Submitted:
10 August 2024
Posted:
13 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
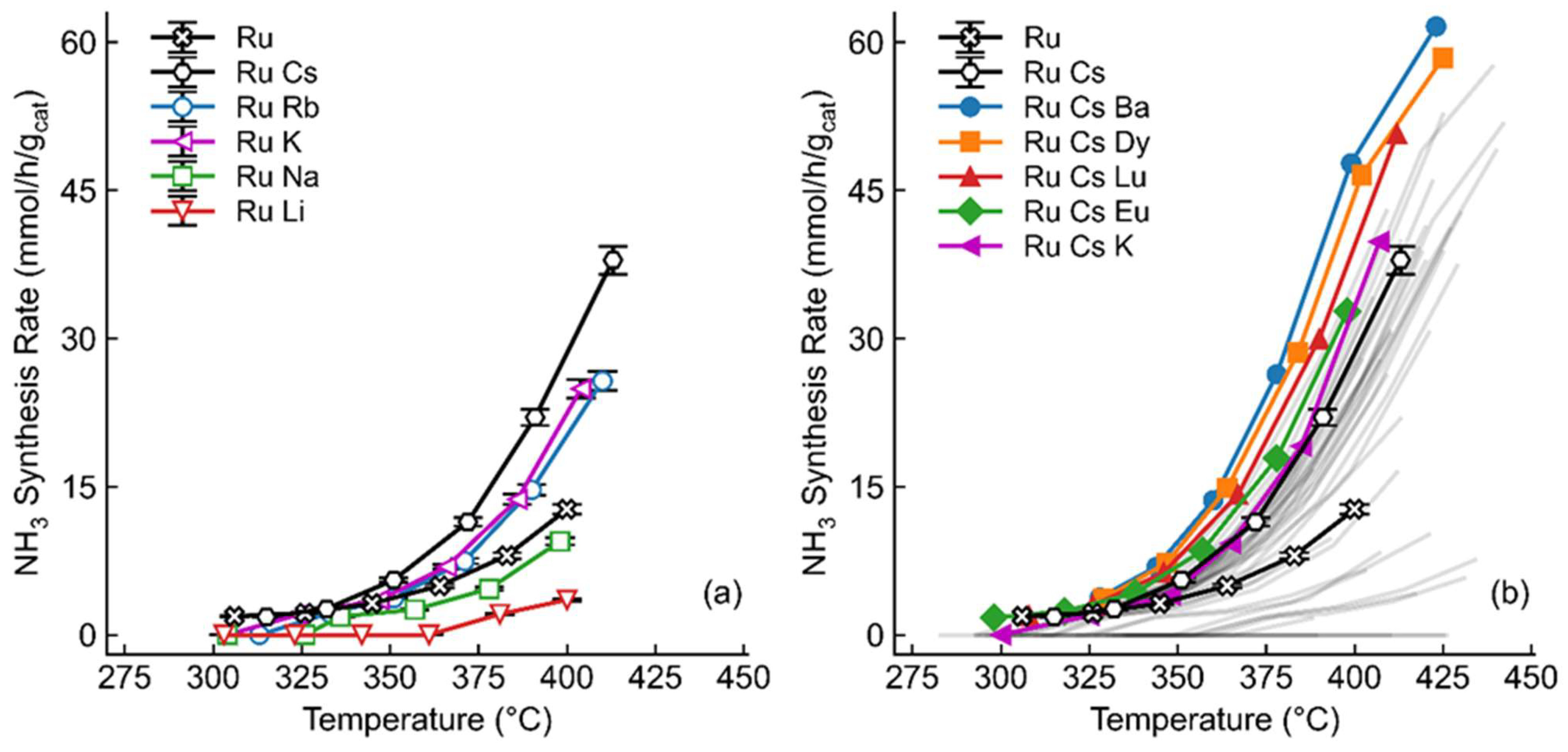
2.1. Screening of Promoters
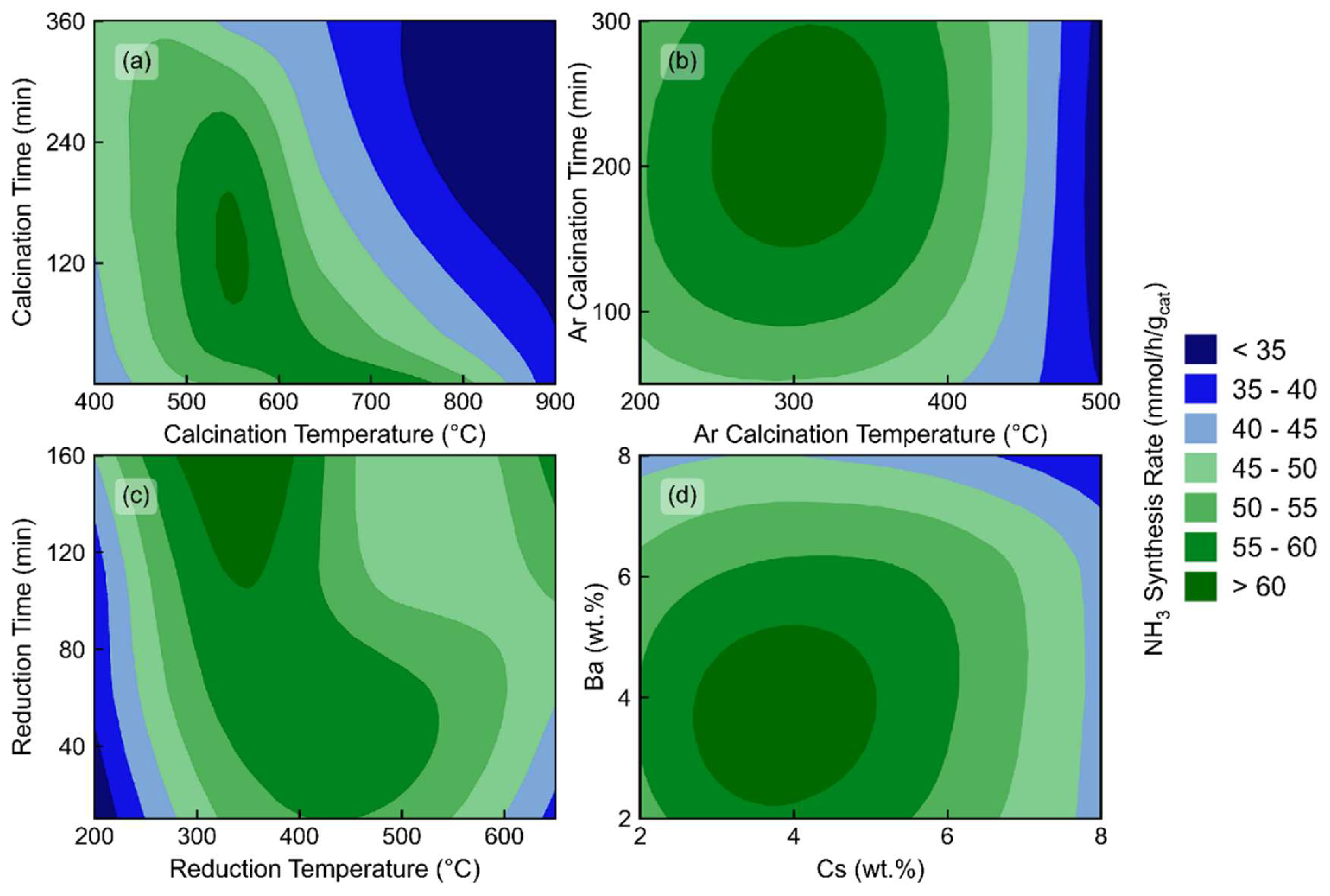
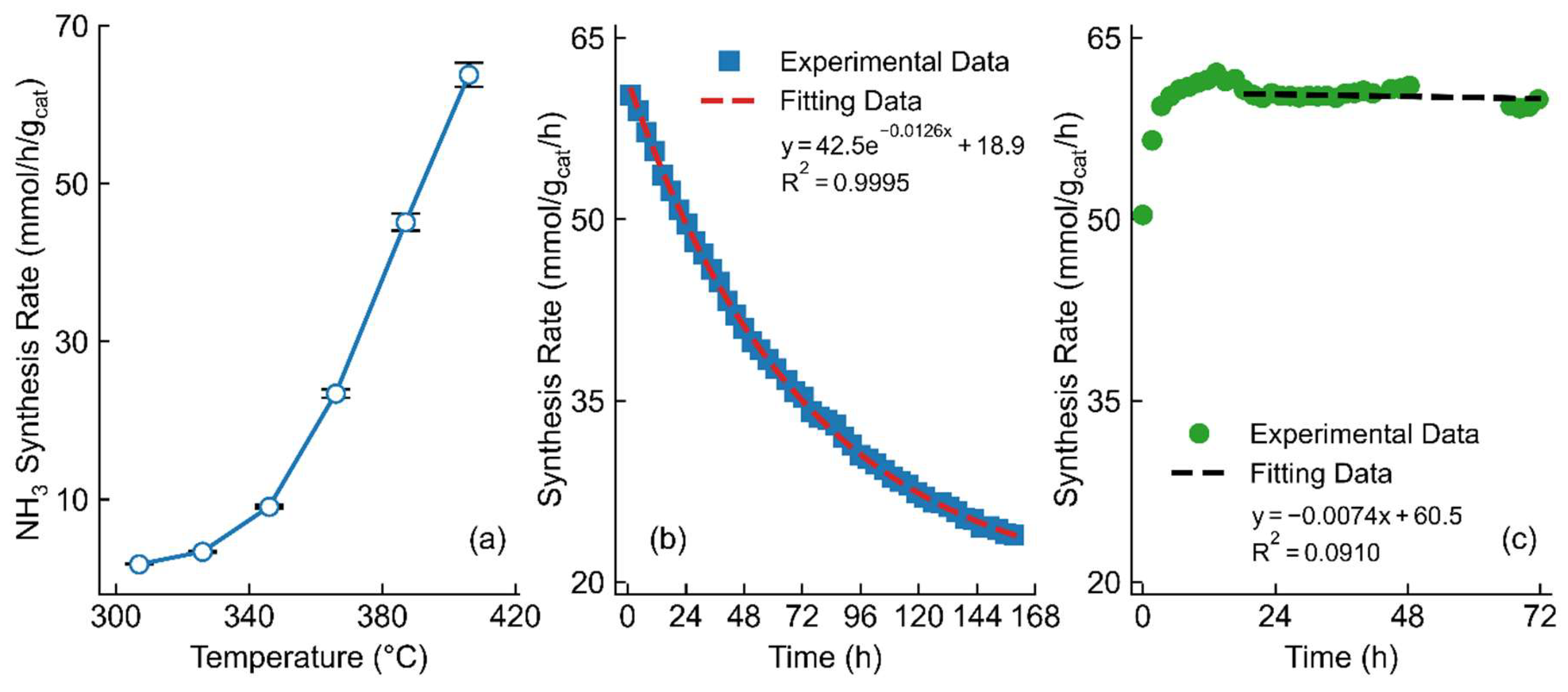
2.2. Optimization of Catalysts
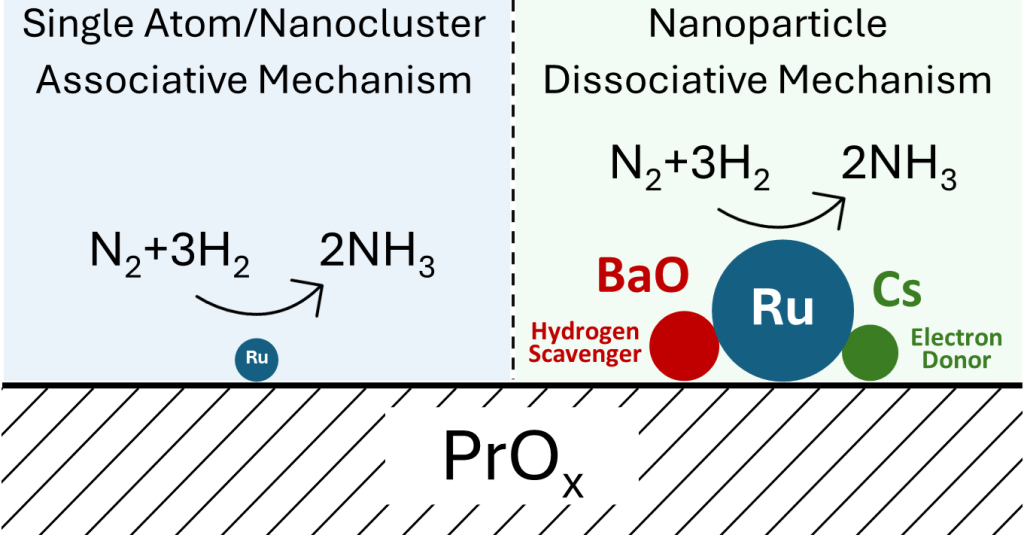
2.3. The Role of the Promoters and the Mechanism
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Evaluation
3.3. Catalyst Characterization
4. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daisley, A.; Hargreaves, J.S.J.; Hermann, R.; Poya, Y.; Wang, Y. A Comparison of the Activities of Various Supported Catalysts for Ammonia Synthesis. Catal Today 2020, 357, 534–540. [CrossRef]
- Ogawa, T.; Yoshida, M.; Ishihara, K. Techno-Economic Analysis on Recent Heterogeneous Catalysts for Ammonia Synthesis. ChemRxiv 2022.
- Knaebel, K.S. Pressure Swing Adsorption System for Ammonia Synthesis 1996.
- Bhadra, S.J. Purification of Ammonia by Pressure Swing Adsorption. Doctoral Dissertation, University of South Carolina, 2012.
- Padinjarekutt, S.; Sengupta, B.; Li, H.; Friedman, K.; Behera, D.; Lecaros, R.; Yu, M. Synthesis of Na+-Gated Nanochannel Membranes for the Ammonia (NH3) Separation. J Memb Sci 2023, 674, 121512. [CrossRef]
- Saadatjou, N.; Jafari, A.; Sahebdelfar, S. Ruthenium Nanocatalysts for Ammonia Synthesis: A Review. Chem Eng Commun 2015, 202, 420–448. [CrossRef]
- Rosowski, F.; Hornung, A.; Hinrichsen, O.; Herein, D.; Muhler, M.; Ertl, G. Ruthenium Catalysts for Ammonia Synthesis at High Pressures: Preparation, Characterization, and Power-Law Kinetics. Appl Catal A Gen 1997, 151, 443–460. [CrossRef]
- Zhang, X.; Liu, L.; Wang, J.; Ju, X.; Si, R.; Feng, J.; Guo, J.; Chen, P. The Role of Lanthanum Hydride Species in La2O3 Supported Ru Cluster Catalyst for Ammonia Synthesis. J Catal 2023, 417, 382–395. [CrossRef]
- Li, C.; Zhang, Z.; Zheng, Y.; Fang, B.; Ni, J.; Lin, J.; Lin, B.; Wang, X.; Jiang, L. Titanium Modified Ru/CeO2 Catalysts for Ammonia Synthesis. Chem Eng Sci 2022, 251, 117434. [CrossRef]
- Imamura, K.; Miyahara, S.; Kawano, Y.; Sato, K.; Nakasaka, Y.; Nagaoka, K. Kinetics of Ammonia Synthesis over Ru/Pr2O3. J Taiwan Inst Chem Eng 2019, 105, 50–56. [CrossRef]
- Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. A Low-Crystalline Ruthenium Nano-Layer Supported on Praseodymium Oxide as an Active Catalyst for Ammonia Synthesis. Chem Sci 2017, 8, 674–679. [CrossRef]
- Li, W.; Wang, S.; Li, J. Highly Effective Ru/BaCeO 3 Catalysts on Supports with Strong Basic Sites for Ammonia Synthesis. Chem Asian J 2019, 14, 2815–2821. [CrossRef]
- Truszkiewicz, E.; Raróg-Pilecka, W.; Schmidt-Szałowski, K.; Jodzis, S.; Wilczkowska, E.; Łomot, D.; Kaszkur, Z.; Karpiński, Z.; Kowalczyk, Z. Barium-Promoted Ru/Carbon Catalyst for Ammonia Synthesis: State of the System When Operating. J Catal 2009, 265, 181–190. [CrossRef]
- Osozawa, M.; Hori, A.; Fukai, K.; Honma, T.; Oshima, K.; Satokawa, S. Improvement in Ammonia Synthesis Activity on Ruthenium Catalyst Using Ceria Support Modified a Large Amount of Cesium Promoter. Int J Hydrogen Energy 2022, 47, 2433–2441. [CrossRef]
- Chen, S.-Y.; Chang, C.-L.; Nishi, M.; Hsiao, W.-C.; Reyes, Y.I.A.; Tateno, H.; Chou, H.-H.; Yang, C.-M.; Chen, H.-Y.T.; Mochizuki, T.; et al. Unraveling the Active Sites of Cs-Promoted Ru/γ-Al2O3 Catalysts for Ammonia Synthesis. Appl Catal B 2022, 310, 121269. [CrossRef]
- Aika, K. Role of Alkali Promoter in Ammonia Synthesis over Ruthenium Catalysts—Effect on Reaction Mechanism. Catal Today 2017, 286, 14–20. [CrossRef]
- Wang, Q.; Guo, J.; Chen, P. The Impact of Alkali and Alkaline Earth Metals on Green Ammonia Synthesis. Chem 2021, 7, 3203–3220. [CrossRef]
- Zeinalipour-Yazdi, C.D.; Hargreaves, J.S.J.; Laassiri, S.; Catlow, C.R.A. A Comparative Analysis of the Mechanisms of Ammonia Synthesis on Various Catalysts Using Density Functional Theory. R Soc Open Sci 2021, 8. [CrossRef]
- Wang, X.; Li, L.; Fang, Z.; Zhang, Y.; Ni, J.; Lin, B.; Zheng, L.; Au, C.; Jiang, L. Atomically Dispersed Ru Catalyst for Low-Temperature Nitrogen Activation to Ammonia via an Associative Mechanism. ACS Catal 2020, 10, 9504–9514. [CrossRef]
- Ghoreishian, S.M.; Shariati, K.; Huh, Y.S.; Lauterbach, J. Recent Advances in Ammonia Synthesis over Ruthenium Single-Atom-Embedded Catalysts: A Focused Review. Chemical Engineering Journal 2023, 467, 143533. [CrossRef]
- Zhang, Y.; Li, J.; Zhou, Y.; Au, C.; Wang, X.; Jiang, L. Recent Progress of Thermocatalytic Ammonia Synthesis via an Associative Mechanism. Fundamental Research 2024. [CrossRef]
- Vieri, H.M.; Badakhsh, A.; Choi, S.H. Comparative Study of Ba, Cs, K, and Li as Promoters for Ru/La2Ce2O7-Based Catalyst for Ammonia Synthesis. Int J Energy Res 2023, 2023, 1–11. [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Advanced Energy and Sustainability Research 2021, 2. [CrossRef]
- Forni, L.; Molinari, D.; Rossetti, I.; Pernicone, N. Carbon-Supported Promoted Ru Catalyst for Ammonia Synthesis. Appl Catal A Gen 1999, 185, 269–275. [CrossRef]
- Aika, K.; Takano, T.; Murato, S. Preparation and Characterization of Chlorine-Free Ruthenium Catalysts and the Promoter Effect in Ammonia Synthesis 3. A Magnesia-Supported Ruthenium Catalyst. J Catal 1992, 136, 126–140. [CrossRef]
- Zheng, J.; Liao, F.; Wu, S.; Jones, G.; Chen, T.; Fellowes, J.; Sudmeier, T.; McPherson, I.J.; Wilkinson, I.; Tsang, S.C.E. Efficient Non-dissociative Activation of Dinitrogen to Ammonia over Lithium-Promoted Ruthenium Nanoparticles at Low Pressure. Angewandte Chemie International Edition 2019, 58, 17335–17341. [CrossRef]
- Lin, B.; Wang, R.; Lin, J.; Ni, J.; Wei, K. Effect of Chlorine on the Chemisorptive Properties and Ammonia Synthesis Activity of Alumina-Supported Ru Catalysts. Catal Letters 2011, 141, 1557–1568. [CrossRef]
- Zeng, H.S.; Inazu, K.; Aika, K. The Working State of the Barium Promoter in Ammonia Synthesis over an Active-Carbon-Supported Ruthenium Catalyst Using Barium Nitrate as the Promoter Precursor. J Catal 2002, 211, 33–41. [CrossRef]
- Ren, Y.; Sun, X.; Huang, J.; Zhang, L.; Zhang, B.; Haruta, M.; Lu, A.-H. Dual-Component Sodium and Cesium Promoters for Au/TS-1: Enhancement of Propene Epoxidation with Hydrogen and Oxygen. Ind Eng Chem Res 2020, 59, 8155–8163. [CrossRef]
- Tikhonov, P.A.; Nakusov, A.T.; Popova, I.O.; Konyukhov, G.S. Investigation of the Surface Structure of Dysprosium and Praseodymium Oxide-Based Polycrystalline Films Sensitive to Ozone and Vapors of Ethanol and Methanol. Glass Physics and Chemistry 2005, 31, 252–258. [CrossRef]
- Ogura, Y.; Sato, K.; Miyahara, S.; Kawano, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Hosokawa, S.; Nagaoka, K. Efficient Ammonia Synthesis over a Ru/La 0.5 Ce 0.5 O 1.75 Catalyst Pre-Reduced at High Temperature. Chem Sci 2018, 9, 2230–2237. [CrossRef]
- Sato, K.; Miyahara, S.; Ogura, Y.; Tsujimaru, K.; Wada, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. Surface Dynamics for Creating Highly Active Ru Sites for Ammonia Synthesis: Accumulation of a Low-Crystalline, Oxygen-Deficient Nanofraction. ACS Sustain Chem Eng 2020, 8, 2726–2734. [CrossRef]
- Bardwell, C.J.; Bickley, R.I.; Poulston, S.; Twigg, M. V. Thermal Decomposition of Bulk and Supported Barium Nitrate. Thermochim Acta 2015, 613, 94–99. [CrossRef]
- Miyajima, K.; Mafuné, F. Thermal Decomposition of Triruthenium Dodecacarbonyl Investigated by Variable-Temperature Mass Spectrometry in the Gas Phase. Chem Phys Lett 2022, 786, 139191. [CrossRef]
- Zhao, X.; Hrbek, J.; Rodriguez, J.A. The Decomposition and Chemistry of Ru3(CO)12 on TiO2(110) Studied with X-Ray Photoelectron Spectroscopy and Temperature Programmed Desorption. Surf Sci 2005, 575, 115–124. [CrossRef]
- Meier, D.C.; Rizzi, G.A.; Granozzi, G.; Lai, X.; Goodman, D.W. Ru 3 (CO) 12 Adsorption and Decomposition on TiO 2. Langmuir 2002, 18, 698–705. [CrossRef]
- Ferro, S. Physicochemical and Electrical Properties of Praseodymium Oxides. International Journal of Electrochemistry 2011, 2011, 1–7. [CrossRef]
- Javaid, R.; Nanba, T. Stability of Cs/Ru/MgO Catalyst for Ammonia Synthesis as a Hydrogen and Energy Carrier. Energies (Basel) 2022, 15, 3506. [CrossRef]
- Schwegmann, S.; Seitsonen, A.P.; Dietrich, H.; Bludau, H.; Over, H.; Jacobi, K.; Ertl, G. The Adsorption of Atomic Nitrogen on Ru(0001): Geometry and Energetics. Chem Phys Lett 1997, 264, 680–686. [CrossRef]
- Peng, X.; Chen, X.; Zhou, Y.; Sun, F.; Zhang, T.; Zheng, L.; Jiang, L.; Wang, X. Size-Dependent Activity of Supported Ru Catalysts for Ammonia Synthesis at Mild Conditions. J Catal 2022, 408, 98–108. [CrossRef]
- Baik, Y.; Kwen, M.; Lee, K.; Chi, S.; Lee, S.; Cho, K.; Kim, H.; Choi, M. Splitting of Hydrogen Atoms into Proton–Electron Pairs at BaO–Ru Interfaces for Promoting Ammonia Synthesis under Mild Conditions. J Am Chem Soc 2023, 145, 11364–11374. [CrossRef]
- Chin, S.Y.; Williams, C.T.; Amiridis, M.D. FTIR Studies of CO Adsorption on Al 2 O 3 - and SiO 2 -Supported Ru Catalysts. J Phys Chem B 2006, 110, 871–882. [CrossRef]
- Rini, A.S.; Radiman, S.; Yarmo, Mohd.A. XPS and TPR Studies of 2%(Ru 1/2 -Sn 1/2 )/Al 2 O 3 Catalyst Synthesized by Microwave Techniques. J Phys Conf Ser 2018, 1120, 012036. [CrossRef]
- Zhang, X.; Liu, L.; Feng, J.; Ju, X.; Wang, J.; He, T.; Chen, P. Ru Nanoparticles on Pr2O3 as an Efficient Catalyst for Hydrogen Production from Ammonia Decomposition. Catal Letters 2022, 152, 1170–1181. [CrossRef]
- Deng, Y.; Yang, L.; Wang, Y.; Zeng, L.; Yu, J.; Chen, B.; Zhang, X.; Zhou, W. Ruthenium Nanoclusters Anchored on Cobalt Phosphide Hollow Microspheres by Green Phosphating Process for Full Water Splitting in Acidic Electrolyte. Chinese Chemical Letters 2021, 32, 511–515. [CrossRef]
- Kubota, J.; Aika, K. Infrared Studies of Adsorbed Dinitrogen on Supported Ruthenium Catalysts for Ammonia Synthesis: Effects of the Alumina and Magnesia Supports and the Cesium Compound Promoter. J Phys Chem 1994, 98, 11293–11300. [CrossRef]
- Zimmermann, F.; Lippert, Th.; Beyer, Ch.; Stebani, J.; Nuyken, O.; Wokaun, A. N=N Vibrational Frequencies and Fragmentation Patterns of Substituted 1-Aryl-3,3-Dialkyl-Triazenes: Comparison with Other High-Nitrogen Compounds. Appl Spectrosc 1993, 47, 986–993. [CrossRef]
- Larkin, P.J. General Outline for IR and Raman Spectral Interpretation. In Infrared and Raman Spectroscopy; Elsevier, 2018; pp. 135–151.
- Rayner-Canham, G.W.; Sutton, D. Identification of ν(N=N) in Metal Arylazo Complexes: The Infrared and Raman Spectra of Some Arylazo Complexes of Rhodium(III). Can J Chem 1971, 49, 3994–3996. [CrossRef]
- Sun, K.; Zou, X.; Sun, X.; Pang, W.; Hao, X.; Xu, Y.; Su, H.-Y. Structure and Reaction Condition Dependent Mechanism for Ammonia Synthesis on Ru-Based Catalyst. Appl Surf Sci 2023, 613, 156060. [CrossRef]
- Zeinalipour-Yazdi, C.D.; Hargreaves, J.S.J.; Laassiri, S.; Catlow, C.R.A. A Comparative Analysis of the Mechanisms of Ammonia Synthesis on Various Catalysts Using Density Functional Theory. R Soc Open Sci 2021, 8. [CrossRef]
- Jacobsen, C.J.H.; Dahl, S.; Hansen, P.L.; Törnqvist, E.; Jensen, L.; Topsøe, H.; Prip, D. V; Møenshaug, P.B.; Chorkendorff, I. Structure Sensitivity of Supported Ruthenium Catalysts for Ammonia Synthesis. J Mol Catal A Chem 2000, 163, 19–26. [CrossRef]
- Logadóttir, Á.; Nørskov, J.K. Ammonia Synthesis over a Ru(0001) Surface Studied by Density Functional Calculations. J Catal 2003, 220, 273–279. [CrossRef]
- Li, Q.; Chen, S.; Wang, Y.; Li, K.; Li, M.; Liu, L.; Wang, F. Elucidating the Dissociative and Associative Mechanisms on the Surface-Anchored Fe3 Cluster under the Effect of External Electric Field. Appl Surf Sci 2023, 623, 157021. [CrossRef]
- Lee, K.; Woo, R.; Woo, H.C.; Ko, G.; Cho, K.; Park, Y.; Choi, M.; Yoon, H.C. Unraveling the Role of MgO in the Ru-Ba/MgO Catalyst for Boosting Ammonia Synthesis: Comparative Study of MgO and MgAlO Supports. J Catal 2024, 434, 115530. [CrossRef]
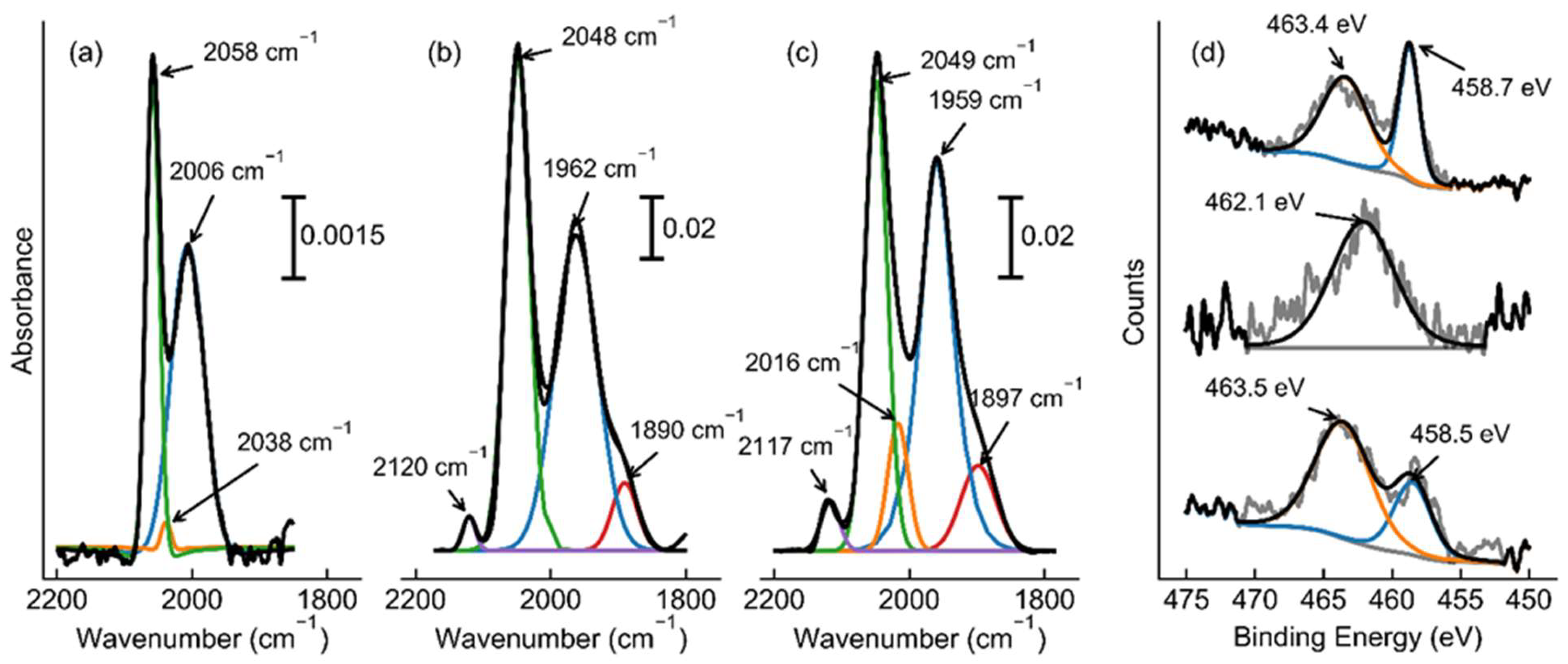
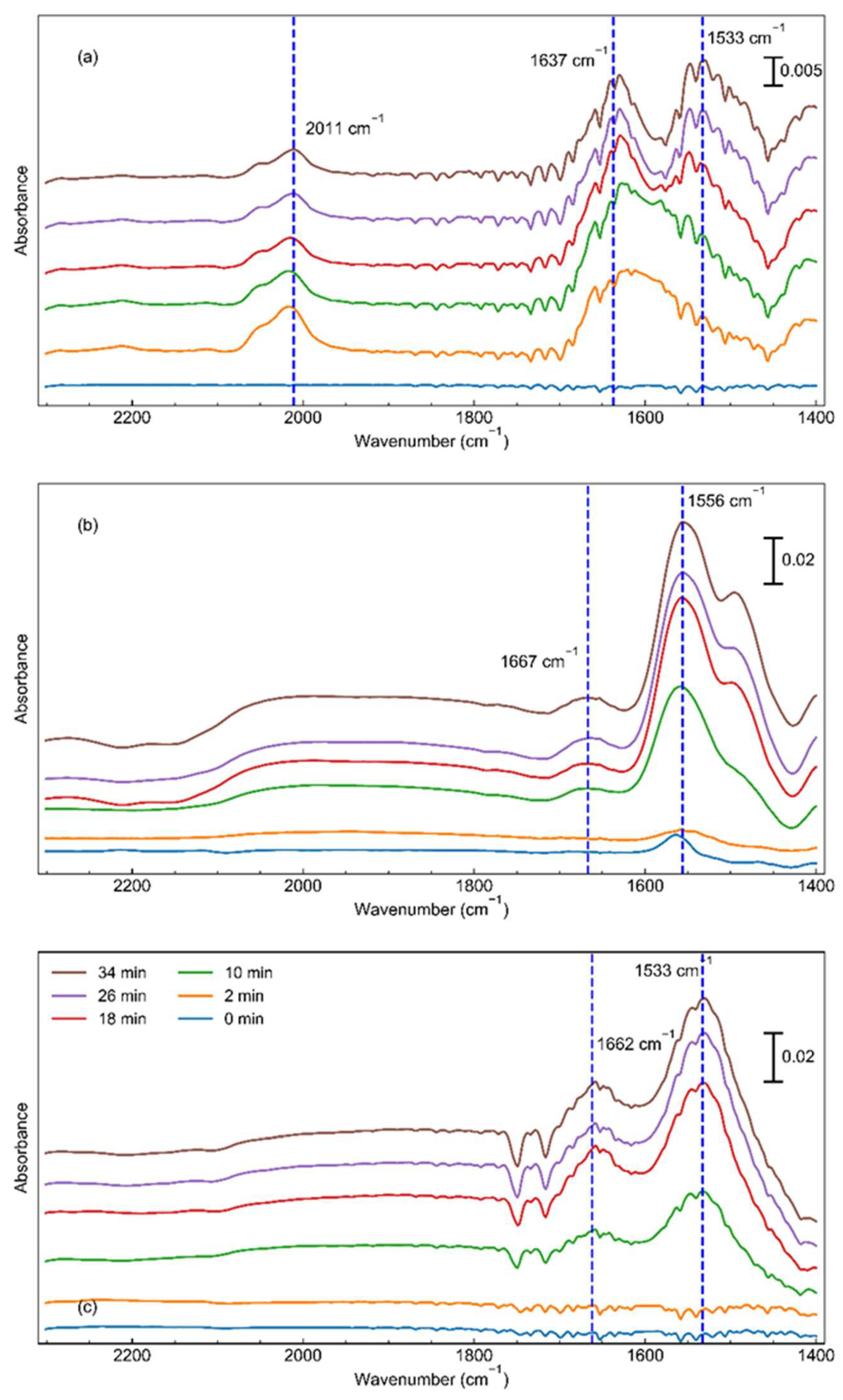
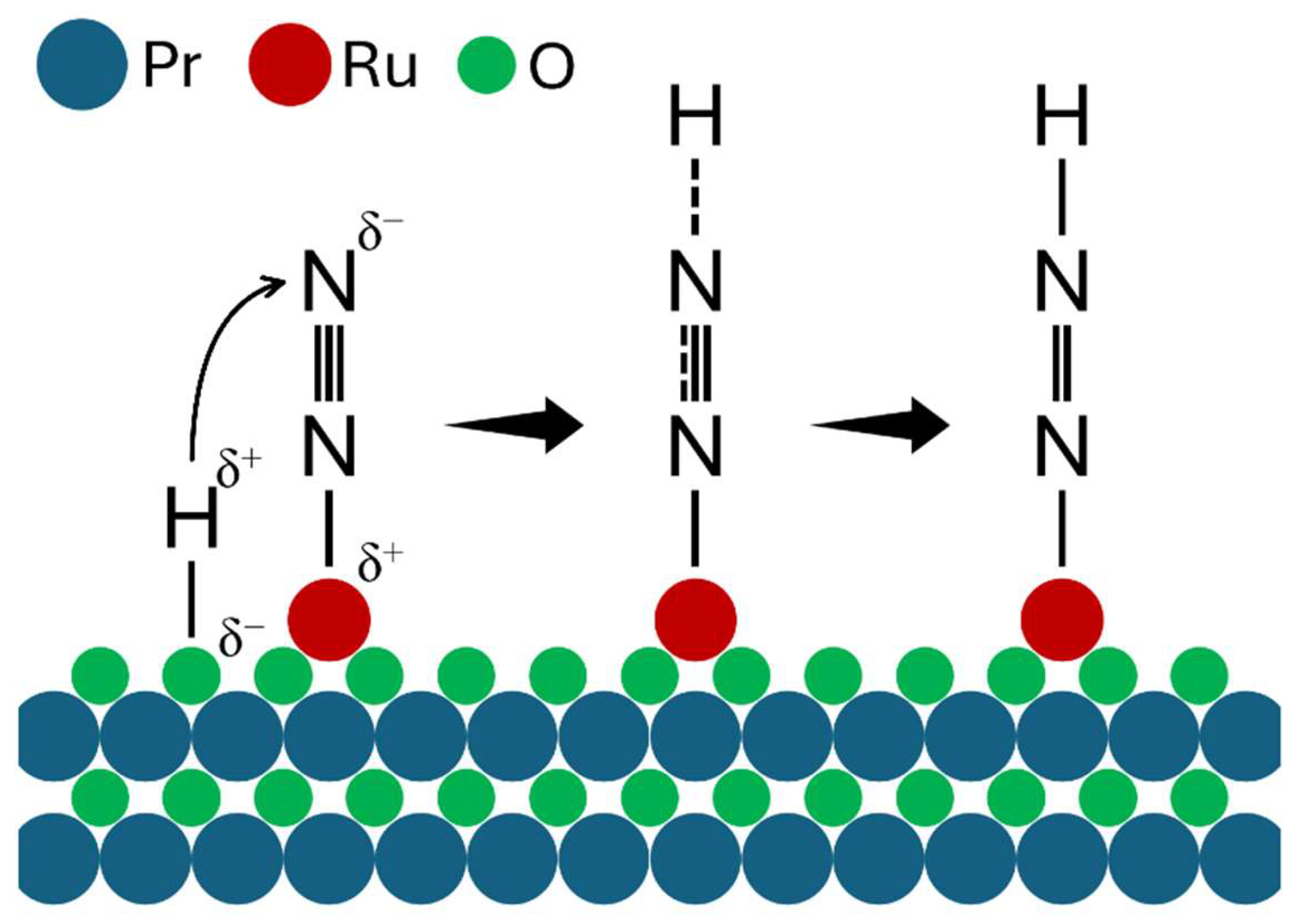
| Catalyst | Dispersion (%) | Chemisorption Particle Size (nm) | BET Surface Area (m2/g) | Pore Volume (cc/g) | TOFRu (s-1) | TOFSurface Ru (s-1) | Apparent Activation Energy (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 1 wt.% Ru/PrOx | ~100 | < 0.9 | 27.1 | 0.745 | 5.95 x 10-2 | 5.95 x 10-2 | 73.9 ± 0.7 |
| 1 wt.% Ru, 2 wt.% Cs/PrOx | 44.2 | 3.0 | 17.6 | 0.199 | 1.63 x 10-1 | 3.69 x 10-1 | 102.3 ± 1.0 |
| 1 wt.% Ru, 4.12 wt.% Cs, 3.86 wt.% Ba/PrOx | 29.3 | 4.6 | 18.1 | 0.361 | 3.06 x 10-1 | 1.04 x 100 | 133.8 ± 1.3 |
| Catalyst | Temperature (°C) | Pressure (bar) | Nitrogen Reaction Order |
Hydrogen Reaction Order |
Ammonia Reaction Order |
|---|---|---|---|---|---|
| 1 wt.% Ru/PrOx | 400 | 30 | 1.05 | 0.01 | -0.38 |
| 1 wt.% Ru, 2 wt.% Cs/PrOx | 400 | 30 | 1.04 | -0.25 | -0.13 |
| 1 wt.% Ru, 4.12 wt.% Cs, 3.86 wt.% Ba/PrOx | 400 | 30 | 0.82 | 0.25 | -0.59 |
| 1 wt.% Ru/PrOx | 360 | 30 | 0.39 | -0.14 | -0.49 |
| 1 wt.% Ru, 2 wt.% Cs/PrOx | 360 | 30 | 0.66 | -0.63 | -0.28 |
| 1 wt.% Ru, 4.12 wt.% Cs, 3.86 wt.% Ba/PrOx | 360 | 30 | 0.72 | -1.08 | -0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





