1. Introduction
The production of sourdough is typically achieved through the fermentation of microorganisms, water, cereals, pseudo cereals, or legumes. It is primarily employed in the manufacture of fermented flour products[
1]. The microbial composition of different sourdoughs varies, with the most dominant strains being lactic acid bacteria (LAB) and yeasts, with LAB dominating and playing a positive role in the quality of sourdough fermented products[
2]. The incorporation of sourdough into fermented dough products can enhance their rheological and functional properties. This is achieved through the action of extracellular polysaccharides, enzymes, organic acids and carbon dioxide produced during the growth and metabolism of the fermenting microorganisms[
3]. Sourdough fermentation is known to not only have a positive effect on the quality and shelf-life of wheat bread but also improve its flavor and aroma[
4,
5]. For instance, The utilization of sourdough in the production of gluten-free bread serves to reduce product hardness and increase bread-specific volume[
6]. Mantzourani et al. indicated that sourdough based on lactobacillus paracasei not only extends the shelf-life of the bread but also enhances its flavour and aroma[
7]. In addition, the presence of sourdough not only alters the texture of bread but also enhances its nutritional value and provides beneficial effects on health[
8,
9,
10]. Some positive effects have been almost definitively proven. The action of organic acids produced during sourdough fermentation results in a reduction in the degree of starch pasting, which in turn leads to a notable decline in the starch digestibility hydrolysis index and the predicted glycaemic index of the bread. This has the beneficial effect of improving blood sugar levels in humans[
11]. In another study, the content of the anti-nutritional factor phytic acid in whole wheat bread reduces the phytase produced by sourdough fermentation, which promotes the digestion and absorption of nutrients[
12].
Fagao (steamed sponge cake) is one of China’s traditional staple foods, prepared with grains such as rice and corn through a process of batter mixing, molding, fermentation, steaming and decorating. The Chinese pronunciation of FAGAO has the meaning of wealth and promotion. It has become an essential pastry for Chinese New Year festivals[
13]. Fagao is similar to sponge cake, but there are differences between the two.
Figure 1 presents a comparison of the two products. While both Fagao and sponge cake are batter products with a uniform dispersion of holes, soft textures, and other characteristics, there are notable differences in their raw materials, leavening ingredients, and cooking methods. Unlike sponge cake, which is cooked by baking, Fagao is steamed to achieve its desired texture. Sponge cake is usually made with cake flour, rich in formula contains eggs and oil, which helps form a porous structure. Fagao is usually made with gluten-free ingredients without eggs and oil. Additionally, Fagao requires fermentation, a process that sponge cake does not undergo. The process of fermentation is of great significance in the production of Fagao. It is responsible for imparting a soft and elastic texture, a uniform honeycomb pore structure, and a distinctive post-fermentation aroma to Fagaos, which is highly regarded by consumers. Yeast is the common starter used in Fagao fermentation.
As the largest planted crop in the world, corn is a common ingredient in Fagao. Corn is rich in nutrients like starch, protein, fat, and linoleic acid[
14]. Corn Fagao is an excellent gluten-free staple for individuals with celiac disease. However, corn Fagao is susceptible to staling, which ultimately results in a reduction in its overall quality. Our previous study has shown that corn Fagao prepared by co-fermentation with sourdough and yeast exhibits better appearance, internal structure, texture and flavour compared to traditional yeast-fermented Fagao[
15]. Thus, the drawback of Fagao may be improved by co-fermentation with a variety of fermented microorganisms. Among the available studies, there are fewer studies on the properties of corn Fagao batter during co-fermentation.
The present study aimed to investigate the corn Fagao batter characteristics and physicochemical properties of corn starch molecules during the co-fermentation with sourdough and yeast. The findings aim to provide theoretical references for co-fermentation with sourdough and yeast to improve the quality of corn-based fermented foods.
2. Materials and Methods
2.1. Materials
The lyophilized powder of Lactobacillus plantarum (1011CFU/g) was procured from Shandong Zhongke Jiayi Biotechnology Co., Ltd. (Shandong, China). The yeast was purchased from Angel Yeast Co., Ltd.(Hubei, China). Corn flour and sugar were obtained from local commercial markets. All the procured chemicals were analytical grade.
2.2. Preparation of Sourdough
A basic sourdough recipe consisting of corn flour (100 g), lactobacillus plantarum powder (1 g), and water (120 g) was used to prepare the sourdough. The ingredients were combined in a mixing bowl. Following this, the dough was proofed in a fermentation cabinet (LHS-100CL, Yiheng Scientific Instrument Co., Shanghai, China) at 30℃ and 75% relative humidity for 10 hours. This proofed dough was designated as sourdough.
2.3. Preparation of Corn Fagao Batter
The corn Fagao batter was prepared according to the methodology reported by Wu et al.[
15]. A basic corn Fagao batter recipe consisting of corn flour (200 g), sugar (24 g), water (240 g), sourdough (20 g) and yeast (2 g)was used to prepare the corn Fagao batter. The ingredients were combined in a mixing bowl until 3 min. Following this, the corn Fagao batter was proofed in a fermentation cabinet at 30℃ and 75% relative humidity for 45 min. Fermented time with 0, 15, 30 and 45 min of co-fermented sourdough and yeast were abbreviated as SY-0, SY-15, SY-30 and SY-45, respectively. The control group consisted of yeast fermentation only, which was recorded as YY-0, YY-15, YY-30 and YY-45. Batter samples were obtained at each of the specified time points for subsequent analysis. Part of the corn Fagao batter with different fermentation conditions was freeze-dried by a vacuum freeze-drier (77530-6L, Labconco Equipment Co., Kansas City, MO, USA), milled into power sifted through an 80-mesh sieve, and stored in a sealed container for further analysis.
2.4. Determination of pH and Total Titratable Acidity(TTA)
The pH and TTA of corn Fagao batter with different fermentation conditions were measured by the modified method by Nagihan et al. [
16]. 10 g of corn Fagao batter with different fermentation conditions was homogenized with 100 mL of distilled water, and pH was measured using a pH meter (PHS-25, Qiwei Instrument Co., Hangzhou, China).
2.5. Determination of Reducing Sugar Content
10 g of corn Fagao batter with different fermentation conditions was homogenized with 100 mL of distilled water. The sample solution consisted of 100 ml of distilled water homogenized with 10 g of corn Fagao batter with different fermentation conditions. The reducing sugar content of the samples was quantified using the 3,5-Dinitrosalicylic acid spectrophotometric method (7230G, Ningbo Shunyu Instrument Co., Ningbo, China).
2.6. Determination of Specific Gravity
The method of testing the specific gravity as described by Charlotte et al. was used with a brief modification[
17]. Following fermentation, the density of each sample was determined in triplicate as the ratio of the batter weight to the water weight filled in a standard container.
2.7. Determination of Viscosity
The viscosity of corn Fagao batter with different fermentation conditions was determined using a viscometer (SNB-1, Tianmei Balance Instrument Co., Shanghai, China) the spindle revolved at 6 rpm in corn Fagao batter with different fermentation conditions (adapted from a method of Cui et al., with slight modifications[
18].)
2.8. Determination of Amylose Content
The amylose content of the dried corn Fagao batter sample was calculated by absorbance according to GB/T 15683-2008.
2.9. The Microstructure of Starch Particles
A total of 0.1 g of corn Fagao batter with different fermentation conditions was dispersed in 10 mL of distilled water. The mixture was then observed and photoed using an optical microscope (E5, Ningbo Shunyu Instrument Co., Ningbo, China) at 1000 times magnification.
2.10. X-ray Diffraction (XRD)
Dried corn Fagao batter sample was subjected to X-ray diffraction (X’Pert PRO-XRD, PANalytical, Netherlands) scanning under the following conditions. The tube flow rate was 40 mA; tube pressure was 40 kV; scanning speed was 2°/min; with starting angle 5°and ending angle of 40°respectively. Origin 2018 software (Origin Lab, Inc., Northampton, MA, USA) was used to analyze the crystalline peak area (Ac) and the total area A to determine the relative crystallinity = (Ac/A)×100[
19].
2.11. Rapid Viscosity Analysis (RVA)
Pasting properties of dried corn Fagao batter sample were evaluated according to AACC 76-21 by a Rapid Visco Analyzer (RVA-4, Newport Scientific Instruments, Inc., Australia).
2.12. Fourier Transform Infrared Spectroscopy (FTIR)
The method of Jiang et al.[
20]was referred to, with minor modifications. The samples prepared by the KBr tablet method were scanned by an infrared absorption spectrometer(WQF-510, Riley Analytical Instruments, Inc., Beijing, China). Omnic software was used to analysis the FTIR spectra and calculate the ratio of peak intensity at 1047/1022 cm
-1 and 995/1022 cm
-1.
2.13. SDS-Polyacrylamide Gel Electrophoresis
Dried corn Fagao batter sample (25 mg) was solubilized in 1 ml sample buffer, boiled for 5 min and centrifuged at 10,000 rpm for 5 min before electrophoresis (24EN, Six One Biotechnology Ltd., Beijing, China). For samples, SDS-PAGE experiments were performed using 4% stacking gel and 15% separating gel. The resulting supernatant (10 µL) was loaded onto the gels, and the electrophoresis was then run at 20 mA until the tracking dye reached the bottom of the gel. Then, the electrophoresis gel was dyed with Coomassie brilliant blue solution for 2 h, and the images were analyzed using Quantity One (Bio-Rad, California, USA) after decolorizing.
2.14. Statistical Analysis
All experiments were repeated three times, analyzed variance using SPSS19.0 software, and expressed results as mean standard deviation (SPSS Inc., IL, USA). Different lowercase letters indicated significant differences between samples (P < 0.05). Origin 2018 software was used to draw the figures.
3. Results
3.1. Effect of Co-Fermentation on the Properties of Corn Fagao Batter
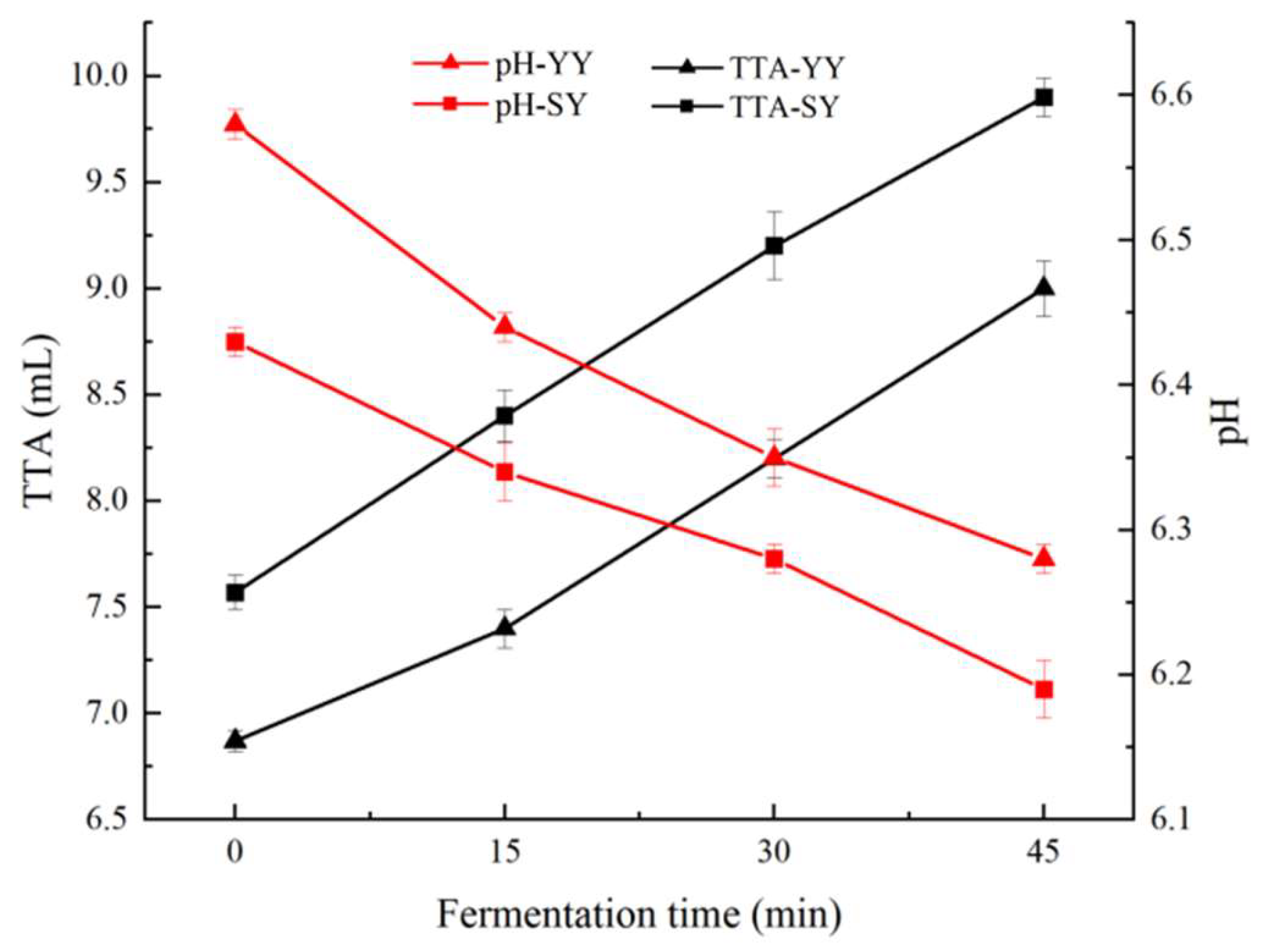
3.1.1. pH and TTA of the Corn Fagao Batter
The effects of fermentation on pH and TTA of corn Fagao batter are shown in
Figure 2. The pH value represents the concentration of hydrogen ions in the corn Fagao batter, thereby reflecting the strength of acidity and alkalinity of the samples. As shown in
Figure 2, the pH declined during the fermentation period in both corn Fagao batter groups. The pH of the sourdough co-fermentation group exhibited a consistently lower value than that of the yeast fermentation group. The pH of the sourdough and yeast co-fermentation was lower than that of the yeast fermentation during early fermentation might be due to lactic acid produced by lactic acid bacteria(LAB), which are the dominant strain in sourdough during the fermentation process[
2]. As co-fermentation progresses, the acidification of LAB promotes yeast fermentation, and similarly, yeast fermentation and metabolites affect the growth and metabolism of LAB[
21]. The process of co-fermentation results in the production of lactic acid, carbon dioxide, and acetic acid, thus resulting in a lower pH of batter than yeast fermentation.
TTA can reflect microbial fermentation acid production, the lower the pH, the higher the TTA. As shown in
Figure 2, it can be seen that the TTA of the two groups of corn Fagao batter showed an increasing trend with fermentation, and the co-fermentation group was always higher than the yeast fermentation group. This may be due to the microorganisms in co-fermentation are more diverse and have a stronger acid production capacity than yeast fermentation. The above results indicate that co-fermentation decreases the pH and accelerates the acidification of corn Fagao batter. The higher acidity of the batter facilitates the activation of amylase activity and endogenous proteases in the corn flour, which results in the formation of more soluble sugars and free amino acids, which is conducive to the improvement of the product’s flavor and texture[
22].
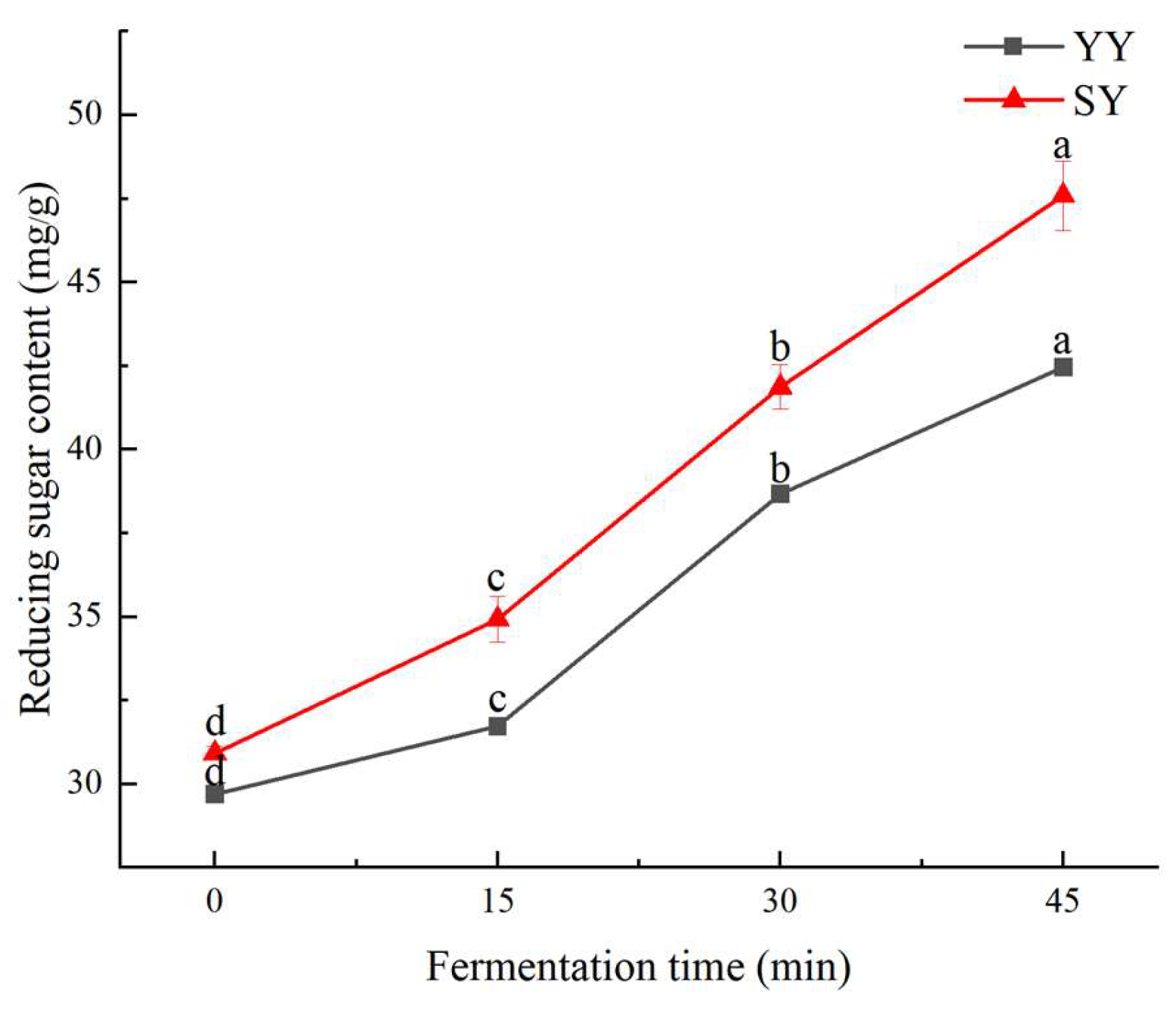
3.1.2. Reducing Sugar Content of Corn Fagao Batter
The generation of products by microorganisms through respiration and fermentation is inextricably linked to the metabolism of sugars in corn Fagao batter. Various enzymes and microorganisms produced during the fermentation process of corn Fagao batter convert some of the polysaccharides and oligosaccharides into reducing sugars. Consequently, the extent of fermentation in corn Fagao batter can be quantified by measuring the reducing sugar content[
23]. As shown in
Figure 3, the reducing sugar content exhibited a gradual increase under the different fermentation methods with the extension of fermentation time.
At the commencement of fermentation, the reducing sugar content of the co-fermentation group was marginally higher than that of the yeast group. This suggests that the increase in reducing sugar content of the corn Fagao batter was due to the addition of sourdough. At 15 min of fermentation, the growth rate of reducing sugar was greater in the co-fermentation group than in the yeast fermentation group, indicating an increase in starch hydrolysis with the addition of sourdough. The growth rate of reducing sugar in the co-fermentation group was found to be less than that observed in the yeast fermentation group at 30 minutes of fermentation. This was attributed to the rapid growth of LAB and yeast, which resulted in a higher rate of reducing sugar consumption, leading to a slower growth rate of reducing sugar in the co-fermentation group. At 45 minutes of fermentation, the reducing sugar content increased under both fermentation methods, but their growth rates were smaller than at 30 minutes of fermentation. At this time, the nutrients present in the corn Fagao batter had been depleted, resulting in a reduction in the overall metabolic hydrolysis and a decline in the growth rate of reducing sugar.
Reducing sugar content in corn Fagao batter systems depends on the dynamic equilibrium between the hydrolysis of starch by acids and enzymes to produce reducing sugar and the consumption of reducing sugar by microbial growth and reproduction. The reducing sugar contents showed an increasing trend during fermentation, indicating that reducing sugar produced by starch hydrolysis and microbial metabolism was higher than those consumed by microbial growth. The increased acidity helps to activate α-amylase present in grains, which converts starch to reducing sugar and increases the reducing sugar content. The higher reducing sugar content in the co-fermentation group than in the yeast fermentation group may be related to the metabolic pathways of the two different microorganisms[
24]. The increase in reducing sugar content may promote the non-enzymatic browning reaction by providing a substrate for caramelization or the Maillard reaction, thereby imparting the product with an enhanced flavour and colour.
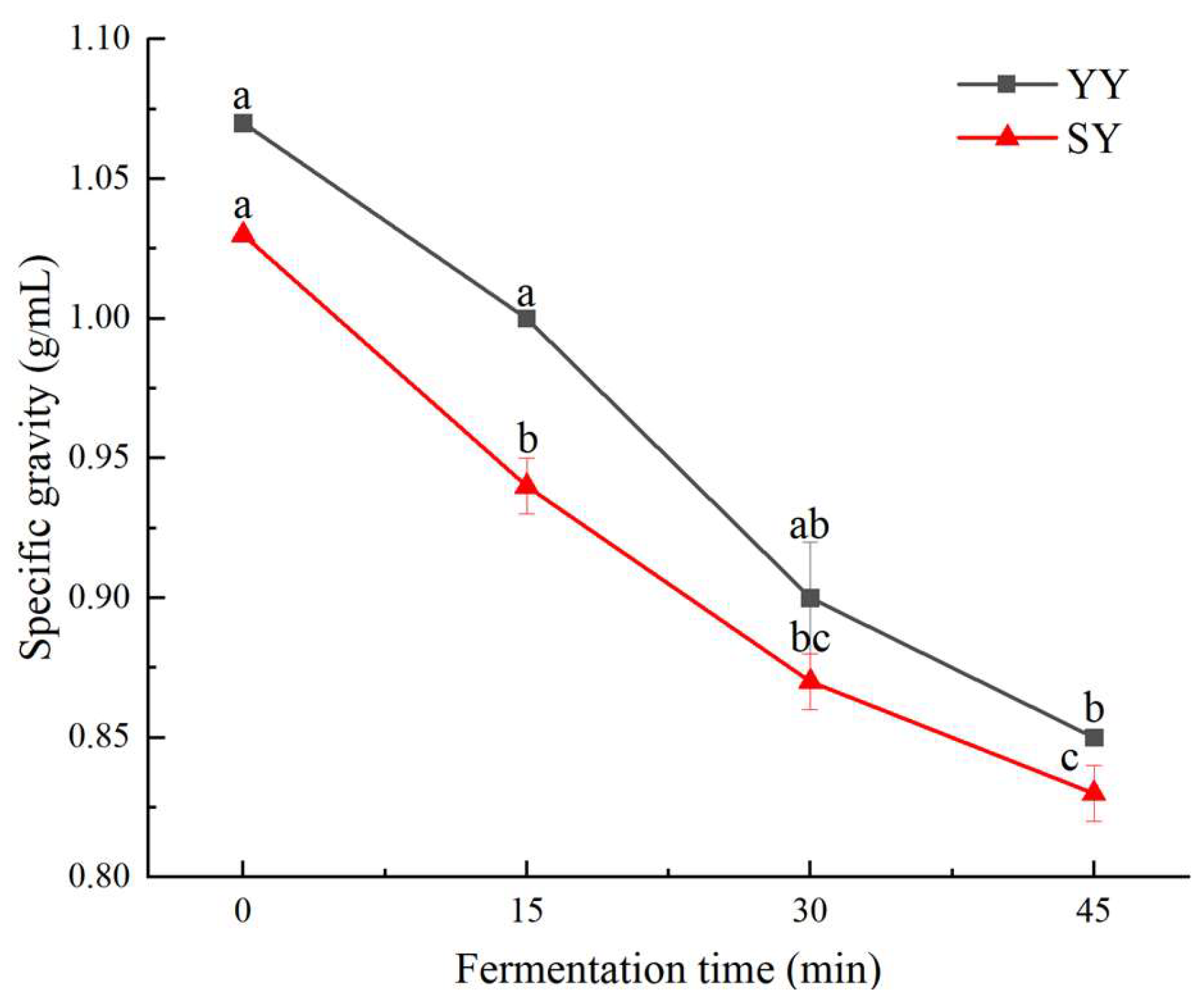
3.1.3. Specific Gravity of Corn Fagao Batter
Specific gravity is an important physical property of the batter, which represents the retention rate of air bubbles in the batter. The specific gravity of the batter is inversely proportional to the volume and fluffiness of the product, the lower the specific gravity of an equal mass of batter, the greater the volume of the product and the fluffier the tissue[
17]. At the same time, the specific gravity can reflect the gas production of the strain utilizing sugars.
As illustrated in
Figure 4, the specific gravity of corn Fagao batter decreased as fermentation progressed. The specific gravity of the co-fermentation group was always lower than that of the yeast fermentation group. On the one hand, sourdough is a rich source of LAB and its fermentation products such as extracellular polysaccharides[
25]. During the fermentation process, yeast provides a range of essential nutrients, including amino acids and vitamins, which are utilized by LAB for fermentation. In turn, LAB facilitates the growth of yeast by supplying reducing sugars[
26,
27]. The fermentation of the two bacteria is mutually beneficial, resulting in increased gas production and a reduction in specific gravity. On the other hand, extracellular polysaccharides, which are fermentation products of sourdough, can increase the viscosity of corn Fagao batter and stabilize the batter bubbles thereby increasing the gas content of the batter[
17]. In the study conducted by Olojede et al.[
28] on the impact of co-fermentation of yeast and pediococcus pentosaceus on sorghum dough, it was observed that the structure of the dough underwent improvement during fermentation, accompanied by an increase in its gas retention capacity. This is similar to the results of this experiment.
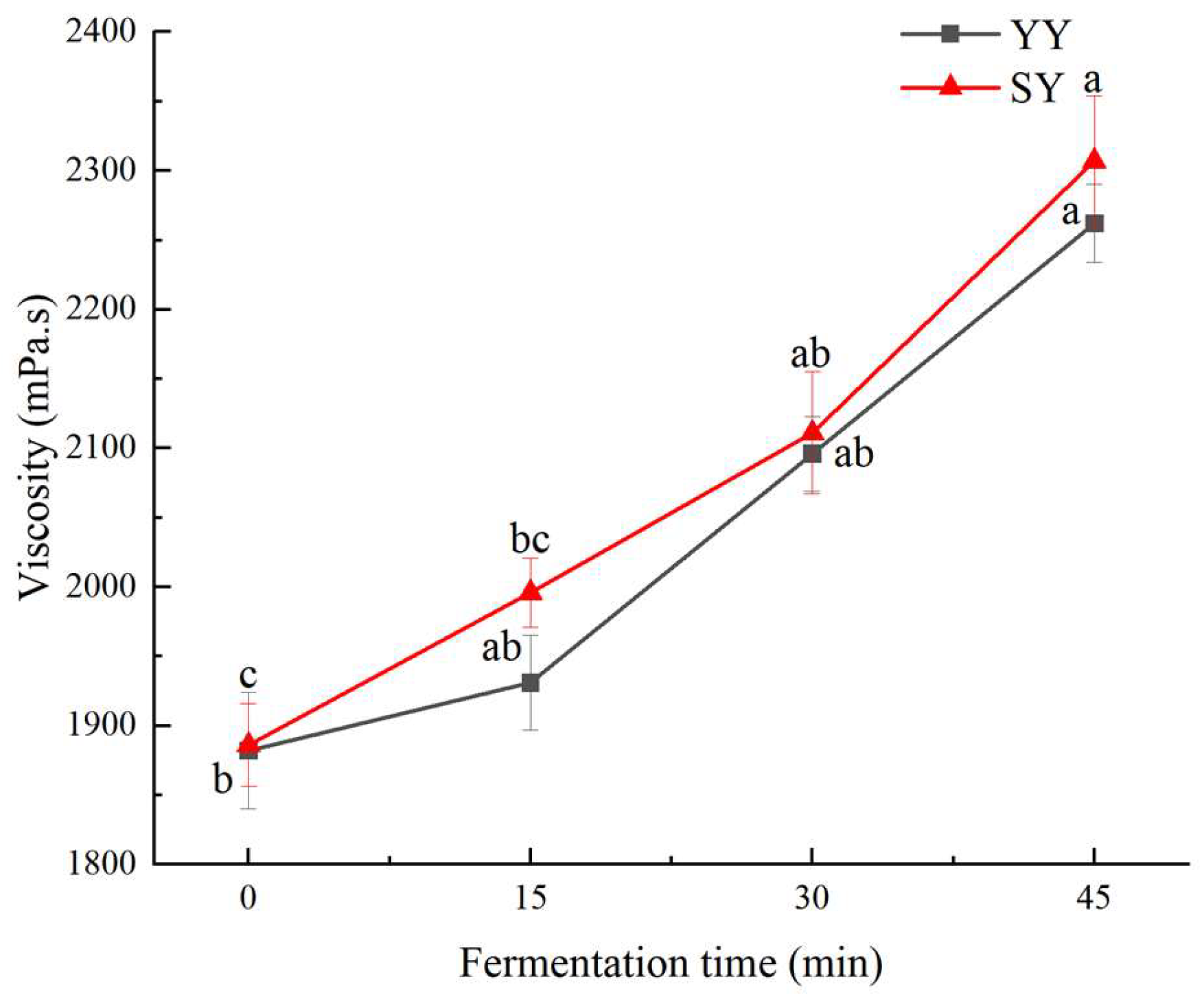
3.1.4. Viscosity of Corn Fagao Batter
Batter viscosity is a crucial indicator of the quality of Fagao. A suitable batter viscosity facilitates the expansion and shaping of the bubbles during the maturing process to ensure that the Fagao has a high specific volume. Higher viscosity can slow down the migration and diffusion of air bubbles, which is conducive to maintaining the stability of the batter. Therefore, an appropriate increase in batter viscosity is conducive to improving the quality of the product.
As shown in
Figure 5, the viscosity of corn Fagao batter showed an upward trend with the fermentation time. This result may be attributed to the penetration of acids and enzymes produced by fermentation into the interior of starch granules, disrupting the chemical bonds between proteins, fats, and starch. This leads to the breakdown and leaching of fats and proteins. The growth of fermenting microorganisms consumes proteins, which in turn purifies the starch to some extent, increasing the batter’s viscosity. The higher viscosity of the co-fermentation group compared to the yeast fermentation group may be due to the greater number of microbial species and numbers in the co-fermentation process. Additionally, LAB fermentation results in the production of polysaccharides containing hydrophilic genes in the carbon chain, which can impede the movement of water molecules and elevate the viscosity of the system. In the study performed by Xu et al.[
29], they concluded that the increase in corn Fagao batter viscosity with fermentation could be attributed to the accumulation of lactic acid produced during the growth and metabolism of the complex strain.
3.2. Effect of Co-Fermentation on the Properties of Starch
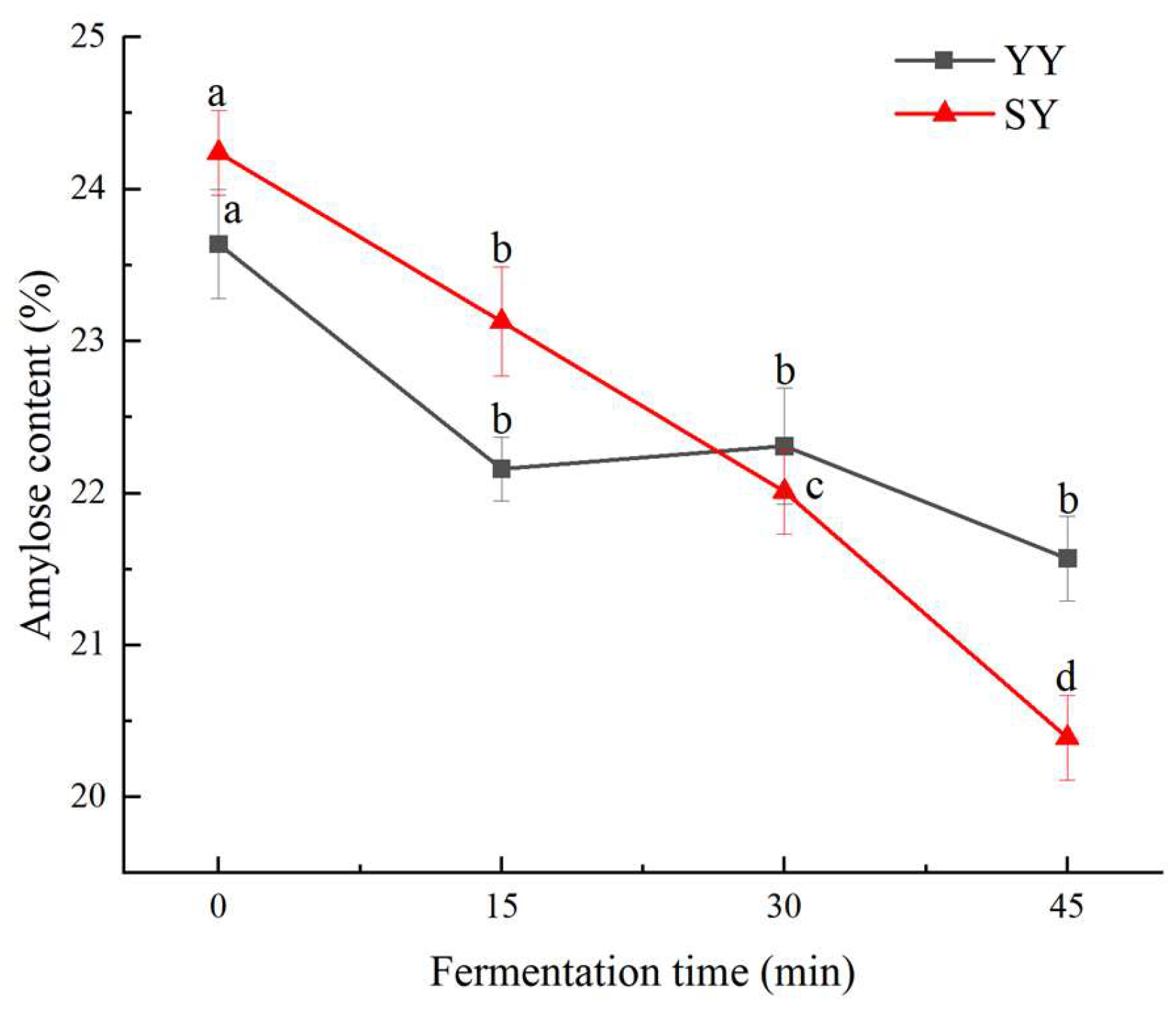
3.2.1. Effect of Co-Fermentation on Amylose Content
The amylose content during the fermentation of corn Fagao batter is illustrated in
Figure 6. As fermentation progressed, the amylose of corn Fagao batter in two groups showed a downward trend. And the amylose content decreased significantly in the co-fermentation group. The co-fermentation group exhibited a higher amylose content than the yeast fermentation group during early fermentation might be due to the degradation of amylopectin into amylose during the fermentation of sourdough, which increases the amylose content after the addition of sourdough. As the fermentation progressed, the amylose content of the co-fermentation group was gradually lower than that of the yeast fermentation. This phenomenon may be due to the greater degradation of amylose by more acids and enzymes produced by the co-fermentation process. The decline in the amylose content during fermentation may be due to the organic acids or enzymes produced by the metabolism of the dominant microorganisms during fermentation worked on the amorphous zone, a relatively loose structure of starch, thus resulting in the dissolution of amylose[
30]. In addition, The α-amylase produced by microbial metabolism degraded the amylose into small molecules such as dextrin or monosaccharides[
31]. Therefore, the amylose content decreased during fermentation. In the study performed by Zhao et al.[
30], they concluded that the reduction in amylose content of wheat starch with fermentation time is due to the metabolites produced by the fermenting microorganisms breaking down the starch into small-molecule sugars and thus obtaining the carbon and energy sources needed for growth and reproduction.
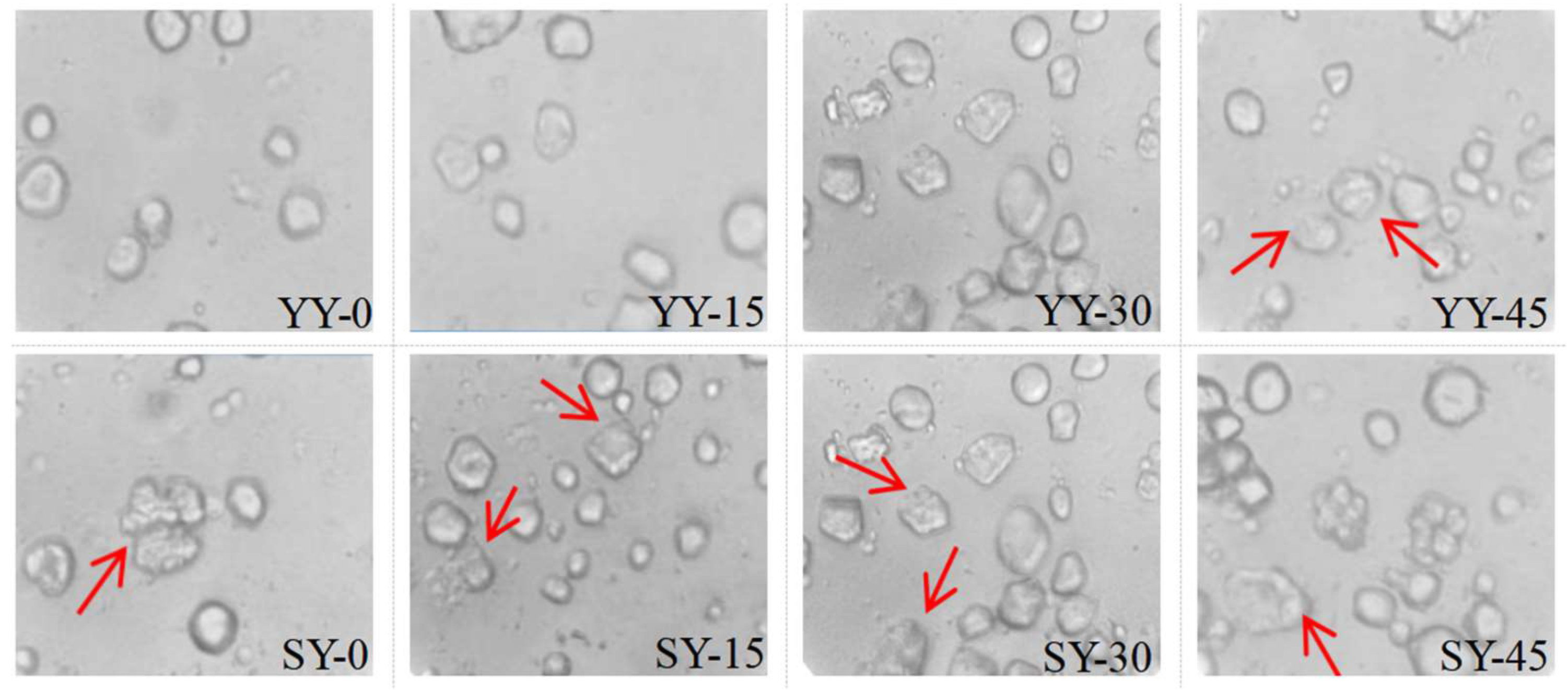
3.2.2. Effect of Co-Fermentation on the Microscopic Structure of Starch
Changes in the microscopic morphology of corn starch during fermentation can explain the effect of different fermentation times on the physicochemical and functional properties. As shown in
Figure 7, corn starch granules exhibited irregularly rounded or polygonal. The starch granules were essentially unchanged during the early fermentation of the yeast. Corn starch granules in the co-fermentation group had disrupted and eroded during the early fermentation, which was likely due to the sourdough itself. In the yeast fermentation group, at the 45-minute, the starch granules began to lose their distinct boundaries, erosion and cracks occurred more extensively on the granule surface, and small holes appeared, leading to the formation of porous network structures. This phenomenon manifested at the 30-minute in the co-fermentation group. The change may be attributed to the hydrolysis of the starch granules by acid and amylase, which are produced during the fermentation process. This results in pores, erosion and cracks on the surface of starch granules[
32]. Furthermore, the fermentation process results in the production of numerous proteases, which facilitate the separation of starch and protein and the formation of holes[
30]. The co-fermentation group may produce more enzymes and show a stronger hydrolyzation towards starch molecules. In general, fermentation destroys starch granules, a phenomenon that becomes more and more apparent as fermentation proceeds. This disruption provides a channel for substances such as water molecules to enter the interior of the starch granules, altering the internal crystalline regions of the granules and thus affecting the physicochemical properties of the starch.
3.2.3. Effect of Co-Fermentation on the Crystalline Structure of Starch
Starch granules are a semi-crystalline biopolymer consisting of crystalline and amorphous regions. The crystalline structure of starch can be classified into A, B, C and V types based on the characteristic peaks of the XRD pattern. The crystalline structures of corn starch are shown in
Figure 8. All corn starches of different fermentation conditions showed A + V hybrid crystalline structures with diffraction peaks at 15, 17, 18, 23
o (2θ) (A-type) and 20
o (2θ) (V-type)[
33]. Microorganism fermentation did not change starch crystalline type, as no new diffraction peaks appeared. Starch hydrolysis by fermentation consists mainly of acid hydrolysis and enzymatic hydrolysis, which hydrolyses the glycoside bonds of starch. The A-type crystalline form is thermally stable and fermentation is not sufficient to change the crystalline form of starch[
34]. Similar results were reported by Zhao et al.[
30], showing that the impact of microbial fermentation on starch is predominantly concentrated within the amorphous zone, and is insufficient to alter the crystalline structure of the starch.
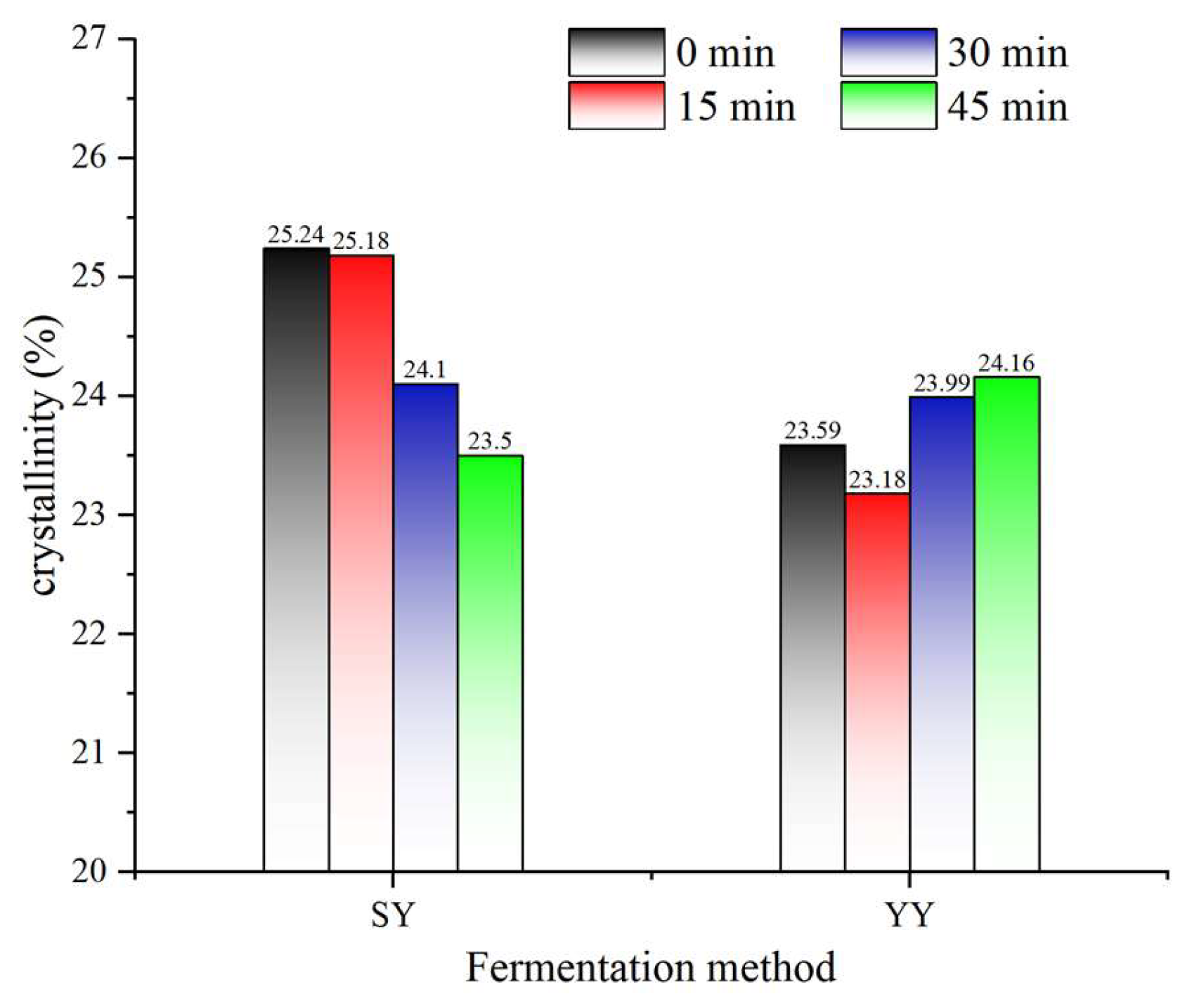
Native starch granules are partially crystalline, exhibiting a degree of crystallinity that typically ranges from 15% to 45%. The amorphous regions of the starch granules are composed mainly of amylose chains and the branching point regions of the amylopectin chains. The crystalline regions are composed of double helices packed into crystalline lattices[
35]. The change in the relative crystallinity of corn starch during fermentation is presented in
Figure 9. A slight increase in the crystallinity of yeast fermentation may be attributed to the amorphous regions of starch being structurally unstable and susceptible to degradation. Yeast fermentation has been observed to preferentially hydrolyze the amorphous regions, resulting in an increase in crystallinity[
31]. The relative crystallinity of corn starch during fermentation showed an upward trend. The hydrolysis of starch by a multitude of acids and enzymes produced by microbial metabolism in sourdough was enhanced. The acids and enzymes penetrate the starch granules, where they hydrolyze the long amylopectin in the crystalline area of the starch granules. This process generates a large number of intermediate and short amylose starches, which alter the molecular structure of starch and result in a reduction in the crystallinity of starch[
21].
3.2.4. Effect of Co-Fermentation on the Pasting Properties of Starch
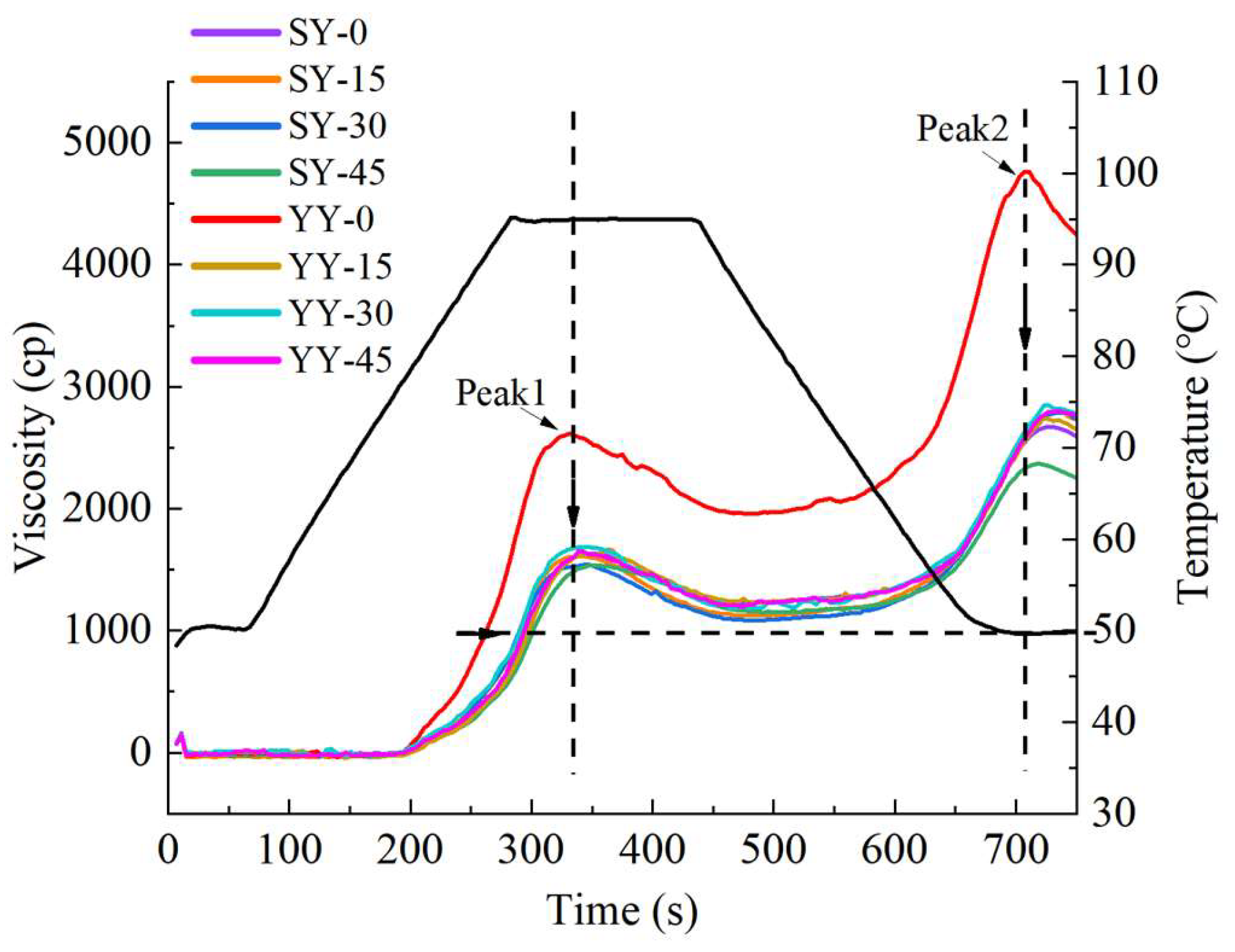
The pasting properties of starch were largely related to the quality of the starch food, and are regarded as a crucial parameter to evaluate the quality of grain food. As described in
Figure 10, fermentation shifted the pasting curve overall downward and rightward at the same time, and the gelatinized temperature increased. Peak 1 (peak viscosity) and peak 2 (setback value) were observed to occur simultaneously in the fermented and non-fermented corn Fagao batter pasting curves. However, the peaks of the fermented corn Fagao batter were all greatly reduced. It shows that the fermented corn flour is not easy to gelatinization and the viscosity is reduced after gelatinization. Products manufactured using fermented corn Fagao batter are more resistant to aging, especially with co-fermentation.
The pasting parameters of different fermentation conditions starch are shown in
Table 1. Peak viscosity, through viscosity, final viscosity, and setback value decreased as fermentation progressed. And the pasting viscosity of the co-fermentation group was lower than that of the yeast fermentation group. The pasting viscosity of starch is mainly influenced by the solubilization of amylose, the length of amylopectin branches and the swelling characteristics of starch granules[
36]. The decrease in pasting viscosity has the following reasons. On the one hand, fermentation results in the hydrolysis of proteins and lipids encapsulated outside the starch, allowing the amylose molecules to come out of the starch granules; On the other hand, fermentation results in the production of a considerable quantity of enzymes and acids, which facilitate the hydrolysis of α-1,4 and α-1,6 glycoside bonds in starch and reduce the spatial site resistance of starch molecules[
31]. They reported that the process of fermentation results in the hydrolysis of the side chains of branched starch, which leads to a reduction in pasting viscosity[
15]. The breakdown value is indicative of the capacity of starch to withstand mechanical shear during the heating process. A lower breakdown value is indicative of enhanced stability of the starch granules. The setback value is indicative of the stability and retrogradation tendency of starch following gelatinisation. A smaller setback value is indicative of a lighter degree of re-crystallization of starch following gelatinisation. The breakdown and setback values depend on the extent of change in viscosity subsequent to starch gelatinization. Factors affecting this include the amylose content, the length of the starch molecular chain, and the molecular weight of the starch[
37]. The setback and breakdown values were found to be reduced for both fermentation methods, indicating that fermentation results in increased thermal stability and resistance to aging of corn Fagao batter. Furthermore, the co-fermentation group was observed to perform better. This result is in agreement with the results of amylose content, microstructure and crystallinity of corn Fagao batter. Similar results were reported by Oyarekua et al.[
38] Additionally, the pasting properties of maize flour exhibited differences across various corn varieties.
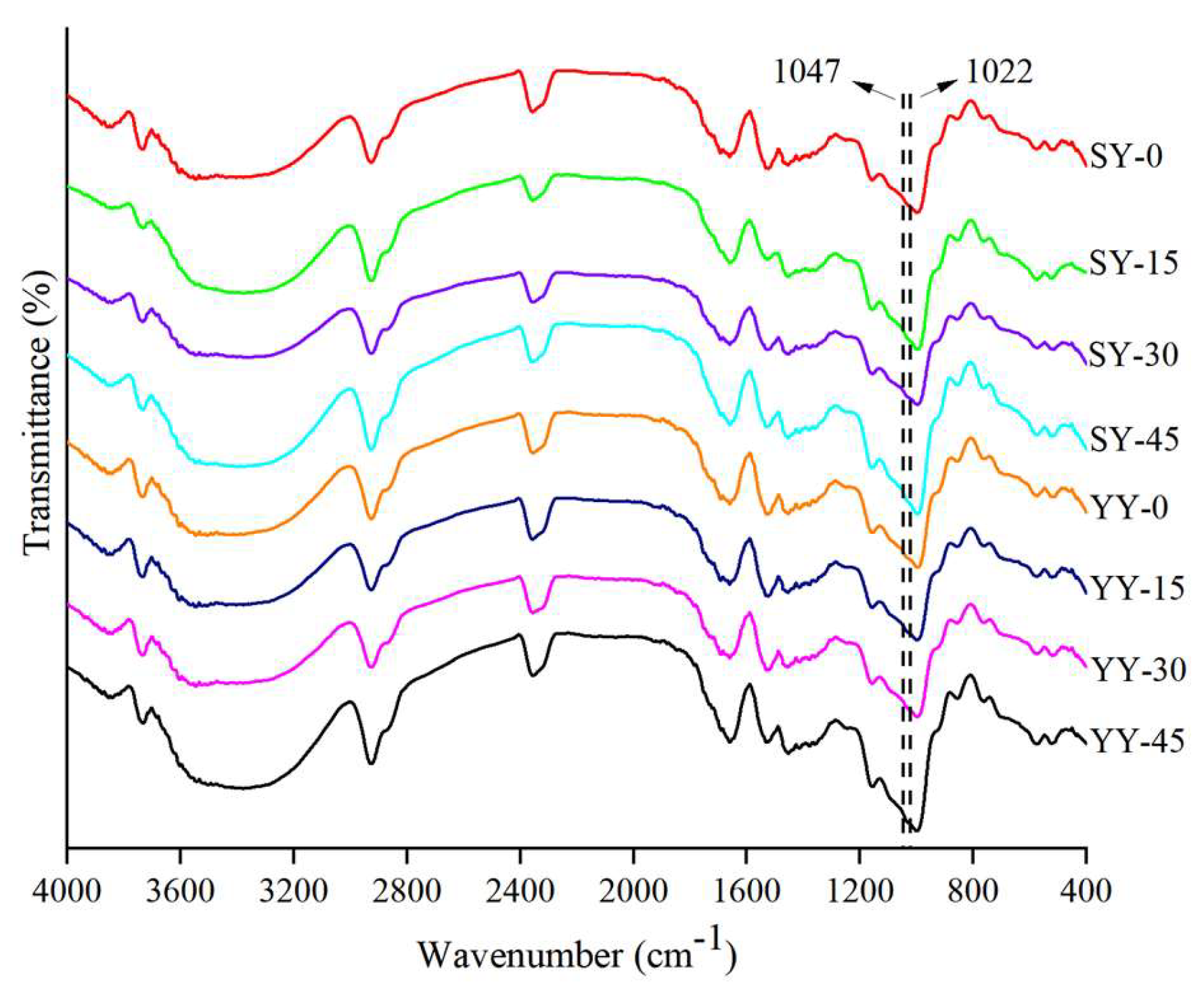
3.2.5. Effect of Co-Fermentation on the Short-Range Ordered Structure of Starch
The short-range ordered structure of starch refers to the structure formed by the orderly stacking of short distances between double helixes, which can reflect the degree of internal structural orderliness of starch chains. In the infrared spectrogram of starch, the different bands of 400-4000 cm
-1 correspond to the different chemical bonds of the starch. The absorption peaks at 2800 -3000 cm
-1 was attributed to the C-H stretching vibration, the absorption peaks at 3000-3600 cm
-1 was assigned to the O-H of starch stretching vibration absorption of the O-H, the absorption peaks at 900-1300 cm
-1 were attributed to C-O, C-C and C-O-H stretching as well as C-O-H bending vibration, and the absorption peaks between this region were sensitive bands for the starch conformation. The absorption peaks at 1047 cm
-1 are sensitive to the molecular crystalline structure and the band at 1022 cm
-1 is linked to the amorphous structure of starch[
39,
40]. The ratio of the integrated area of absorption bands at 1,047/1,022 cm
-1 is generally used to quantify the internal changes of the starch molecule in the degree of short-range order, respectively. The short-range ordered structure had a positive correlation with the 1,047/1,022 cm
-1 ratio[
41].
Figure 11.
FTIR diffraction patterns of corn starches with different fermentation methods.
Figure 11.
FTIR diffraction patterns of corn starches with different fermentation methods.
As shown in
Table 2, as fermentation progressed under different fermentation conditions, the 1047/1022 cm
-1 ratio showed a decreasing tendency, suggesting that ultrasound irradiation might act on starch by weakening the short-range crystallinity. The enzymes produced during fermentation break down large starch molecules, disrupting the crystalline structure of starch and producing short-chain starch molecules, which results in a decrease in the orderliness of starch. The 1047/1022 cm
-1 ratio in the co-fermentation group exhibited a notable decline from 0.962 to 0.950, which was a more pronounced reduction in orderliness than that observed under yeast fermentation. This finding suggests that co-fermentation may disrupt the ordered structure of starch to a greater extent than yeast fermentation. Similar results were reported by Qi et al.[
42].
3.3. Effect of Co-Fermentation on the Relative Molecular Weight of Proteins
Corn proteins can be divided into zein, glutenin, globulin, and albumin. Zein is the predominant protein in corn, comprising 60-70% of the total protein content. The structural function of zein is analogous to that of glutenin, which enables it to form a viscoelastic network[
43]. Zein proteins are classified into four protein subunits, α-zein, β-zein, γ-zein, and δ-zein, based on their molecular weight, solubility, and charge. The predominant subunit is α-zein, with a molecular weight of 21-25 kDa, followed by β-zein, with a molecular weight of 15-17 kDa, γ-zein, with a molecular weight of 27 kDa, and a small portion of δ-zein[
44]. Protein distribution has a great influence on the structure, physicochemical properties and rheological properties of starch[
45].
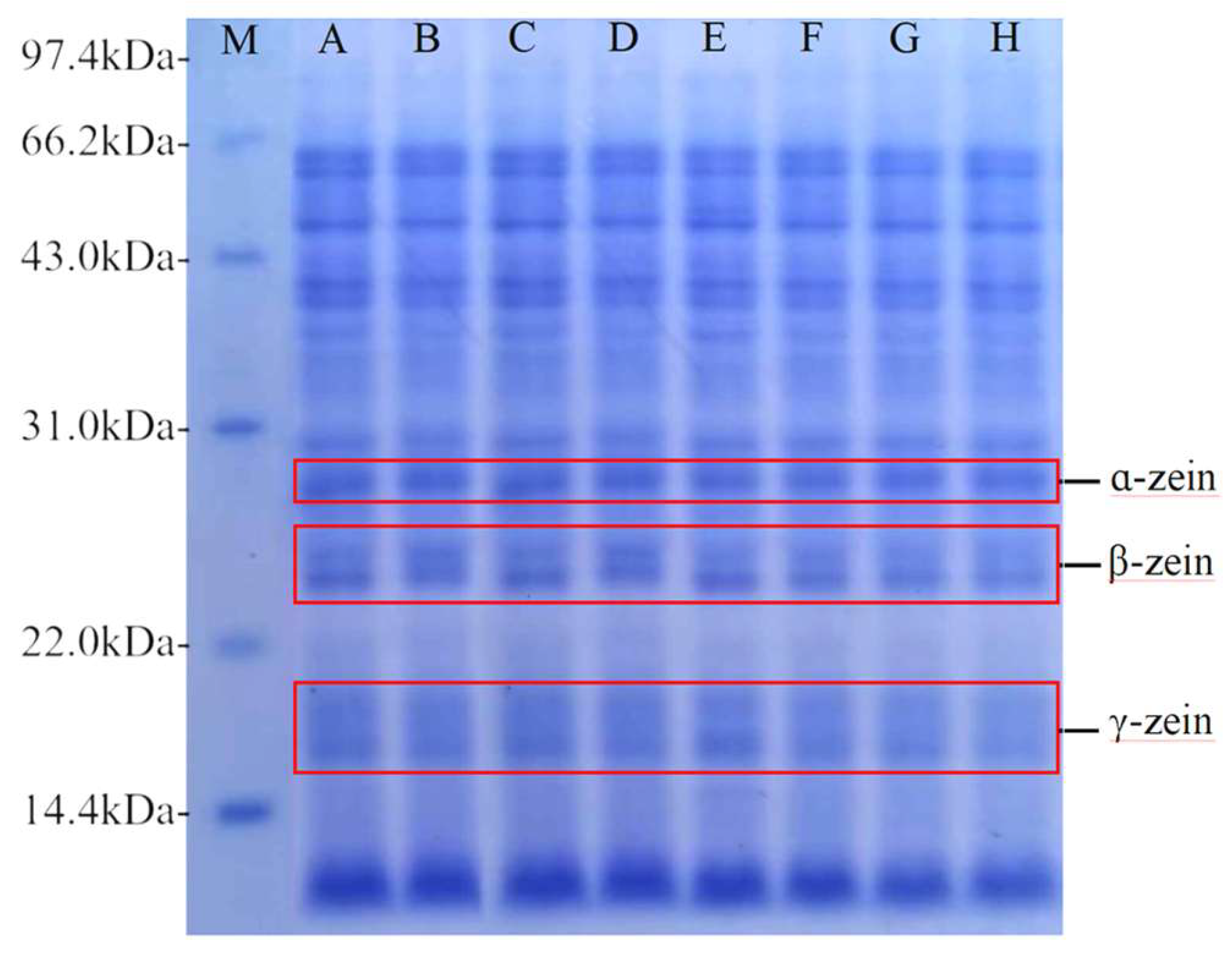
The bands of each subunit of corn protein can be clearly seen from
Figure 12. The molecular weights corresponding to each subunit were mainly concentrated in the range of 12.03 kDa, 16.64 kDa, 23.97 kDa, 28.33 kDa, and 36.78 kDa~86.11 kDa. As fermentation progressed, the number of subunit bands remained unaltered, but the area occupied by each subunit band exhibited a varying degree of reduction.
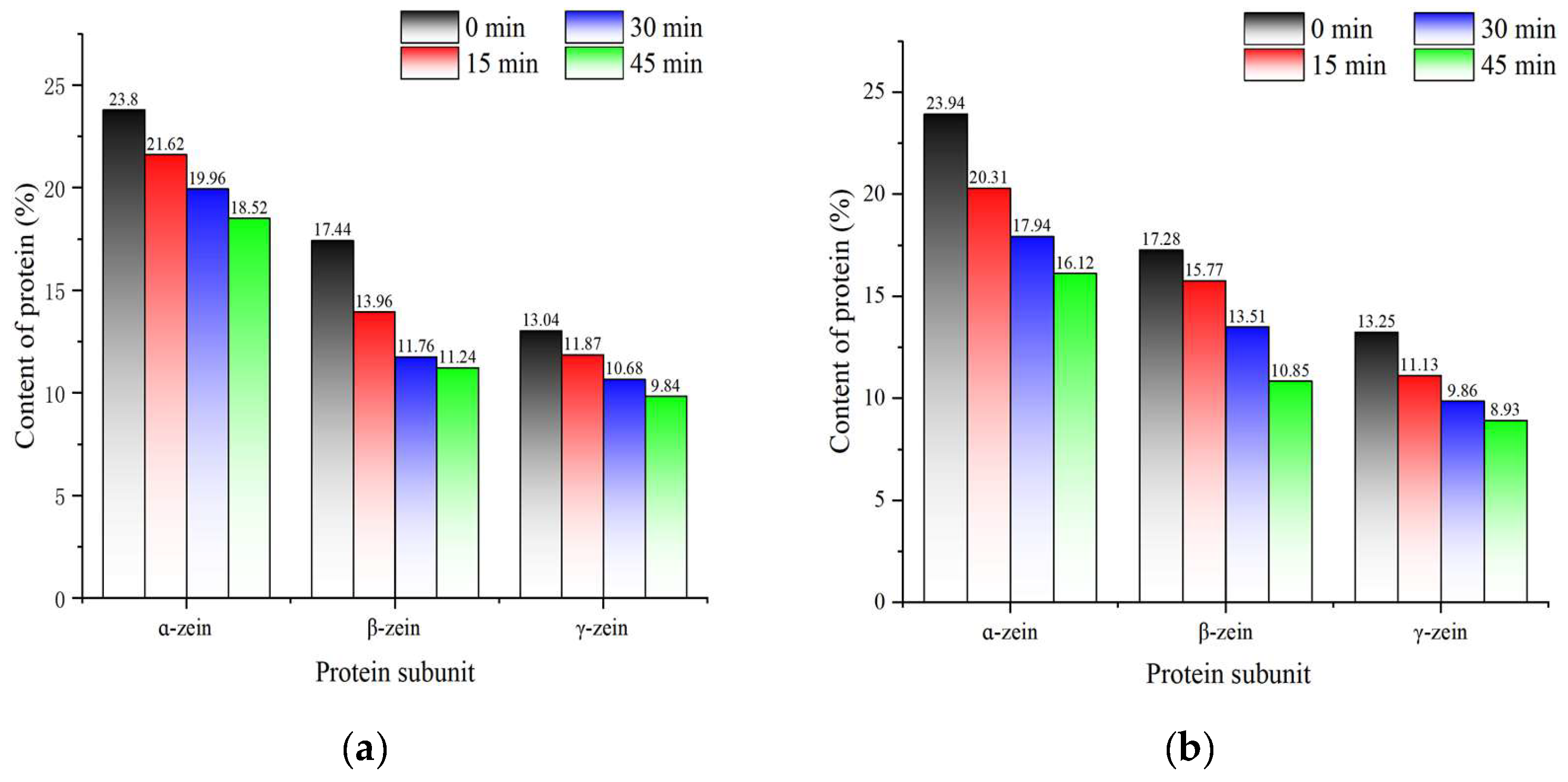
Figure 13 shows the results of the quantitative analysis of ɑ-zein, β-zein, and γ-zein by Quantity One. At the beginning of fermentation, the contents of α-, β-, and γ-zein were similar between the two fermentation conditions. As fermentation progressed, there was a decline in the contents of each subunit, with co-fermentation resulting in a more pronounced reduction in protein subunit contents than yeast fermentation. Proteases produced by fermentation hydrolyse proteins to produce small molecules such as peptides, which are a source of nitrogen for the growth and metabolism of the strain. This process results in a reduction in corn protein content during fermentation[
46]. In contrast, synergistic fermentation leads to the production of more proteases. The results of this analysis were consistent with the results of the viscosity of corn Fagao batter and its microscopic structure.
4. Conclusions
In this study, alterations in the physicochemical properties of corn Fagao batter were examined during the sourdough-yeast co-fermentation. Compared with the control sample, the sourdough-yeast co-fermentation enhanced the acid-producing capacity, gas production and viscosity of corn Fagao batter, which may contribute to the volume and flavor of corn Fagao batter. In contrast, The co-fermented batter shows a more pronounced increase in reducing sugar content, higher hydrolysis of starch and less amylose content. During the fermentation process, the starch granules lost their distinct boundaries, erosion and cracks occurred more extensively on the granule surface, and small holes appeared, leading to the integrity of the starch granules being damaged. This phenomenon manifested more seriously in the co-fermentation group. The crystallinity was less than yeast fermented batter, even though the crystal structure type of starch did not change obviously. Fourier transform infrared analysis indicated the 1047/1022 cm-1 ratio in the co-fermentation group exhibited a notable decline from 0.962 to 0.950, which suggests that co-fermentation may disrupt the ordered structure of starch to a greater extent than yeast fermentation. The peak viscosity, minimum viscosity, final viscosity, decay value and recovery value of corn Fagao batter are reduced by co-fermentation, which can improve the thermal stability of corn Fagao batter and slow down the aging. Co-fermentation also results in a more pronounced reduction in protein subunit contents than yeast fermentation. In conclusion, co-fermentation can promote the hydrolysis of starch and protein, and change the crystallinity of starch. This may improve the sensory quality of the Fagao product and delay the staling process. The results of this study not only explained the quality improvement of corn Fagao made from the co-fermentation method but also offered theoretical references for co-fermentation with sourdough and yeast to other corn-based fermented foods.
Author Contributions
Y.L.: conceptualization, data curation, formal analysis, visualization, writing—review and editing, supervision, conceptualization, methodology, funding acquisition, project administration. Q.Y.: writing—original draft preparation, data curation, investigation, formal analysis, validation. Z.W.: writing—original draft preparation, data curation, investigation. X.L.: conceptualization, supervision, writing—review and editing, supervision, formal analysis. K.L.: supervision, conceptualization, methodology, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Henan Province Key R&D Special Project (231111111800).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Henan University of Technology for supplying the experimental instruments to us.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alkay, Z.; Falah, F.; Cankurt, H.; Dertli, E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods 2024, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Ripari, V.; Gänzle, M.G.; Berardi, E. Evolution of sourdough microbiota in spontaneous sourdoughs started with different plant materials. Int J Food Microbiol 2016, 232, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gauchez, H.; Loiseau, A.L.; Schlich, P.; Martin, C. Impact of aging on the overall liking and sensory characteristics of sourdough breads and comparison of two methods to determine their sensory shelf life. J Food Sci 2020, 85, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, N.; Angelis, M.D.; Calasso, M.; Quinto, M.; Mentana, A.; Minervini, F.; Cappelle, S.; Gobbetti, M. Microbial cell-free extracts affect the biochemical characteristics and sensorial quality of sourdough bread. Food Chem 2017, 237, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, R.; Torrieri, E.; Pepe, O.; Masi, P.; Cavella, S. Effect of Sourdough with Exopolysaccharide (EPS)-Producing Lactic Acid Bacteria (LAB) on Sensory Quality of Bread during Shelf Life. Food Bioprocess Technol 2015, 8, 691–701. [Google Scholar] [CrossRef]
- Gharekhani, M.; Nami, Y.; Aalami, M.; Hejazi, M.A. Sourdoughs fermented by autochthonous Lactobacillus strains improve the quality of gluten-free bread. Food Sci Nutr 2021, 9, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a novel Lactobacillus paracase starter on sourdough bread quality. Food Chem 2019, 271, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen-Bicak, H.; Arici, M.; Yaman, M.; Karasu, S.; Sagdic, S. Effect of Different Fermentation Condition on Estimated Glycemic Index, In Vitro Starch Digestibility, and Textural and Sensory Properties of Sourdough Bread. Foods 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Angelis, M.D.; Cagno, R.D.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int J Food Microbiol 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Cagno, R.D.; Angelis, M.D. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Caponio, G.R.; Difonzo, G.; de Gennaro, G.; Calasso, M.; De Angelis, M.; Pasqualone, A. Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected? Front Nutr 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT-Food Sci Technol 2018, 91, 557–567. [Google Scholar] [CrossRef]
- Yao, J.; Bu, E.S.; Shan, Z. F. A piece of Fagao, a lot of flavours. Chinese Handicraft 2014, 2, 38–40. (In Chinese) [Google Scholar]
- Wei, C.; Ge, Y.; Zhao, S.; Liu, D.; Jiliu, J.; Wu, Y.; Hu, X.; Wei, M.; Wang, Y.; Wang, W.; Wang, L.; Cao, L. Effect of Fermentation Time on Molecular Structure and Physicochemical Properties of Corn Ballast Starch. Front Nutr 2022, 9, 12. [Google Scholar] [CrossRef]
- Wu, Z.H.; Lv Y, G.; Chen, J.; Wang, L. Study on the cooperative fermentation of microwave gluten-free corn sponge cake by sourdough. Cereals & Oils 2023, 36, 118–123. (In Chinese) [Google Scholar]
- Caglar, N.; Ermis, E.; Durak, M.Z. Spray-dried and freeze-dried sourdough powders: Properties and evaluation of their use in breadmaking. J Food Eng 2021, 292, 7. [Google Scholar] [CrossRef]
- Stemler, C.D.; Scherf, K.A. Lipases as cake batter improvers compared to a traditional emulsifier. LWT-Food Sci Technol 2023, 174, 8. [Google Scholar] [CrossRef]
- Cui, L.; Chen, J.; Wang, Y.; Xiong, Y.L. The Effect of Batter Characteristics on Protein-Aided Control of Fat Absorption in Deep-Fried Breaded Fish Nuggets. Foods 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Zhao, L.; Lin, L.; Wang, J.; Liu, Q.; Wei, C. Changes in kernel morphology and starch properties of high-amylose brown rice during the cooking process. Food Hydrocolloids 2017, 66, 227–236. [Google Scholar] [CrossRef]
- Jiang, H.H.; Zhang, Y.Y.; Hong, Y.; Bi, Y.; Gu, Z.B.; Cheng, L.; Li, Z.F.; Li, C.M. Digestibility and changes to structural characteristics of green banana starch during in vitro digestion. Food Hydrocolloids 2015, 49, 192–199. [Google Scholar] [CrossRef]
- Tu, Y.; Huang, S.; Chi, C.; Lu, P.; Chen, L.; Li, L.; Li, X. Digestibility and structure changes of rice starch following co-fermentation of yeast and Lactobacillus strains. Int J Biol Macromol 2021, 184, 530–537. [Google Scholar] [CrossRef]
- Calvert, M.D.; Madden, A.A.; Nichols, L.M.; Haddad, N.M.; Lahne, J.; Dunn, R.R.; McKenney, E.A. A review of sourdough starters: ecology, practices, and sensory quality with applications for baking and recommendations for future research. PeerJ 2021, 9, 37. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Gioulatos, S.; Tsakalidou, E.; Kalantzopoulos, S. Interactions between Saccharomyces cerevisiae and lactic acid bacteria in sourdough. 2006, 41, 2429–2433. [Google Scholar] [CrossRef]
- Katina, K.; Maina, N.H.; Juvonen, R.; Flander, L.; Johansson, L.; Virkki, L.; Laitila, A. In situ production and analysis of Weissella confusa dextran in wheat sourdough. Food Microbiol 2009, 26, 734–743. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int J Biol Macromol 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Loretan, T. The diversity and technological properties of yeasts from indigenous traditional South African fermented milks: University of the Free State; 1999.
- Roostita, R.; Fleet, G.H. The occurrence and growth of yeasts in Camembert and blue-veined cheeses. International Journal of Food Microbiology 1996, 28, 393–404. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT-Food Sci Technol 2020, 120, 8. [Google Scholar] [CrossRef]
- Xu, M.; Zhai, A. Effect of different lactic acid bacteria fermentation on maize meal property. China Brewing 2009, 3, 53–55. (In Chinese) [Google Scholar]
- Zhao, T.; Li, X.; Zhu, R.; Ma, Z.; Liu, L.; Wang, X.; Hu, X. Effect of natural fermentation on the structure and physicochemical properties of wheat starch. Carbohydr Polym 2019, 218, 163–169. [Google Scholar] [CrossRef]
- Reyes, I.; Cruz-Sosa, F.; Roman-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Structural changes of corn starch during Saccharomyces cerevisiae fermentation. Starch-Starke 2016, 68, 961–971. [Google Scholar] [CrossRef]
- Qi, X.; Yang, S.; Zhao, D.; Liu, J.; Wu, Q.; Yang, Q. Changes in Structural and Physicochemical Properties of Corn Flour after Fermentation with Lactobacillus plantarum Y1. Starch-Starke 2020, 72, 8. [Google Scholar] [CrossRef]
- Chang, F.; He, X.; Huang, Q. Effect of lauric acid on the V-amylose complex distribution and properties of swelled normal cornstarch granules. J Cereal Sci 2013, 58, 89–95. [Google Scholar] [CrossRef]
- Bian, X.; Chen, J.R.; Yang, Y.; Yu, D.H.; Ma, Z.Q.; Ren, L.K.; Zhang, N. Effects of fermentation on the structure and physical properties of glutinous proso millet starch. Food Hydrocolloids 2022, 123, 11. [Google Scholar] [CrossRef]
- Van Der Maarel, M.J.; Van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the alpha-amylase family. Journal of biotechnology 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res Int 2010, 43, 399–413. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches A review. Carbohydr Polym 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Oyarekua, M.A. Effect of co-fermentation on nutritive quality and pasting properties of maize/cowpea/sweet potato as complementary food. 2013, 13, 7171–7191. [Google Scholar] [CrossRef]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Ye, X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason Sonochem 2019, 59, 8. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Kong, X.; Wu, D.; Chen, S.; Liu, D.; Ye, X. Proanthocyanidins from Chinese berry leaves modified the physicochemical properties and digestive characteristic of rice starch. Food Chem 2021, 335, 7. [Google Scholar] [CrossRef]
- Cao, X.; Tong, J.; Ding, M.; Wang, K.; Wang, L.; Cheng, D.; Gao, X. Physicochemical properties of starch in relation to rheological properties of wheat dough. Food Chem 2019, 297, 9. [Google Scholar] [CrossRef]
- Qi, Q.T.; Hong, Y.; Zhang, Y.Y.; Gu, Z.B.; Cheng, L.; Li, Z.F.; Li, C.M. Combinatorial effect of fermentation and drying on the relationship between the structure and expansion properties of tapioca starch and potato starch. Int J Biol Macromol 2020, 145, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.A.; Pauchard, L.; Allain, C.; Mangavel, C.; Zeng, M.; Lourdin, D. Influence of dehydration rate on the vitrification of corn protein [Article]. J Appl Polym Sci 2008, 110, 1–7. [Google Scholar] [CrossRef]
- Anderson, T.J.; Lamsal, B.P. Zein Extraction from Corn, Corn Products, and Coproducts and Modifications for Various Applications: A Review. Cereal Chem 2011, 88, 159–173. [Google Scholar] [CrossRef]
- Błaszczak, W.; Valverde, S.; Fornal, J.; Amarowicz, R.; Lewandowicz, G.; Borkowski, K. Changes in the microstructure of wheat, corn and potato starch granules during extraction of non-starch compounds with sodium dodecyl sulfate and mercaptoethanol. 2003, 53, 63–73. [Google Scholar] [CrossRef]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Composition and activity of microbiota in sourdough and their effect on bread quality and safety. 2021, 129–172. [Google Scholar]
Figure 1.
Fagao and sponge cake. (a) Fagao; (b) sponge cake.
Figure 1.
Fagao and sponge cake. (a) Fagao; (b) sponge cake.
Figure 2.
The changes in pH and TAA of corn Fagao batter.
Figure 2.
The changes in pH and TAA of corn Fagao batter.
Figure 3.
The changes in reducing sugar content of corn Fagao batter.
Figure 3.
The changes in reducing sugar content of corn Fagao batter.
Figure 4.
The changes in Specific gravity content of corn Fagao batter.
Figure 4.
The changes in Specific gravity content of corn Fagao batter.
Figure 5.
The changes in viscosity of corn Fagao batter.
Figure 5.
The changes in viscosity of corn Fagao batter.
Figure 6.
Amylose content of corn Fagao batter during fermentation.
Figure 6.
Amylose content of corn Fagao batter during fermentation.
Figure 7.
Optical micrograph of starch granules during fermentation.
Figure 7.
Optical micrograph of starch granules during fermentation.
Figure 8.
XRD patterns of corn starch granules during fermentation.
Figure 8.
XRD patterns of corn starch granules during fermentation.
Figure 9.
Changes in starch crystallinity of corn Fagao batter with different fermentation.
Figure 9.
Changes in starch crystallinity of corn Fagao batter with different fermentation.
Figure 10.
Pasting properties curve of corn starches with different fermentation methods.
Figure 10.
Pasting properties curve of corn starches with different fermentation methods.
Figure 12.
Gel electrophoresis of corn protein with different fermentation methods. M represents low molecular weight marker; A, B, C and D represent samples of yeast fermented for 0, 15, 30 and 45 minutes; E, F, G and H represent samples of co-fermented with sourdough and yeast for 0, 15, 30 and 45 minutes.
Figure 12.
Gel electrophoresis of corn protein with different fermentation methods. M represents low molecular weight marker; A, B, C and D represent samples of yeast fermented for 0, 15, 30 and 45 minutes; E, F, G and H represent samples of co-fermented with sourdough and yeast for 0, 15, 30 and 45 minutes.
Figure 13.
The change of ɑ-, β- and γ- zein content in different fermentation methods. (a) Changes in the content of protein subunits during yeast fermentation; (b) Changes in the content of protein subunits during co-fermentation with sourdough and yeast.
Figure 13.
The change of ɑ-, β- and γ- zein content in different fermentation methods. (a) Changes in the content of protein subunits during yeast fermentation; (b) Changes in the content of protein subunits during co-fermentation with sourdough and yeast.
Table 1.
Pasting properties of corn starches with different fermentation methods.
Table 1.
Pasting properties of corn starches with different fermentation methods.
| |
Peak Viscosity /cp |
Through Viscosity /cp |
Breakdown/cp |
Final Viscosity /cp |
Setback/cp |
Pasting temperature/℃ |
| SY-0 |
1632 |
1228 |
404 |
2441 |
1213 |
77.4 |
| SY-15 |
1612 |
1121 |
491 |
2556 |
1435 |
78.35 |
| SY-30 |
1542 |
1082 |
460 |
2572 |
1490 |
77.5 |
| SY-45 |
1538 |
1146 |
392 |
2120 |
974 |
78.25 |
| YY-0 |
2616 |
1958 |
658 |
4044 |
2086 |
76.65 |
| YY-15 |
1665 |
1225 |
440 |
2512 |
1287 |
79.05 |
| YY-30 |
1687 |
1184 |
503 |
2605 |
1421 |
63.65 |
| YY-45 |
1653 |
1196 |
457 |
2624 |
1428 |
66.8 |
Table 2.
The short-range ordered structure of corn starches with different fermentation methods.
Table 2.
The short-range ordered structure of corn starches with different fermentation methods.
| Sample |
1047/1022cm-1
|
Sample |
1047/1022cm-1
|
| SY-0 |
0.962±0.004ab
|
YY-0 |
0.965±0.005a
|
| SY-15 |
0.955±0.001ab
|
YY-15 |
0.962±0.007ab
|
| SY-30 |
0.953±0.007ab
|
YY-30 |
0.960±0.003ab
|
| SY-45 |
0.950±0.004b
|
YY-45 |
0.956±0.000ab
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).