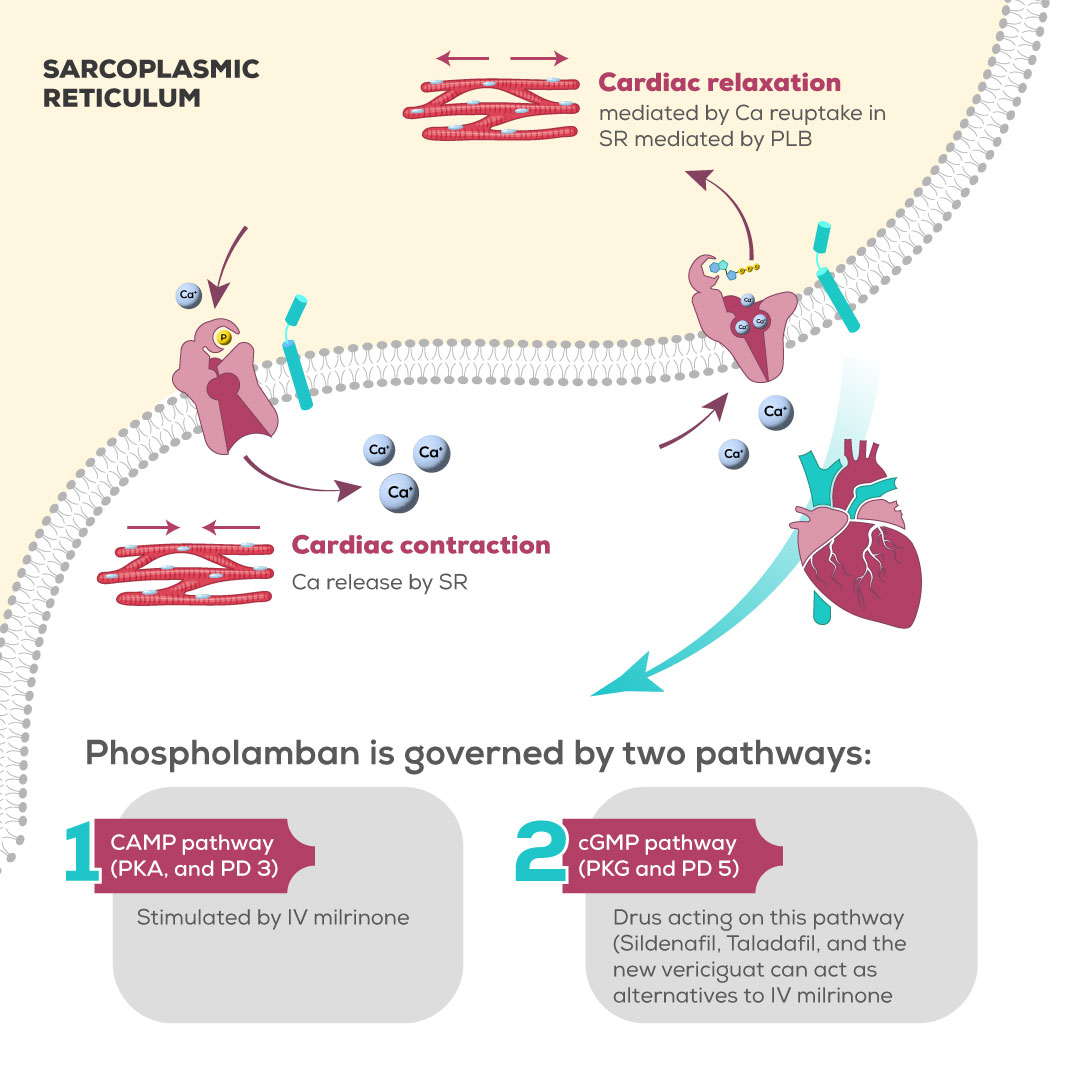
Background:
Lusitropic defect is a specific problem in pediatric heart failure.
Lusitropic effect describes the myocardial relaxation rate during the cardiac cycle. It is increasingly recognized that the pathogenesis of familial dilated cardiomyopathies (an important cause of pediatric heart failure) does not only involve inotropic defects but also suppression of Lusitropy. [
1]
Different pathways to Lusitropy:
While cardiac contraction is initiated by calcium efflux from the sarcoplasmic reticulum, cardiac relaxation is mediated by calcium reuptake by the SR. The channel through which calcium re-enters the SR is termed sarco/endoplasmic reticulum Ca2 + -ATPase (SERCA2a); its activity is largely governed by an associated protein called phospholamban (PLB). Phosphorylation of phospholamban by cAMP generation is crucial in the process of the reuptake of calcium and is the rate-controlling step of cardiac relaxation. cAMP protein Kinase achieves PLB phosphorylation [
2],
Despite the knowledge that PLB is mainly governed by cAMP; it is increasingly recognized that cGMP exerts similar effects on PLB. A study by Mattiazzi and colleagues showed that there are three phosphorylation loci on PLB, the particular site of action of cAMP, is also affected by cGMP leading to the same effect and thereby mediating Lusitropy [
3].
Milrinone is currently the only recognized medication exerting lusitropic effects on the heart muscle., This review of literature aims to shed light on the possible repurposing of medications, thought to be exclusively vasodilators, such as sildenafil, by using them to improve Lusitropy.
Main Body:
Milrinone remains a drug with no alternatives!
IV milrinone has been the primary treatment for individuals with advanced HF for many years. Milrinone is a medication that inhibits phosphodiesterase-3 and acts as an inodilator. It has effects on heart inotropy, lusitropy, and peripheral vasodilation. Continuous intravenous milrinone can serve as a transitional treatment for patients with advanced heart failure who are unable to be gradually withdrawn from inotropes. [
4]
This therapy can be used as a temporary measure until heart transplantation, long-term mechanical circulatory support, or palliative care can be provided. Milrinone is especially favored in patients who have both pulmonary hypertension and inodilator property, since it can decrease pulmonary artery pressures and enhance right ventricular function. Nevertheless, the process of managing it is linked to logistical difficulties and financial expenses. Safe administration typically necessitates chronic central venous catheterization and home-infusion services, as well as regular laboratory monitoring. Complications, such as infection (bacteremia) and thrombosis, are frequently observed. To date, there are no obvious oral alternatives to milrinone, and the addition of oral alternatives can be a game changer in patients with advanced heart failure. [
5]
-Repurposing of Sildenafil and Taladafil, can they be used as oral lusitropic agents?
Old and new evidence of the possible repurposing of Sildenafil and Taladafil in heart failure.
There are conflicting data on the role of activation of PKG in the context of failing heart.
Catecholamine resistance is a characteristic feature of a failing heart and may be observed both in living organisms and in isolated ventricular myocytes. The mechanisms underlying the weakened effects of catecholamines in heart failure (HF) are diverse and involve decreased activity of adenylate cyclase, as well as increased activity of G-protein receptor kinase (GRK2) and intracellular protein phosphatases (PP1 and PP2A). These factors collectively result in a reduction in cAMP-dependent signaling and impaired phosphorylation of PKA-dependent targets. [
6]
From the above, it is thought that PKG activation induces catecholamine unresponsiveness, but in theory and as mentioned earlier, PKG phosphorylates PLB leading to similar effects as PKA activation. [
7]
Several studies have explored the effect of Phosphodiesterase 5 inhibitors such as Sildenafil and Taladafil, in the context of heart failure.
Intriguingly, there is a bimodal fashion, in the studies exploring this hypothesis, more than 10 years ago, several studies studied the effect of sildenafil, on heart failure with or without pulmonary hypertension, and after a gap of five or six years, researchers have resumed the exploitation of the PKG hypothesis, but via Taladafil.
Both Ferrera and colleagues, as well as Bishu et al, have demonstrated, that Sildenafil can improve diastolic relaxation. Bishu et al added Brain Natriuretic peptide to potentiate the effects of sildenafil. [
8,
9]
Belyavskskiy et al, proved that PD 5 inhibitors are beneficial for heart failure in the context of pulmonary hypertension, while Lawless et al, demonstrated that catecholamine response and systolic and diastolic functions are enhanced by Taladafil. The model of the latter study did not involve a context of elevated pulmonary vascular resistance, which signifies that the effect of PD 5 inhibition, is independent of its vasodilator potential, and might operate via inherent intracardiac mechanisms. [
10,
11]
These results, gradually refute, the fixed belief that PD 5 inhibition with subsequent PKG stimulation is linked to catecholamine resistance and worsening heart failure and might open up new hopes for oral cheap alternatives to milrinone in patients with end-stage heart failure.
New therapeutic targets, by direct stimulation of PKG: the “guat” family
The name Vericiguat, the suffix "(Guat)" is derived from "guanine," a reference to the drug's mechanism of action involving guanylate cyclase. Vericiguat stimulates sGC, an enzyme that converts guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP). Ideally, nitric oxide (NO) activates sGC; however, vericiguat can directly stimulate sGC even when NO availability is reduced, as seen in HF. Any severe impairment in the NO-sGC-cGMP pathway can increase oxidative stress, decrease cGMP production, and ultimately lead to the death of cardiomyocytes, resulting in fibrosis. Therefore, maintaining cardiovascular functions is critical for the stability of this pathway. [
12,
13]
Vericiguat has proven to be successful in improving myocardial function by increasing cGMP levels, which promotes cardiac relaxation.
Higher cGMP levels facilitate vasodilation and improve ventricular filling, by reducing intracellular calcium concentrations during diastole, a lusitropic effect identical to that of milrinone. Additionally, increased levels of cGMP activate protein kinase G (PKG), which is essential for phosphorylating titin, a protein crucial in regulating myocardial stiffness. By enhancing phosphorylation, vericiguat can reduce myocardial hypertrophy and fibrosis, which may be beneficial for long-term cardiac remodeling and essentially promote lusitropy. [
14]
Vericiguat has undergone extensive evaluation in adults with heart failure and is currently approved for the treatment of chronic heart failure with reduced ejection fraction in stabilized patients following a decompensation event that requires intravenous therapy. [
15]
At present, vericiguat is being investigated for its potential use in treating pediatric heart failure as part of the ongoing VALOR study. This study is assessing the efficacy, safety, and pharmacokinetics of Vericiguat in pediatric patients ranging from 28 days to 18 years who have heart failure, with the goal of shedding light on the potential benefits and appropriate dosing in this age group. [
16]
Conclusion:
Lusitropic effect, is an important cornerstone in the management of advanced heart failure. For years, patients with this disabling stage, have been hospital-bound to receive intravenous milrinone, which is regarded indispensable. Exploiting other intra-myocardial pathways of lusitropy, via cGMP rather than cAMP, can be a game-changer in delaying the need for heart transplantation. This could be either by repurposing vasodilators as Taladafil and Sildenafil or by emerging heart failure specific medications as Vericiguat.
Figure 1 summarizes the facts and hypotheses brought by this review.
Abbreviations: Ca: Calcium, cAMP: cyclic adenine monophosphate, cGMP: cyclic guanine monophosphate, PD: Phosphodiesterase, PK: Protein Kinase, PLB: phospholamban, SR: Sarcoplasmic reticulum,
List of abbreviations
| ATP |
Adenine Triphosphate |
| cAMP |
Cyclic Adenine Monophosphate |
| cGMP |
Cyclic Guanine Monophosphate |
| PKA/G |
Protein Kinase (cAMP or cGMP mediated) |
| PLB |
Phospholamban |
| SERCA |
Sarco-Endoplasmic Reticulum Calcium ATPase |
| SR |
Sarcoplasmic reticulum |
Author Contributions
Conceptualization, AFA; Methodology, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS; software, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS; investigation, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS; resources, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS, data curation, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS; writing—original draft preparation, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS; writing—review and editing, AFA, BR, JTE, MMZ, MWS, NEH, YMH, PS; supervision, AFA; project administration, AFA; funding acquisition, (none). All authors have read and agreed to the published version of the manuscript.”
Institutional Review Board Statement
not applicable as this study is a hypothesis/Review article.
Informed Consent Statement
not applicable as this study is a viewpoint/editorial
Data Availability Statement
All data is made available within the manuscript.
Acknowledgement
To the peacekeepers in every part of the world, in every community, every family and every tiny relationship. Peace keeping might sometimes look like weakness, but it requires utmost strength,
Conflicts of Interest
The authors declare no conflict of interest. The manuscript is submitted under Creative Commons Licensing CC-BY-NC-ND.
References
- Marston, S.; Pinto, J.R. Suppression of lusitropy as a disease mechanism in cardiomyopathies. Front. Cardiovasc. Med. 2023, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marston, S. Recent studies of the molecular mechanism of lusitropy due to phosphorylation of cardiac troponin I by protein kinase A. J. Muscle Res. Cell Motil. 2023, 44, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mattiazzi, A.; Kranias, E.G. The role of CaMKII regulation of phospholamban activity in heart disease. Front. Pharmacol. 2014, 5 JAN, 1–11. [Google Scholar] [CrossRef]
- Furck, A.K.; Bentley, S.; Bartsota, M.; Rigby, M.L.; Slavik, Z. Oral Enoximone as an Alternative to Protracted Intravenous Medication in Severe Pediatric Myocardial Failure. Pediatr. Cardiol. 2016, 37, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- ILONZE, O.J.; PANG, P.S. Nebulized Milrinone: Choosing Next Steps Wisely. J. Card. Fail. 2024, 30, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.K.; Zheng, H.; Mahata, S.; Liu, X.; Patel, K.P. Effect of heart failure on catecholamine granule morphology and storage in chromaffin cells. J. Endocrinol. 2016, 230, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Arcones, A.C.; Murga, C.; Penela, P.; Inserte, J.; Mayor, F. G protein–coupled receptor kinase 2 at crossroads of metabolic and cardiovascular diseases. Curr. Opin. Endocr. Metab. Res. 2021, 16, 75–85. [Google Scholar] [CrossRef]
- Ferreira-Melo, S.E.; Demacq, C.; Lacchini, S.; Krieger, J.E.; Irigoyen, M.C.; Moreno, H. Sildenafil preserves diastolic relaxation after reduction by L-NAME and increases phosphodiesterase-5 in the intercalated discs of cardiac myocytes and arterioles. Clinics 2011, 66, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Bishu, K.; Hamdani, N.; Mohammed, S.F.; Kruger, M.; Ohtani, T.; Ogut, O.; Brozovich, F. V.; Burnett, J.C.; Linke, W.A.; Redfield, M.M. Sildenafil and B-Type Natriuretic Peptide Acutely Phosphorylate Titin and Improve Diastolic Distensibility In Vivo. Circulation 2011, 124, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Belyavskiy, E.; Ovchinnikov, A.; Potekhina, A.; Ageev, F.; Edelmann, F. Phosphodiesterase 5 inhibitor sildenafil in patients with heart failure with preserved ejection fraction and combined pre- and postcapillary pulmonary hypertension: a randomized open-label pilot study. BMC Cardiovasc. Disord. 2020, 20, 408. [Google Scholar] [CrossRef] [PubMed]
- Lawless, M.; Caldwell, J.L.; Radcliffe, E.J.; Smith, C.E.R.; Madders, G.W.P.; Hutchings, D.C.; Woods, L.S.; Church, S.J.; Unwin, R.D.; Kirkwood, G.J.; et al. Phosphodiesterase 5 inhibition improves contractile function and restores transverse tubule loss and catecholamine responsiveness in heart failure. Sci. Rep. 2019, 9, 6801. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Ayalasomayajula, S.; Blaustein, R.O.; Gheyas, F. Vericiguat, a novel <scp>sGC</scp> stimulator: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2023, 16, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, A.; Meyer, M.; Blaustein, R.O.; Trujillo, M.E.; Kauh, E.; Roessig, L.; Boettcher, M.; Becker, C. Clinical Pharmacokinetic and Pharmacodynamic Profile of Vericiguat. Clin. Pharmacokinet. 2024, 63, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event. JAMA Cardiol. 2021, 6, 706. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, B.; Cuppini, E.; Fumanelli, J.; Di Candia, A.; Sabatino, J.; Sirico, D.; Vida, V.; Padalino, M.; Di Salvo, G. Chronic Heart Failure in Children: State of the Art and New Perspectives. J. Clin. Med. 2023, 12, 2611. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).