay of coronary intervention for those found to have STEMI. While CT practices vary institutionally, the present data concerning the utility of protocolized standardized CT imaging after OHCA resuscitation call for additional prospective investigation to better understand the utility of post-arrest CT evaluation.
5. Ultrasonography
Ultrasound is an appealing imaging modality for cardiac arrest resuscitation. It is a mobile, non-invasive, inexpensive, real-time imaging modality that can be potentially utilized during arrest management. While image quality and interpretation are clinician dependent, it is a modality for which emergency medicine and critical care physicians have grown in proficiency over recent years. Ultrasound can be utilized for three major roles within cardiac arrest care: rhythm diagnosis, evaluation of underlying arrest etiology, and real-time assessment of CPR performance.
5.1. Rhythm Diagnosis
Presenting arrest rhythm is important in guiding subsequent treatment and prognosis. Initial rhythm is included in nearly every model of prognostication, and in many models, it is the single most consequential predictor of outcome [
33,
34]. Arrest rhythm is nearly always derived from ECG analysis, except in rare cases of fine VF; and in most PEA arrests, further discrimination of rhythm can be made using ultrasonography.
While shockable rhythms almost always present as such on ECG, ultrasound occasionally reveals an underlying rhythm not detected on the ECG. Although it is rare, it is a known phenomenon that fine VF can present electrocardiographically as asystole or even PEA. Ultrasound discovery of fine ventricular fibrillation (VF) may indicate responsiveness to defibrillation (despite an evidently non-shockable ECG) and subsequently improved outcome [
35,
36].
All cases of PEA can be sub-classified as true PEA and pseudo-PEA (pPEA), a state in which organized cardiac activity can be detected despite a pulseless state and PEA electrical rhythm. Pseudo-PEA is more likely to be associated with various reversable outcomes and generally has a higher chance of survival, though it depends on the exact underlying etiology [
37,
38].
5.2. Arrest Etiology
5.2.1. Shockable Arrest
ACLS guideline treatment protocols are initially organized around presenting rhythm. All arrest patients receive CPR, and then patients with shockable rhythms receive defibrillation and specific pharmacologic interventions, while patients with non-shockable rhythms require further evaluation for potential non-cardiac etiologies. For VF arrests, it is often assumed that the underlying physiology is more likely than not myocardial ischemia [
39], although there are other etiologies associated with VF including channelopathies and valvular disease [
40]. Ventricular tachycardia (VT) is frequently associated with structural heart disease including either acute ischemia or electrical activation from previous scarring related to prior ischemia; although VT can also be caused by channelopathies, cardiomyopathies and other structural defects. Following resuscitation from VF or VT arrest, cardiac ultrasonography may demonstrate regional wall motion abnormalities, which helps confirm the diagnosis of coronary ischemia. Sonographic evidence of wall motion abnormalities, in the setting of shockable arrest, should prompt clinicians to consider timely angiography for further investigation and/or treatment.
5.2.2. Non-Shockable Arrest
Where echocardiography plays a largely confirmatory role in shockable arrest, it plays a potentially more important diagnostic role in non-shockable arrest, specifically PEA. PEA and pPEA often both represent heterogeneous underlying disease processes that lack rhythm specific treatment. The treatment of PEA, in addition to CPR and epinephrine, depends on the underlying physiology. PEA is classically taught to have reversable causes in some cases, many of which may be diagnosed by echocardiographic investigation, leading to subsequent focused treatment.
Resuscitative ultrasound protocols have been developed to search for sonographic signs of a reversable cause during cardiac arrest. These include the CASA protocol [
41], FEER protocol [
42], PEA protocol [
43], and RUSH exam [
44] among others. These strategies likely offer tradeoff of varying sensitives (through more comprehensive images), at the expense of speed; no head-to-head comparison of these protocols has ever been conducted and thus the clinician must decide on the extent of sonographic investigation based on clinical resources, and pre-test suspicion for an underlying etiology.
A comprehensive form of echocardiographic investigation is exemplified by the Motol University approach using an “ABC” checklist [
45]. The “A” represents airway evaluation, which is to say one could use ultrasonography to confirm ETT placement, however this is rarely done in practice. “B” represents lungs evaluation to assess for tension pneumothorax, pulmonary edema and other abnormalities. C” denotes cardiac evaluation which is frequently the first and most impactful set of images in various protocols. A subxiphoid view can provide information about pericardial effusion, PE (RV>LV, D sign, Mcconell’s sign), MI (wall motion abnormality) and hypovolemia (collapsing/underfilled IVC). In PEA, more than any other arrest rhythm, the ability to diagnose the underling etiology in real-time allows for arrest-specific treatment thus potentially improving the chance of survival.
5.3. CPR Quality
Intra-arrest ultrasound also plays an important role in the real-time assessment of initial arrest outcome as well as CPR quality. Some studies have suggested that ultrasound may be superior to manual palpation for determining ROSC. Clinicians cannot reliably palpate low systolic pressures, generally taught to be less than 60mmHg, at the femoral artery. However, ultrasound is more sensitive to flow indicating ROSC. Peak systolic velocities (PSV) greater than 20 cm/s have increased accuracy compared to manual palpation [
46] and there is evidence to suggest that ultrasound pulse check durations are comparable to manual pulse checks [
47].
While ultrasound images may provide important data during CPR, some question whether CPR quality, and ultimately survival, suffers as a result of attempts to obtain clear images of the chest and potentially increase CPR pause times. Evidence on this question is limited.
Regarding the impact of ultrasound on CPR quality, Lien et al. 2023, in a retrospective study of 3300 CPR pauses, showed that using ultrasound during intra-arrest care did not lower the compression fraction; although ultrasound use reduced the rate of ROSC and failed to improve survival (although it did not worsen it) [
48]. Conversely, in a prospective study of 110 pauses, ultrasound during CPR significantly increased CPR pause length, by a mean of 6 seconds [
49]. It is possible that ultrasound use increases CPR pause time, which is a serious consideration given the therapeutic value of minimizing CPR interruptions [
50].
Regarding the effects of ultrasound on arrest survival
, a meta-analysis from 2023 that examined ultrasound use during CPR, found “no difference in rate of return of spontaneous circulation (RR 0.83, 95% CI 0.24–1.66, p=0.60) (very low certainty of evidence) and a significant decrease in rate of survival to hospital discharge (RR 0.44, 95% CI 0.22–0.88, p=0.02) (very low quality of evidence)” [
51]. Contemporary data suggest that ultrasound use has not been associated with improved cardiac arrest outcomes, but given the low quality of data, and the theoretical benefits of ultrasound, more research must be conducted before changing current use of ultrasound in cardiac arrest care.
5.4. Transesophageal Echocardiography
Transesophageal Echocardiography (TEE) is a more invasive modality of ultrasonography that has a number of advantages over transthoracic echocardiography (TTE). TEE does not require access to the chest which is complicated by ongoing CPR. As a result, TEE can allow for continuous imaging of the heart, unlike TTE which is usually intermittent, in tandem with CPR and pulse checks. As a result, TEE can be performed during active CPR, which ultimately allows for real-time continuous visualization of CPR quality. While few emergency physicians are currently trained to interpret TEE images, skilled operators can provide feedback on “Area of Maximal Compression” (where compressions are having primary impact on myocardial tissue), Left Ventricular Outflow Tract (LVOT) opening, and heart contractility [
52], all of which are potential determinants of survival. Finally, there is evidence that TEE may have higher sensitivity to identify reversible causes of arrest [
53].
5.5. Prognostication
While the diagnostic role of ultrasound requires more research, its ability to prognosticate ROSC and survival is more clear, especially in PEA. The distinction between PEA and pPEA is of particular importance when trying to prognosticate survival; a distinction that is best differentiated through ultrasound. A meta-analysis of 11 studies, including 777 PEA patients, found a substantially increased rate of ROSC (RR = 4.35 [95% confidence interval [CI], 2.20–8.63; p<0,00001]) for pPEA compared to true PEA [
54]. In addition to predicting ROSC, the discovery of pPEA significantly prognosticates both survival to hospital admission [
55] (|) as well as survival to hospital discharge [
55]). While the diagnosis of pPEA is not integrated into any widely accepted Termination of Resuscitation (TOR) guidelines, discovering pPEA denotes a significantly better prognosis, of which clinicians should be mindful.
6. Serum Biomarkers
Serum biomarkers represent an additional source of information in cardiac arrest management. IV access is nearly always obtained at some point during resuscitation, and therefore blood sampling is both common and practical. A number of commonly ordered laboratory tests and less common biomarkers may have potential diagnostic value during resuscitation and post-arrest care, described below.
6.1. Electrolytes
6.1.1. Potassium
Serum potassium level is a critical data point for resuscitation decision making. Hyperkalemia is a well-described cause of PEA and is especially common in patients with pre-existing renal dysfunction [
56]. Other common causes of hyperkalemia include overdose syndromes from medications such as ACE inhibitors, rhabdomyolysis, and tumor lysis syndrome [
57].
Hypokalemia is most often caused from diuretic use, although also caused by diarrhea, DKA, and alcohol use. At levels less than 3mmol/L [
58], which classically causes u-waves, hypokalemia can cause cardiac arrest by hyperpolarizing cardiac membranes leading to early after depolarizations that devolve to Torsades de Pointes and subsequent VT.
6.1.2. Sodium
Sodium plays an essential role in myocardial action potentials however, interestingly, sodium derangements are uncommon primary causes of cardiac arrest. Conversely, sodium levels are frequently abnormal after cardiac arrest, and the degree of derangement may predict outcomes in some instances [
59,
60]. While hyponatremia can represent both volume overload, such as in cases of congestive heart failure, as well as dehydration, the determination of fluid status during resuscitation is probably best made clinically and/or with echocardiography.
6.1.3. Calcium
Calcium handling is essential for both action potential and myofibril contraction physiology. In a retrospective case-control analysis of 770 patients, hypocalcemia was correlated with higher odds of cardiac arrest. Specifically, Ca levels lower than 8.95 mg/dL were associated with 2.3-fold increase in odds of SCA comparing to levels higher than 9.55 mg/dL (OR= 2.33, 95% CI: 1.17–4.61) [
61]. Hypocalcemia can prolong QT intervals which is associated with Torsades de Pointes and shockable rhythms. Hypercalcemia does not seem to be a common cause of cardiac arrest [
62].
6.1.4. Magnesium
Hypomagnesaemia has been associated with higher incidence of arrest, perhaps by prolonging the QT interval. In a prospective study of 9820 patients, chronic hypomagnesaemia was associated with increased risk of arrest [
63]. Hypomagnesemia predisposes the myocardium to Premature Ventricular Contractions (PVCs) which can devolve into Torsades de Pointes and subsequently shockable rhythm [
64].
6.1.5. Glucose
Glucose is one of the most immediately available laboratory results during arrest resuscitation, being frequently obtained by EMS via point of care testing. Hypoglycemia presents the greater risk for cardiac arrest, compared to hyperglycemia. Profound hypoglycemia (<15mg/dL) can cause reflex catecholamine release that causes sinus tach that then devolves into AV dissociation, bradycardia and finally cardiac arrest [
65].
Hyperglycemia, as an isolated diagnosis, is not known to cause cardiac arrest. However, sequela of hyperglycemia, particularly DKA, which is associated with other aforementioned electrolyte derangements, can lead to cardiac arrest [
66]. Like many other electrolyte abnormalities, glucose derangements are frequently noted on peri-arrest laboratory work, and are associated with poor outcomes, but it is not always possible to diagnose the abnormality as the causal etiology of arrest [
67,
68].
6.2. Liver Biomarkers
The liver is a highly perfused organ, and thus demonstrates a high degree of ischemic injury during cardiac arrest. Prognostically, clotting times (PT/PTT/INR) may inform decisions regarding coagulopathy reversal, especially if hemorrhage is thought to be the cause of cardiac arrest. Conversely, sub-therapeutic clotting times may suggest thrombosis (MI or PE) as the cause of arrest.
Liver damage is both a common and poor prognosticator of cardiac arrest outcomes. A study of 374 post-arrest patients demonstrated that acute liver failure (ALF) (defined as a bilirubin >1.2 mg/dL and an international normalized ratio ≥1.5.) was present in 56% of post-arrest patients, while hypoxic hepatitis (defined as an aminotransferase level >1000 IU/L) developed in 7% of these patients and 6% of patients developed both conditions. Patients who developed hypoxic hepatitis, but not necessarily acute liver failure, had an increased incidence of poor neurological outcomes in this study [
69]. In a separate study (which defined ALF as an INR > 1.5), patients with ALF at admission had substantially increased odds of 28-day mortality (Odds =10.6, 95% CI 1.36–83.04, p= 0.024) [
70].
6.3. Lactate
Many studies have investigated the role of lactate in cardiac arrest evaluation, given its correlation to hypoperfused states. Lactate is frequently elevated in cardiac arrest patients when tested shortly after resuscitation. Prognostically, a meta-analysis of 23 studies involving 6720 cardiac arrest found that higher lactate concentrations were associated with worse outcomes. Lactate clearance rates provide more predictive prognostic information than single point measurements [
71,
72].
6.4. Cardiac Biomarkers
Multiple biomarkers used in clinical practice may help diagnose cardiac etiology of arrest.
6.4.1. Troponin
Troponin levels are frequently elevated after cardiac arrest. While elevated troponin levels are commonly associated with myocardial ischemia, classically suggesting the need for early angiography, multiple studies have failed to find a troponin cut off that predicts the need for angiography in the cardiac arrest population [
73,
74]. Physiologically, this is likely due to troponin elevations inherent in arrest-related ischemia, as well as iatrogenic myocardial injury from CPR and defibrillation. The inability of troponin levels to aptly discriminate the need for PCI may also be because of a fairly well-established element of ischemic signs-CAD dissociation in cardiac arrest. For example, it has been demonstrated that around 30% of non-STEMI arrests are found to have significant CAD, requiring PCI [
17]; conversely, there are some patients with STE on post-ROSC ECG who do not have underlying coronary obstruction [
6].
6.4.2. Brain Natriuretic Peptide
Unlike Troponin levels which are commonly elevated by peri-arrest processes, brain natriuretic peptide (BNP) levels may provide more useful information. In a smaller trial of 70 patients, Lee et al. demonstrated two useful characteristics of BNP levels. First, it has some ability to discriminate between cardiac etiologies and non-cardiac etiologies (Odds 2.1 of cardiac etiology with BNP ≥100 pg/mL). Second, BNP levels do not seem to vary after receiving CPR, which implies that CPR and arrest itself does not elevate the BNP level [
75]. That BNP levels are associated with cardiac etiology is unsurprising considering the significant association with BNP levels, heart failure and risk of SCD [
76].
6.4.3. D-Dimer
One of the significant etiologies of arrest, particularly PEA, is massive pulmonary embolism (PE). Difficult to diagnose clinically, PEs classically present without physical exam findings and frequently do not have ECG findings. PEs are definitively diagnosed with chest CT evaluation, but this first requires resuscitation and stabilization. D-dimer assessment provides indirect evidence of clotting and thus potentially thromboembolic events. D-dimer elevations are frequently falsely elevated by advanced age, malignancy and chest trauma [
77] which are similarly risk factors for cardiac arrest [
78]; thus, dimers are at risk of being falsely elevated in cardiac arrest patients. Similar to troponin levels, CPR can iatrogenically elevate D-dimer levels. Asano et al. demonstrated the D-dimer levels were elevated in 97.7% of arrest patients, and that D-dimer levels correlated with duration of CPR [
79].
6.5. Infection Biomarkers
Complete blood count, different microbial cultures, and procalcitonin are important indicators of ongoing infectious processes. Interpreting these results may be challenging given that infection may present as a cause or sequelae of cardiac arrest.
6.5.1. White Blood Cell Count
Nearly universally obtained after cardiac arrest, the Compete Blood Count (CBC) provides useful diagnostic and prognostic information after cardiac arrest. As cardiac arrest is a profoundly inflammatory state (due to CPR trauma, ischemia-reperfusion injury etc.) it frequently results in leukocytosis. Interestingly, the most common admission white count is in the normal range (about 60% of post-arrest patients), however leukocytosis (about 30% of patients) is a common finding [
80]. Like many-post arrest derangements, worsening leukocytosis, especially neutrophilia and monocytosis, are associated with worse outcomes [
80]
6.5.2. Blood Cultures
Blood cultures are a useful adjunct to help differentiate true infection from reactive leukocytosis. Rech et al., in a retrospective single center study, found a true bacteremia rate of about 16% in post cardiac arrest patients; and bacteremic patients had worse mortality [
81]. Coba et al., in a retrospective single center study, found a higher rate of bacteremia (38%), and interestingly found that bacteremia patients most often presented with non-shockable arrest. It is worth noting that the presence of bacteremia presents its own diagnostic challenges. It is known that sepsis can, itself, cause cardiac arrest in nearly 10% of septic patients [
82]. Conversely, it is well documented that sepsis is a common consequence of cardiac arrests with estimates ranging from 13 to 27% [
83]. The key to differentiating sepsis as cause or consequence lies in history, lab timing and clinical reasoning.
6.6. Toxicology Screening
Substance abuse remains an important cause of OHCA. A study by Rittenberger et al., found that drug overdose was the cause of nearly 15% of arrests, and the contributing drugs were most often opiates, benzodiazepines, or both; with some contribution from cocaine, methadone, marijuana and ethanol [
84]. Interestingly, overdose patients in this study were less likely to present in PEA (Odds 0.58 [0.31 – 1.07] p=0.08). A recent study Winkel et al., evaluated the utility of creating a toxicological profile using mass spectrometry for cardiac arrest patients. The authors found that 82% had at least one drug detected at SCA, polypharmacy was common (19%), and commonly abused drugs were encountered in 16% of SCA, but ultimately the clinical utility of complex toxicological screen was low [
85].
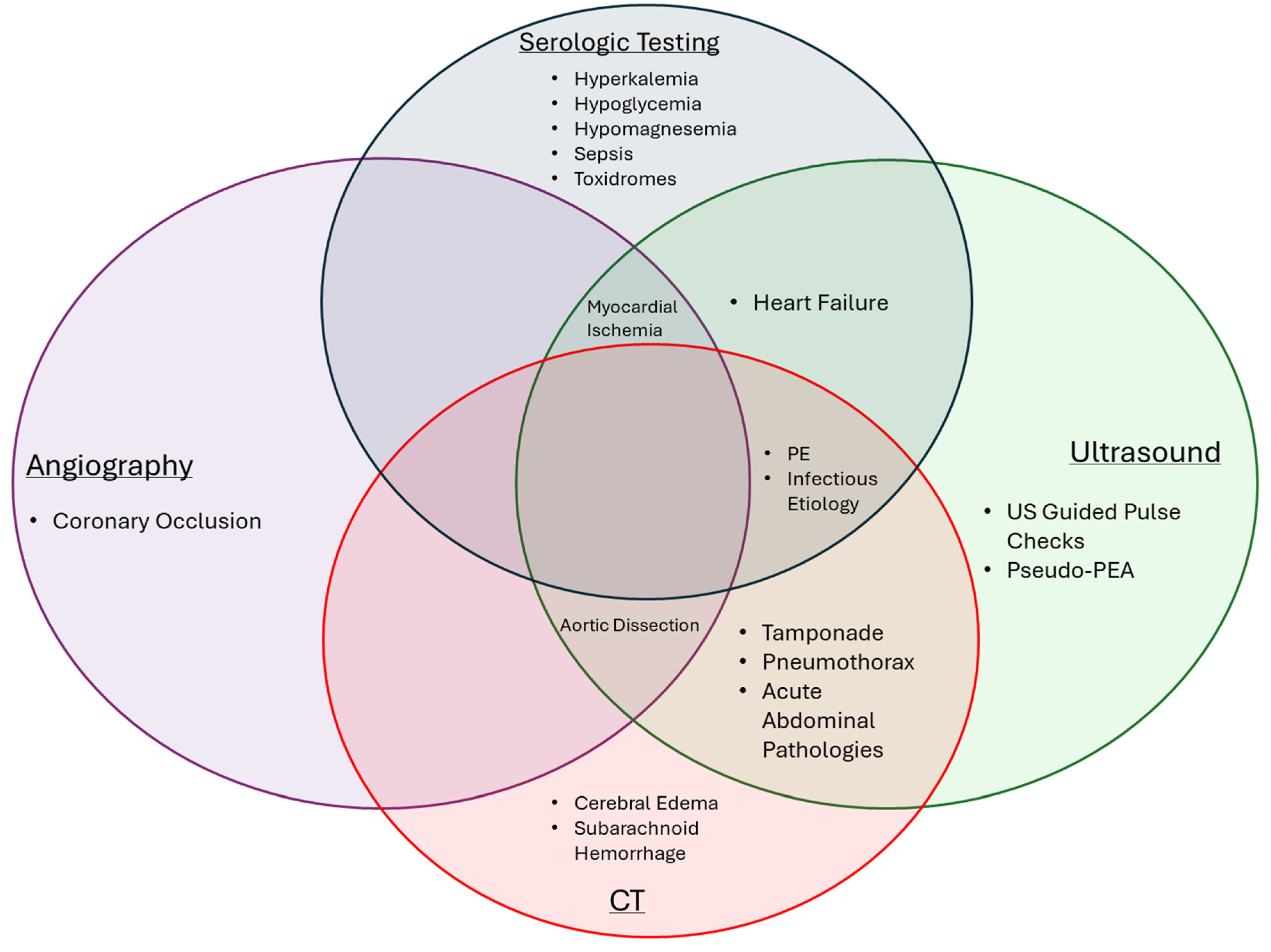
7. Conclusions
OHCA is a complex syndrome that requires an elaborate chain of collaboration between bystanders, EMS, and hospital teams. High-quality resuscitation, post-resuscitation diagnosis, and subsequent procedural or medical interventions remain the cornerstone elements to the care of OHCA patients. A combination of serological testing, ultrasonography, CT imaging, and angiography provide essential data points for diagnosing and treating underlying causes of cardiac arrest (
Figure 1). These elements require further research and development to better understand modalities that help in post-ROSC diagnosis and populations that would benefit from available interventions. There remains great potential to improve post-resuscitation care in OHCA, given the consistently high mortality worldwide. In this review, we focused on evolving evidence and research tailored around important diagnostic modalities used in post-resuscitation care, and we look forward to further investigations that will better guide post-arrest care for OHCA and improve outcomes.
Author Contributions
Conceptualization, S.M.H., W.R. and B.S.A.; investigation, S.M.H., W.R. and B.S.A.; writing—original draft preparation, S.M.H. and W.R.; writing—review and editing, B.S.A.; supervision, B.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Andoni Elola, E.A., Unai Irusta, Erik Alonso, Pamela Owens, Mary Chang, Ahamed Idris. ECG characteristics of Pulseless Electrical Activity associated with Return of Spontaneous Circulation in Out-of-Hospital Cardiac Arrest. Resuscitation 2018, 130, e54. [CrossRef]
- Elola, A.; Aramendi, E.; Irusta, U.; Picon, A.; Alonso, E.; Owens, P.; Idris, A. Deep Neural Networks for ECG-Based Pulse Detection during Out-of-Hospital Cardiac Arrest. Entropy (Basel) 2019, 21. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabanas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Staer-Jensen, H.; Nakstad, E.R.; Fossum, E.; Mangschau, A.; Eritsland, J.; Draegni, T.; Jacobsen, D.; Sunde, K.; Andersen, G.O. Post-Resuscitation ECG for Selection of Patients for Immediate Coronary Angiography in Out-of-Hospital Cardiac Arrest. Circ Cardiovasc Interv 2015, 8. [Google Scholar] [CrossRef]
- Baldi, E.; Schnaubelt, S.; Caputo, M.L.; Klersy, C.; Clodi, C.; Bruno, J.; Compagnoni, S.; Benvenuti, C.; Domanovits, H.; Burkart, R.; et al. Association of Timing of Electrocardiogram Acquisition After Return of Spontaneous Circulation With Coronary Angiography Findings in Patients With Out-of-Hospital Cardiac Arrest. JAMA Netw Open 2021, 4, e2032875. [Google Scholar] [CrossRef]
- E Baldi, S.S., M.L Caputo, C Klersy, C Clodi, J Bruno, S Compagnoni, C Benvenuti, R Burkart, R Fracchia, M Holzer, A Auricchio, S Savastano. Post-ROSC electrocardiogram timing in the management of out-of-hospital cardiac arrest: results of an international multicentric study (PEACE study). European Heart Journal 2020, 41. [CrossRef]
- Sharma, A.; Miranda, D.F.; Rodin, H.; Bart, B.A.; Smith, S.W.; Shroff, G.R. Do not disregard the initial 12 lead ECG after out-of-hospital cardiac arrest: It predicts angiographic culprit despite metabolic abnormalities. Resusc Plus 2020, 4, 100032. [Google Scholar] [CrossRef]
- Lee, S.E.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Joung, B. Combined ECG, Echocardiographic, and Biomarker Criteria for Diagnosing Acute Myocardial Infarction in Out-of-Hospital Cardiac Arrest Patients. Yonsei Med J 2015, 56, 887–894. [Google Scholar] [CrossRef]
- Park, M.J.; Choi, Y.J.; Shim, M.; Cho, Y.; Park, J.; Choi, J.; Kim, J.; Lee, E.; Kim, S.Y. Performance of ECG-Derived Digital Biomarker for Screening Coronary Occlusion in Resuscitated Out-of-Hospital Cardiac Arrest Patients: A Comparative Study between Artificial Intelligence and a Group of Experts. J Clin Med 2024, 13. [Google Scholar] [CrossRef]
- Gentile, F.R.; Baldi, E.; Klersy, C.; Schnaubelt, S.; Caputo, M.L.; Clodi, C.; Bruno, J.; Compagnoni, S.; Fasolino, A.; Benvenuti, C.; et al. Association Between Postresuscitation 12-Lead ECG Features and Early Mortality After Out-of-Hospital Cardiac Arrest: A Post Hoc Subanalysis of the PEACE Study. J Am Heart Assoc 2023, 12, e027923. [Google Scholar] [CrossRef]
- Riganello, F.; Zubler, F.; Haenggi, M.; De Lucia, M. Heart rate complexity: An early prognostic marker of patient outcome after cardiac arrest. Clin Neurophysiol 2022, 134, 27–33. [Google Scholar] [CrossRef]
- Wanha, W.; Kolodziejczak, M.; Kowalewski, M.; Januszek, R.; Kuzma, L.; Jaguszewski, M.; Tomaniak, M.; Darocha, S.; Kupczynska, K.; Dobrowolski, P.; et al. Out-of-hospital cardiac arrest: Do we have to perform coronary angiography? Cardiol J 2023, 30, 1026–1037. [Google Scholar] [CrossRef]
- Hollenbeck, R.D.; McPherson, J.A.; Mooney, M.R.; Unger, B.T.; Patel, N.C.; McMullan, P.W., Jr.; Hsu, C.H.; Seder, D.B.; Kern, K.B. Early cardiac catheterization is associated with improved survival in comatose survivors of cardiac arrest without STEMI. Resuscitation 2014, 85, 88–95. [Google Scholar] [CrossRef]
- Dumas, F.; Bougouin, W.; Geri, G.; Lamhaut, L.; Rosencher, J.; Pene, F.; Chiche, J.D.; Varenne, O.; Carli, P.; Jouven, X.; et al. Emergency Percutaneous Coronary Intervention in Post-Cardiac Arrest Patients Without ST-Segment Elevation Pattern: Insights From the PROCAT II Registry. JACC Cardiovasc Interv 2016, 9, 1011–1018. [Google Scholar] [CrossRef]
- Khan, M.S.; Shah, S.M.M.; Mubashir, A.; Khan, A.R.; Fatima, K.; Schenone, A.L.; Khosa, F.; Samady, H.; Menon, V. Early coronary angiography in patients resuscitated from out of hospital cardiac arrest without ST-segment elevation: A systematic review and meta-analysis. Resuscitation 2017, 121, 127–134. [Google Scholar] [CrossRef]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.D.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N Engl J Med 2019, 380, 1397–1407. [Google Scholar] [CrossRef]
- Patterson, T.; Perkins, G.D.; Perkins, A.; Clayton, T.; Evans, R.; Dodd, M.; Robertson, S.; Wilson, K.; Mellett-Smith, A.; Fothergill, R.T.; et al. Expedited transfer to a cardiac arrest centre for non-ST-elevation out-of-hospital cardiac arrest (ARREST): a UK prospective, multicentre, parallel, randomised clinical trial. Lancet 2023, 402, 1329–1337. [Google Scholar] [CrossRef]
- Viana-Tejedor, A.; Andrea-Riba, R.; Scardino, C.; Ariza-Sole, A.; Baneras, J.; Garcia-Garcia, C.; Jimenez Mena, M.; Vila, M.; Martinez-Selles, M.; Pastor, G.; et al. Coronary angiography in patients without ST-segment elevation following out-of-hospital cardiac arrest. COUPE clinical trial. Rev Esp Cardiol (Engl Ed) 2023, 76, 94–102. [Google Scholar] [CrossRef]
- Kern, K.B.; Radsel, P.; Jentzer, J.C.; Seder, D.B.; Lee, K.S.; Lotun, K.; Janardhanan, R.; Stub, D.; Hsu, C.H.; Noc, M. Randomized Pilot Clinical Trial of Early Coronary Angiography Versus No Early Coronary Angiography After Cardiac Arrest Without ST-Segment Elevation: The PEARL Study. Circulation 2020, 142, 2002–2012. [Google Scholar] [CrossRef]
- Hauw-Berlemont, C.; Lamhaut, L.; Diehl, J.L.; Andreotti, C.; Varenne, O.; Leroux, P.; Lascarrou, J.B.; Guerin, P.; Loeb, T.; Roupie, E.; et al. Emergency vs Delayed Coronary Angiogram in Survivors of Out-of-Hospital Cardiac Arrest: Results of the Randomized, Multicentric EMERGE Trial. JAMA Cardiol 2022, 7, 700–707. [Google Scholar] [CrossRef]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. N Engl J Med 2021, 385, 2544–2553. [Google Scholar] [CrossRef]
- Helfer, D.R.; Helber, A.R.; Ferko, A.R.; Klein, D.D.; Elchediak, D.S.; Deaner, T.S.; Slagle, D.; White, W.B.; Buckler, D.G.; Mitchell, O.J.L.; et al. Clinical factors associated with significant coronary lesions following out-of-hospital cardiac arrest. Acad Emerg Med 2022, 29, 456–464. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Bottiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Bradley J.; Petek, D.E.H., Lindsay G Panah, Philip E Dormish, Sean R Mendez, Elizabeth K Stevenson, David Cardona Estrada, Michael G Silverman. Abstract 375: Utility of Routine Early Head Computed Tomography Following Non-Traumatic Out-Of Hospital Cardiac Arrest. Circulation 2019, 140. [CrossRef]
- Viniol, S.; Thomas, R.P.; Konig, A.M.; Betz, S.; Mahnken, A.H. Early whole-body CT for treatment guidance in patients with return of spontaneous circulation after cardiac arrest. Emerg Radiol 2020, 27, 23–29. [Google Scholar] [CrossRef]
- Cankaya Gokdere, D.; Emektar, E.; Corbacioglu, S.K.; Yuzbasioglu, Y.; Ozturk, C.; Cevik, Y. The Role of Brain CT in Patients with Out-of-Hospital Cardiac Arrest with Return of Spontaneous Circulation. Am J Emerg Med 2022, 52, 143–147. [Google Scholar] [CrossRef]
- Adel, J.; Akin, M.; Garcheva, V.; Vogel-Claussen, J.; Bauersachs, J.; Napp, L.C.; Schafer, A. Computed-Tomography as First-line Diagnostic Procedure in Patients With Out-of-Hospital Cardiac Arrest. Front Cardiovasc Med 2022, 9, 799446. [Google Scholar] [CrossRef]
- Tam, J.; Soufleris, C.; Ratay, C.; Frisch, A.; Elmer, J.; Case, N.; Flickinger, K.L.; Callaway, C.W.; Coppler, P.J.; University of Pittsburgh Post-Cardiac Arrest, S. Diagnostic yield of computed tomography after non-traumatic out-of-hospital cardiac arrest. Resuscitation 2023, 189, 109898. [Google Scholar] [CrossRef]
- Branch, K.R.H.; Gatewood, M.O.; Kudenchuk, P.J.; Maynard, C.; Sayre, M.R.; Carlbom, D.J.; Edwards, R.M.; Counts, C.R.; Probstfield, J.L.; Brusen, R.; et al. Diagnostic yield, safety, and outcomes of Head-to-pelvis sudden death CT imaging in post arrest care: The CT FIRST cohort study. Resuscitation 2023, 188, 109785. [Google Scholar] [CrossRef]
- Branch, K.R.H.; Strote, J.; Gunn, M.; Maynard, C.; Kudenchuk, P.J.; Brusen, R.; Petek, B.J.; Sayre, M.R.; Edwards, R.; Carlbom, D.; et al. Early head-to-pelvis computed tomography in out-of-hospital circulatory arrest without obvious etiology. Acad Emerg Med 2021, 28, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Halablab, J.M., Anahita Ghorbani, Aarthi Kaviyarasu, William Reis, Benjamin, S. Abella, Oscar, J. L. Mitchell. Computed tomography assessment after out-of-hospital cardiac arrest resuscitation. Acad Emerg Med 2024, 31, 2. [CrossRef]
- Martinell, L.; Nielsen, N.; Herlitz, J.; Karlsson, T.; Horn, J.; Wise, M.P.; Unden, J.; Rylander, C. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit Care 2017, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Carrick, R.T.; Park, J.G.; McGinnes, H.L.; Lundquist, C.; Brown, K.D.; Janes, W.A.; Wessler, B.S.; Kent, D.M. Clinical Predictive Models of Sudden Cardiac Arrest: A Survey of the Current Science and Analysis of Model Performances. J Am Heart Assoc 2020, 9, e017625. [Google Scholar] [CrossRef]
- Romolo, J. Gaspari, J.A., Jacob Baxter, Drew Clare, John DeAngelis, Timothy Gleeson, Powell, L. Graham, John, E. Hipskind, Ryan Joseph, Monica Kapoor, Tobias Kummer, Margaret, R. Lewis, Stephanie Midgley, Robert, J. Lindsay, Offdan Narvaez-Guerra, Jason, T. Nomura, Mark, D. Scheatzle, Nikolai Schnittke, Michael, A. Secko, Trent, T. She, Zachary P. Soucy, Jeffrey Stowell, Rebecca G. Theophanous, Jordan Tozer, Tyler J. Yates, Andrew Balk. Visualization of occult ventricular fibrillation by echocardiography during cardiac arrest: A multicenter trial. Acad Emerg Med 2024, 31, 1. [CrossRef]
- Limb, C.; Siddiqui, M.A. Apparent asystole: are we missing a lifesaving opportunity? BMJ Case Rep 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Devia Jaramillo, G.; Navarrete Aldana, N.; Rojas Ortiz, Z. Rhythms and prognosis of patients with cardiac arrest, emphasis on pseudo-pulseless electrical activity: another reason to use ultrasound in emergency rooms in Colombia. Int J Emerg Med 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, R.; Price, S.; Steiger, H.V.; Seeger, F.H.; Ilper, H.; Ackermann, H.; Rudolph, M.; Uddin, S.; Weigand, M.A.; Muller, E.; et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitation 2010, 81, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Luqman, N.; Sung, R.J.; Wang, C.L.; Kuo, C.T. Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol 2007, 119, 283–290. [Google Scholar] [CrossRef]
- John R. Giudicessi, M.J.A. Abstract 14531: Etiology of Sentinel Non-Ischemic Ventricular Fibrillation Cardiac Arrest Across the Age Spectrum. Circulation 2018, 136.
- Gardner, K.F.; Clattenburg, E.J.; Wroe, P.; Singh, A.; Mantuani, D.; Nagdev, A. The Cardiac Arrest Sonographic Assessment (CASA) exam - A standardized approach to the use of ultrasound in PEA. Am J Emerg Med 2018, 36, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, R.; Walcher, F.; Seeger, F.H. Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med 2007, 35, S150–S161. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Cibinel, G.A.; Portale, G.; Forte, P.; Giannuzzi, R.; Pignataro, G.; Silveri, N.G. The proposal of an integrated ultrasonographic approach into the ALS algorithm for cardiac arrest: the PEA protocol. Eur Rev Med Pharmacol Sci 2010, 14, 77–88. [Google Scholar] [PubMed]
- Seif, D.; Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. Bedside ultrasound in resuscitation and the rapid ultrasound in shock protocol. Crit Care Res Pract 2012, 2012, 503254. [Google Scholar] [CrossRef]
- Durila, M. Reversible causes of cardiac arrest 4 “Ts” and 4 “Hs” can be easily diagnosed and remembered following general ABC rule, Motol University Hospital approach. Resuscitation 2018, 126, e7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Li, T.; Becker, L.B.; Owens, C.; Singh, N.; Gold, A.; Nelson, M.J.; Jafari, D.; Haddad, G.; Nello, A.V.; et al. Femoral artery Doppler ultrasound is more accurate than manual palpation for pulse detection in cardiac arrest. Resuscitation 2022, 173, 156–165. [Google Scholar] [CrossRef]
- Badra, K.; Coutin, A.; Simard, R.; Pinto, R.; Lee, J.S.; Chenkin, J. The POCUS pulse check: A randomized controlled crossover study comparing pulse detection by palpation versus by point-of-care ultrasound. Resuscitation 2019, 139, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lien, W.C.; Chong, K.M.; Chang, C.H.; Cheng, S.F.; Chang, W.T.; Ma, M.H.; Chen, W.J. Impact of Ultrasonography on Chest Compression Fraction and Survival in Patients with Out-of-hospital Cardiac Arrest. West J Emerg Med 2023, 24, 322–330. [Google Scholar] [CrossRef]
- Clattenburg, E.J.; Wroe, P.; Brown, S.; Gardner, K.; Losonczy, L.; Singh, A.; Nagdev, A. Point-of-care ultrasound use in patients with cardiac arrest is associated prolonged cardiopulmonary resuscitation pauses: A prospective cohort study. Resuscitation 2018, 122, 65–68. [Google Scholar] [CrossRef]
- Uppiretla, A.K.; G, M.G.; Rao, S.; Don Bosco, D.; S, M.S.; Sampath, V. Effects of Chest Compression Fraction on Return of Spontaneous Circulation in Patients with Cardiac Arrest; a Brief Report. Adv J Emerg Med 2020, 4, e8. [Google Scholar] [CrossRef]
- Ventorp, S.; Wagner, H.; Härdig, B.M. Does Point-of-care Ultrasound Improve Survival when Used During Cardiac Arrest? A Systematic Review and Meta-analysis. Medical Research Archives 2023, 11. [Google Scholar] [CrossRef]
- Teran, F.; Prats, M.I.; Nelson, B.P.; Kessler, R.; Blaivas, M.; Peberdy, M.A.; Shillcutt, S.K.; Arntfield, R.T.; Bahner, D. Focused Transesophageal Echocardiography During Cardiac Arrest Resuscitation: JACC Review Topic of the Week. J Am Coll Cardiol 2020, 76, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Arntfield, R.; Pace, J.; Hewak, M.; Thompson, D. Focused Transesophageal Echocardiography by Emergency Physicians is Feasible and Clinically Influential: Observational Results from a Novel Ultrasound Program. J Emerg Med 2016, 50, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zheng, Z.; Jiang, L.; Gao, Y.; Xu, J.; Jin, X.; Chen, Q.; Zhang, M. The predictive value of bedside ultrasound to restore spontaneous circulation in patients with pulseless electrical activity: A systematic review and meta-analysis. PLoS One 2018, 13, e0191636. [Google Scholar] [CrossRef]
- Kedan, I.; Ciozda, W.; Palatinus, J.A.; Palatinus, H.N.; Kimchi, A. Prognostic value of point-of-care ultrasound during cardiac arrest: a systematic review. Cardiovasc Ultrasound 2020, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.S.; Chattopadhyay, A.; Lu, T.P.; Liao, S.H.; Chang, C.M.; Lee, Y.C.; Lo, W.E.; Wu, J.J.; Hsieh, V.C.; Hu, S.Y.; et al. Effect of end-stage kidney disease on the return of spontaneous circulation in Taiwanese adults with out-of-hospital cardiac arrest. Sci Rep 2023, 13, 7905. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ Arrhythm Electrophysiol 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Ornato, J.P.; Gonzalez, E.R.; Starke, H.; Morkunas, A.; Coyne, M.R.; Beck, C.L. Incidence and causes of hypokalemia associated with cardiac resuscitation. Am J Emerg Med 1985, 3, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Shida, H.; Matsuyama, T.; Komukai, S.; Irisawa, T.; Yamada, T.; Yoshiya, K.; Park, C.; Nishimura, T.; Ishibe, T.; Yagi, Y.; et al. Early prognostic impact of serum sodium level among out-of-hospital cardiac arrest patients: a nationwide multicentre observational study in Japan (the JAAM-OHCA registry). Heart Vessels 2022, 37, 1255–1264. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, M.S.; Kwon, W.Y.; Shin, J.; Suh, G.J.; Jung, Y.S.; Song, W.J.; Yeo, G.; Jo, Y.H.; Investigators, S.C. Hypernatremia is associated with poor long-term neurological outcomes in out-of-hospital cardiac arrest survivors. Am J Emerg Med 2022, 59, 30–36. [Google Scholar] [CrossRef]
- Yarmohammadi, H.; Uy-Evanado, A.; Reinier, K.; Rusinaru, C.; Chugh, H.; Jui, J.; Chugh, S.S. Serum Calcium and Risk of Sudden Cardiac Arrest in the General Population. Mayo Clin Proc 2017, 92, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Guimard, C.; Batard, E.; Lavainne, F.; Trewick, D. Is severe hypercalcemia immediately life-threatening? Eur J Emerg Med 2018, 25, 110–113. [Google Scholar] [CrossRef]
- Kieboom, B.C.; Niemeijer, M.N.; Leening, M.J.; van den Berg, M.E.; Franco, O.H.; Deckers, J.W.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Serum Magnesium and the Risk of Death From Coronary Heart Disease and Sudden Cardiac Death. J Am Heart Assoc 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Negru, A.G.; Pastorcici, A.; Crisan, S.; Cismaru, G.; Popescu, F.G.; Luca, C.T. The Role of Hypomagnesemia in Cardiac Arrhythmias: A Clinical Perspective. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; Daphna-Iken, D.; Chen, Y.S.; VanderWeele, J.; Jethi, K.; Fisher, S.J. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013, 62, 3570–3581. [Google Scholar] [CrossRef]
- Hifumi, T.; Kiriu, N.; Kato, H.; Inoue, J.; Koido, Y. Survival after prolonged resuscitation from cardiac arrest due to diabetic ketoacidosis using extracorporeal life support. Am J Emerg Med 2013, 31, 892 e891–e892. [Google Scholar] [CrossRef] [PubMed]
- Wongtanasarasin, W.; Phinyo, P. Dextrose Administration and Resuscitation Outcomes in Patients with Blood Sugar Less Than 150 mg/dL during Cardiopulmonary Resuscitation: An Observational Data Analysis. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Wongtanasarasin, W.; Ungrungseesopon, N.; Phinyo, P. Association between Intra-Arrest Blood Glucose Level and Outcomes of Resuscitation at the Emergency Department: A Retrospective Study. Journal of Clinical Medicine 2022, 11, 3067. [Google Scholar] [CrossRef]
- Iesu, E.; Franchi, F.; Zama Cavicchi, F.; Pozzebon, S.; Fontana, V.; Mendoza, M.; Nobile, L.; Scolletta, S.; Vincent, J.L.; Creteur, J.; et al. Acute liver dysfunction after cardiac arrest. PLoS One 2018, 13, e0206655. [Google Scholar] [CrossRef]
- Delignette, M.C.; Stevic, N.; Lebosse, F.; Bonnefoy-Cudraz, E.; Argaud, L.; Cour, M. Acute liver failure after out-of-hospital cardiac arrest: An observational study. Resuscitation 2024, 197, 110136. [Google Scholar] [CrossRef]
- Zhou, B.C.; Zhang, Z.; Zhu, J.J.; Liu, L.J.; Liu, C.F. Blood Lactate or Lactate Clearance: Which Is Robust to Predict the Neurological Outcomes after Cardiac Arrest? A Systematic Review and Meta-Analysis. Biomed Res Int 2018, 2018, 8014213. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.W.; Andersen, L.W.; Giberson, T.; Gaieski, D.F.; Abella, B.S.; Peberdy, M.A.; Rittenberger, J.C.; Callaway, C.W.; Ornato, J.; Clore, J.; et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med 2014, 42, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.A.; Wares, C.M.; Mayer, K.A.; Runyon, M.S.; Studnek, J.R.; Ward, S.L.; Kraft, K.M.; Heffner, A.C. Troponin Marker for Acute Coronary Occlusion and Patient Outcome Following Cardiac Arrest. West J Emerg Med 2015, 16, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Agusala, V.; Khera, R.; Cheeran, D.; Mody, P.; Reddy, P.P.; Link, M.S. Diagnostic and prognostic utility of cardiac troponin in post-cardiac arrest care. Resuscitation 2019, 141, 69–72. [Google Scholar] [CrossRef] [PubMed]
- S.O. Hwang, K.C., H. Kim, K. Lee. Clinical implications of elevated plasma b-type natriuretic peptide in cardiac arrest. Annals of Emergency Medicine 2004, 44. [CrossRef]
- Berger, R.; Huelsman, M.; Strecker, K.; Bojic, A.; Moser, P.; Stanek, B.; Pacher, R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002, 105, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Kabrhel, C.; Mark Courtney, D.; Camargo, C.A., Jr.; Plewa, M.C.; Nordenholz, K.E.; Moore, C.L.; Richman, P.B.; Smithline, H.A.; Beam, D.M.; Kline, J.A. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med 2010, 17, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, H.L.; Jonsson, M.; Hedayati, E.; Hedman, C.; Djarv, T. Poorer survival after out-of-hospital cardiac arrest among cancer patients: a population-based register study. Eur Heart J Acute Cardiovasc Care 2023, 12, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Kurabayashi, M.; Yamauchi, Y.; Sasano, T. Relationship between D-dimer level upon emergency room arrival and the duration of cardiac arrest in patients with witnessed out-of-hospital cardiac arrest. Heart and Vessels 2021, 36, 731–737. [Google Scholar] [CrossRef]
- Smith, R.J.; Sarma, D.; Padkins, M.R.; Gajic, O.; Lawler, P.R.; Van Diepen, S.; Kashani, K.B.; Jentzer, J.C. Admission Total Leukocyte Count as a Predictor of Mortality in Cardiac Intensive Care Unit Patients. JACC Adv 2024, 3, 100757. [Google Scholar] [CrossRef]
- Colon Hidalgo, D.; Menich, B.E.; Lovett, S.; Rech, M.A. The incidence and characteristics of bacteremia in cardiac arrest. Heart Lung 2022, 52, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Duazo, C.; Hsiung, J.C.; Qian, F.; Sherrod, C.F.; Ling, D.A.; Wu, I.J.; Hsu, W.T.; Liu, Y.; Wei, C.; Tehrani, B.; et al. In-hospital Cardiac Arrest in Patients With Sepsis: A National Cohort Study. Front Med (Lausanne) 2021, 8, 731266. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.W.; Fitzgerald, J.C.; Weiss, S.L.; Nadkarni, V.M.; Sutton, R.M.; Berg, R.A. Sepsis-associated in-hospital cardiac arrest: Epidemiology, pathophysiology, and potential therapies. J Crit Care 2017, 40, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Lynch, M.J.; Kristan, J.; Morgan, P.; Gerstel, S.J.; Callaway, C.W.; Rittenberger, J.C.; Pittsburgh Post-Cardiac Arrest, S. Recreational drug overdose-related cardiac arrests: break on through to the other side. Resuscitation 2015, 89, 177–181. [Google Scholar] [CrossRef]
- Stampe, N.K.; Glinge, C.; Rasmussen, B.S.; Bhardwaj, P.; Linnet, K.; Jabbari, R.; Paludan-Muller, C.; Hassager, C.; Kjaergaard, J.; Tfelt-Hansen, J.; et al. Toxicological profile using mass spectrometry in sudden cardiac arrest survivors admitted to a tertiary centre. Resuscitation 2024, 198, 110197. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).