Submitted:
10 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Durrheim, D.N; Wynd, S.; Liese, B.; Gyapong, J.O. Lymphatic filariasis endemicity–an indicator of poverty? Trop Med Int Health 2004, 9(8), pp. 843-845. [CrossRef]
- Resolution A. RES/70/1. Transforming our world: the 2030 agenda for sustainable development. Seventieth United Nations General Assembly 2015, 25, pp. 86-97.
- World Health Organization. Validation of elimination of lymphatic filariasis as a public health problem. 2017.
- Dreyer, G.; Norões, J.; Figueredo-Silva, J.; Piessens, W.F. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today 2000, 16(12), pp. 544-8.
- Consoli, R.A.G.B; Oliveira, R.L. Principais mosquitos de importância sanitária do Brasil, 1rd ed.; Memórias do Instituto Oswaldo Cruz – Fiocruz: Rio de Janeiro, Brasil, 1994; pp. 228.
- Rocha, A.; Barbosa, C.S.; Brandão Filho, S.P.; Oliveira, C.M.F.; Almeida, A.M.P; Gomes, Y.M. Primeiro workshop interno dos serviços de referência do Centro de Pesquisas Aggeu Magalhães da Fundação Oswaldo Cruz. Rev Soc Bras Med Trop 2009, 42, pp. 228-34.
- Fontes, G.; Leite, A.B.; de Lima, A.R.V.; Freitas, H.; Ehrenberg, J.P.; da Rocha, E.M.M. Lymphatic filariasis in Brazil: epidemiological situation and outlook for elimination. Parasites Vectors 2012, 5(1), pp. 1-11.
- Nascimento, J.B.; Brandão, E.; da Silva, F.D.; Bernart, F.D.; Rocha, A. The ituation of Lymphatic Filariasis in the municipality of Paulista, Pernambuco, Brazil. Rev Pat Trop 2018, 47(4), pp. 217-224.
- Xavier, A.; Oliveira, H.; Aguiar-Santos, A.; Barbosa Júnior, W.; da Silva, E.; Braga, C.; Bonfim, C.; Medeiros, Z. Assessment of transmission in areas of uncertain endemicity for lymphatic filariasis in Brazil. PLoS Negl Trop Dis 2019, 13(11), e0007836–e. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Moreno, M.; Fuller, D.O.; Cardenas, G.; Petrie, W.D..; Beier, J.C. Urbanization favors the proliferation of Aedes aegypti and Culex quinquefasciatus in urban areas of Miami-Dade County, Florida. Sci Rep 2021, 11(1), pp. 22989. [CrossRef]
- Moise, I.K.; Riegel, C.; Muturi, E.J. Environmental and social-demographic predictors of the southern house mosquito Culex quinquefasciatus in New Orleans, Louisiana. Parasites Vectors 2018, 11(1), pp. 249.
- Ximenes, R.A.A.; Martelli, C.M.T.; Souza, W.V.; Lapa, T.M.; Albuquerque, M.F.M.; Andrade, A.L.S.S.; Morais Neto, O.L.; Silva, S.A.; Lima, M.L.C; Portugal, J.L. Vigilância de doenças endêmicas em áreas urbanas: a interface entre mapas de setores censitários e indicadores de morbidade. Cad Saúde Pública 1999, 15(1), pp. 53-62.
- Domínguez-Berjón, M.F.; Borrell, C.; Cano-Serral, G.; Esnaola, S.; Nolasco, A.; Pasarín, M.I.; Ramisc, R.; Saurinag, C.; Escolar-Pujolarh, A. Constructing a deprivation index based on census data in large Spanish cities(the MEDEA project). Gac Sanit 2008, 22(3), pp. 179-87.
- Braga, C.; Ximenes, R.A.A.; Albuquerque, M.F.P.M.; Souza, W.V.; Miranda, J.; Brayner, F.; Alves, L.; Silva, L.; Dourado, I.. Avaliação de indicador sócio-ambiental utilizado no rastreamento de áreas de transmissão de filariose linfática em espaços urbanos. Cad Saúde Pública 2001, 17(5) pp. 1211-8.
- Weiss, P.S.; Michael, E.; Richards, F.O. Simulating a Transmission Assessment Survey: An evaluation of current methods used in determining the elimination of the neglected tropical disease, Lymphatic Filariasis. Int J Infect Dis 2021, 102, pp. 422-8.
- Wanji, S.; Amvongo-Adjia, N.; Koudou, B.; Njouendou, A.J.; Chounna Ndongmo, P.W.; Kengne-Ouafo, J.A.; Datchoua-Poutcheu, F.R.; Fovennso, B.A.; Tayong, D.B.; Fombad, F.F.; Fischer, P.U.; Enyong, P.I.; Bockarie, M. Cross-reactivity of filariais ICT cards in areas of contrasting endemicity of Loa loa and Mansonella perstans in Cameroon: implications for shrinking of the lymphatic filariasis map in the Central African Region. PLoS Negl Trop Dis 2015, 9(11), e0004184. [Google Scholar] [CrossRef] [PubMed]
- Riches, N.; Badia-Rius, X.; Mzilahowa, T.; Kelly-Hope, L.A. A systematic review of alternative surveillance approaches for lymphatic filariasis in low prevalence settings: Implications for post-validation settings. PLoS Negl Trop Dis 2020, 14(5), e0008289. [Google Scholar] [CrossRef] [PubMed]
- Dobbin Jr, J.; Cruz, A. Inquéritos de filariose em alguns municípios do Litoral-Mata de Pernambuco. Rev Bras Malariol Doenças Trop 1967, 19, pp.45-51.
- Censo Populacional do Instituto Brasileiro de Geografia e Estatística. Available online: http://www.censo2010.ibge.gov.br (accessed on 23 May 2020).
- Kaiser, H.F. An index of factorial simplicity. Psychometrika 1974, 39(1), pp. 31-6.
- Bartlett, M.S. The effect of standardization on a χ 2 approximation in factor analysis. Biometrika 1951, 38(3/4), pp. 337-44.
- Arndt, S.; Turvey, C.; Andreasen, N.C. Correlating and predicting psychiatric symptom ratings: Spearmans r versus Kendalls tau correlation. J Psychiatr Res 1999, 33(2), pp. 97-104.
- Carvalho, F.R.D. Análise fatorial. Dissertação de Mestrado em Matemática apresentada à Faculdade de Ciências e Tecnologia, Universidade de Coimbra, Coimbra, 2013.
- Sharma, S. Applied multivariate techniques. 1996.
- Dean, C.; Lawless, J.F.; Willmot, G.E. A Mixed Poisson-Inverse-Gaussian Regression Model. Can J Statistics 1989, 17(2), pp. 171-81.
- Sakamoto, Y.; Ishiguro, M.; Kitagawa, G. Akaike information criterion statistics. 1rd ed.; KTK Scientific Publishers; D. Reidel; Sold and distributed in the U.S.A. and Canada by Kluwer Academic Publishers. 1986.
- Ramesh, A.; Cameron, M.; Spence, K.; Spaans, R.H. Melo-Santos, M.A.V.; Paiva, M.H.S.; Guedes, D.R.D.; Barbosa, R.M.R.; Oliveira, C.M.F.; Sá, A.; Jeffries, C.L.; Castanha, P.M.S.; Oliveira, P.A.S.; Walker, T.; Alexander, N.; Braga, C. Development of an urban molecular xenomonitoring system for lymphatic filariasis in the Recife Metropolitan Region, Brazil. PLoS Negl Trop Dis 2018, 12(10), e0006816.
- Forattini, O.P. Entomologia médica. USP: São Paulo, Brasil, 1965; pp. 506.
- Eder, M.; Cortes, F.; Teixeira de Siqueira Filha, N.; Araújo de França, G.V.; Degroote, S.; Braga, C.; Ridde, V.; Martelli, C.M.T. Scoping review on vector-borne diseases in urban areas: transmission dynamics, vectorial capacity and co-infection. Infect Dis Poverty 2018; 7(1), pp. 1-24.
- Graves, P.M.; Sheridan, S.; Fuimaono, S.; Lau, C.L. Demographic, socioeconomic and disease knowledge factors, but not population mobility, associated with lymphatic filariasis infection in adult workers in American Samoa in 2014. Parasites Vectors 2020, 13(1), pp. 125-143.
- Zerbo, A.; Castro Delgado, R.; Arcos González, P. Exploring the dynamic complexity of risk factors for vector-borne infections in sub-Saharan Africa: Case of urban lymphatic filariasis. J Biosaf Biosecur 2021, 3(1), pp. 17-21.
- Riches, N.; Badia-Rius, X.; Mzilahowa, T.; Kelly-Hope, L.A. A systematic review of alternative surveillance approaches for lymphatic filariasis in low prevalence settings: Implications for post-validation settings. PLoS Negl Trop Dis 2020, 14(5), :e0008289.
- Simonsen, P.E.; Mwakitalu, M.E. Urban lymphatic filariasis. Parasitol Res 2013, 112(1), pp. 35-44.
- Slater, H.; Michael, E. Mapping, bayesian geostatistical analysis and spatial prediction of lymphatic filariasis prevalence in Africa. PLoS One 2013, 8(8), e71574. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.C.; Mkwanda, S.; Mzilahowa, T.; Bockarie, M.J.; Kelly-Hope, L.A. Quantifying filariasis and malaria control activities in relation to lymphatic filariasis elimination: a multiple intervention score map (MISM) for Malawi. Trop Med Int Health 2014, 19(2), pp. 224-35.
- Allik, M.; Leyland, A.; Ichihara, M.Y.T.; Dundas, R. Creating small-area deprivation indices: a guide for stages and options. J Epidemiol Community Health 2020, 74(1), pp. 20-5.
- Xavier, M.D.N.; Santos, E.M.M.; Silva, A.; Gomes Júnior, P.P.; Barbosa, R.M.R.; Oliveira, C.M.F. Field evaluation of sticky BR-OVT traps to collect culicids eggs and adult mosquitoes inside houses. Rev Soc Bras Med Trop 2018, 51(3), pp. 297-303.
- Santos, S.A.; Barbosa, R.M. Immature Aedes mosquitoes colonize Culex quinquefasciatus breeding sites in neighborhoods in the municipality of Olinda, State of Pernambuco. Rev Soc Bras Med Trop 2014, 47(6), pp. 775-7.
- Lupenza, E.; Gasarasi, D.B.; Minzi, O.M. Lymphatic filariasis, infection status in Culex quinquefasciatus and Anopheles species after six rounds of mass drug administration in Masasi District, Tanzania. Infect Dis Poverty 2021, 10(1), pp. 20.
- Bonfim, C.; Netto, M.J.; Pedroza, D.; Portugal, J.L.; Medeiros, Z. A socioenvironmental composite index as a tool for identifying urban areas at risk of lymphatic filariasis. Trop Med Int Health 2009, 14(8), pp. 877-84.
- Bonfim, C.; Aguiar-Santos, A.M.; Pedroza Jr, D..; Costa, T.R.; Portugal, J.L.; Oliveira, C.; Medeiros, Z. Social deprivation index and lymphatic filariasis: a tool for mapping urban areas at risk in northeastern Brazil. Int Health 2009, 1(1), pp. 78-84.
- Xavier. A.; Bonfim, C.; Barbosa Júnior, W.; Bezerra, G.; Oliveira, C.; Uchikawa, R.; da Silva, F.; Aguiar-Santos, A.; Medeiros, Z. Influence of social and environmental factors for Culex quinquefasciatus distribution in Northeastern Brazil: a risk index. Int J Environ Health Res 2023, 33(12), pp. 1580-1590. [CrossRef]
- Bonfim, C.; Alves, A.; Costa, T.R.; Alencar, F.; Pedroza, D.; Portugal, J.L.; Medeiros, Z. Spatial analysis and privation index to identify urban areas with a high risk of lymphatic filariasis. Trop Med Int Health 2011, 16(6), pp. 748-755.
| Variable | Definition | Indicator |
|---|---|---|
| WATS | Households with inadequate water supply | Proportion of households without internal water plumbing and without access to public water supply network relative to the total number of permanent private households |
| BATHR | Households without exclusive use bathrooms for residents | Proportion of households without showers or bathtubs and exclusive use of toilet facilities for household residents. |
| GARB | Households with inadequate garbage collection | Proportion of households with garbage collection by public or private company services relative to the total number of permanent private households. |
| ELEC | Households without electricity | Proportion of households without any type of electricity supply |
| HOUS | Households with 6 or more residents | Proportion of households with 6 or more residents |
| SEW | Households without sewage system | Proportion of households without drainage system for waste from the bathroom or toilet. |
| ISEW | Households with inadequate sewage systems | Proportion of households without plumbing for waste from the bathroom or toilet, connected to a collection system that leads to a general drainage system in the area, region, or municipality, even if the system does not have a sewage treatment plant. |
| ILIND | Illiterate individuals who are household heads | Proportion of household heads who either did not know how to read and write, those who learned but forgot due to an unconsolidated literacy process, and those who could only sign their own name. |
| RACE | Resident individuals self-declared as black race or ethnicity | Proportion of resident individuals self-declared as black race or ethnicity |
| REND | Individuals responsible with no positive income | Proportion of individuals responsible for permanent private households with no positive income, meaning no type of earnings in value. |
| Variable | Acronyms | Pearson Correlation | Spearman Correlation | ||
|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | ||
| Proportion of households without public water supply | WATS | 0,34 | 0,00 | 0,41 | 0,00 |
| Proportion of households without exclusive-use bathrooms for residents | BATHR | 0,06 | 0,54 | 0,34 | 0,00 |
| Proportion of households without any kind of sewage system | SEW | 0,06 | 0,54 | -0,13 | 0,21 |
| Proportion of households without adequate sewage system | ISEW | 0,10 | 0,30 | 0,10 | 0,30 |
| Proportion of households without garbage collected by sanitation services | GARB | 0,37 | 0,00 | 0,32 | 0,00 |
| Proportion of households without electricity | ELEC | 0,03 | 0,74 | 0,23 | 0,02 |
| Proportion of households with 6 or more residents | HOUS | 0,16 | 0,11 | 0,22 | 0,03 |
| Proportion of illiterate individuals responsible for the household | ILIND | 0,26 | 0,01 | 0,10 | 0,30 |
| Proportion of resident individuals of black race/color | RACE | 0,02 | 0,80 | 0,08 | 0,40 |
| Proportion of household heads with no positive income | REND | -0,10 | 0,31 | 0,09 | 0,37 |
| Per capita household income | RENDo | -0,21 | 0,03 | -0,34 | 0,00 |
| Per capita income of household heads | RENDp | -0,16 | 0,11 | -0,32 | 0,00 |
| KMO (0,68¹) | Variáveis | WATS | ISEW | GARB | HOUS | ILIND | RENDo | RENp |
|---|---|---|---|---|---|---|---|---|
| 0,56 | WATS | 1 | 0,01 | 0,23 | 0,27 | 0,46 | -0,05 | 0,06 |
| 0,60 | ISEW | 1 | 0,10 | 0,49 | 0,24 | -0,24 | -0,20 | |
| 0,87 | GARB | 1 | 0,25 | 0,47 | -0,36 | -0,30 | ||
| 0,68 | HOUS | 1 | 0,54 | -0,32 | -0,30 | |||
| 0,75 | ILIND | 1 | -0,53 | -0,53 | ||||
| 0,59 | RENDo | 1 | 0,93 | |||||
| 0,57 | RENp | 1 |
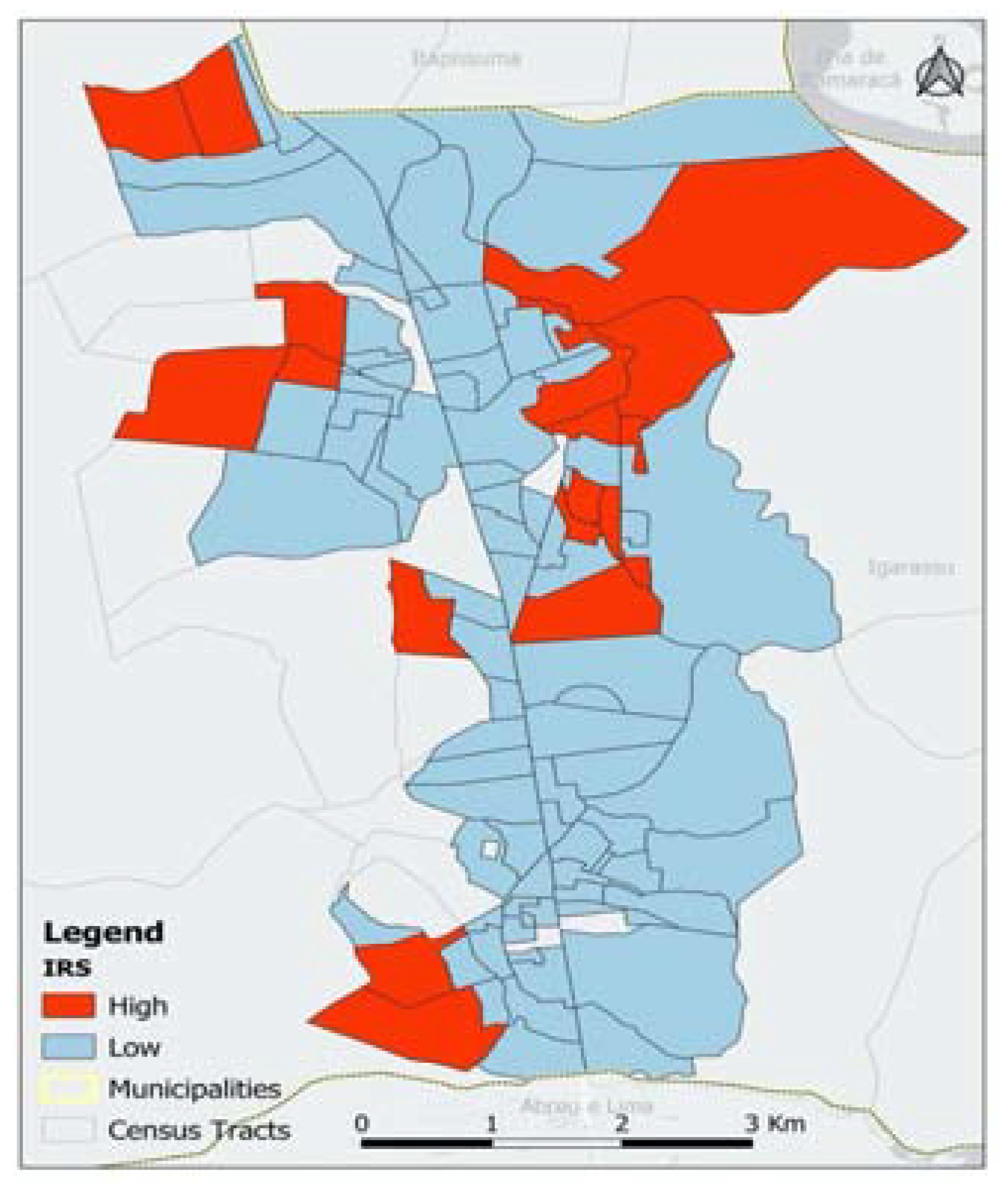
| Strata | SDI | Model with 4 bands | Model with 2 bands | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| min | max | Coeff.¹ | p-value | N | % | Coeff.¹ | p-value | N | % | |||
| Very low risk (1)² | 0,00 | 0,20 | 4,51 | 0,00 | 12 | 11,7% | 4,68 | 0,00 | 82 | 79,6% | ||
| Low risk (2) | 0,21 | 0,34 | -0,13 | 0,68 | 37 | 35,9% | - | - | - | - | ||
| Medium risk (3) | 0,35 | 0,48 | 0,45 | 0,16 | 33 | 32,0% | - | - | - | - | ||
| High risk (4) | 0,51 | 1,00 | 0,93 | 0,01 | 21 | 20,4% | 0,81 | 0,00 | 21 | 20,4% | ||
| Dispersion parameter | - | - | 1,66 | 0,01 | - | - | 1,82 | 0,00 | - | - | ||
| Source: Authors, 2023¹ Parameter estimates given by the regression model for the respective risk stratum/dispersion parameter.² Risk stratum 1 represents the intercept of the regression model. | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





