Submitted:
13 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials and Methods
2.1.1. Materials
2.1.2. Instrumentations
2.1.3. Methods
- Sodium sulfadiazine (C10H9N4NaO2S; sfz)
- (i)
- Synthesis of sodium phenyl dithiocarbamate (pl -dtc)
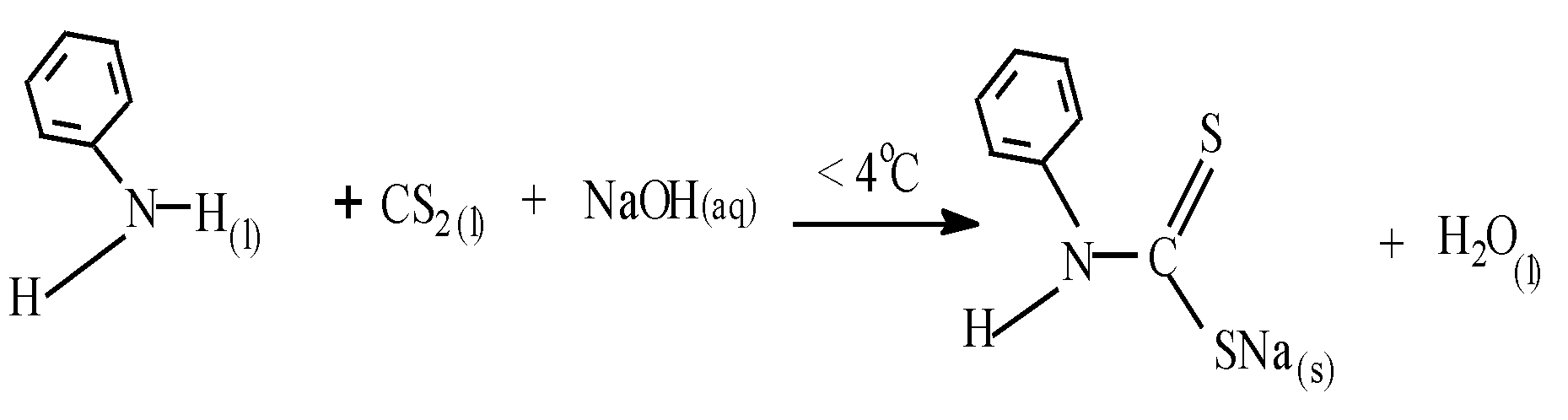
- (ii)
- Synthesis of oxovanadium(IV) coordination compound of [VO(sfz)(pl-dtc)]
- (iii)
- Synthesis of zinc(II) coordination compound of [Zn(sfz)(pl-dtc)]
2.2. Crystal Growth and Crystallographic Activities
| Crystal data | |
|---|---|
| Formula | C7 H12 N Na O3 S2 |
| Formula weight | 245.29 |
| Crystal system | orthorhombic |
| Space group | Pbcn (No. 60) |
| a (Å) | 28.6599(5) |
| b (Å) | 6.9376(1) |
| c (Å) | 11.3162(2) |
| V (Å3) | 2250.01(6) |
| Z | 8 |
| Dcalc (g cm-3) | 1.448 |
| μ(CuKa) (mm-1) | 4.552 |
| F(000) | 1024 |
| Crystal size (mm) | 0.14 x 0.20 x 0.27 |
| Crystal habit | Colourless polygon crystal |
| Temperature (K) | 120 |
| Radiation (Å) | CuKa 1.54184 |
| Theta Min-Max (°) | 3.1, 67.1 |
| Dataset | -29:34; -8:8; -11:13 |
| Tot. & unique data | 12884, 2001 |
| R(int) | 0.031 |
| Observed I > 2σ(I) | 1852 |
| Nref, Npar | 2001, 156 |
| R, wR2 | 0.0225, 0.0603 |
| S | 1.10 |
| Min, Max residual density (e/Å3) | -0.18, 0.28 |
| CCDC | 2237964 |
2.3. Antibacterial Screening and Mechanism of Action of Antibacterial Agents
3. Results and Discussion
3.1. Physicochemical Properties
| Compounds | Molecular formulae | Colour and state of matter | Assay/Yield (%) | Melting point (0°C) | Molar conductivity (Ω-1 cm2 mol-1) |
|---|---|---|---|---|---|
| Na-sfz | C10H9N4NaO2S | white solid | ≥ 98 | > 300 | 8.16 |
| pl-dtc | C7H6NS2Na | whitish yellow crystals | 90 | 72 | 1.09 |
| [VO(sfz)(pl-dtc)] | C17H15N5S3O3Na2V | green solid | 75 | 250 | 0.74 |
| [Zn(sfz)(pl -dtc)] | C17H15N5S3O2Na2Zn | white solid | 72 | 216-218 | 0.01 |
1.2. Infrared Spectroscopy
- Coordination compound of [VO(sfz)(pl-dtc)]
- Coordination compound of [Zn(sfz)(pl-dtc)]
| ν(N-H)asy | ν(N-H)sy | ν(C=N) | ν(SO2)asy | ν(SO2)sy | ν(C-N) | ν(CS2) | ν(V=O) | ν(M-N) | ν(M-S) | |
|---|---|---|---|---|---|---|---|---|---|---|
| sfz | 3404(m) | 3268 (m) | 1577(m) | 1227(m) | 1115(m) | 1453(shp) | 985 (sm) | |||
| pl-dtc | 3409(m) | 3277(m) | 1225(m) | 1112(m) | 1517(sm) | 981(sm) | ||||
| [VO(sfz)(pl-dtc)] | 3401(shp) | 3227(shd) | 1615(m) | 1223(m) | 1145(m) | 1528(sm) | 1002(sm) | 937(shp) | 395(m) | 466(m) |
| [Zn(sfz)(pl-dtc)] | 3400(m) | 3266(sm) | 1578(m) | 1246(sm) | 1141(shp) | 1487(m) | 963(sm) | 391(shd) | 551(m) |
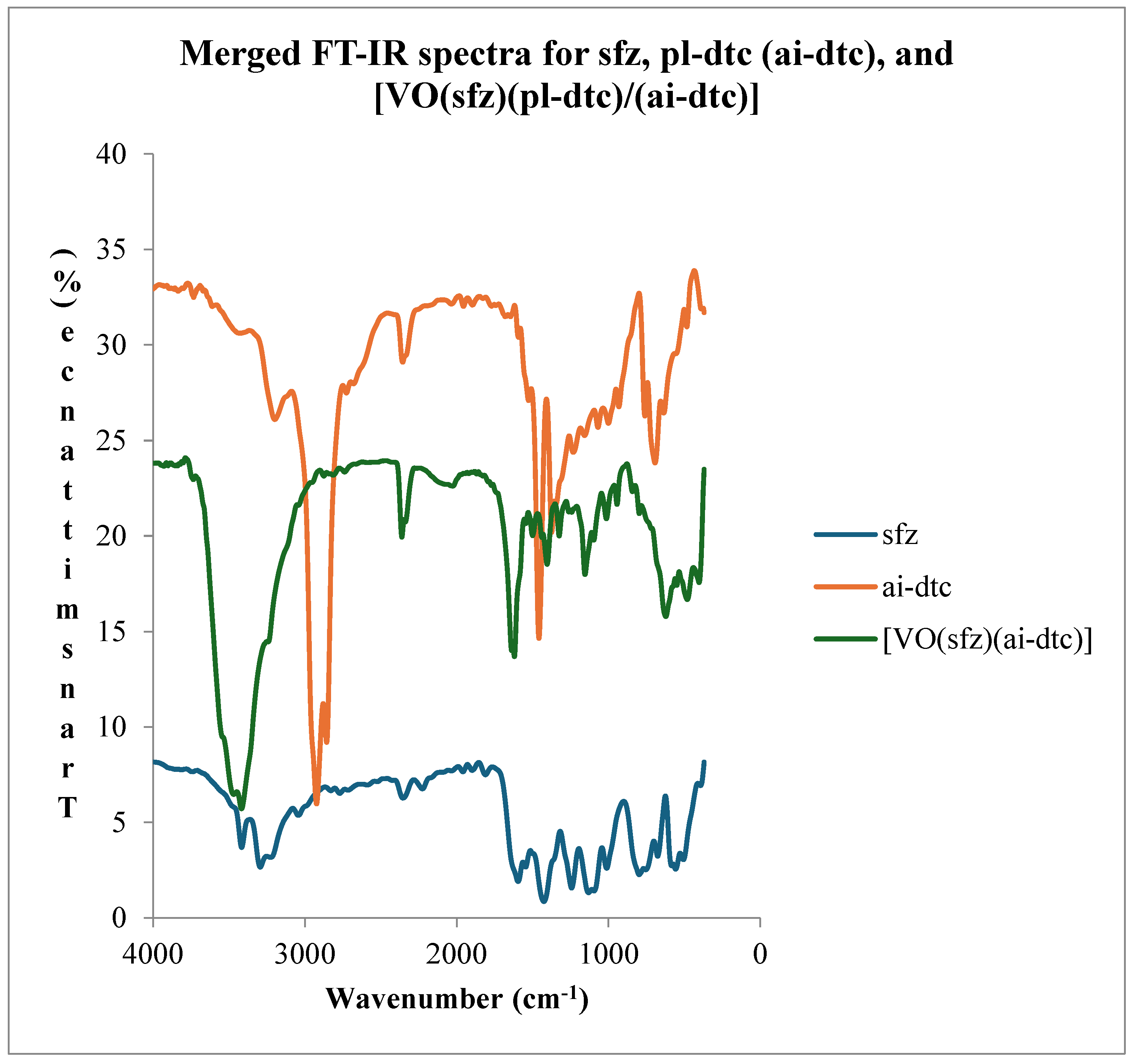
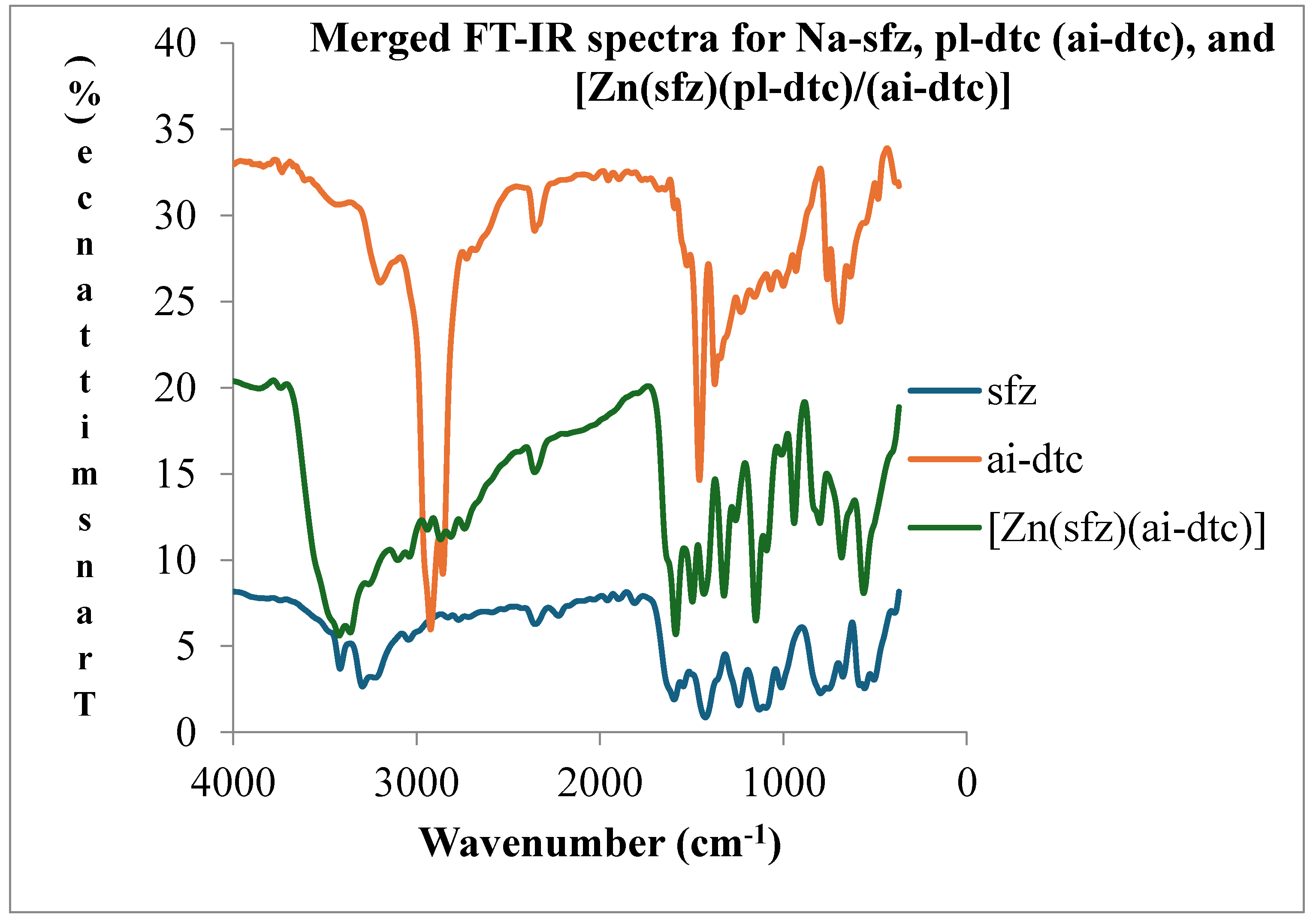
3.3. Electronic Spectroscopy
| π-π* (nm) | n-π* (nm) | d-d transitions (nm) | ||||
| N-C=S | S-C=S | |||||
| sfz | 274 | 319 | 322 | |||
| pl-dtc | 246 | 317 | 381 | |||
| Band I | Band II | Band III | ||||
| [VO (sfz)(pl-dtc)] | 319 | 322 | 355 | 828 738 |
617 | 396 |
| [Zn(sfz)(pl-dtc)] | 235 256 |
292 | 382 | |||
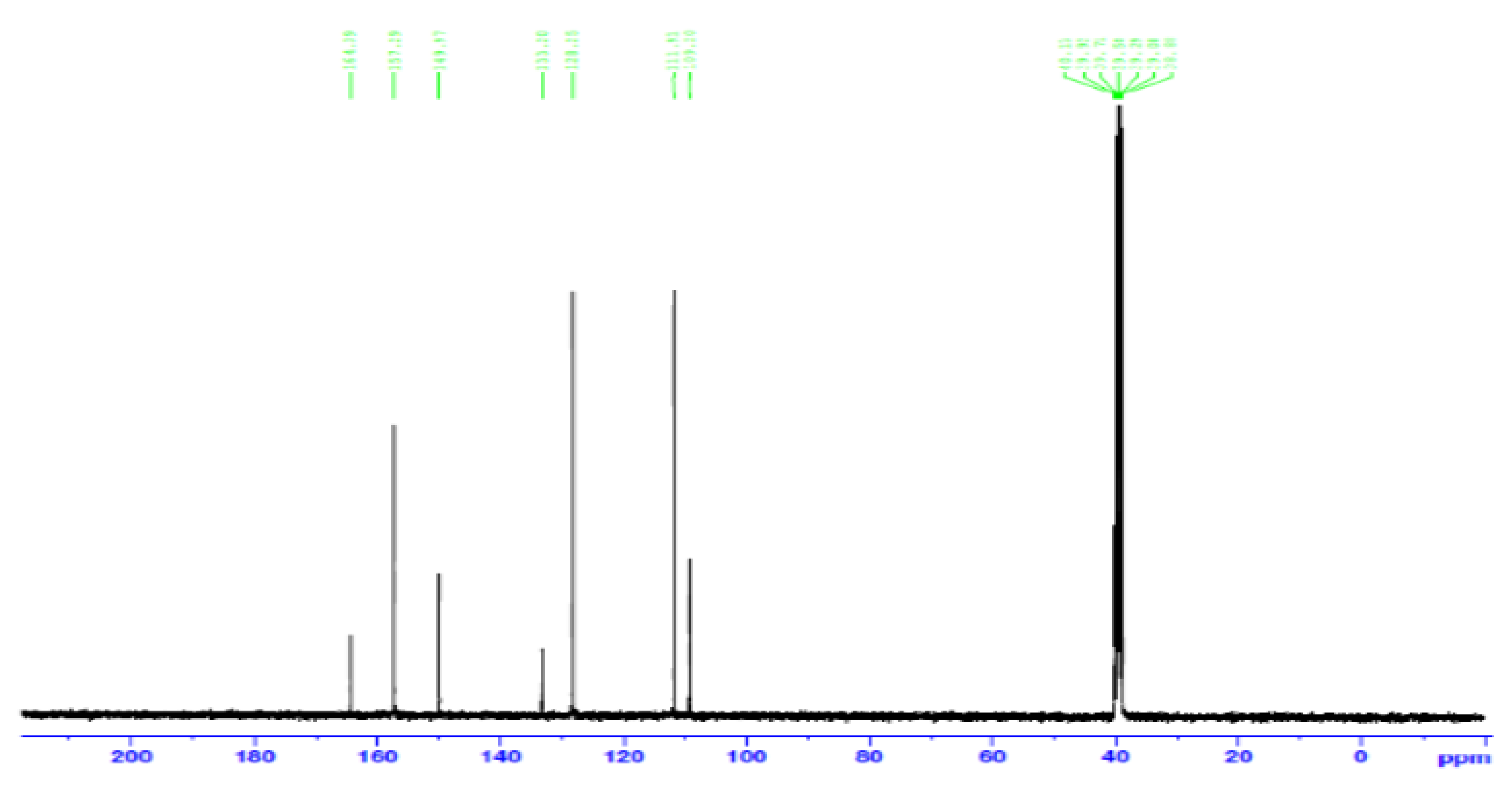
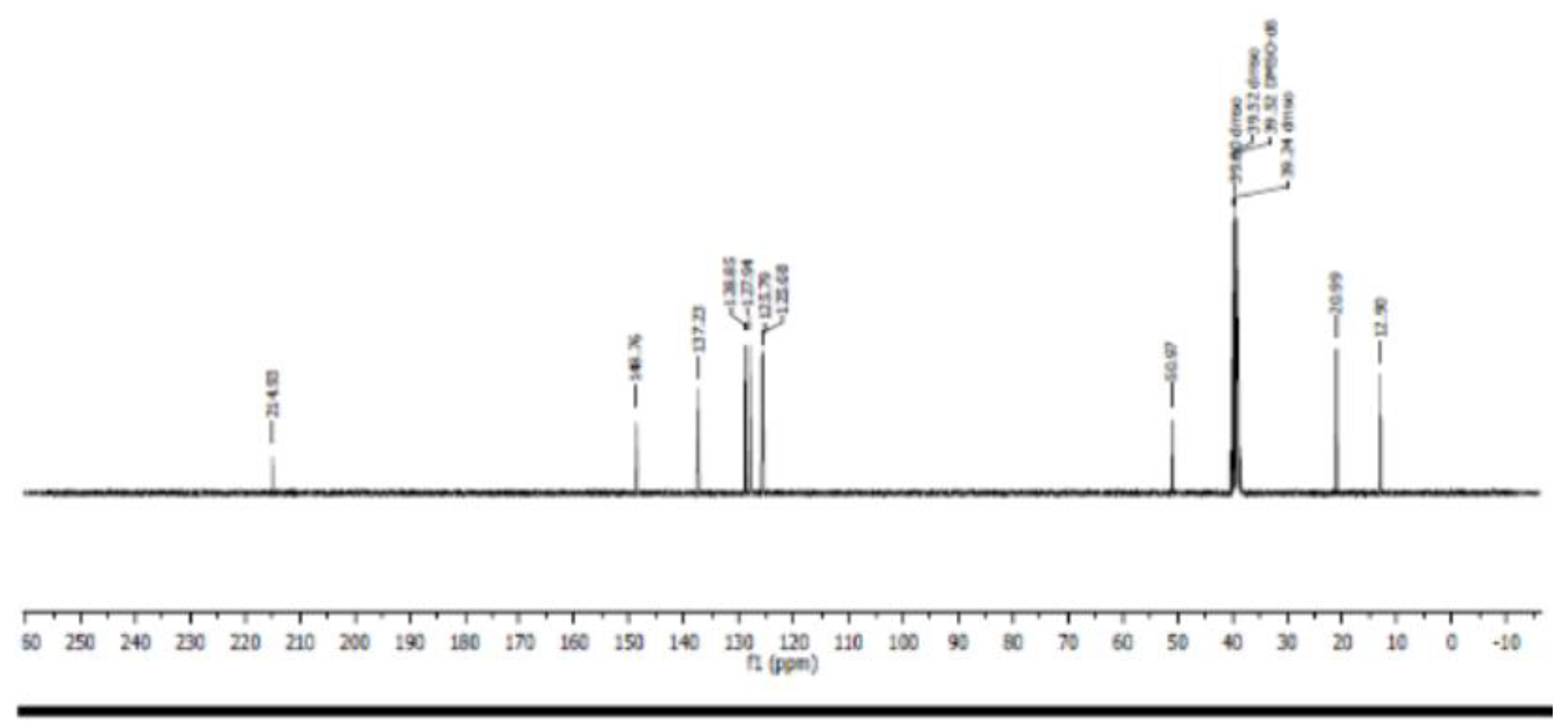
3.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
- Proton NMR (1H NMR)
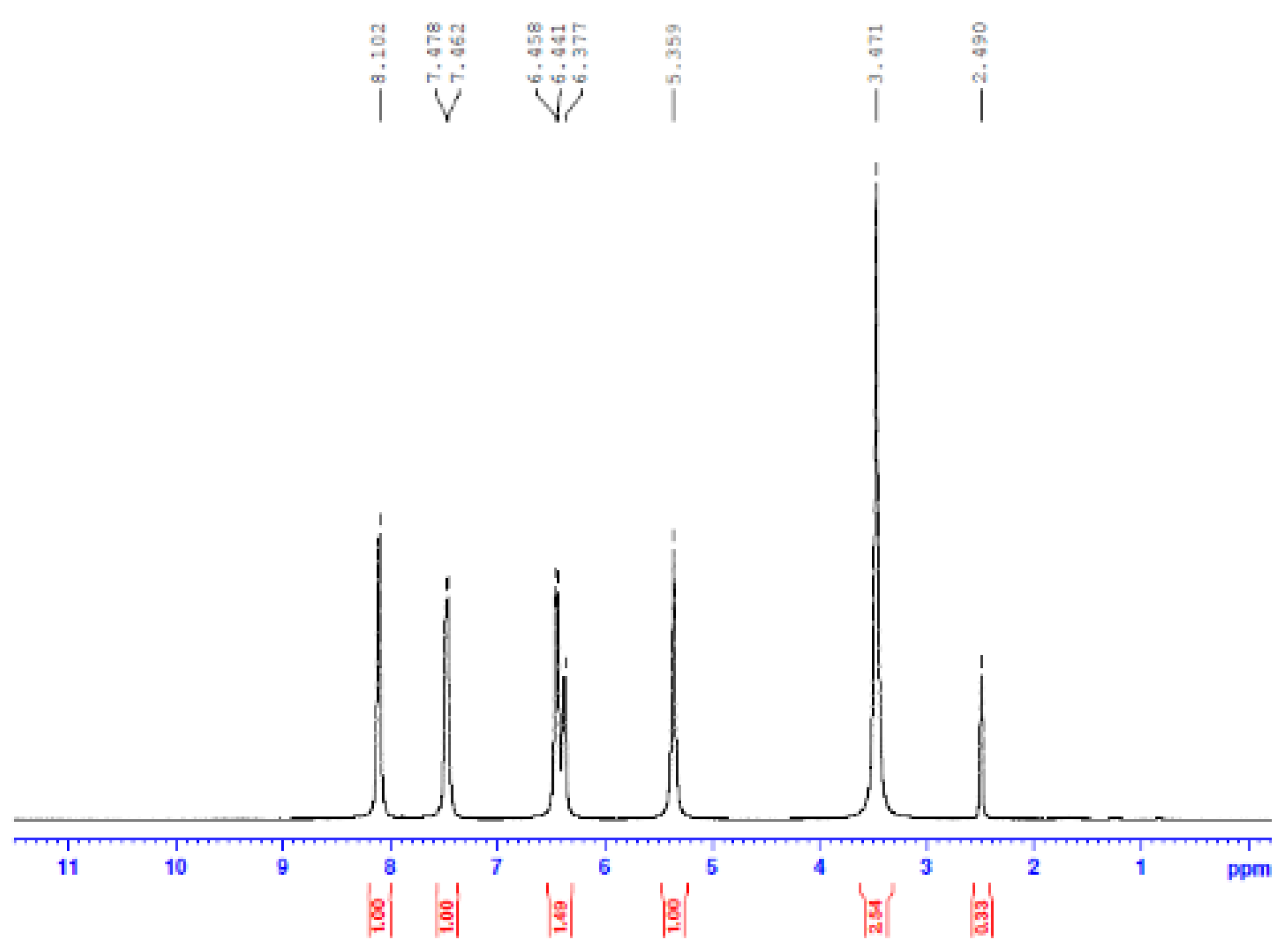
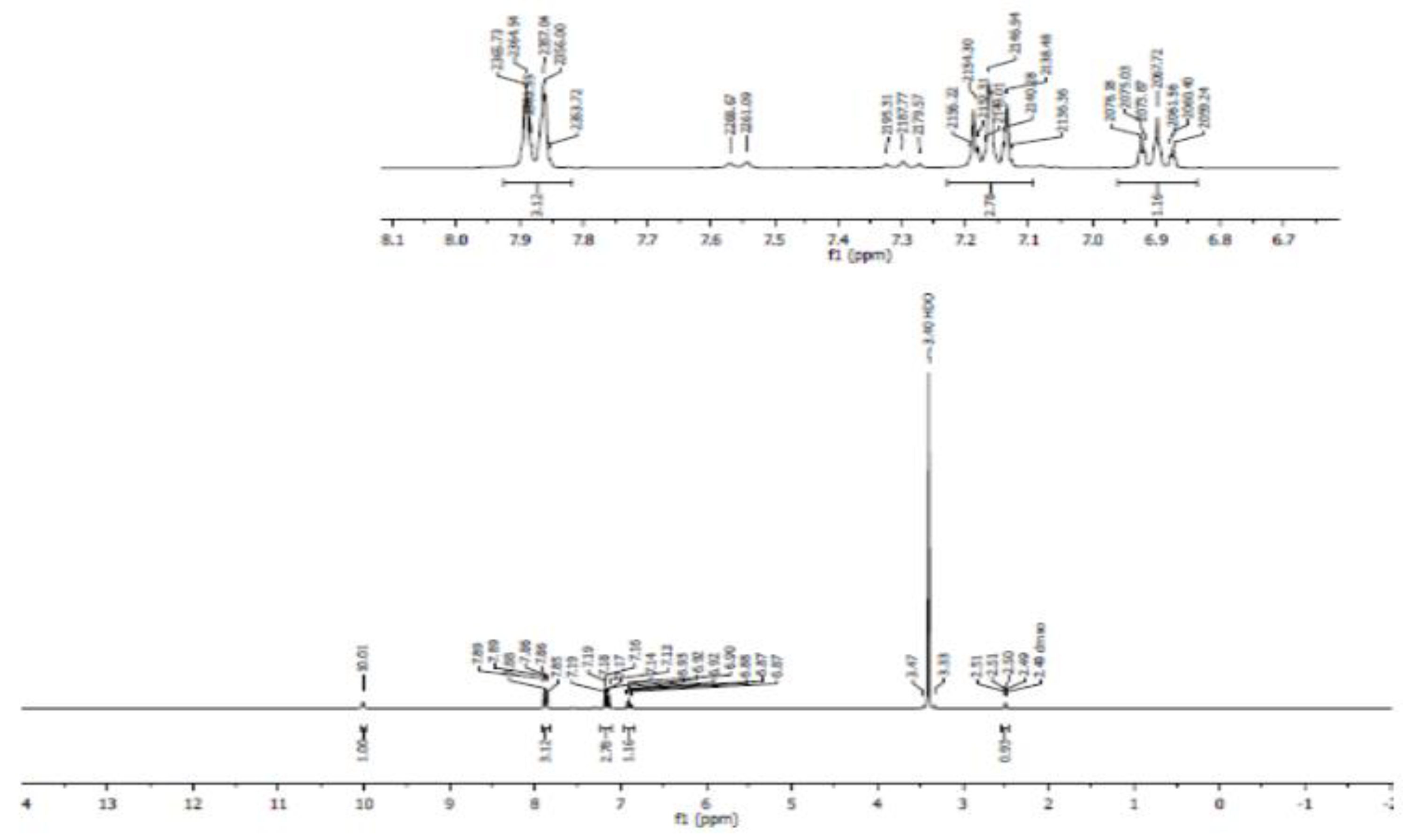
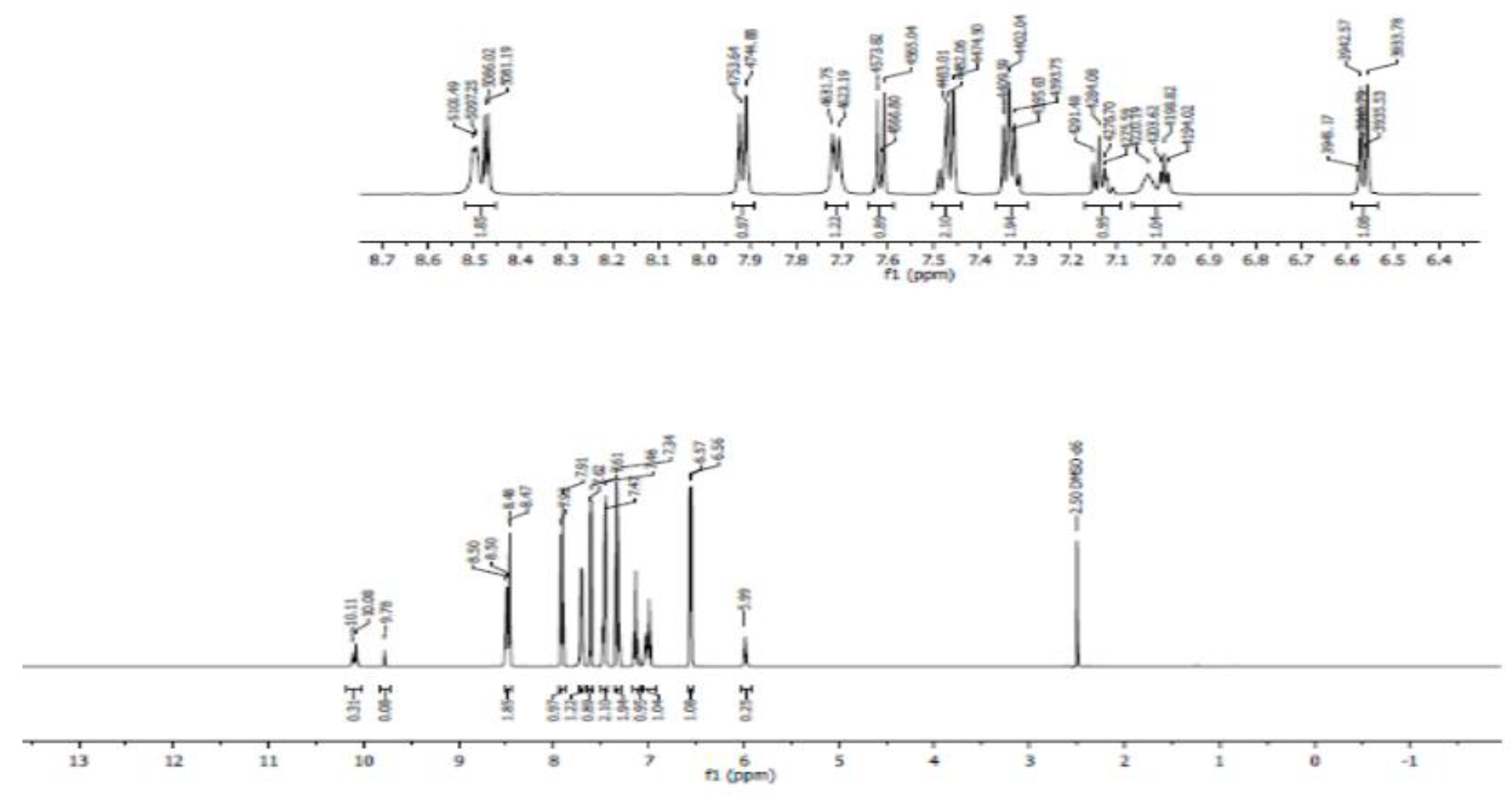
- Carbon 13 NMR (13C NMR) and hybridization
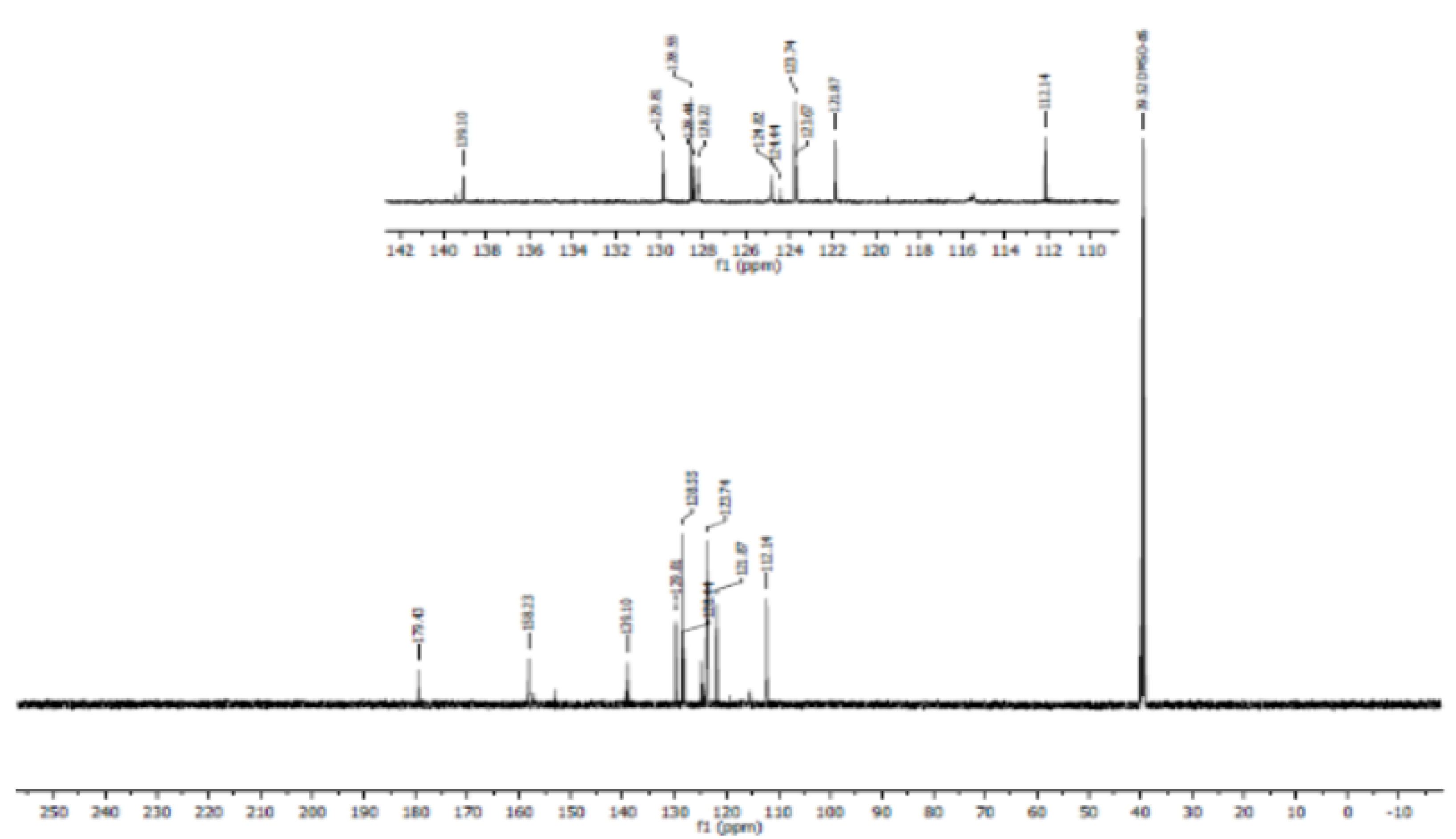
3.5. Crystal Data, Data Collection and Refinements of pl-dtc
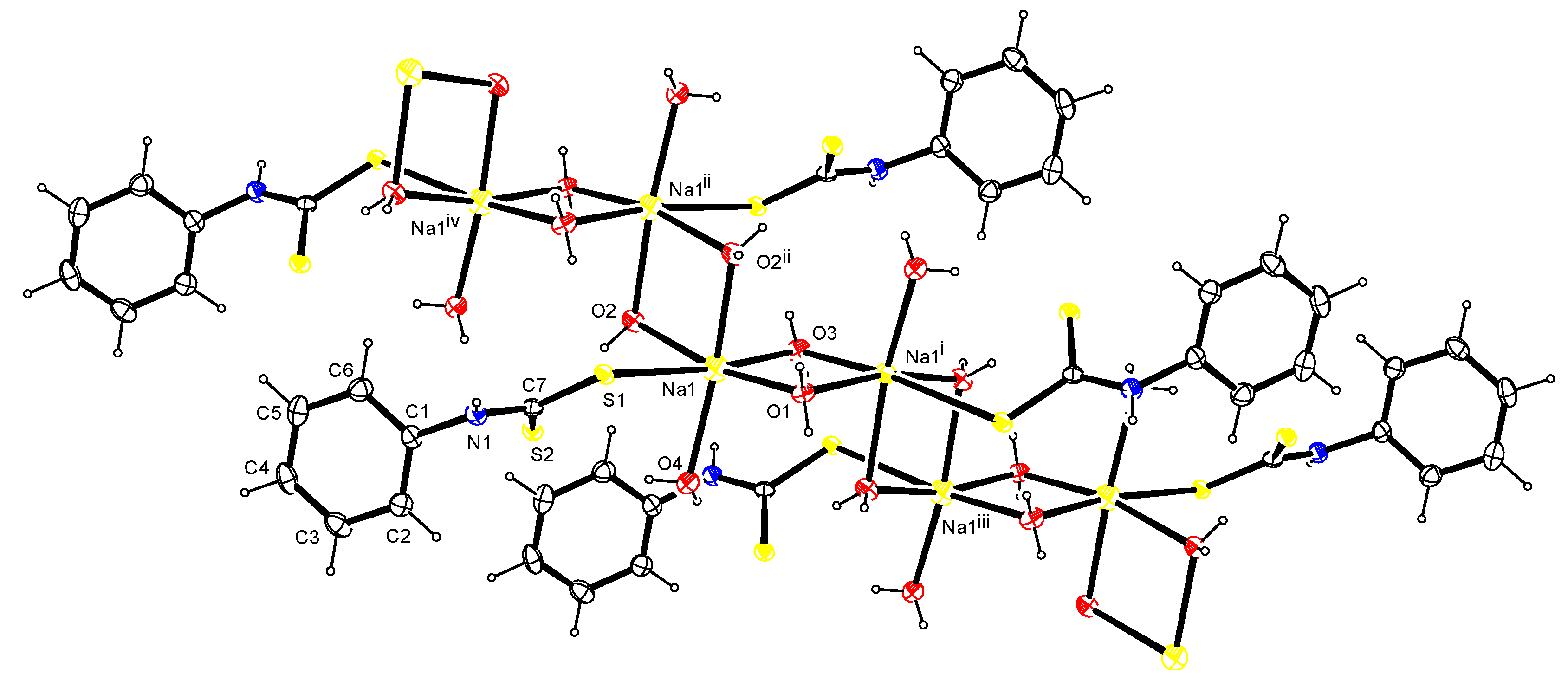
| Lengths (Å) | Angles (°) | ||
|---|---|---|---|
| S1―Na1 | 3.0235(6) | Na1―S1―C7 | 109.73(5) |
| S1―C7 | 1.7353(15) | Na1―O1―Na1i | 93.70(5) |
| S2―C7 | 1.7102(13) | Na1―O2―Na1ii | 96.37(4) |
| Na1―O1 | 2.3562(11) | Na1―O3―Na1i | 92.83(5) |
| Na1―O2 | 2.3891(12) | S1―Na1―O1 | 94.73(3) |
| Na1―O3 | 2.3730(11) | S1―Na1―O2 | 84.96(3) |
| Na1―O4 | 2.3757(13) | S1―Na1―O3 | 172.90(3) |
| Na1―O2ii | 2.4247(13) | S1―Na1―O4 | 81.85(3) |
| N1―C1 | 1.4397(19) | S1―Na1―O2ii | 86.21(3) |
| N1―C7 | 1.3305(18) | O1―Na1―O2 | 167.50(4) |
| O1―Na1―O3 | 86.74(4) | ||
| O1―Na1―O4 | 91.95(3) | ||
| O1―Na1―O2ii | 83.88(3) | ||
| O2―Na1―O3 | 92.11(4) | ||
| O2―Na1―O4 | 100.36(4) | ||
| O2―Na1―O2ii | 83.63(4) | ||
| O3―Na1―O4 | 105.07(3) | ||
| O2ii―Na1―O3 | 87.04(3) | ||
| O2ii―Na1―O4 | 167.00(4) | ||
| C1―N1―C7 | 124.97(12) | ||
| D―H…A | D―H (Å) | H…A (Å) | D…A (Å) | D―H…A (°) |
|---|---|---|---|---|
| N1―H1…O4i | 0.84(2) | 2.24(2) | 3.0205(16) | 156.7(17) |
| O1―H1A…S1ii | 0.83(2) | 2.43(2) | 3.2446(9) | 169.3(18) |
| *O2―H2A…S2 | 0.84(2) | 2.39(2) | 3.2162(12) | 172.0(18) |
| O2―H2B…S1iii | 0.77(2) | 2.59(2) | 3.3478(11) | 167.2(18) |
| *O3―H3A…S1iv | 0.80(2) | 2.52(2) | 3.3152(9) | 177.5(17) |
| *O4―H4A…S2 | 0.80(2) | 2.54(2) | 3.3145(12) | 163(2) |
| *O4―H4B…S2iv | 0.80(2) | 2.52(2) | 3.2789(12) | 158(2) |
3.6. Antibacterial Studies and Mechanism of Action
| Compound | S.aureus MRSA252 | E.faecalis ATCC 19433 | E. coli MC4100 | P.aeruginosa PAO1 |
|---|---|---|---|---|
| VOSO4. H20 | 0 | 0 | 0 | 0 |
| ZnCl2 | 14 | 0 | 9 | 0 |
| sfz | 17 | ND | 16.3 | ND |
| pl-dtc | 8 | ND | 0 | ND |
| [VO(sfz)(pl-dtc)] | 0 | NA | 8 | NA |
| [Zn(sfz)(pl-dtc)] | 0 | NA | 10 | 10 |
| Meropenem | NA | NA | NA | 30 |
| Tetracycline | 30 | NA | 28 | NA |
| Vancomycin | NA | 22 | NA | NA |
| DMSO | 0 | 0 | 0 | 0 |
4. Conclusion and Future Perspective
Appendix A. Supplementary material
Data availability
Acknowledgements
Conflict of interest
References
- Odularu, A. T.; Ajibade, P. A. Dithiocarbamates: Challenges, Control, and Approaches to Excellent Yield, Characterization and Their Biological Applications. Bioinorg. Chem. Appl. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Xu, S.; Wu, X. Synthesis, Characterization, and Anticancer Activity of Dithiocarbamate Ruthenium(II) Complexes. New Heteroleptic Palladium(II) Dithiocarbamates: Synthesis, Characterization, Packing and Anticancer Activity against Five Different Cancer Cell Lines. Phos. Sulf. Sil. 2017, 192, 1219–1223. [Google Scholar] [CrossRef]
- Ajibade, P. A.; Idemudia, O. G.; Okoh, A. I. Synthesis, Characterization and Antibacterial Studies of Metal Complexes of Sulfadiazine with N-Alkyl-N-Phenyldithiocarbamate. Bull. Chem. Soc. Ethiop. 2013, 27, 77–84. [Google Scholar] [CrossRef]
- Khan, S. Z.; Amir, M. K.; Ullah, I.; Aamir, A.; Pezzuto, J. M.; Kondratyuk, T.; Bélanger-Gariepy, F.; Ali, A.; Khan, S. Zia-ur-Rehman. Appl. Organometal. Chem. 2016, 30, 392–398. [Google Scholar] [CrossRef]
- Tarique, M. Coordination Aspects of Dimethyldithiocarbamate Ligand toward the First Series Transition Metal Ions. Chem. Sci. Trans. 2012, 1, 257–260. [Google Scholar] [CrossRef]
- Tarique, M. Cr(III), Mn(III), Fe(III), Co(II) and Zn(II) Complexes with Disobutyldithiocarbamato Ligand. E-J. Chem. 2011, 8, 2020–2023. [Google Scholar]
- Kanchi, S.; Singh, P.; Bisetty, K. Dithiocarbamates as Hazardous Remediation Agent: A Critical Review on Progress in Environmental Chemistry for Inorganic Species Studies of 20 th Century. Arab. J. Chem. 2014, 7, 11–25. [Google Scholar] [CrossRef]
- Sağlik, B. N.; Özkay, Y.; Özkay, U. D.; Genҫer, H. K. Synthesis and Biological Evaluation of Some Novel Dithiocarbamate Derivatives. J. Chem. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Yurttas, L.; Özkay, Y.; Demirci, F.; Gӧger, G.; Yildirim, S. U.; Mohsen, U. S.; Öztürk, O.; Kaplancikli, Z. A. Synthesis, Anticandidal Activity and Cytotoxicity of Some Thiazole Derivatives with Dithiocarbamates Side Chains. Turk. J. Chem. 2014, 38, 815–824. [Google Scholar] [CrossRef]
- Rehder, D. The Role of Vanadium in Biology. Metallomics. 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Arafat, Y.; Ali, S.; Shahzadi, S.; Shahd, M. Preparation, Characterization and Antimicrobial Activities of Bimetallic Complexes of Sarcosine with Zn(II) and Sn(IV). Bioinorg. Chem. Appl. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Chapuis, G. “Super flip- A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions,” J. Applied Crystal. 2007, 40, 780–790. [Google Scholar] [CrossRef]
- Petříček, V.; Dŭsek, M.; Palatinus, L. Structure Determination Software Programs, Institute of Physics, Praha, Czech Republic, 2007.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Groom, C.T.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hadjar, F.; Tahar, B.; Eddine, D. A.; Sofiane, D. Antioxidant and Antimicrobial Activity of Some Transition Metal Complexes with Non-Natural Amino Acids Used as Ligand. J. Mater. Environ. Sci. 2018, 9, 2153–2157. [Google Scholar]
- Bellú, S.; Hure, E.; Trapé, M.; Rizzotto, M. The Interaction between Mercury(II) and Sulfathiazole. Quím. Nova. 2003, 26, 188–192. [Google Scholar] [CrossRef]
- Athar, A.; Khan, A.; Khan, F.; Khan, S.; Zia-ul-Haq; Amanulla, M.; Khan, S. Synthesis, Spectral Characterization and Antibacterial Study of Novel Schiff Base Complexes Derived from 4-Nitrobenzenesulfonamide and 5-Chloro-2-Hydroxybenzaldehyde. Am-Eur. J. Agric. Ext. Sci. 2015, 15, 2263–2268. [Google Scholar]
- Doadrio, A. L.; Sotelo, J.; Fernández-Ruano, A. Synthesis and Characterization of Oxovanadium(IV) Dithiocarbamates with Pyridine. Quim. Nova. 2002, 25, 525–528. [Google Scholar] [CrossRef]
- Prakasam, B. A.; Lahtinen, M.; Peuronen, A.; Muruganandham, M.; Kolehmainen, E.; Haapaniemi, E.; Sillanpää. Synthesis, NMR Spectra and Structural Studies on Mixed Ligand Complexes of Pd(II) Dithiocarbamates: First Structural Report on Palladium(II) Dithiocarbamates with SCN Ligand. J. Mol. Str. 2016, 1108, 195–202. [Google Scholar] [CrossRef]
- Abbas, S. A. Synthesis, Characterization and Biological Activity of Some Nickel(II) Mixed Ligands Complexes of Dithiocarbamate and 1, 10-Phenanthroline. Eur. J. Chem. 2017, 8, 367–370. [Google Scholar] [CrossRef]
- Berto, S.; Alladio, E.; Daniella, P. G.; Laurenti, E.; Bono, A.; Sgarlata, C.; Valora, G.; Cappai, R.; Lachowicz, J. I.; Nurchi, V. M. Oxovanadium(IV) Coordination Compounds with Kojic Acid Derivatives in Aqueous Solution. Mole. 2019, 24, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Reddy, K. V.; Bajpai, A.; Khare, K.; Nagaraja, V. Synthesis and Characterization of Bisdithiocarbamates from Weak Nitrogen Bases and Its Metal Complexes. Int. Res. J. Pure App. Chem. 2015, 7, 78–91. [Google Scholar] [CrossRef]
- Khedr, F.; Saad, F. A. Synthesis, Structural Characterization, and Antimicrobial Efficiency of Sulfadiazine, Azomethine Dyes and Their Bi-Homonuclear Uranyl Complexes for Chemotherapeutic Use. Turk. J. Chem. 2015, 39, 267–280. [Google Scholar] [CrossRef]
- Athar, A.; Khan, A.; Khan, F. S.; Zia-ul-Haq; Amanulla, M.; Khan, S. Synthesis, Spectral Characterization and Antibacterial Study of Novel Schiff Base Complexes Derived from 4-Nitrobenzenesulfonamide and 5-Chloro-2-Hydroxybenzaldeyde. Amer-Euras. J. Agric. Environ. Sci. 2015, 15, 2263–2268. [Google Scholar]
- Kartina, D.; Wahab, A. W.; Ahmad, A.; Irfandi, R.; Raya, I. In vitro Antibacterial and Anticancer Activity of Zn(II) Valine dithiocarbamate Complexes. 3rd Int. Conf. Sci. J. Phys. 2019, 1341, 1–7. [Google Scholar]
- Sathiyaraj, E.; Peruma, M. V.; Nagarajan, E. R.; Ramalingan, C. Functionalized Zinc(II) Dithiocarbamates: Synthesis, Spectral and Molecular Structures of Bis(N-Cyclopropyl-N-4-Metoxybenzylditiocarbamato-S,S’)Zinc(II). J. Saudi Arab. Chem. Soc. 2018, 55, 527–537. [Google Scholar] [CrossRef]
- Haas, R. M.; Arshad, M.; Anthony, J.; Altmann, P. J.; Pöthig, A.; Köhler, F. H.; Hess, C. R. Six-and Seven-Coordinate Fe(II) and Zn(II) Compounds Ligated by Unsymmetrical Xanthene-Based Ligands: Characterization and Magnetic Properties. Inorg. Chem. Front. 2016, 3, 616–629. [Google Scholar] [CrossRef]
- Ahamad, M. M.; SureshKumar, E. V.; Rao, R. M.; Phebe, P. Synthesis, Characterization, Antifungal and Antibacterial Activity of Dithiocarbamate Metal Complexes. Arch. Appl. Sci. 2016, 8, 61–64. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





