1. Introduction
Human rabies is a lethal, neurological, neglected viral zoonotic disease and a persistent global problem [
1]. The only method to fight the illness both before and after exposure is by vaccination. The disease is fatal after the onset of symptoms. Many rabies vaccines for use in humans and animals have been developed over the years, with varied degrees of safety and efficacy, since Pasteur first crude nerve tissue vaccine in 1885 [
2,
3]. Currently, inactivated rabies vaccinations derived from cell cultures are widely utilized worldwide. However, these vaccines are cost ineffective and exorbitant for vaccination of humans and animals in developing countries [
2]. Even though rabies is vaccine preventable disease, still it claims around 60000 lives every year and 40% of the cases are among the children below the age of 15 years [
4]. India accounts for 59.9% of rabies deaths in Asia and 35% of global death burden [
5]. As per Government of India approximation, various rabies vaccine manufactures in India produce about 50 million doses of rabies vaccine to meet the demand of 48 million doses annually. However, more han 30% of the rabies vaccine produced in India is exported to other countries across the world to fetch higher price. It is estimated that every year, more than 15 million people worldwide receive post-bite vaccinations, preventing thousands of rabies-related fatalities [
6].
Since aluminum (Al) is a strong immune system stimulant, it is the most widely used adjuvant in vaccines, albeit the exact process is still unknown [
7,
8]. There has been evidence that Al, a neurotoxic that has been shown in experiments, can affect the human neurological system [
9,
10,
11]. Apart from its long-term use as adjuvant for over 80 years, the safety of Al as adjuvant rests largely on assumptions rather than experimental evidences. Furthermore, it is yet unclear how Al adjuvants interact with the immune system [
12,
13]. However, experimental studies unequivocally demonstrate that Al adjuvant likely possesses the ability to cause significant immunological issues in people. Specifically, using Al as an adjuvant increases the likelihood of autoimmunity, chronic brain inflammation, and related neurological problems, which could have serious and pervasive negative health effects [
9]. Inactivated rabies vaccines withstand diverse quality control tests during in-process and as well as final lot stage including the tests for purity, potency and safety including test for residual live rabies virus, etc. specified as per the national or international compendia or guidelines. This panel of tests is mandatory for market release of the vaccine. However, the test for residual live rabies virus assay is considered very critical for the safety assessment of vaccine batches. Therefore, detection of the presence of residual live rabies virus is of the paramount importance in case of inactivated rabies vaccines. National Institute of Biologicals (NIB) being a National Control Laboratory (NCL) is mandated for quality evaluation of biologicals to be marketed in India. To ensure that the rabies virus is effectively inactivated in rabies vaccine preparations, the safety of both non-adjuvanted inactivated rabies vaccinations and aluminum phosphate adjuvanted rabies vaccines was investigated in this work. Additionally, studies also conducted to examine the potential toxicity or interference caused by the vaccine Al adjuvant in both animals and in vitro cell culture.
2. Materials and Methods
2.1. Animals Used and Ethical Approval
Swiss outbred mice (3-4 weeks old, weighing 12-15gm) maintained at Animal Facility of National Institute of Biologicals (NIB) (Noida, Uttar Pradesh, India) under specific pathogen free conditions were used in the test [
14]. The mice were kept in polycarbonate cages with a 12:12 day/night cycle, controlled humidity and temperature, and corncob bedding. A balanced pellet diet (Golden Feed, Delhi) was given to the mice. The procedures carried out on mice for this study strictly adhered to the approvals given by the Institutional Animal Ethics Committee (IAEC), and all animals were housed and maintained in accordance with the guidelines of the Committee for Control and Supervision of Experiments on Animals (CCSEA).
2.2. Rabies Vaccine Preparations under Study
The three batches each of six different inactivated rabies vaccine preparations marketed in India, out of which five were non-adjuvanted inactivated cell culture rabies vaccine (coded as RV1, RV2, RV3, RV4, RV5) and one was aluminum phosphate adjuvanted inactivated cell culture rabies vaccine (coded as ARV) studied for safety for residual live virus and interreference of aluminum phosphate adjuvant in the test for residual live virus in inactivated cell culture rabies vaccine as part of safety test for quality evaluation at NCL level. The composition of the vaccines was given in
Table 1.
2.3. Assay for Residual Live Virus in Rabies Vaccine by Mouse Inoculation Test (MIT)
Residual live virus was tested in vaccine preparations by MIT as described earlier [
14]. Group of 10 mice were used for each vaccine preparation and each mouse was intracerebrally injected with 0.03ml of undiluted vaccine preparation. Post inoculation, mice were observed everyday till 14 days for appearance of any specific symptoms of illness or neurological complications indicating rabies such as ruffled fur, trembling, paralysis or death [
14,
15]. The mice died during the test were further investigated to confirm the presence of the residual live rabies virus.
2.4. Harvesting of Mouse Brain and Preparation of Brain Suspension
To confirm the presence of live rabies virus, the mice brains were harvested and brain suspensions were prepared as per the standard method [
14,
16]. The brain of mice died after intracerebral inoculation of the vaccine were harvested and kept at 2-8ºC in 5ml of 50% suspension of glycerol (Sigma-Aldrich, Cat no. G55I6) in phosphate buffer saline (PBS). The 20% (w/v) brain suspension was prepared by grinding the brain harvest in sterile mortar and pestle under aseptic conditions in PBS followed by centrifugation at 150g for 5 mins to remove the gross particles. This 20% brain suspension was stored at -80ºC till further use.
2.5. Confirmation of Live Rabies Virus in Mouse Brain by Direct Slide Fluorescent Antibody Test (FAT)
To confirm the presence of live rabies virus, the impression smear was prepared from brain harvested from dead mice (cerebrum, cerebellum and brain stem) on a glass slide as well as from the 20% mouse brain suspension and FAT was performed as described earlier [
14,
17]. The impression smear was fixed at -20ºC in 100% acetone (SDFCL, Cat. No. 20003) for 30 minutes. After the fixation, polyclonal antibody FITC (Fluorescein Isothiocyanate; Millipore, Cat. No. 5100) conjugate (dilution of 1:40 in PBS) was applied over the smear followed by incubation at 37°C for 30 minutes. The slides were washed with PBS and allowed to dry in air. Few drops of glycerol were applied on the smear which were then covered and observed under fluorescent microscope. A rabies virus positive smear was prepared from the rabies Challenge Virus Standard (CVS) mouse brain suspension (CVS IP-18, B.N. 1/10) obtained from Central Research Institute (CRI), Kasauli, H.P. India; served as control.
2.6. Detection of Live Rabies Virus in Dead Mouse Brain Suspension by Virus Amplification in BHK 21 and MNA Cell Lines
BHK 21 (ATCC no. CCL-10) and Mouse neuroblastoma (Neuro-2a) (ATCC, CCL131) cell lines were used to check the rabies virus infection in the mouse brain suspension. The cells were cultured in DMEM (Genetix, Cat. No. CC3001.05L) with FBS (Himedia B.No. 0000276896). Briefly, in a 96-well plate, 100μl of 20% mouse brain suspension (20%) in PBS (pH 7.4 + 0.02) was added to 200μl of 2×10
5 cells/ml followed by incubation at 37°C and 5% CO
2 for 4 hours. The rabies challenge virus standard (CVS) mouse brain suspension (CVS IP-18, B.N. 1/10) sourced from Central Research Institute, Kasauli, H.P. (India) was used for positive control. Cells were sensitized by treatment with 1% aqueous DEAE-dextran solution (Sigma-Aldrich, Cat. No. 30461) for 10 mins prior to addition of brain suspension to the cells. Following this, 200μl of fresh medium was added to each well after the supernatant from each well was removed. After further incubation of 72 hours, the supernatant was removed and cells were fixed with 80% cold acetone (-20°C) for 15 mins. The plates were dried and cells were stained with 50μl/well of polyclonal antibody FITC (fluorescein isothiocyanate) conjugate (Millipore, Cat. No. 5100) with 1:40 dilution in PBS followed by incubation 37°C for 30 minutes [
14]. After staining, the cells were observed under florescent microscope (Nikon, Eclipse Ti-S).
2.7. Determination of Aluminum Content in Adjuvanted Rabies Vaccine
Aluminum content in the vaccine preparations was determined using pharmacopeial standard protocol [
18]. Vaccine preparation (ARV) was thoroughly mixed then and 1ml of ARV was added to a 50ml conical flask. For blank control, 1ml of 18.2 mΩ Milli Q (Millipore) was taken in another 50ml conical flask. Further 2ml of 18.2 mΩ Milli Q water, 1ml of sulphuric acid, 0.1ml of nitric acid and few glass beads were added to each flask. Heated the solution in flasks, till white fumes evolve, followed by cooling for few minutes. Added 10ml of 18.2 mΩ Milli Q water and 02 drops of 0.1% methyl orange solution (w/v). The solution in each flask was neutralized with sodium hydroxide solution (10M) till yellow color appeared followed by addition of 10 ml of 0.01M EDTA and 10ml of acetate buffer solution (pH 4.4 + 0.2). The solution was boiled gently and added 0.1ml of 1-pyridyl-2-azonapthol (PAN) (0.1% w/v). The solution was titrated against 0.01M copper sulphate until color changed to purple brown and the volume of copper sulphate solution (0.01M) consumed was noted.
The aluminum (Al3+) content in ARV was calculated by the using the formula:
Aluminum (Al3+) content = {(VB –VS) x 0.2698} / A
Where, ‘VB’ is the volume copper sulphate solution (0.01M) consumed in blank, ‘VS’ is the volume copper sulphate solution (0.01M) consumed in sample (ARV), ‘A’ is the volume of sample (ARV) taken for the test and 1ml of 0.1M disodium edetate is equivalent of 0.2698 mg of aluminum (Al3+).
2.8. Determination of Aluminum Adjuvant Toxicity in BHK 21 Cell Line
The cell suspension (50,000 cells) was added to each well in six wells plate followed by addition of 1ml of vaccine dilution (ARV) (
Table 2) containing aluminum concentration ranging from 273.3µg/ml to 1.7µg/ml to respective wells in 6 well plates in triplicates. Similarly, vaccine dilutions of RV were prepared which served as negative control; as well to check the matrix effect for specificity in the assay. After this, 1ml of assay medium (DMEM supplemented with 5% FBS) was added to all wells. All plates were incubated at 37°C and 5% CO
2 for 72hrs. After incubation, 250µl of alamar Blue (Sigma) was added to each well and plates were further incubated for 4 hours. After incubation the plates were read for fluorescence at wavelength of 530nm for excitation and 590nm for emission in multimode reader (Tecan, spark control Magellan V3.0). After reading the plates in microplate reader, IC
50 value was calculated using GraphPad Prism software (Version 6.0) by plotting the relative fluorescent units (RFUs) against the natural log (LN) transformation of the concentration of aluminum phosphate adjuvant.
2.9. Statistical Analysis
Three separate experiments were conducted to accomplish the alamar blue assay. Using GraphPad Prism software (version 6.0), the IC
50 value was calculated using non-linear regression analysis and the log inhibition vs. normalized response-variable slope. A nonlinear four-parameter logistic (4 PL) model was fitted using the relative fluorescence units (RFU) that were obtained from the reader. To "fit the curve," the following four parameters must be estimated: A, B, C, and D; where the half value of the maximal effective concentration (IC
50) was represented by C, the slope by B, and the higher and lower asymptotes, respectively, by A and D [
19,
20]. The model equation is as follows:
y = D+ (A-D)/(1+(x/C)^B)
Where, y = dependent variable; x = independent variable.
3. Results
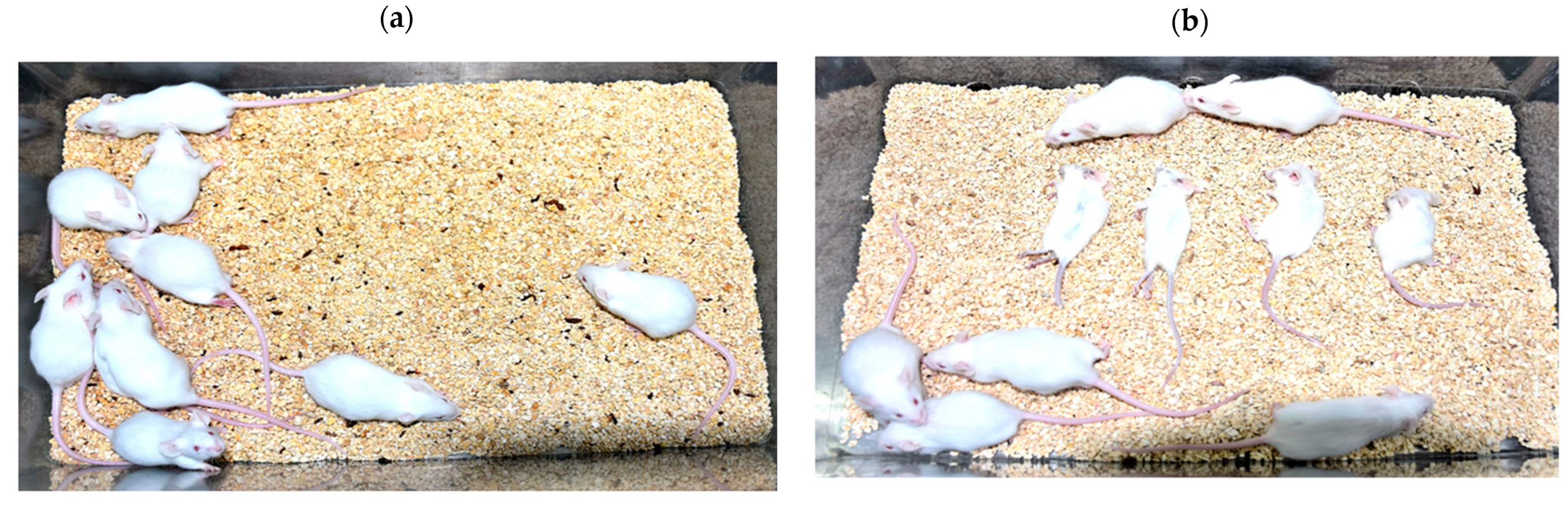
3.1. ARV Failed to Pass Indian Pharmacopoeia Acceptance Criteria for Mouse Inoculation Test
The Indian Pharmacopoeia monograph standard criteria for MIT states that in absence of residual live virus, none of the animal should die due to neurological complications. No symptoms of neurological complications or illness were observed in the mice inoculated with nom-adjuvanted inactivated rabies vaccine preparations (RV) (
Figure 1). However, the mice inoculated with ARV displayed rabies like symptoms i.e., ruffled fur, trembling, paralysis post intracerebral inoculation and all mice died between days 04 to 10. The day 04 observations are shown in the
Figure 1. The Indian Pharmacopoeia monograph standard criterion was defeated in case of ARV and results indicated the presence of residual live rabies virus in ARV. Further investigations were carried out to confirm the cause of death of mice inoculated with ARV.
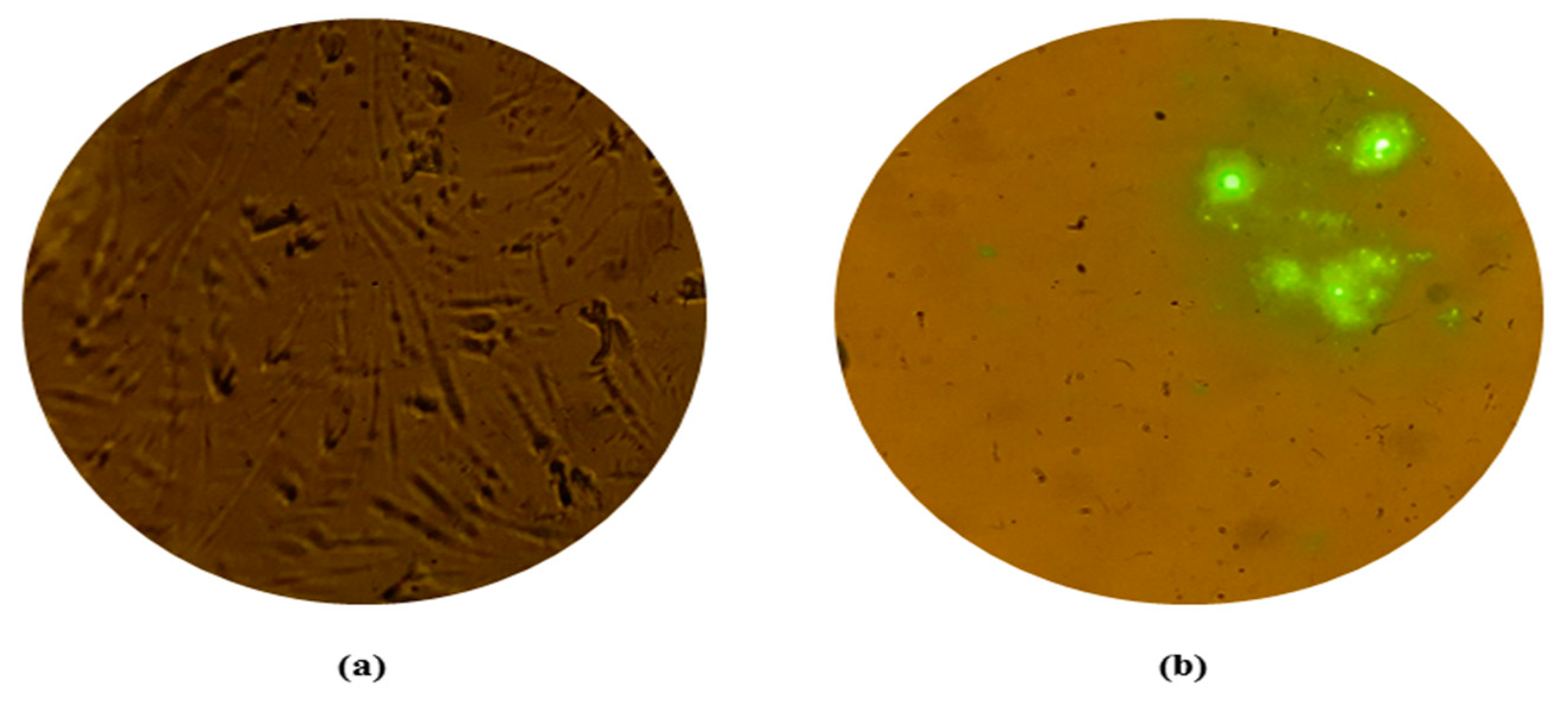
3.2. Absence of Fluorescence in Dead Mouse Brain Impression Smears Indicating Absence of Residual Live Rabies Virus
No fluorescence was observed in mouse brain impression smears or smears prepared from brain suspension from the mouse died after inoculation of ARV [
Figure 2]. However, rabies positive control smear prepared from rabies CVS mouse brain suspension showed green fluorescence. The absence of fluorescence in brain harvested from dead mice (MIT test) inoculated with ARV clearly indicated absence of residual live virus. This led to further investigations regarding death of mice inoculated with ARV.
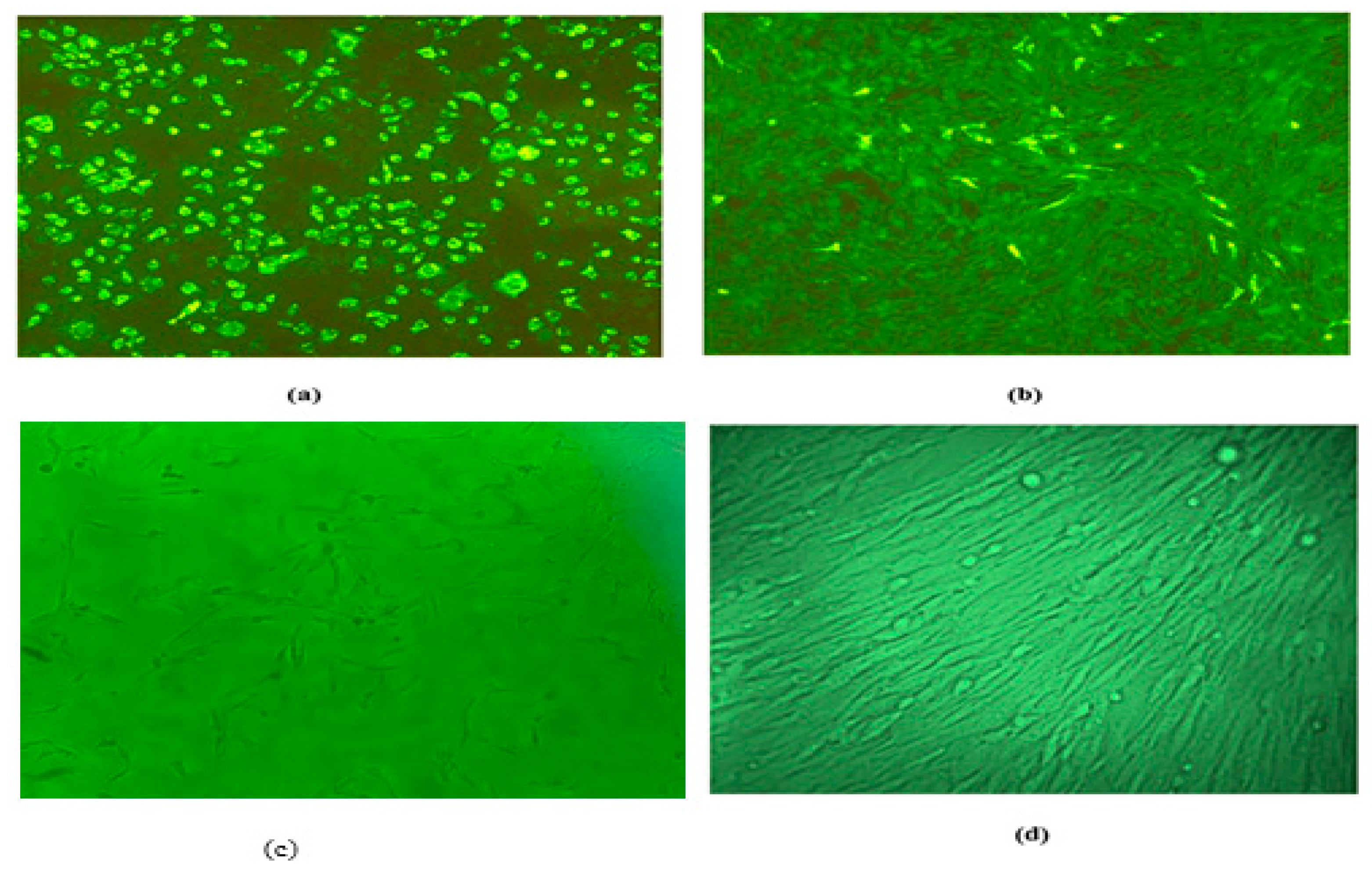
3.3. Absence of Live Rabies Virus Confirmed by Virus Amplification Test in BHK 21 Cell Lines
Live rabies virus was not detected by virus amplification test carried out using mouse brain suspension in BHK 21 cells and MNA cells. After amplification cells lines were stained with FITC, no fluorescence was observed both on MNA cells and BHK 21 [
Figure 3] cells infected with brain suspension of mouse died after inoculation with aluminum phosphate adjuvanted rabies vaccine (ARV). Green fluorescence was observed in both MNA cells and BHK 21 cells infected with brain suspension of mouse inoculated with rabies challenge virus standard. These results further confirmed absence of residual live rabies virus.
3.4. Aluminum Content in ARV
The mean value of aluminum content for ARV sample investigated in this study was found to be 0.82mg/ml.
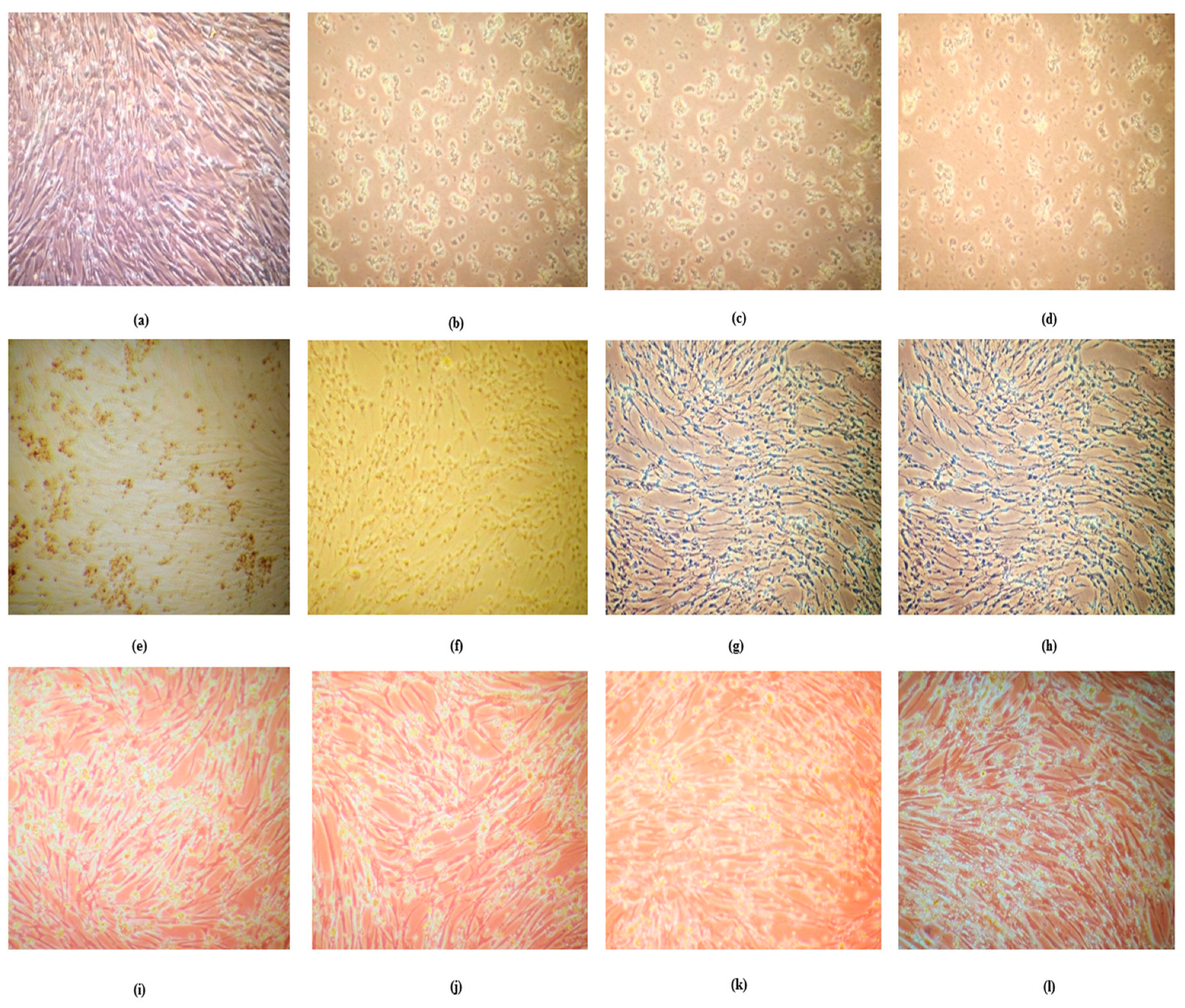
3.5. Aluminum Adjuvant Toxicity in BHK 21 Cell Line Even at Lower Concentrations
The BHK 21 cells after incubation of 72 hours and before addition of alamar blue were observed for cell morphology under phase contrast microscopic (10X) [
Figure 4]. The number of viable cells were more in untreated wells and the well-treated with RV which were kept as control, however the cells treated with ARV showed changes in the cell morphology and higher dead cells which were directly proposal to the concentration with aluminum content in ARV.
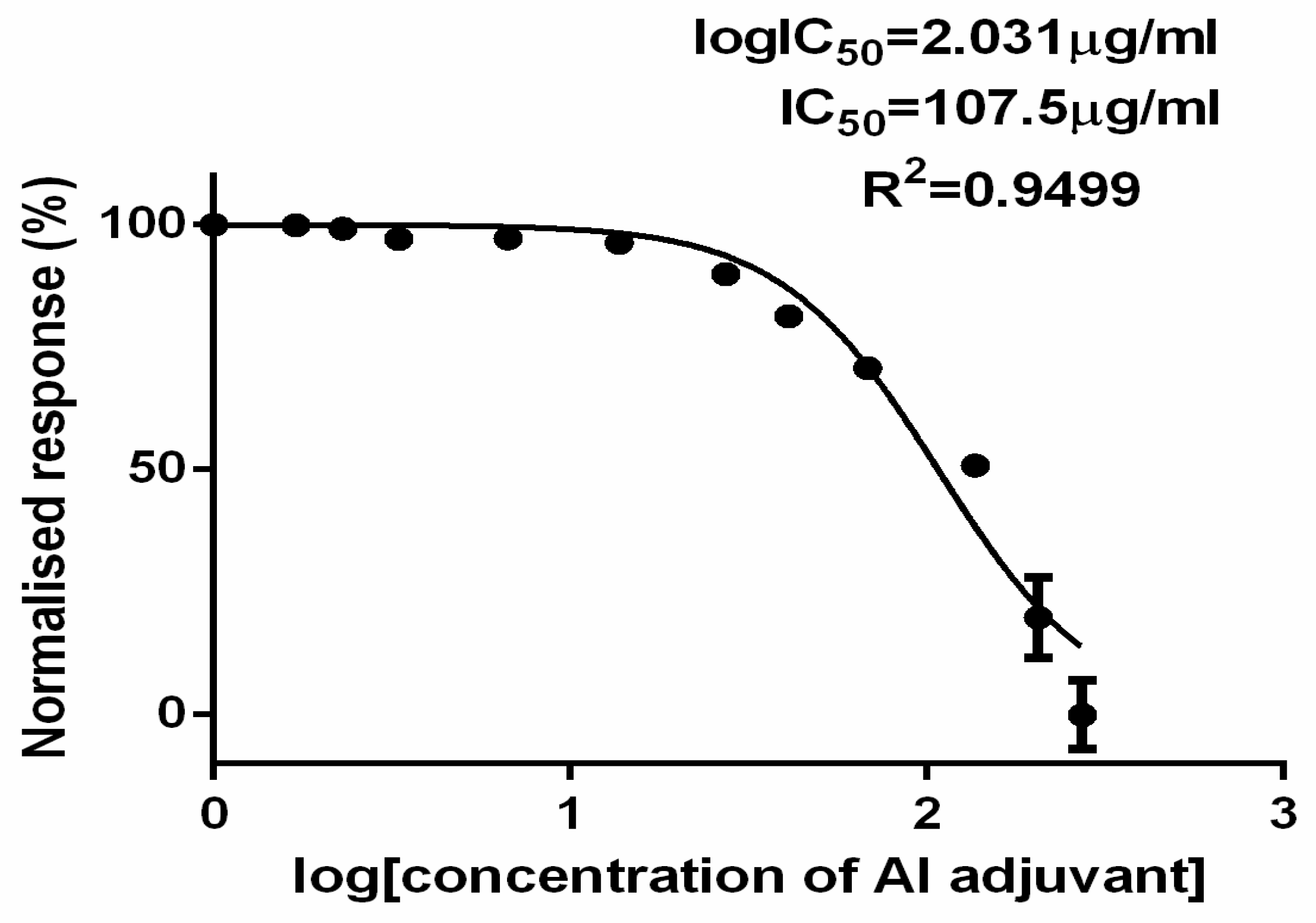
The dose response curve [
Figure 5] shows the normalized response to the logarithmic (LN) aluminum concentration, clearly demonstrating the toxicity of aluminum phosphate adjuvant in rabies vaccine. The IC
50 value of the aluminum phosphate adjuvanted rabies vaccine in this investigation was 2.3µg/ml. Consequently, it may be inferred that the sample's effective concentration to stop the 50% development of BHK cells was 2.3µg/ml, as indicated by the regression coefficient value of 0.95. This provided an explanation of the model's best fit for the experiment with confidence interval (CI) at 95% (
Table 3).
4. Discussion
Over 150 countries worldwide are affected by the disease rabies. More over a third of the world's cases of rabies occur in India, which also accounts for 59.9% of Asian cases and 35% of cases worldwide. Indian rabies vaccine manufacturers, produce around 50 million doses of rabies vaccine to meet the annual demand of 48 million doses. While, Indian manufacturers focus on export of the doses, it leads to shortfall in domestic supply in the country itself. In the end of 2019, Indian government has directed the Indian manufacturers of rabies vaccines to find a way to address the critical issue of vaccine shortage [
6,
21,
22]. The rabies vaccines that are presently on the market globally are based on fixed strains of the virus, including the Flury low egg passage (LEP), L-Pasteur 2061 strain, Pitman Moore (PM) strain, and Kissling strain of the Challenge Virus Standard (CVS)[
23]. The vaccination preparations include liquid adjuvants in some cases and lyophilized inactivated virus in others. The inactivated rabies vaccine is made using a sophisticated manufacturing process and is produced in a variety of cell cultures, including human diploid cell culture (HDCV), purified chick embryo cell vaccine (PCEC), purified Vero cell vaccine, etc. The various processes involved at every stage of production of rabies vaccine can affect the quality, safety and efficacy of the final product. Therefore, a robust and extensive quality control characterization by manufacturer as well as independently by National Control Laboratory (NCL) is of utmost importance to ensure the quality, safety and efficacy of each lot of rabies vaccine. One of the most important quality checks to guarantee the safety of the rabies vaccination is to look for residual live virus in the inactivated rabies vaccine preparations. The various national and international regulatory and pharmacopeial guidelines recommend in vivo test for detection of residual live virus involving intracerebral inoculation in mice and is a gold standard method [
24]. Indian Pharmacopoeia also recommends use of mice to ensure the complete inactivation of rabies virus in the vaccines.
In this study, the interference of the aluminum adjuvant in test for residual live rabies virus which is one the critical parameter in ensuring safety of inactivated cell culture rabies vaccine was reported. This study concluded that death of mice observed was not due to rabies virus in the vaccine but due to the toxic element in the vaccine preparation owing to aluminum phosphate adjuvant. This is an exception to the compendial method for detection of residual live virus for effective inactivation virus in rabies vaccines. Any neurological complication or death after day 4 of intracerebral inoculation of rabies vaccine in mice were considered as failure of test due to the presence of residual live rabies virus. There is no mention about the exclusion in pharmacopeial guidelines about the interference of aluminum adjuvant in test for residual live rabies virus or virus inactivation by MIT method on the final lot of inactivated rabies vaccine for human use, specifically in case of independent testing by National Control Laboratories (NCLs) [
25].
The first cell culture rabies vaccine, human diploid cell rabies vaccine (HDCV), was created in 1967 and is considered the gold standard rabies vaccine. However, being expensive over the other cell culture rabies vaccines have made it unaffordable in rabies endemic countries of Africa and Asia including India [
26]. In 1988, the new rabies vaccine for humans made from cell cultures, known as adsorbed rabies vaccine, received a license for pre- and post-exposure prophylaxis [
27]. An affordable version of HDCV adsorbed to aluminum adjuvant was licensed in India in the year 2002 [
26]. While aluminum (Al) can effectively stimulate the immune system and improve the immunological response to an antigen, the scientific literature does not seem to be certain about the safety of aluminum adjuvants. According to a US Food and Drug Administration assessment, the absence of clinical trials that could effectively address concerns about vaccine safety can be attributed to the fact that regulatory bodies have not historically considered vaccines to be harmful [
28].
Aluminum adjuvants can cause autoimmunity and cognitive impairment in adults who are likely to be sensitive [
29]. According to reports, individuals who received vaccinations containing aluminum hydroxide exhibited a very long persistence of aluminum hydroxide nanoparticles loaded macrophages at the site of prior intramuscular immunization, forming a granulomatous lesion known as macrophagic myofasciitis (MMF). These individuals also showed a delayed onset of diffuse myalgia, chronic fatigue, and cognitive dysfunctions [
30]. According to a study, oral administration of several aluminum compounds for two months caused neurodegeneration in mice, with the degree of manifestation varying depending on the chemical type of aluminum. The various aluminum compounds have a neurotoxic effect on the morphofunctional integrality of the hippocampal region as well as the central nervous system when taken orally at a dose of Al3+ = 0.1 mg/kg of body weight per day [
31].
This effect of the aluminum adjuvant was also thought to be responsible for the death of all 10 mice in the ARV group and was found to be neurotoxic even at a intracerebral dose of 24.6µg/0.03ml per mouse. The toxicity of aluminum adjuvant in ARV was also confirmed in BHK 21 cells in 6 wells cell culture plate by alamar blue cell cytotoxicity assay wherein, the IC
50 value of aluminum adjuvant in ARV was found to be 2.3µg/ml. However, on examination in phase contrast microscopy, the BHK 21 cells showed shrinkage and deformation in cell morphology even at aluminum adjuvant concentration of 1.7µg/ml. According to a literature report, Al causes stress on the endoplasmic reticulum (ER) and the formation of reactive oxygen species (ROS), which weakens the antioxidant defenses of neuronal cells and encourages neuronal death through a p53-independent route [
31]. The side effects due to the aluminum adjuvant might be the reasons that aluminum adsorbed rabies vaccine was not approved for intradermal administration; even when the universal switch to intradermal delivery in 2018 on WHO recommendation which have improved the affordability and accessibility by dramatically reducing the cost of vaccination for healthcare providers [
32,
33]. The 40% of the cases of rabies are among the children less than 15 years of age [
4]. Theoretically the dose of Al in vaccines is centered on the efficacy of the aluminum content for production of antibody titers, not o the safety aspects. However, a study has deciphered that body weight should also be considered while administering aluminum adsorbed vaccines to infants [
34]. Same study also concluded that current vaccine schedule put infants at high risk of acute as well as chronic toxic levels of aluminum. Furthermore, the infant population may have Al intolerance due to genetic or other reasons. As a result, before such vaccines are put on the market, a thorough assessment of the negative health effects of vaccination in the pediatric population is vital. Children are particularly vulnerable to aluminum-adsorbed vaccine-induced complications. The majority of tissue samples from post-mortem brains of patients diagnosed with autism spectrum disorder (ASD) were found to possess significant quantities of aluminum [
35]. Aluminum may have significant and pervasive negative health effects due to its potential for autoimmunity, chronic brain inflammation, and related neurological issues. The aluminum content in ARV sample investigated in this study was found to be 0.820 mg/single human dose which is well within the compendial limit of 1.25 mg/single human dose by various regulatory agencies like WHO, European Union, Indian Pharmacopoeia, and this content was derived from data related to aluminum content required per dose to enhance the vaccine immune response and effectiveness, but safety considerations are still undercarpet [
36,
37]. Although the evidences on the safety as well as concerns over aluminum adjuvants both are overpoweringly reported yet, associated concern continue to be fueled by poor study design and outcomes from such studies. As newer vaccine Al adjuvants are established, safety concerns both pre- and post-licensure become vital to assure public safety.
A detailed evaluation to assess the overall impact of aluminum is urgently needed wherein studies should be designed to investigate the effects of aluminum adjuvants alone and in combination with other vaccine components which can be potentially toxic (e.g., thiomersal, β-propiolactone, formaldehyde, polysorbate 80, glutaraldehyde, etc.) in different age groups. The safety of aluminum adjuvanted vaccine is particularly important for mandating regimes of vaccination for age group based civilian populations [
37]. Therefore, in our opinion, the benefits of aluminum adjuvant should not be overrated at the cost of underestimated potential risk of adverse effects. It is believed that the present study will pose a question in mind of regulatory authorities or vaccine manufactures to undertake a much-needed assessment before considering aluminum adjuvants pertaining to its respective safety in vaccines.
5. Conclusions
The study presented the challenges in ensuring the safety of aluminum adjuvated inactivated rabies vaccine at level of National Control Laboratory considering the fatality of the rabies disease. Alternative cell culture based methods shall be considered after necessary analytical comparability with MIT and thorough investigation shall be done to ensure the presence of any residual live rabies virus in inactivated rabies vaccine before market release. Further, this study also underlined the aluminum phosphate adjuvant toxicity at intracerebral dose of 24.6µg/0.03ml per mouse. However, the IC50 of aluminum phosphate was found at 2.3µg/ml in BHK 21 cells, highlighting the concerns over the safety of aluminum adjuvants in vaccines at current recommended limit of less than 1.25mg/SHD by the various regulatory agencies in combination with potentially toxic vaccine constituents.
Author Contributions
Conceptualization, S.C., S.Y.; methodology, S.C., J.M., S.Y.; software, J.M., S.C.; formal analysis, S.C., G.B. H.K., F.S.; investigation, S.C., J.M., F.S., H.K., G.B.; resources, S.Y., H.C., A.A.; writing—original draft preparation, J.M., S.C.; writing—review and editing, S.C. S.Y., and H.C. All authors have read and agreed to the publish the work.
Funding
Study was funded by seed grant of National Institute of Biologicals (Ministry of Health and Family Welfare), Government of India. Grant No.: NIB/VV/CCRV/2014.
Institutional Review Board Statement
All animal subjects used in the study were housed and maintained according to guidelines of Committee for Control and Supervision of Experiments on Animals guidelines (CCSEA) of Govt. India, and the procedures performed on mice were in strict compliance with the approvals granted by the Institutional Animal Ethics Committee (IAEC). The animal study protocol was approved by the Institutional Animal Ethics Committee of National Institute of Biologicals (Protocol code NIB/IAEC/2010/10). No human subjects were used in the research work.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated and the original contributions are included in the Article.
Acknowledgments
The authors would like to thank CDSCO (Central Drugs Standard Control Organization), Ministry of Health and Family Welfare, Government of India for providing the vaccine samples to National Institute of Biologicals. This article is a revised and expanded version of a poster (P1.101) entitled ‘Toxicity of Aluminum Adjuvant in Rabies Vaccine: A Case Study’ presented in 17th Vaccine Congress, Glasgow, Scotland, 24-27 September 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moreira, B.L.C.; Gimenez, A.P.L.; Inagaki, J.M.F.; Raboni, S.M. Inactivated rabies vaccines: Standardization of an in vitro assay for residual viable virus detection. PLoS Neglected Tropical Diseases 2020, 14, e0008142. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, B.J.; Madhusudana, S.N.; Ashwathnarayana, D.H.; Sampath, G.; Datta, S.S.; Sudarshan, M.K.; Venkatesh, G.M.; Muhamuda, K.; Bilagumba, G.; Shamanna, M. A comparative study on the immunogenicity, safety and tolerance of purified duck embryo vaccine (PDEV) manufactured in India (Vaxirab) and Switzerland (Lyssavac-N): A randomized simulated post-exposure study in healthy volunteers. Vaccine 2007, 25, 8405–8409. [Google Scholar] [CrossRef]
- Vashishtha, V.M.; Choudhury, P.; Kalra, A.; Bose, A.; Thacker, N.; Yewale, V.N.; Bansal, C.P.; Mehta, P.J. Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years — India, 2014 and updates on immunization. Indian Pediatrics 2014, 51, 785–800. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Driving progress towards rabies elimination: New WHO recommendations on human rabies immunization and results of Gavi’s Learning Agenda on rabies & 2nd international meeting of the Pan-African Rabies Control Network (PARACON). Meeting Report; 12-14 September 2018, Johannesburg, South Africa. Geneva: WHO, 2019.
- World Health Organization. Rabies: Epidemiology and burden of disease. https://www.who.int/teams/control-of-neglected-tropical-diseases/rabies/epidemiology-and-burden. [accessed on 07 July, 2024].
- World Health Organization. Rabies: Key Facts. https://www.who.int/news-room/fact-sheets/detail/rabies [accessed on 07 July, 2024].
- Tritto, E.; Mosca, F.; De Gregorio, E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009, 27, 3331–3334. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Soullie, T.; Van Nimwegen, M.; Willart, M.A.M.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. The Journal of Experimental Medicine 2008, 205, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? Journal of Inorganic Biochemistry 2011, 105, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Li, D.; Tomljenovic, L. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy 2014, 6, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kumar, M. Development in immunoprophylaxis against rabies for animals and humans. Avicenna J Med Biotechnol. 2010, 2, 3–21. [Google Scholar] [PubMed]
- Kamoltham, T.; Thinyounyong, W.; Phongchamnaphai, P.; Phraisuwan, P.; Khawplod, P.; Banzhoff, A.; Malerczyk, C. Pre-Exposure Rabies Vaccination Using Purified Chick Embryo Cell Rabies Vaccine Intradermally is Immunogenic and Safe. The Journal of Pediatrics 2007, 151, 173–177. [Google Scholar] [CrossRef]
- Haupt, W. Rabies – risk of exposure and current trends in prevention of human cases. Vaccine 1999, 17, 1742–1749. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Fooks, A.R. ; Abela-Ridder, B; editors. Laboratory Techniques in Rabies, 5th edition. Vol. 1. Geneva; World Health Organization; 2018.
- Indian Pharmacopoeia. Rabies vaccine, Human. Indian Pharmacopoeia Commission: 2022; vol.3:3682-85.
- Meslin, F.X.; Kaplan, M.M. ; Koprowsky, H; editors. Laboratory Techniques in Rabies. 4th edition. Geneva; World Health Organization; 1996.
- Dean, D.J.; Ableseth, M.K. ; Atanasiu. P. The fluorescent antibody test. In: Meslin, F.X.; Kaplan, M.M.; Koprowsky, H; editors. Laboratory Techniques in Rabies. 4th edition. Geneva; World Health Organization; 1996.
- Indian Pharmacopoeia. General Chapters: Test methods. Indian Pharmacopoeia Commission 2018; 1:2.3.9: pp 137-8.
- Shalini, S.; Sharma, A.; Mishra, N.N.; Sharma, R.K. , Chander, H. Anvikar, A.R.; Chand, S. Cost effective and reliable cell based ELISA as an alternative method of flow cytometry for assessment of binding activity of Vedolizumab. Heliyon 2023, 9, e13570. [Google Scholar] [CrossRef]
- Chand, S.; Vaish, U.; Sharma, A.; Mishra, N.N.; Prasad, J.P.; Mahajan, R.V. A reliable assay for ensuring the biological activity of Anti-T lymphocyte immunoglobulin as an alternate to compendial flow cytometer method. Biologicals 2020, 65, 33–38. [Google Scholar] [CrossRef] [PubMed]
- The Hindu Buisness line-Supply of anti-rabies vaccine set to receive a shot in the arm. 2019 (https://www.thehindubusinessline.com/companies/supply-of-anti-rabies-vaccine-set-to-receive-a-shot-in-the-arm/article29962844.ece,) [accessed 10 June 2023].
- Kole, A.K.; Roy, R.; Kole, D.C. Human rabies in India: a problem needing more attention. Bulletin of the World Health Organization 2014, 92, 230. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.E.; Rupprecht, C.E.; Fishbein, D.; Hanlon, C.A.; Lumlertdacha, B.; et al. Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008, 57, 1–28. [Google Scholar] [PubMed]
- WHO Technical Report Series, No. 941. WHO Expert Committee on Biological Standardization. Recommendations for inactivated rabies vaccine for human use produced in cell substrates and embryonated eggs. WHO TRS 2007, 941 Annexure -2:83-132.
- Chand, S.; Meena, J.; Sheikh, F.; Bindra, G.; Yadav, S.; Kasana, H.; Chander, H. Toxicity of Aluminum Adjuvant in Rabies Vaccine: A Case Study. 17th Vaccine Congress, Glasgow, Scotland, 24-27 September 2023. Available at SSRN: https://ssrn.com/abstract=4586090. [CrossRef]
- Sudarshan, M.K.; Bhardwaj, S.; Mahendra, B.J.; Sharma, H.; Sanjay, T.V.; Ashwathnarayana, D.H.; Bilagumba, G. An immunogenicity, safety and post-marketing surveillance of a novel adsorbed human diploid cell rabies vaccine (Rabivax®) in Indian subjects. Human Vaccines 2008, 4, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control (CDC. Current Trends Rabies Vaccine, Adsorbed: A New Rabies Vaccine for Use in Humans. MMWR. Morb Mortal Wkly Report. 1988, 37, 217–18. [Google Scholar]
- Tomljenovic, L.; Shaw, C. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus 2012, 21, 223–230. [Google Scholar] [CrossRef]
- Crépeaux, G.; Heidi, H.; David, M.O.; Curmi, P.; Tzavara, E.; Giros, B.; Shaw, C.A.; Gherardi, R.K.; Cadusseau, J. Assessment of the neurotoxic effects of aluminum hydroxide vaccine adjuvant injections in mice. Toxicology Letters 2014, 229, S189. [Google Scholar] [CrossRef]
- Kuznetsova, I.A.; Areshidze, D.A.; Kozlova, M.A. The influence of different aluminium compounds on the hippocampal morphofunctional state and conditioning in mice. Toxicology and Environmental Health Sciences 2017, 9, 215–221. [Google Scholar] [CrossRef]
- Rizvi, S.H.M.; Parveen, A.; Verma, A.K.; Ahmad, I.; Arshad, M.; Mahdi, A.A. Aluminium Induced Endoplasmic Reticulum Stress Mediated Cell Death in SH-SY5Y Neuroblastoma Cell Line Is Independent of p53. PloS ONE 2014, 9, e98409. [Google Scholar]
- Hampson, K.; Cleaveland, S.; Briggs, D. Evaluation of Cost-Effective Strategies for Rabies Post-Exposure Vaccination in Low-Income Countries. PLoS Neglected Tropical Diseases 2011, 5, e982. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Position paper on rabies vaccines. Weekly Epidemiological Record 2018, 93, 201–220. [Google Scholar]
- Lyons-Weiler, J.; Ricketson, R. Reconsideration of the immunotherapeutic pediatric safe dose levels of aluminum. Journal of Trace Elements in Medicine and Biology 2018, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminium in brain tissue in autism. Journal of Trace Elements in Medicine and Biology 2018, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, S.; Bufali, S.; Skibinski, D.A.; O'Hagan, D.T.; Singh, M. Aluminum Adjuvant Dose Guidelines in Vaccine Formulation for Preclinical Evaluations. J. Pharm. Sci. 2012, 101, 17–20. [Google Scholar] [CrossRef]
- Tomljenovic, L.; Shaw, C.A. Aluminum Vaccine Adjuvants: Are they Safe? Current Medicinal Chemistry 2011, 18, 2630–2637. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).