Introduction
Japan’s health care system has been very strong, delivering long life expectancy at relatively low cost [1]. However, costs have risen in recent years and are projected to increase further due to expensive medical advances and the ageing population [2]. Japanese citizens currently expect to reach an age of 82 among men and 88 among women in 2020, increasing to 85 among men and 91 among women by 2060 [3]. Seniors do not necessarily burden the economy though – if fit and healthy, elderly adults represent a valuable and often-utilized asset, for example for additional labor or childcare assistance [4]. However, health care spending scales up when seniors become ill, especially since this increases the likelihood of them requiring social care, for example through nursing homes [5,6].
While increasing health expenditures were not a major concern in times of economic growth, the stagnating economy and increasing inflation challenge the public as well as private medical care sector. The pressure on hospital management intensifies, adding to the existing issues of skilled labor force shortages and high mental loads of medical professionals. Thus, Japanese hospitals need to become more cost-efficient and reduce the number of patient admissions [7]. This demand has been already acknowledged by the government and health professionals [8,9]. The Japan Vision: Health Care 2035 included the roadmap to a “Tobacco-free” Tokyo Olympics 2020 and a “Tobacco-free” society by 2035, suggesting a policy option for tobacco tax increase.8
Although the Japanese health sector performs well above the OECD average, several factors put pressure on hospital resources. These include high air pollution mortality, and myocardial infarctions mortality [10]. To improve health outcomes, reducing behavioral risks such as alcohol abuse and smoking should be prioritized. Minimizing smoking-related risks should be a cost-effective measure and initial success has already been shown in Japan. While up to 77% of male adults smoked cigarettes in the 1950s, overall prevalence declined to 17% in 2019[11,12]. This may be due to introduction of strict measures for reducing smoking in public places in combination with the Japanese enthusiasm for technological innovations. Japan was one of the earliest markets in which alternatives to traditional combustible cigarettes were made available in the form of Heated tobacco products (HTPs).
By heating tobacco using a complex battery-powered device system, the inhalation of burnt contaminants that are cancerogenic can be avoided and the toxicological absorption into a smoker's body or the environment may be significantly reduced [13,14]. Other advantages involve less odor and higher social acceptability [15,16]. Both characteristics are attractive to the Japanese population, especially urban and well-off citizens. Given its singularity in Japan – the government has not yet legalized any other similar non-combustible products, such as snus, nicotine pouches, or liquid alternatives – the Japanese HTP market can be considered as a model for analyzing the comparative risks related to HTP or combustible smoking. Four HTP producers compete in the Japanese market with four different technology platforms, which fosters innovation in the Japanese HTP market [17]. Despite this market evolvement on the supply side, demand slowed down in the past years. In 2019, prevalence of HTP use was 3%. Of all tobacco users, only 7% of males and 5% of females consume both cigarettes and HTPs [18]. Both – the low share of dual use and a rapidly declining smoking prevalence since HTP introduction – point to the substitution potential of HTPs. This hypothesis is backed by a Japanese study finding that HTPs contributed to reducing cigarette sales [19].
Nevertheless, smoking prevalence still was at 17% in 2019, causing a significant number of preventable disease incidents. Reducing smoking prevalence would help mitigate pressure on health costs and hospital resources. Measures to target specific subgroups may be essential, especially that still resist the overall trend towards for smoking cessation. For example, smoking prevalence was 27% among male adults in 2019 and among low-educated men and women 58% and 35% respectively in 2016 [20]. In addition, smoking behavior differs substantially between regions, potentially being correlated with economic strength, demography, and cultural differences. Smoking prevalence remains the highest in Hokkaido (27% in 2016) and Tohoku (21%), that are in the north of Japan, while Chugoku (21%), Shikoku (21%) and Kyushu (21%) are located in the south-west [21].
Based on real world population data and published epidemiological data, we will examine the implications of switching to HTPs on health outcomes in the form of patients of non-communicable diseases attributable to smoking. To the best of our knowledge, despite the exceptional opportunity resulting from the special market situation, this research has not yet been performed in Japan.
Methods
The research consists of three steps. First, based on a literature review, we establish the association between cigarette smoking and smoking induced diseases such as lung cancer, cardiovascular diseases, or COPD, and health care cost. Then, based on a literature review we determine the impact of switching from cigarettes to HTPs on associated health hazard. This literature includes published epidemiological data and studies on the potential of HTPs. Finally, changes in risks are translated into corresponding changes in health outcomes, survival rates, and associated changes in health care costs.
Smoking Attributable Function
A literature review was conducted on the disease burden related to smoking and the reduced disease burden associated with HTP use instead of smoking. To estimate the burden of disease of smoking, epidemiologists rely on the Population Attributable Fraction (PAF) which is the country-specific proportion of incidents, attributable to a certain risk factor. As the risk factor of interest is smoking, the PAF can then be called Smoking Attributable Fraction (SAF). In order to determine the number of patients who developed a disease due to smoking, we use disease-specific relative risks from meta-studies by Thun et al. (2000) and Gandini et al. (2008).45,46 With that we do not rely on a single source for relative risks, which tend to vary over studies. To estimate Japan’s Population Attributable Fractions, we follow the approach used in “The Preventable Risk Integrated Model” a WHO-supplied tool from Scarborough et al. (2016). To do this, we calculate the number of patients by smoking status and disease, weighted by their respective relative risks.37
Risk Reduction Potential of HTPs
Published evidence was used to determine the effect of so called reduced-risk products, particularly HTPs. A study by Forster et al. (2017) indicates an absolute risk reduction of 97% [22]. The authors investigated the nine toxicants proposed by the WHO Study Group on Tobacco Product Regulation (TobReg) for mandated reduction in cigarette emissions. They find an overall average reduction of 97.1%. Li et al. (2019) estimated 80% fewer harmful constituents in the releases from HTPs [23]. There is a number of studies that found the content of HTP smoke containing 70–95% of the concentration of nicotine and toxicant exposure found in traditional cigarette smoke [24,25,26,27] Another study conducted by Nutt et al. (2014) employed a rather unusual method: a multi-criteria decision analysis model of the relative importance of different types of harm related to the use of nicotine-containing products [28]. This expert-based analysis concluded that the risk related to the use of HTPs is 96% lower compared to cigarettes. The study by Zhang et al. (2023) showed significantly lower exhaled levels of carbon monoxide with HTPs compared to cigarettes [29]. Lower toxicant levels are confirmed by Bekki et al. (2017) of the National Institute of Public Health Japan who found HTP smoke containing one hundredth of carbon monoxide compared to combustible cigarette smoke [30].
Translating Reduced Emissions into Health Impacts
Given the recent introduction of HTPs, there is no longitudinal epidemiologic study allowing the analysis of the impact of reduced emissions on individual or public health. However, public health researchers suggest that beneficial health impacts are plausible. The Dutch National Institute for Public Health and the Environment (RIVM) derives the change in cumulative exposure (CCE) of HTPs and cigarettes, which is then translated into an estimate of the health impact – a change in expected life span [31]. Assuming an absolute risk reduction of 90%, the life expectancy of HTP consumers would be closer to the life expectancy of non-smokers than to smokers. In a detailed modeling assessment, Stephens (2017) compared relative harmfulness of different nicotine products with a model based on exposure data and cancer potencies [32]. The calculated lifetime cancer risk of the HTP was one to two orders of magnitude lower compared to combustible cigarettes. The meta-studies of Znyk et al. (2021), Uphadyay et al. (2023) and Yayan et al. (2024) provide literature reviews about the impact of Reduced-Risk Products on the individual and public health [33–35]. Among other health impacts, Znyk et al. (2021) found that HTP use is correlated with a decreased risk of lung cancer and cardiovascular diseases. Yayan et al. (2024) noted that respiratory damage caused by e-cigarettes and cardiovascular health consequences could be less severe compared to conventional cigarettes.
Results
Reported at
Table 3, the annual number of smoking attributable patients would be reduced by more than 12 million cases (-26%) in the baseline scenario. Stroke cases as well as IHD cases would be reduced by -29%, i.e. more than seven million less stroke cases and more than two million less IHD cases than those in in the status quo.
Savings in associated health care costs are summarized in
Table 4. According to our estimates, total health care costs of smoking attributable diseases could decline from 1,778 bn JPY to 1,324 bn JPY (-26%). The highest savings of approximately 247 bn JPY (-29%) could be generated by the reduced numbers of stroke cases.
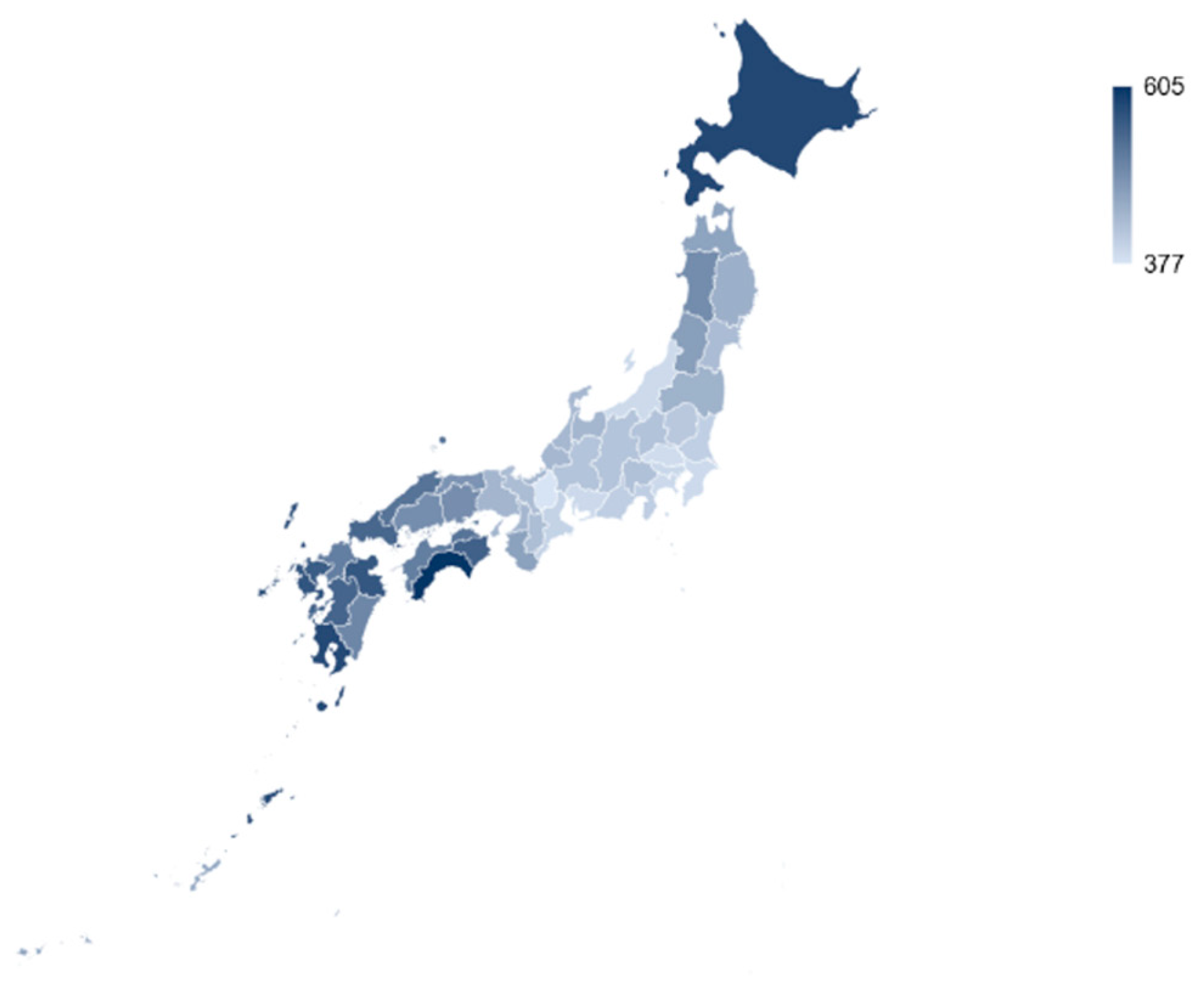
The regional breakdown of savings is reported in
Figure 1. To account for differences in population size, cost savings are displayed in million JPY per 100,000 people with darker colors representing increased savings. Prefectures located in the north and south of Japan could benefit significantly more from smokers switching to HTPs compared to the middle and eastern prefectures.
Figure 1.
Savings in health costs by prefecture (in Million JPY per 100.000 people).
Figure 1.
Savings in health costs by prefecture (in Million JPY per 100.000 people).
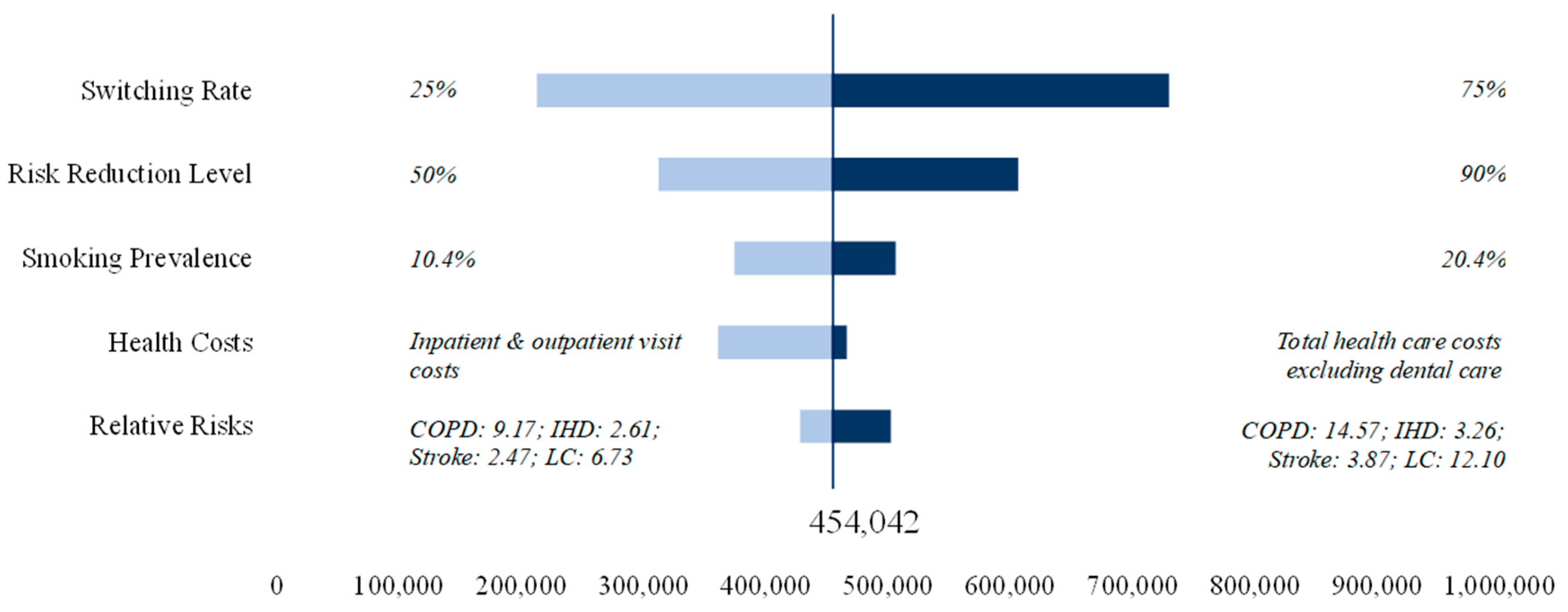
Figure 2 reports the results of the sensitivity analysis for expected cost savings. Switching rate and risk reduction are the two parameters that influence the results of the baseline scenario most. With a risk reduction of 90%, cost savings could amount to 607 bn JPY. Similar savings of 730 bn JPY could be achieved with a switching rate of 75% and base-case risk reduction. For health care costs we use costs for inpatient and outpatient visits as our lower bound and total health care costs excluding dental care as our upper bound. A variation in smoking prevalence would influence the results of the baseline scenario marginally.
Discussion
The Japanese health care system has long been acclaimed for its performance in terms of delivering the world’s longest average life expectancy at relatively low cost [47]. However, in recent years, Japan has been struggling with rising health care costs, mainly due to its ‘super ageing’ population [48]. Even now, hospital doctors suffer from overwork and burnouts [49]. Considering further increase in life expectancy in the future decades, health costs are expected to rise significantly. One way for the Japanese government to decrease the share of its GDP that is spent on health care is to reduce risks related to smoking. Switching smokers to HTPs seem to offer an effective means for reducing health care risks and related health care cost.
Similar to other countries, public health policy traditionally focuses on smoking cessation, and cessation treatments have been covered under universal health insurance in Japan since 2006. In April 2006, Chuikyo (Central Social Insurance Medical Council), which is an advisory body to the Minister of Health, Labor and Welfare that deliberates on revisions to Japan's health insurance system and medical fees, included outpatient smoking cessation guidance (nicotine dependence management fee) in the National Formulary. This was the first time in history that the Chuikyo had decided on insurance coverage based on cost-effectiveness considerations. The following four points were discussed at that time: 1) Verification of cost-effectiveness was required for insurance coverage, 2) Domestic economic evaluation research was required, 3) It was not about reducing costs, and good cost-effectiveness is accepted, and 4) Model analysis is not regarded as evidence [50]. However, the discussions within the government stagnated regarding the cost-effectiveness evaluation until the trial implementation of cost-effectiveness evaluation in 2016 [51].
One recent example is the CureappSC digital smoking cessation app that received reimbursement in November 2020 [52]. The app is used together with a portable device that measures carbon monoxide concentration in a patient’s breath [53]. However, actual adoption is limited in Japan[54] Even though public health policy encourages cessation on many communication levels, this approach has seen limited success.
One reason is that many smokers do not necessarily find smoking cessation desirable even if they are aware of the risk it imposes on health. In Japan, only one quarter of smokers actually want to quit [55]. Moreover, approximately 86% of those that reported to quit smoking relapse back into their harmful behavior [56]. Exclusive policy focus on cessation therefore may not be a sustainable strategy and other, new solutions need to be found to reduce smoking-related disease burdens – such as facilitating a switch to risk-reduced products such as HTPs.
Our model indicates a reduction in the incidence of smoking attributable diseases and a related reduction in cost. According to our baseline estimates, a 50% switch of smokers to HTPs could reduce the number of smoking attributable diseases such as lung cancer or heart diseases by 12 million patients annually. The associated savings in direct health care costs would amount to 454 bn JPY a year (3 billion USD) or -26%.
The impact estimated through our model for the Japanese context compares well to other reports. Igarashi A, et al. (2016) estimate 6% lower medical costs if the smoke cessation drug varenicline would increasingly be used for cessation [57]. In comparison, our model predicts HTP-related health care cost reduction of 26%. The difference can be explained due to the low cessation success rate of varenicline, of which only 14% of individuals undergoing a quit attempt succeed [58]. Our estimated reduction in health outcomes for Japan also point to similar findings when compared to studies conducted in other countries [59–62].
At a regional level, highest potential savings could be realized in the prefectures in the South and West of Japan, for instance within Shikoku or Kyushu islands (e.g. Kochi and Kagoshima). These rather rural areas have a lower regional gross product but a high smoking prevalence. Physician shortages in those remote areas are of increasing concern [63,64]. Reducing physician utilization by reducing health risks may be one effective remedy. Considering the heterogeneous prevalence rates across population groups and the uneven age and working population distribution across prefectures, a one-size-fits-all, centralized approach to tobacco control may not be the best answer for the Japanese government. To leave no one behind, health authorities need to employ multiple tools for reducing the negative health impacts of smoking – including both cessation and behavioral change with a device innovation such as switching to HTPs.
Policy Implications
Implementing cost-efficient measures beneficial to public health and economy should be the primary goal of the Japanese government. Despite strict policies, smoking behaviors have not been eliminated completely in Japan. Encouraging smokers to switch from smoking traditional cigarettes to reduced-risk products and preventing young adults from initiating tobacco use may be complimentary and effective policies to reduce the negative health consequences and health care cost. In addition, raising awareness of the health risks associated with each tobacco product enables the consumer to make an informed, independent and rational choice.
Most importantly, price controls in the form of tobacco taxes have proven to be the most effective regulatory measure [65]. A well-designed tobacco tax mix should favor reduced-risk products to incentivize smokers to switch. A harm-based approach by taxing according to the external health risk of a product may be justified [66]. The current tax plans of the Japanese government collide with this theorem, however, as they neglect any differences in harm and seem to maximize fiscal returns instead of maximizing the strategic impact on health [67]. Finally, investing into longitudinal epidemiological studies remains important to gain more knowledge about the health risks of reduced-risk products [68]. This allows governments to enable evidence-based regulatory decisions.
Limitations
The simulation rests on several assumptions that are central to the model outcomes. Mainly, we assume the HTPs are a substitute for traditional tobacco products. Although this assumption is backed by the literature, results would change if HTPs would attract non-smokers. In that case population risks would rather increase than decrease with unintentional consequences for health outcomes and costs. In addition, long term risks of HTPs are not yet fully assessed. While the applied rate of 70% risk reduction seems reasonable, some long-term harms might be underestimated. Another assumption is, that reduction in toxicological risks as shown in several studies represents a meaningful reduction in effective health risks for the users. However, we also might have underestimated some of the benefits of switching to HTPs. For instance, a Japanese study conducted during COVD-19 found out that compared with cigarette-only users, HTP-only users were more likely to quit smoking all together [69].
Another reason why our study might underestimate the benefits from switching is that only four smoking attributable diseases were considered. Smoking also increases the risk for many other illnesses such as psoriasis, rheumatoid arthritis, or inflammatory bowel disease which were not covered in our analysis [70–72]. Furthermore, only direct health care costs were taken into consideration for our cost estimates. However, indirect costs such as productivity losses are even more relevant. Globally, the total burden of smoking was estimated to be 1,852 billion USD (in purchasing power parity) in 2012. Only 467 billion USD (25%) of this amount can be associated with health care expenditures [73].
Conclusions
This analysis for Japan indicated that switching from smoking to heated tobacco products could be associated with annual savings of 26% or 454 bn JPY. The second finding involves the number of 12 million prevented patients, both inpatients and outpatients. Saved hospital resources could be more efficiently allocated to relieve the burden on nursing staff and would most likely be used for patients with non-smoking related illnesses.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was funded by Health Outcomes Strategies GmbH.
Disclosures
The authors declare no conflicts of interest.
References
- OECD. Japan needs to improve the efficiency of its health care system, says OECD. Available from: . Accessed April 06, 2024.
- Inoue S, Xu H, Kobayashi M. Forecasting of Future Medical Care Expenditure in Japan Using a System Dynamics Model. INQUIRY J. Health Care Organ. Provis. Financ. 2022, 59. [Google Scholar]
- National Institute of Population and Social Security Research. Estimated future population of Japan, Tokyo 2021. Available from : https://www.ipss.go.jp/pp-zenkoku/j/zenkoku2023/pp2023_gaiyou.pdf, Accessed April 07, 2024.
- Sun Y. Positive and negative effects of the “power of grandparents” participating in childcare: A comparative study of Japan and China. Institute of Human Sciences, Ritsumeikan University. 2018. Available from: https://www.ritsumeihuman.com/en/essay/positive-and-negative-effects-of-the-power-of-grandparents-participating-in-childcare-a-comparative-study-of-japan-and-china/. Accessed. April 06, 2024.
- Hosokawa R, Ojima T, Myojin T, Aida J, Kondo K, Kondo N. Associations between Health care Resources and Healthy Life Expectancy: A Descriptive Study across Secondary Medical Areas in Japan. Int. J. Environ. Res. Public Health 2020, 17, 6301. [Google Scholar] [CrossRef]
- Ibuka Y, Chen SH, Ohtsu Y, Izuminda N. Medical Spending in Japan. Fiscal Studies. 2016, 37, 561–592. [Google Scholar] [CrossRef]
- Coady D, Clements BJ, Gupta S. Chapter 11: Challenges in Reforming the Japanese Health Care System. The Economics of Public Health Care Reform in Advanced and Emerging Economies. USA: International Monetary Fund. 2012.
- Health Care 2035 Advisory Panel. The Japan Vision: Health Care 2035. 2015. Available from: https://www.mhlw.go.jp/seisakunitsuite/bunya/hokabunya/shakaihoshou/hokeniryou2035/assets/file/health care2035_proposal_150703_summary_en.pdf. Accessed April 06, 2024.
- UCA News. Why are nurses underpaid in Japan? 2023. Available from: https://www.ucanews.com/news/why-are-nurses-underpaid-in-japan/103217. Accessed April 06, 2024.
- OECD. Health at a Glance 2023 Country Note Japan. Available from: https://www.oecd.org/japan/health-at-a-glance-Japan-EN.pdf. Accessed April 08, 2024.
- Forey B, Hamling J, Thornton A, Lee P. International Smoking Statistics Japan. P N Lee Statistics & Computing Ltd. 2016. Available from: http://www.pnlee.co.uk/Downloads/ISS/ISS-Japan_161220.pdf. Accessed April 06, 2024.
- Ministry of Health, Labour and Welfare. National Health and Nutrition Survey. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html. Accessed April 02, 2024.
- Gravely S, Fong GT, Sutanto E, et al. Perceptions of Harmfulness of Heated Tobacco Products Compared to Combustible Cigarettes among Adult Smokers in Japan: Findings from the 2018 ITC Japan Survey. Int. J. Environ. Res. Public Health. 2020, 17, 2394. [Google Scholar] [CrossRef]
- Bekki K, Inaba Y, Uchiyama S, Kunugita N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J UOEH. 2017, 39, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Otsuka Y, Kaneita Y, Itani O, Matsumoto Y. Why Do Physicians in Japan Use e-Cigarettes and/or Heated Tobacco Products? A Cross-Sectional Survey. Hygiene. 2024, 4, 1–13. [Google Scholar]
- Xu SS, Meng G, Yan M, et al. Reasons for Regularly Using Heated Tobacco Products among Adult Current and Former Smokers in Japan: Finding from 2018 ITC Japan Survey. Int. J. Environ. Res. Public Health. 2020, 17, 8030. [Google Scholar] [CrossRef]
- Yasuda Y. Why do Prices Drop Amidst Inflation? Economics Design Inc. 2023. Available from: https://econ.news/wp-content/uploads/2023/11/DiscussionPaper_001_Eng.pdf. Accessed April 08, 2024.
- Ministry of Health, Labour and Welfare. National Health and Nutrition Survey. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html. Accessed April 02, 2024.
- Stoklosa M, Cahn Z, Liber A, Nargis N, Drope J. Effect of IQOS introduction on cigarette sales: evidence of decline and replacement. Tob Control. 2020, 29, 381–387. [Google Scholar]
- Tanaka H, Mackenbach JP, Kobayashi Y. Widening Socioeconomic Inequalities in Smoking in Japan, 2001–2016. Journal of Epidemiology. 2021, 31, 369–377. [Google Scholar] [CrossRef]
- Tanaka H, Mackenbach JP, Kobayashi Y. Widening Socioeconomic Inequalities in Smoking in Japan, 2001–2016. Journal of Epidemiology. 2021, 31, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Forster M, Fiebelkorn S, Yurteri C, et al. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regulatory Toxicology and Pharmacology. 2018, 93, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Li X, Luo Y, Jiang X, Zhang H, et al. Chemical Analysis and Simulated Pyrolysis of Tobacco Heating System 2.2 Compared to Conventional Cigarettes. Nicotine & Tobacco Research. 2019, 21, 111–118. [Google Scholar]
- Auer R, Concha-Lozano N, Jacot-Sadowski I, Cornuz J, Berthet A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Jaccard G, Tafin Djoko D, Moennikes O, Jeannet C, Kondylis A, Belushkin M. Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul Toxicol Pharmacol. 2017, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos K, Yannovits N, Sarri T, Voudris V, Poulas K, Leischow S. Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction. 2018, 113, 2099:2106. [Google Scholar]
- Farsalinos K, Yannovits N, Sarri T, Voudris V, Poulas K. Nicotine Delivery to the Aerosol of a Heat-Not-Burn Tobacco Product: Comparison With a Tobacco Cigarette and E-Cigarettes. Nicotine & Tobacco Research. 2018, 20, 1004–1009. [Google Scholar]
- Nutt D, Phillips L, Balfour D, Curran H, et al. Estimating the Harms of Nicotine-Containing Products Using the MCDA Approach. Eur Addict Res. 2014, 20, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang X, Sun Y, Cheung YTD, et al. Cigarettes, heated tobacco products and dual use: exhaled carbon monoxide, saliva cotinine and total tobacco consumed by Hong Kong tobacco users. Tobacco Control 2023.
- Bekki K, Inaba Y, Uchiyama S, Kunugita N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J UOEH. 2017, 39, 201–207.
- Slob W, Soeteman-Hernández L, Bil W, Staal Y, Stephens W, Talhout R. A Method for Comparing the Impact on Carcinogenicity of Tobacco Products: A Case Study on Heated Tobacco Versus Cigarettes. Risk Analysis. 2020, 40, 1355–1366. [Google Scholar] [CrossRef]
- Stephens, W. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob Control. 2018, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zynk M, Jurewicz J, Kaleta D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health. 2021, 18, 6651. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay S, Rahman M, Johanson G, Palmberg L, Ganguly K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics. 2023, 11. [Google Scholar]
- Yayan J, Franke K, Biancosino C, Rasche K. Comparative systematic review on the safety of e-cigarettes and conventional cigarettes. Food and Chemical Toxicology 2024, 185.
- Ministry of Health Labour and Welfare. Handbook of Health and Welfare Statistics 2022, Tokyo 2023; available at: https://www.mhlw.go.jp/english/database/db-hh/2-2.html.
- Scarborough et al. The preventable risk Integrated Model. National Library of Medicine. 2016. available at: https://pubmed.ncbi.nlm.nih.gov/25328757/.
- Ministry of Health Labour and Welfare. Handbook of Health and Welfare Statistics 2022, Tokyo 2023; available at: https://www.mhlw.go.jp/english/database/db-hh/5-1.html.
- Djurdjevic S, Lee PN, Weitkunat R, Sponsiello-Wang Z, Lüdicke F, Baker G. Modeling the Population Health Impact of Introducing a Modified Risk Tobacco Product into the U.S. Market. Health Care (Basel). 2018, 16, 47. [Google Scholar]
- Ministry of Health Labour and Welfare. National Health and Nutrition Survey 2019, Tokyo 2020; available at: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/.
- Stoklosa M, Cahn Z, Liber A, Nargis N, Drope J. Effect of IQOS introduction on cigarette sales: evidence of decline and replacement. Tob Control. 2020, 29, 381–387. [Google Scholar]
- Davigo M, Klerx W, van Schooten F, Opperhuizen A, Remels A, Talhout R. Impact of More Intense Smoking Parameters and Flavor Variety on Toxicant Levels in Emissions of a Heated Tobacco Product. Nicotine & Tobacco Research 2023, 238.
- UK Committee on Toxicity. Statement on the toxicological evaluation of novel heat-not-burn tobacco products. 2017. Available from: https://cot.food.gov.uk/sites/default/files/heat_not_burn_tobacco_statement.pdf. Accessed April 02, 2024.
- Jaccard G, Tafin Djoko D, Moennikes O, Jeannet C, Kondylis A, Belushkin M. Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul Toxicol Pharmacol. 2017, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA, 2000; 584, 706–712.
- Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer 2008, 122, 155–164.
- Reich M, Ikegami N, Shibuya K, Takemi K. 50 years of pursuing a healthy society in Japan. Lancet 2011, 378, 1051–1053. [Google Scholar] [CrossRef]
- Muramatsu N, Akiyama H. Japan: Super-Aging Society Preparing for the Future. Gerontologist 2011, 51, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Economist Intelligence Unit. Health system sustainability in Japan, Tokyo 2020; available at: https://impact.economist.com/perspectives/sites/default/files/health_system_sustainability_in_japan_en_whitepaper.pdf.
- Fukuda, T. Regarding the policy use of health economic evaluation: An example of insurance coverage of smoking cessation treatment. Monthly IHEP 2007, 152, 39–43. (in Japanese). [Google Scholar]
- Mahlich J, Kamae I, Rossi B () A new Health Technology Assessment System for Japan? Simulating the Potential Impact on the Price of Simeprevir, Int J Technol Assess Health Care. 2017, 33, 121–127.
- Demiya-Dillenburger S, Isshiki M, Mahlich J. Telemedicine in Japan: Challenges and Opportunities. In: Walzer, S. (eds) Digital Health care in Germany. Contributions to Economics. Springer, Cham 2022.
- Masaki K, Tateno H, Nomura A, Muto T, Suzuki S, Satake K, Hida E, Fukunaga K. A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. npj Digit. Med. 2020, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T. , Saito, J., Odawara, M. et al. Smoking cessation interventions and implementations across multiple settings in Japan: a scoping review and supplemental survey. Implement Sci Commun 2023, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. National Health and Nutrition Survey. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html. Accessed April 02, 2024.
- Hagimoto A, Nakamura M, Morita T, Masui S, Oshima A. Smoking cessation patterns and predictors of quitting smoking among the Japanese general population: a 1-year follow-up study. Addiction. 2010, 105, 164–173.
- Igarashi A, Goto R, Suwa K, Yoshikawa R, Ward AJ, Moller J. Cost-Effectiveness Analysis of Smoking Cessation Interventions in Japan Using a Discrete-Event Simulation. Appl Health Econ Health Policy. 2016, 14, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Benli AR, Erturhan S, Oruc MA, Kalpakci P, Sunay D, Demirel Y. A comparison of the efficacy of varenicline and bupropion and an evaluation of the effect of the medications in the context of the smoking cessation programme. Tob Induc Dis, 2017; 15, 10. [CrossRef] [PubMed]
- Wilson N, Petrović-van der Deen F, Cleghorn C, Gartner C, Blakely T. Vaping very likely to be improving health in NZ – but how does this compare with other options? Public Health Communication Centre Aotearoa. 2019. Available from: https://www.phcc.org.nz/briefing/vaping-very-likely-be-improving-health-nz-how-does-compare-other-options. Acessed April 08, 2024.
- Moscone, F. Does switching from tobacco to reduced-risk products free up hospital resources? British Journal of Health care Management. 2024, 29. [Google Scholar] [CrossRef]
- Espinosa Herrera A. Economic Gains of Transitioning Towards Reduced-Risk Products: Evidence from Mexico. 2024. [CrossRef]
- Cabuay CJ, An economic evaluation of the use of non-combusted alternatives using a cost of illness approach: The Philippine case. DLSU - Angelo King Institute for Economic and Business Studies: DLSU-AKI Working Paper Series 2023:11-088.
- Matsumoto M, Inoue K, Bowman R, Noguchi S, Toyokawa S, Kajii E. Geographical distributions of physicians in Japan and US: Impact of health care system on physician dispersal pattern. Health Policy. 2010, 96, 255–261. [CrossRef] [PubMed]
- Matsuda, S. Health Policy in Japan – Current Situation and Future Challenges. JMA J. 2019, 2, 1–10. [Google Scholar] [PubMed]
- WHO. The economic and health benefits of tobacco taxation. 2015. Available from: https://www.who.int/publications/i/item/WHO-NMH-PND-15.6#. Accessed April 08, 2024.
- Yoo S, Hong W, Yi D. To heat or burn? Evidence from heated tobacco product adoption in South Korea. Applied Economics. 2024, 1–14. [Google Scholar]
- The Japan News. Government proposal to increase taxes on heated cigarettes to the same level as cigarettes for defense funding. Available from: https://www.yomiuri.co.jp/economy/20231212-OYT1T50109/. Accessed April 12, 2024.
- Braznell S, Campbell J, Gilmore A. What Can Current Biomarker Data Tell Us About the Risks of Lung Cancer Posed by Heated Tobacco Products? Nicotine & Tobacco Research. 2023, 26, 270–280. [Google Scholar]
- Yamamoto T, Abbas H, Kanai M, et al. Factors associated with smoking behaviour changes during the COVID-19 pandemic in Japan: a 6-month follow-up study. Tobacco Control 2022. [CrossRef]
- Naldi L, Mercuri SR. Smoking and psoriasis: from epidemiology to pathomechanisms. J Invest Dermatol. 2009, 129, 2741–3.
- Chang K, Yang SM, Kim SH, Han KH, Park SJ, Shin JI. Smoking and rheumatoid arthritis. Int J Mol Sci. 2014, 15, 22279–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, BC. , Weng, MT., Chang, CH. et al. Effect of smoking on the development and outcomes of inflammatory bowel disease in Taiwan: a hospital-based cohort study. Sci Rep 2022, 12, 7665. [Google Scholar] [CrossRef] [PubMed]
- Goodchild M, Nargis N, Tursan d'Espaignet EGlobal economic cost of smoking-attributable diseases. Tobacco Control 2018, 27, 58–64. [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare, Japan. National Health and Nutrition Survey 2016, Table 91: Proportion of Habitual Smokers Aged 20 and Over by Sex and Regional Block, Age-Adjusted. Available from: https://www.e-stat.go.jp/dbview?sid=0003234812. Accessed 2024.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).