Submitted:
13 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
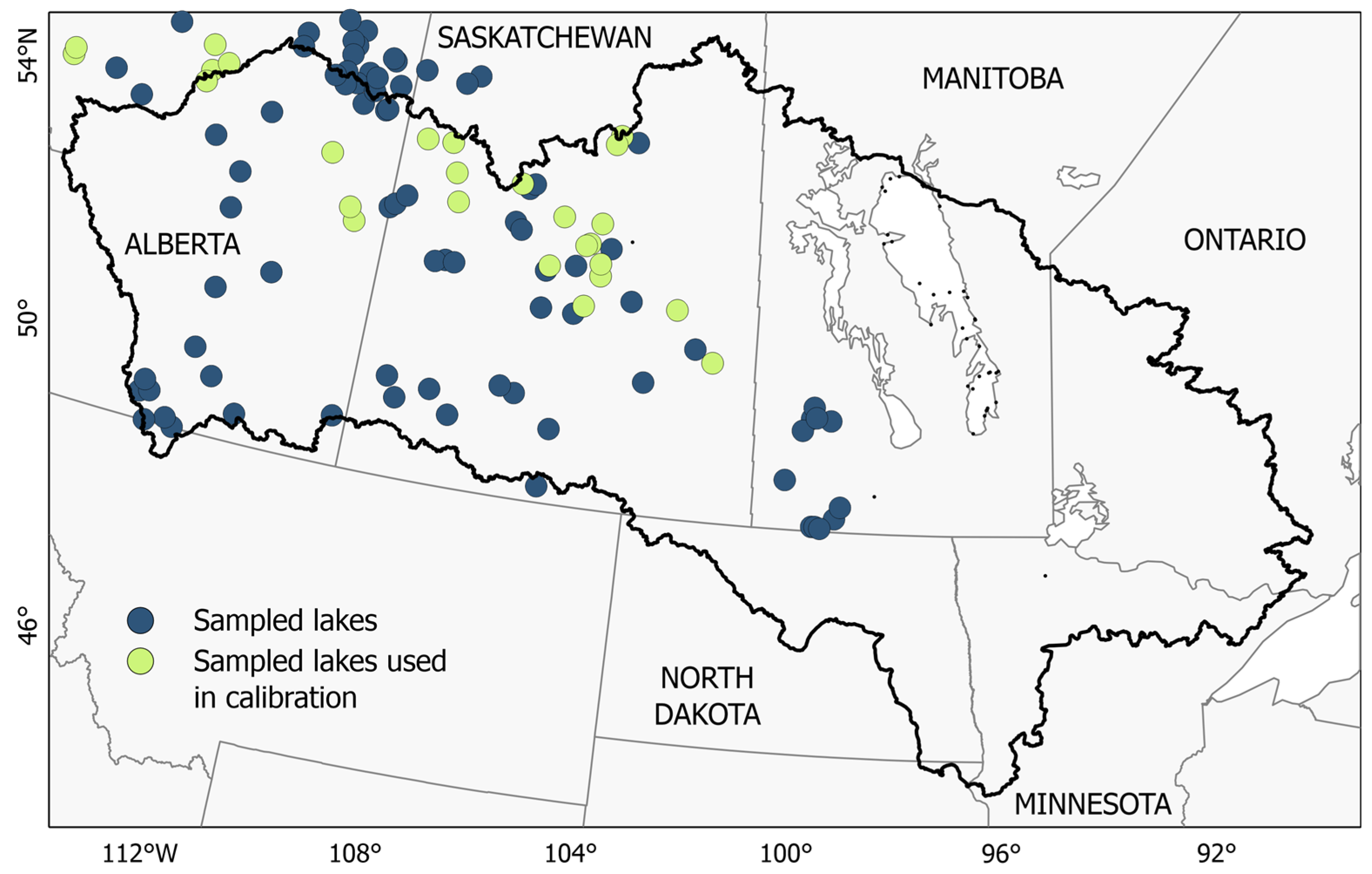
2.1. Ground-Based Dataset
2.2. Landsat Image Acquisition, Processing, and Analysis
2.2.1. In Situ and Satellite Match Up Considerations
2.2.2. Atmospheric Correction
- Conversion to at-sensor spectral radiance: Landsat 8 OLI Level 1 data, comprising raw digital numbers (DN) ranging from 0 to 65,000, were used. Converting DN values to a common radiometric scale by calculating radiance is the first step in analyzing images from different sensors and platforms [51,52]. The DN values were converted to Top-of-Atmosphere (TOA) radiance using Equation (1) (Table 2; [51]).
- Calculation of Rayleigh scattering effects on at-sensor spectral radiance: To remove Rayleigh scattering from the TOA radiance, Equation (2) was applied (Table 2; [53]). The Rayleigh pressure (Pr) was calculated using Equation (3) (Table 2; [54,55]), Rayleigh optical thickness (τr) was determined using Equation (4) (Table 2; [53]), and ozone transmittance (τoz) was computed using Equation (5) (Table 2; [56,57]). Finally, Rayleigh-corrected TOA radiance (Lr) was obtained by subtracting it from Lλ using Equation (6) (Table 2).
- Conversion of Rayleigh-corrected radiances to partially-corrected BOA reflectance: The Rayleigh-corrected TOA radiance was then converted to Rayleigh-corrected Bottom-of-Atmosphere (BOA) reflectance (Rrc) using Equation (7) (Table 2), which was used to develop Chl-a retrieval models. This step offers multiple advantages: it eliminates the impact of varying solar zenith angles due to different image acquisition times, accounts for differences in Earth-Sun distances, and compensates for varying exoatmospheric solar irradiances [52].
2.3. Wildfire Correction
2.4. Chl-a Retrieval Model Development
2.5. Chl-a Retrieval Model Evaluation
3. Results
3.1. Landsat Image Acquisition, Processing, and Analysis
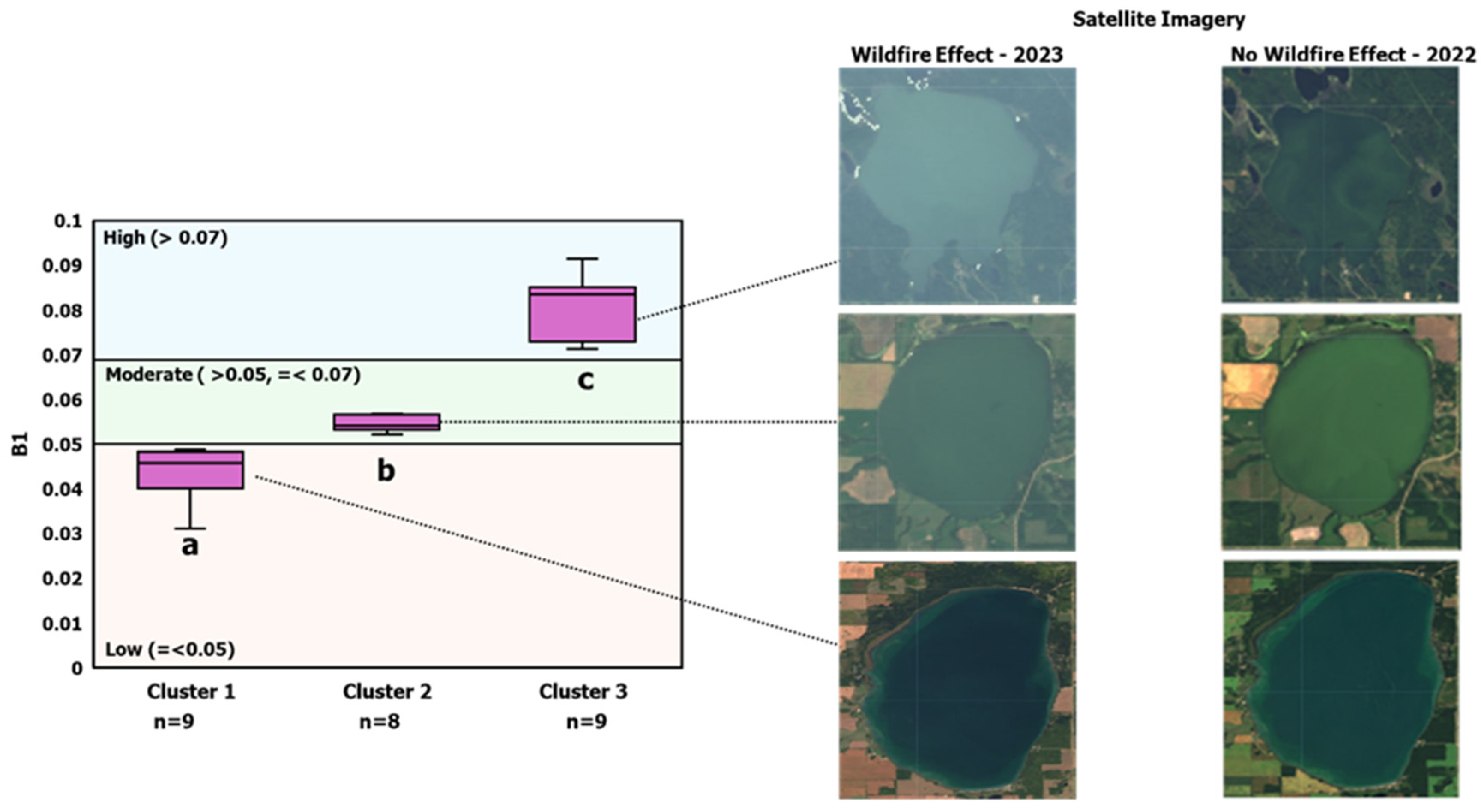
3.2. Clustering the Impact of Wildfires on Remote Sensing Imagery
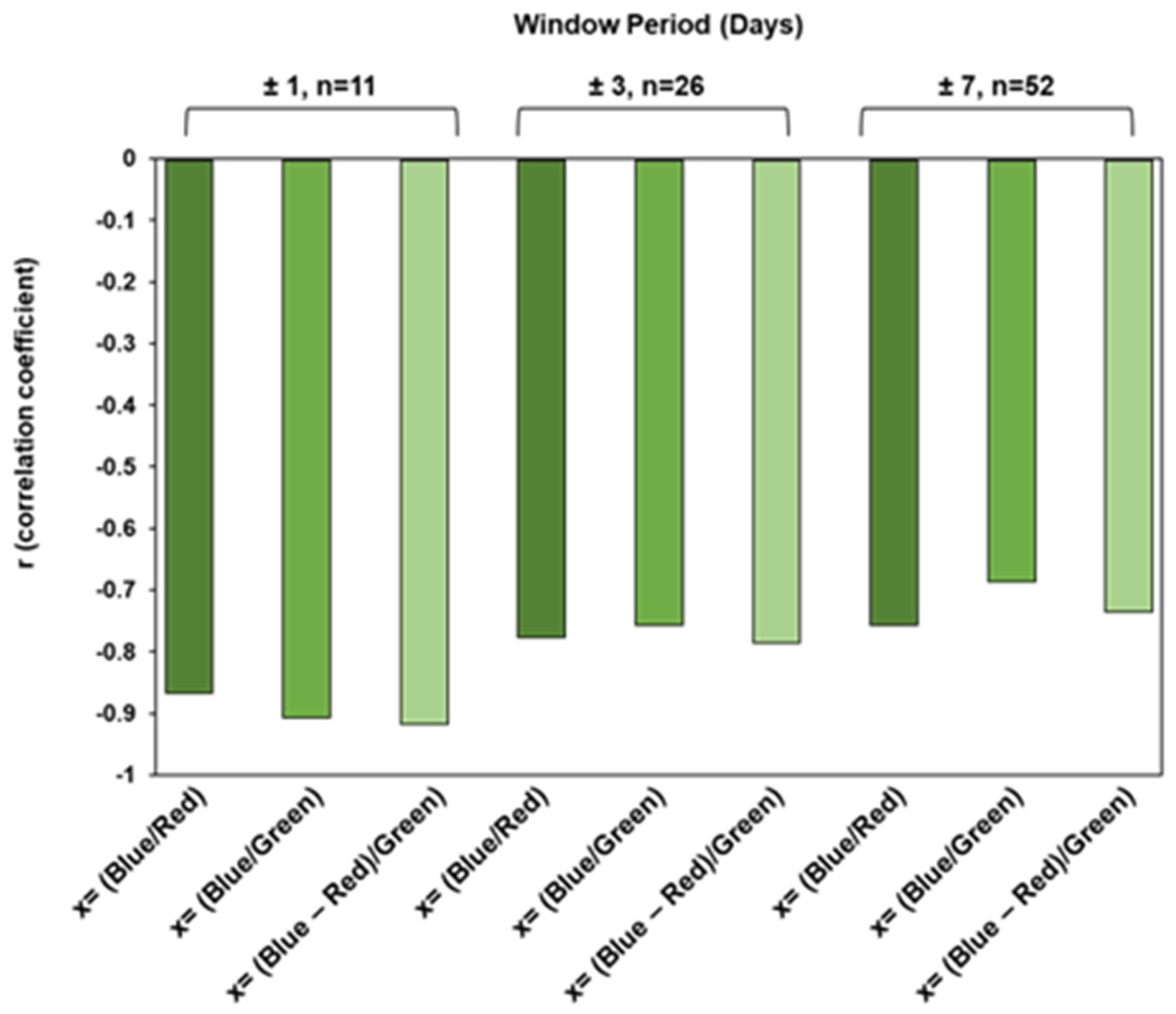
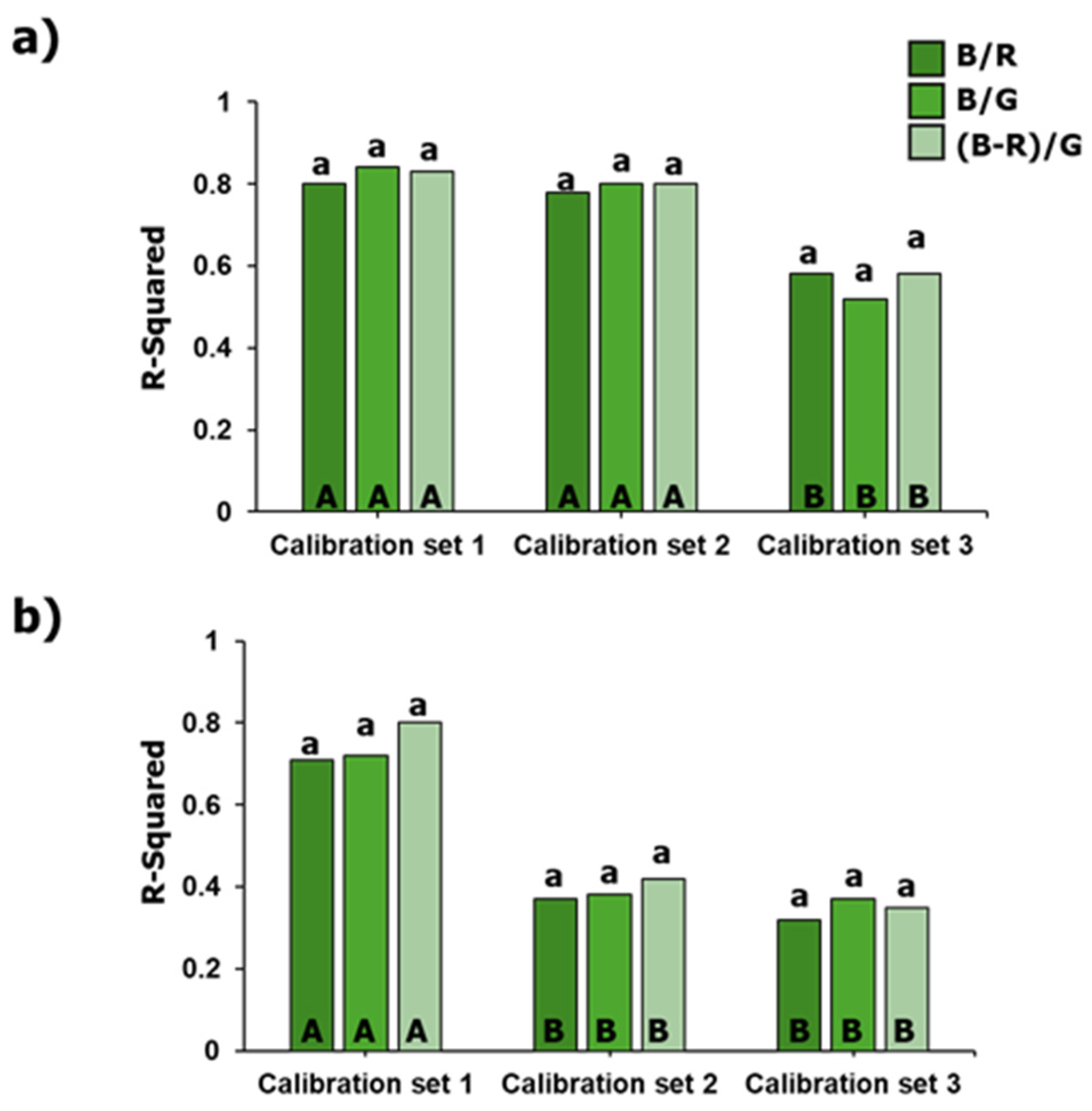
3.3. Performance of Chl-a Retrieval Models with Partial Atmospheric Correction
- Calibration set 1: Includes cluster 1 (low wildfire interference).
- Calibration set 2: Includes clusters 1 and 2 (low and moderate wildfire interference).
- Calibration set 3: Includes all clusters (low, moderate, and high wildfire interference).
3.4. Performance of Chl-a Retrieval Models with Full Atmospheric Correction
3.5. Comparison of Chl-a Retrieval Modeling
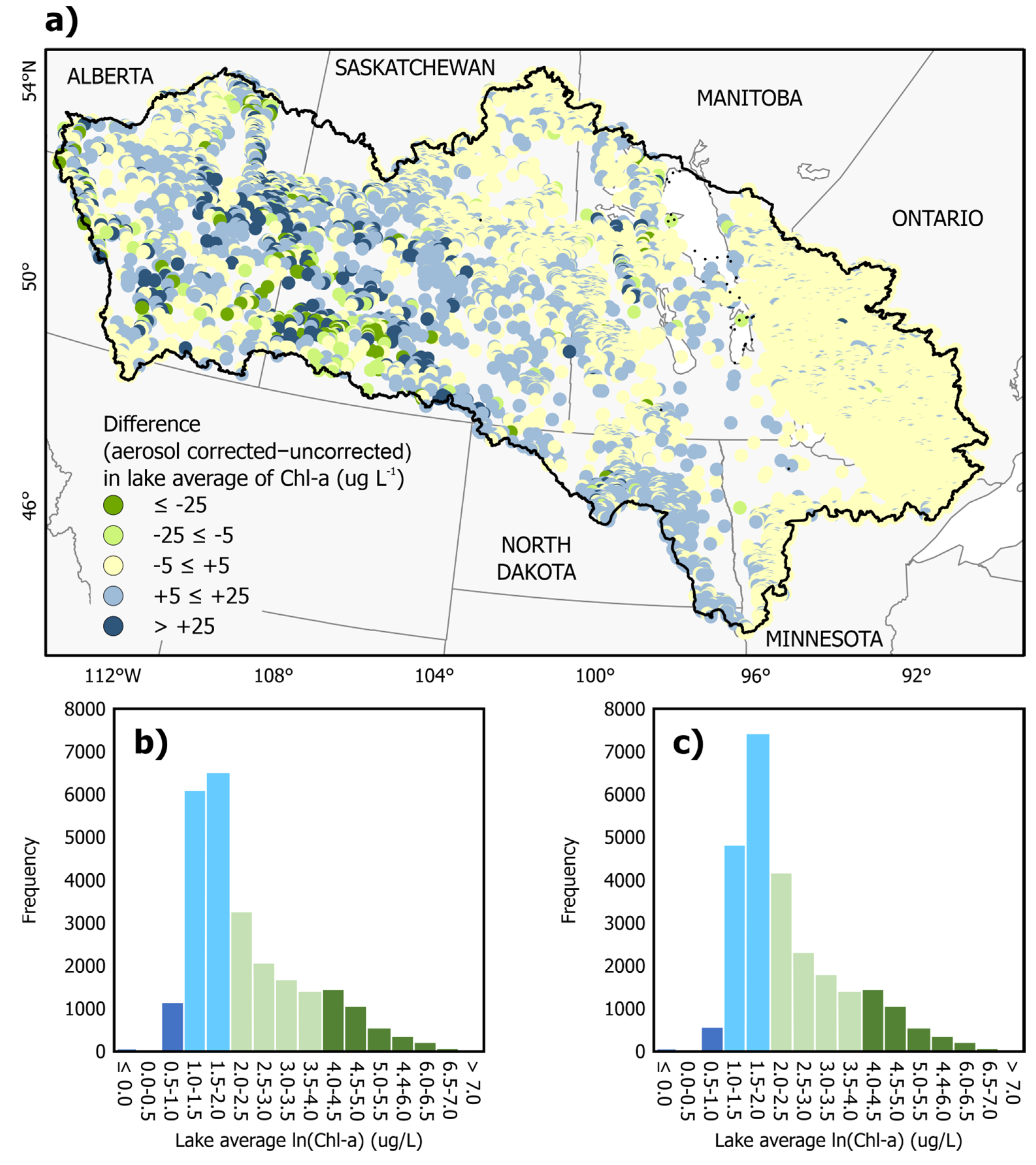
3.6. Effect of Aerosol Band Filtering on Lake Chl-a Concentrations Across the Lake Winnipeg Watershed
4. Discussion
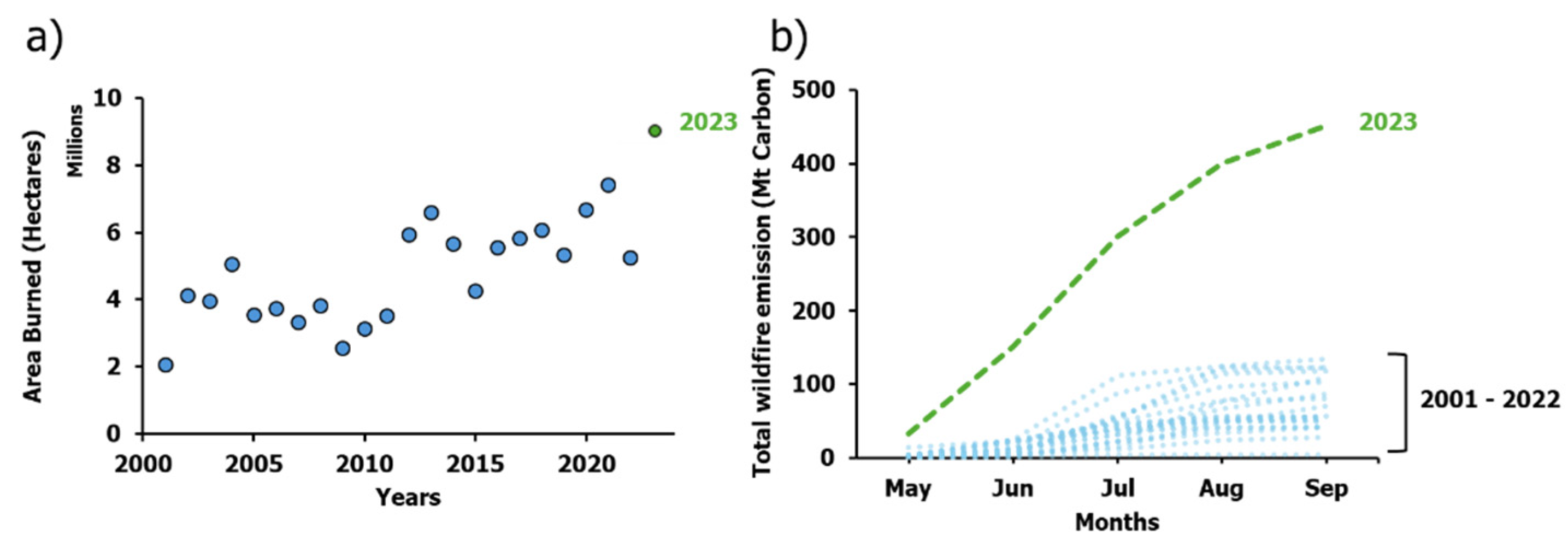
4.1. Removing Effects of Severe Smoke
4.2. Evaluating Chl-a retrieval Model Performance after Removal of Smoke Effects
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downing, J.A. Limnology and oceanography: two estranged twins reuniting by global change. Inland waters 2014, 4, 215–232. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev.-Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and cyanotoxins in a changing environment: Concepts, controversies, challenges. Water, 2021, 13, 2463. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Klinger, P.; Heege, T.; Oppelt, N. Multi-sensor satellite and in situ monitoring of phytoplankton development in a eutrophic-mesotrophic lake. Sci. Total Environ. 2018, 612, 1200–1214. [Google Scholar] [CrossRef]
- Sòria-Perpinyà, X.; Vicente, E.; Urrego, P.; Pereira-Sandoval, M.; Ruíz-Verdú, A.; Delegido, J.; Soria, J.M.; Moreno, J. Remote sensing of cyanobacterial blooms in a hypertrophic lagoon (Albufera of València, Eastern Iberian Peninsula) using multitemporal Sentinel-2 images. Sci. Total Environ. 2020, 698, 134305. [Google Scholar] [CrossRef]
- Mishra, S.; Stumpf, R.P.; Schaeffer, B.A.; Werdell, P.J.; Loftin, K.A.; Meredith, A. Measurement of cyanobacterial bloom magnitude using satellite remote sensing. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Boyer, J.N.; Kelble, C.R.; Ortner, P.B.; Rudnick, D.T. Phytoplankton bloom status: Chlorophyll a biomass as an indicator of water quality condition in the southern estuaries of Florida, USA. Ecol. Indic. 2009, 9, 56–67. [Google Scholar] [CrossRef]
- Olmanson, L.G. , Brezonik, P.L., Bauer, M.E. Remote sensing for regional lake water quality assessment: capabilities and limitations of current and upcoming satellite systems. In Advances in Watershed Science and Assessment, Younos, T., Parece, T., Eds.; Springer International Publishing, Switzerland, 2015; The Handbook of Environmental Chemistry 33, pp. 111– 140. [CrossRef]
- Papenfus, M.; Schaeffer, B.; Pollard, A.I.; Loftin, K. Exploring the potential value of satellite remote sensing to monitor chlorophyll-a for US lakes and reservoirs. Environ. Monit. Assess. 2020, 192, 808. [Google Scholar] [CrossRef] [PubMed]
- Randolph, K.; Wilson, J.; Tedesco, L.; Li, L.; Pascual, D.L.; Soyeux, E. Hyperspectral remote sensing of cyanobacteria in turbid productive water using optically active pigments, chlorophyll a and phycocyanin. Remote Sens. Environ. 2008, 112, 4009–4019. [Google Scholar] [CrossRef]
- Binding, C.E.; Greenberg, T.A.; McCullough, G.; Watson, S.B.; Page, E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J. Gt. Lakes Res. 2018, 44, 436–446. [Google Scholar] [CrossRef]
- Brezonik, P.; Menken, K.D.; Bauer, M. Landsat-based remote sensing of lake water quality characteristics, including chlorophyll and colored dissolved organic matter (CDOM). Lake Reserv. Manag. 2005, 21, 373–382. [Google Scholar] [CrossRef]
- Hou, X.; Feng, L.; Dai, Y.; Hu, C.; Gibson, L.; Tang, J.; Lee, Z.; Wang, Y.; Cai, X.; Liu, J.; Zheng, Y.; Zheng, C. Global mapping reveals increase in lacustrine algal blooms over the past decade. Nat. Geosci. 2022, 15, 130–134. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote sensing of inland waters: Challenges, progress, and future directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Paltsev, A.; Creed, I.F. Are northern lakes in relatively intact temperate forests showing signs of increasing phytoplankton biomass? Ecosystems 2022, 25. [Google Scholar] [CrossRef]
- Paltsev, A.; Creed, I.F. Multi-decadal changes in phytoplankton biomass in northern temperate lakes as seen through the prism of landscape properties. Glob. Change Biol. 2022, 28, 2272–2285. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Liu, P.; Liu, Y.; Yang, S.; Feng, S. A 30-year assessment of phytoplankton blooms in Erhai lake using Landsat imagery: 1987 to 2016. Remote Sens. 2017, 9, 1265. [Google Scholar] [CrossRef]
- Sass, G.Z.; Creed, I.F.; Bayley, S.E.; Devito, K.J. Understanding variation in trophic status of lakes on the Boreal Plain: A 20-year retrospective using Landsat TM imagery. Remote Sens. Environ. 2007, 109, 127–141. [Google Scholar] [CrossRef]
- Hong, G.; Zhang, Y. Haze removal for new generation optical sensors. Int. J. Remote Sens. 2018, 39, 1491–1509. [Google Scholar] [CrossRef]
- Pinardi, M.; Stroppiana, D.; Caroni, R.; Parigi, L.; Tellina, G.; Free, G.; Giardino, C.; Albergel, C.; Bresciani, M. Assessing the impact of wild fires on water quality using satellite remote sensing: The Lake Baikal case study. Front. Remote Sens. 2023, 4, 1107275. [Google Scholar] [CrossRef]
- Raoelison, O.D.; Valenca, R.; Lee, A.; Karim, S.; Webster, J.P.; Poulin, B.A.; Mohanty, S.K. Wildfire impacts on surface water quality parameters: Cause of data variability and reporting needs. Environ. Pollut. 2023, 317, 120713. [Google Scholar] [CrossRef]
- Murphy, S.F.; Alpers, C.N.; Anderson, C.W.; Banta, J.R.; Blake, J.M.; Carpenter, K.D.; Clark, G.D.; Clow, D.W.; Hempel, L.A.; Martin, D.A.; Meador, M.R.; Mendez, G.O.; Mueller-solger, A.B.; Stewart, M.A.; Payne, S.E.; Peterman, C.L.; Ebel, B.A. A call for strategic water-quality monitoring to advance assessment and prediction of wildfire impacts on water supplies. Front. Water. 2023, 5, 1144225. [Google Scholar] [CrossRef]
- Paul, M.J.; Leduc, S.D.; Lassiter, M.G.; Moorhead, L.C.; Noyes, P.D.; Leibowitz, S.G. Wildfire induces changes in receiving waters: A review with considerations for water quality management. Water Resour Res. 2022, 58, 1–28. [Google Scholar] [CrossRef]
- Robinne, F.; Miller, C.; Parisien, M.; Emelko, M.B.; Bladon, K.D.; Silins, U.; Flannigan, M. A global index for mapping the exposure of water resources to wildfire. Forests 2016, 7, 22. [Google Scholar] [CrossRef]
- Williams, A.P.; Livneh, B.; McKinnon, K.A.; Hansen, W.D.; Mankin, J.S.; Cook, B.I.; Smerdon, J.E.; Varuolo-Clarke, A.M.; Bjarke, N.R.; Juang, C.S.; Lettenmaier, D.P. Growing impact of wildfire on western US water supply. PNAS 2022, 119, e2114069119. [Google Scholar] [CrossRef]
- Kganyago, M.; Ovakoglou, G.; Mhangara, P.; Alexandridis, T.; Odindi, J.; Adjorlolo, C.; Mashiyi, N. Validation of atmospheric correction approaches for Sentinel-2 under partly-cloudy conditions in an African agricultural landscape. In Remote Sensing of Clouds and the Atmosphere XXV, Proceedings of SPIE Vol, 11531(115310B), 1–19, 21-25 September 2020. [CrossRef]
- Pinto, C.T.; Jing, X.; Leigh, L. Evaluation analysis of Landsat Level-1 and Level-2 data products using in situ measurements. Remote Sens. 2020, 12, 2597. [Google Scholar] [CrossRef]
- Hayes, N.M.; Haig, H.A.; Simpson, G.L.; Leavitt, P.R. Effects of lake warming on the seasonal risk of toxic cyanobacteria exposure. Limnol. Oceanogr. Lett. 2020, 5, 393–402. [Google Scholar] [CrossRef]
- Mackeigan, P.W.; Taranu, E.; Pick, F.R.; Beisner, B.E.; Gregory-eaves, I. Both biotic and abiotic predictors explain significant variation in cyanobacteria biomass across lakes from temperate to subarctic zones. Limnol. Oceanogr. 2023, 68, 1360–1375. [Google Scholar] [CrossRef]
- Erratt, K.J.; Creed, I.F.; Trick, C.G. Comparative effects of ammonium, nitrate and urea on growth and photosynthetic efficiency of three bloom-forming cyanobacteria. Freshw. Biol. 2018, 63, 626–638. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie Und Physiologie Der Pflanzen, 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine : Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Velastegui-Montoya, A.; Montalván-Burbano, N.; Carrión-Mero, P.; Rivera-Torres, H.; Sadeck, L.; Adami, M.G. Google Earth Engine: A global analysis and future trends. Remote Sens. 2023, 15, 3675. [Google Scholar] [CrossRef]
- Chu, H.; He, Y.; Nisa, W.; Jaelani, L.M. Multi-reservoir water quality mapping from remote sensing using spatial regression. Sustainability, 2021, 13, 6416. [Google Scholar] [CrossRef]
- Jin, H.; And, S.F.; Chen, C. Mapping of the spatial scope and water quality of surface water based on the Google Earth Engine cloud platform and Landsat time series. Remote Sens. 2023, 15, 4986. [Google Scholar] [CrossRef]
- Katlane, R.; El, B.; Dhaoui, O.; Kateb, F.; Chehata, N. Monitoring of sea surface temperature, chlorophyll, and turbidity in Tunisian waters from 2005 to 2020 using MODIS imagery and the Google Earth Engine. Reg. Stud. Mar. Sci. 2023, 66, 103143. [Google Scholar] [CrossRef]
- Kwong, I.H.Y.; Wong, F.K.K.; Fung, T. Automatic mapping and monitoring of marine water quality parameters in Hong Kong using Sentinel-2 image time-series and Google Earth Engine cloud computing. Front. Mar. Sci. 2022, 9, 871470. [Google Scholar] [CrossRef]
- Sagan, V.; Peterson, K.T.; Maimaitijiang, M.; Sidike, P.; Sloan, J.; Greeling, B.A.; Maalouf, S. ; Adams, Monitoring inland water quality using remote sensing: Potential and limitations of spectral indices, bio-optical simulations, machine learning, and cloud computing. Earth-Sci. Rev. 2020, 205, 103187. [Google Scholar] [CrossRef]
- Bresciani, M.; Cazzaniga, I.; Austoni, M.; Sforzi, T.; Buzzi, F.; Morabito, G.; Giardino, C. Mapping phytoplankton blooms in deep subalpine lakes from Sentinel-2A and Landsat-8. Hydrobiologia, 2018, 824, 197–214. [Google Scholar] [CrossRef]
- Katsoulis-dimitriou, S.; Lefkaditis, M.; Barmpagiannakos, S. Comparison of iCOR and Rayleigh atmospheric correction methods on Sentinel-3 OLCI images for a shallow eutrophic reservoir. PeerJ , 2022, 10, e14311. [Google Scholar] [CrossRef]
- Kuhn, C.; de Matos Valerio, A.; Ward, N.; Loken, L.; Sawakuchi, H.O.; Kampel, M.; Richey, J.; Stadler, P.; Crawford, J.; Striegl, R.; Vermote, E.; Pahlevan, N.; Butman, D. Performance of Landsat-8 and Sentinel-2 surface reflectance products for river remote sensing retrievals of chlorophyll-a and turbidity. Remote Sens. Environ. 2019, 224, 104–118. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. A new approach to quantify chlorophyll-a over inland water targets based on multi-source remote sensing data. Sci. Total Environ. 2024, 906, 167631. [Google Scholar] [CrossRef]
- Dallosch, M.A.; Creed, I.F. Optimization of Landsat Chl-a retrieval algorithms in freshwater lakes through classification of optical water types. Remote Sens. 2021, 13, 4607. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Robertson, L. An algorithm for detecting trophic status ( chlorophyll- a), cyanobacterial-dominance, surface scums and floating vegetation in inland and coastal waters. Remote Sens. Environ. 2012, 124, 637–652. [Google Scholar] [CrossRef]
- Matthews, M.W.; Odermatt, D. Improved algorithm for routine monitoring of cyanobacteria and eutrophication in inland and near-coastal waters. Remote Sens. Environ. 2015, 156, 374–382. [Google Scholar] [CrossRef]
- Pahlevan, N.; Smith, B.; Schalles, J.; Binding, C.; Cao, Z.; Ma, R.; Alikas, K.; Kangro, K.; Gurlin, D.; Hà, N.; Matsushita, B.; Moses, W.; Greb, S.; Lehmann, M.K.; Ondrusek, M.; Oppelt, N.; Stumpf, R. Seamless retrievals of chlorophyll-a from Sentinel-2 (MSI) and Sentinel-3 (OLCI) in inland and coastal waters: A machine-learning approach. Remote Sens. Environ. 2020, 240, 111604. [Google Scholar] [CrossRef]
- Tao, M.; Duan, H.; Cao, Z.; Loiselle, S.A.; Ma, R. A Hybrid EOF Algorithm to improve MODIS cyanobacteria phycocyanin data quality in a highly turbid lake: Bloom and nonbloom condition. IEEE J. Sel. Top. Appl. Earth Observ. Remote Sens. 2017, 10, 4430–4444. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Duan, H.; Loiselle, S.; Zhang, M.; Xu, J. A novel MODIS algorithm to estimate chlorophyll a concentration in eutrophic turbid lakes. Ecol. Indic. 2016, 69, 138–151. [Google Scholar] [CrossRef]
- Chander, G.; Markham, B. Revised Landsat-5 TM radiometric calibration procedures and post calibration dynamic ranges. IEEE Trans. Geosci. Remote Sensing 2003, 41, 2674–2677. [Google Scholar] [CrossRef]
- Chander G, Markham B, Helder, D. Summary of current radiometric calibration coefficients for Landsat MSS, TM, ETM+, and EO-1 ALI sensors. Remote Sens. Environ. 2009, 113, 893–903. [CrossRef]
- Gilabert, M.A.; Conese, C.; Maselli, F. An atmospheric correction method for the automatic retrieval of surface reflectances from TM images. Int. J. Remote Sens. 1994, 15, 2065–2086. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Radiative Transfer; Dover Publications: New York, USA, 1960; ISBN 9780486605906. [Google Scholar]
- Bucholtz, A. Rayleigh-scattering calculations for the terrestrial atmosphere. Appl Opt. 1995, 34, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Jorge, D.S.F.; Barbosa, C.C.F.; Carvalho, L.A.S. De, Affonso, A.G.; Novo, F.D.L.L.; E. M. L. D. M. SNR (Signal-To-Noise Ratio) impact on water constituent retrieval from simulated images of optically complex Amazon lakes. Remote Sens. 2017, 9, 644. [Google Scholar] [CrossRef]
- Sturm, B. The atmospheric correction of remotely sensed data and the quantitative determination of suspended matter in marine water surface layers. In Remote Sensing in Meteorology, Oceanography and Hydrology; Cracknell, A.P., Ed.; Ellis Horwood Limited: Chichester, UK, 1981; Chapter 11; ISBN 0-85312-212-1. [Google Scholar]
- Vermote, E.; Justice, C.; Claverie, M.; Franch, B. Preliminary analysis of the performance of the Landsat 8 / OLI land surface reflectance product. Remote Sens. Environ. 2016, 185, 46–56. [Google Scholar] [CrossRef]
- Allan, M.G.; Hamilton, D.P.; Hicks, B.J.; Brabyn, L. Landsat remote sensing of chlorophyll a concentrations in central North Island lakes of New Zealand. Int. J. Remote Sens. 2011, 32, 2037–2055. [Google Scholar] [CrossRef]
- Han, L.; Jordan, K.J. Estimating and mapping chlorophyll-a concentration in Pensacola Bay, Florida using Landsat ETM + data. Int. J. Remote Sens. 2005, 26, 5245–5254. [Google Scholar] [CrossRef]
- Brivio, P.A.; Giardino, C.; Zilioli, E. Determination of chlorophyll concentration changes in Lake Garda using an image-based radiative transfer code for Landsat TM images. Int. J. Remote Sens. 2001, 22, 487–502. [Google Scholar] [CrossRef]
- Bocharov, A.V.; Tikhomirov, O.A.; Khizhnyak, S.D.; Pakhomov, P.M. Monitoring of chlorophyll in water reservoirs using satellite data. J. Appl. Spectrosc. 2017, 84, 291–295. [Google Scholar] [CrossRef]
- Boucher, J.O.B.; Weathers, K.A.C.W.; Norouzi, H.A.N.; Steele, B.E.S. Assessing the effectiveness of Landsat 8 chlorophyll a retrieval algorithms for regional freshwater monitoring. Ecol. Appl. 2018, 28, 1044–1054. [Google Scholar] [CrossRef]
- Maeda, E.E.; Lisboa, F.; Kaikkonen, L.; Kallio, K.; Koponen, S.; Brotas, V.; Kuikka, S. Temporal patterns of phytoplankton phenology across high latitude lakes unveiled by long-term time series of satellite data. Remote Sens. Environ. 2019, 221, 609–620. [Google Scholar] [CrossRef]
- Matthews, M.W. A current review of empirical procedures of remote sensing in inland and near-coastal transitional waters. Int. J. Remote Sens. 2011, 32, 6855–6899. [Google Scholar] [CrossRef]
- Mayo, M.; Gitelson, A.; Yacobi, Y.Z.; Ben-Avraham, Z. Chlorophyll distribution in Lake Kinneret determined from Landsat Thematic Mapper data. Int. J. Remote Sens. 1995, 16, 175–182. [Google Scholar] [CrossRef]
- Makarau, A.; Richter, R.; Müller, R.; Reinartz, P. Haze detection and removal in remotely sensed multispectral imagery. IEEE Trans. Geosci. Remote Sens. 2014, 52, 5895–5905. [Google Scholar] [CrossRef]
- Huang, S.; Li, D.; Zhao, W.; Liu, A.Y. Haze removal algorithm for optical remote sensing image based on multi-scale model and histogram characteristic. IEEE Access 2019, 7, 104179–104196. [Google Scholar] [CrossRef]
- Riordan, B.; Verbyla, D.; Mcguire, A.D. Shrinking ponds in subarctic Alaska based on 1950-2002 remotely sensed images. J. Geophys. Res. 2006, 111, G04002. [Google Scholar] [CrossRef]
- Guindon, B.; Zhang, Y. Robust haze reduction: An integral processing component in satellite-based land cover mapping. Symposium on Geospatial Theory, Processing and Applications, Proceedings of the ISPRS Commission IV Symposium. Ottawa, Canada, 8 - 12 July, 2002.
- Neagoe, I.C.; Vaduva, C.; Datcu, M. Haze and smoke removal for visualization of multispectral images: a DNN physics aware architecture. IEEE International Geoscience and Remote Sensing Symposium IGARSS. Brussels, Belgium, July 12-16, 2021. Pp. 2102–2105. [CrossRef]
- Jaelani, L.M.; Limehuwey, R.; Kurniadin, N.; Pamungkas, A. Estimation of TSS and Chl - a concentration from Landsat 8 - OLI: The effect of atmosphere and retrieval algorithm. IPTEK, J. Technol. Sci. 2016, 27, 16–23. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Fang, L.; A, Y.; Ren, S.; Men, J.; Wang, G. Remote sensing retrieval and driving analysis of phytoplankton density in the large storage freshwater lake: A study based on random forest and. J. Contam. Hydrol. 2024, 261, 104304. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, X.; Li, F.; Cochrane, M.A.; Ciren, P. Detection of fire smoke plumes based on aerosol scattering using VIIRS data over global fire-prone regions. Remote Sens. 2021, 13, 196. [Google Scholar] [CrossRef]
- Chegoonian, A.M.; Pahlevan; N. , Zolfaghari, K.; Leavitt, P.R.; Davies, J.; Baulch, H.M.; Duguay, C.R. Comparative analysis of empirical and machine learning models for Chl a extraction using Sentinel-2 and Landsat OLI Data: Opportunities, limitations, and challenges. Can J Remote Sens. 2023, 49, 1. [Google Scholar] [CrossRef]
| Sensor | Bands | Wavelengths (mm) | Resolution (m) |
|---|---|---|---|
| Landsat 8 OLI | Band 1: Coastal/Aerosol | 0.43-0.45 | 30 |
| Band 2: Blue | 0.45-0.51 | 30 | |
| Band 3: Green | 0.53-0.59 | 30 | |
| Band 4: Red | 0.64-0.67 | 30 | |
| Band 5: Near Infrared (NIR) | 0.85-0.88 | 30 | |
| Band 6: Shortwave Infrared (SWIR) | 1.57-1.65 | 30 |
| (1) | |
| where, is the TOA radiance for band , is the raw digital number (DN) for band , is the multiplicative rescaling factor for band and is the additive rescaling factor for band . |
|
| (2) | |
| where, is the Rayleigh path radiance, is the exo-atmospheric solar irradiance constant for band , is the solar zenith angle in degrees, is the satellite view angle in dgrees, is the Rayleigh phase function (Equation (3)), is the Rayleigh optical thickness for band (Equation (4)), and is the ozone transmittance for band (Equation (5)). |
|
| (3) | |
| where, is obtained from in which is the depolarization factor, and is the scattering angle in degrees (180 - ). |
|
| (4) | |
| (5) | |
| where, is the ozone optical thickness for band . |
|
| (6) | |
| where, is the Rayleigh-corrected radiance for band . |
|
| (7) | |
| where, is the partially corrected BOA reflectance for band , and d is the Earth–Sun distance in astronomical units. |
|
| (8) | |
| where, is the coefficient of determination, is the residual sum of square, and is the total sum of square. |
|
| (9) | |
| where, is Root Mean Square Error, is the predicted value, is the observed value, and n is the number of samples. |
|
| (10) | |
| where, is Normalized Root Mean Square Error, is the standard deviation of the observed values. |
|
| (11) | |
| where, is Mean Absolute Error |
|
| (12) |
| A. Partial atmospheric correction (Rayleigh-corrected reflectance) | |||||||||||||
|
Calibration set (Sample Size) |
Predictor | Correlation (r) | Chl-a Models | Train | Test | ||||||||
| R2 | RMSE | NRMSE | Bias | MAE | R2 | RMSE | NRMSE | Bias | MAE | ||||
|
1 (n = 9) |
x = (B/R) | -0.90 | ln(Chl-a) = -2.796*x+7.685 | 0.80 | 0.48 | 0.41 | 0 | 0.41 | 0.79 | 0.53 | 0.37 | 0.07 | 0.45 |
| x = (B/G) | -0.93 | ln(Chl-a) = -6.36*x+9.923 | 0.84 | 0.51 | 0.37 | 0 | 0.48 | 0.87 | 0.18 | 0.3 | -0.03 | 0.17 | |
| x = (B-R)/G | -0.95 | ln(Chl-a) = -5.988*x+5.61 | 0.83 | 0.43 | 0.37 | 0 | 0.38 | 0.99 | 0.10 | 0.08 | 0.05 | 0.08 | |
|
2 (n = 17) |
x = (B/R) | -0.89 | ln(Chl-a) = -2.646*x+7.342 | 0.78 | 0.52 | 0.45 | 0 | 0.40 | 0.74 | 0.64 | 0.46 | -0.09 | 0.58 |
| x = (B/G) | -0.90 | ln(Chl-a) = -4.631*x+8.017 | 0.80 | 0.45 | 0.42 | 0 | 0.38 | 0.77 | 0.68 | 0.43 | -0.01 | 0.55 | |
| x = (B-R)/G | -0.90 | ln(Chl-a) = -4.58*x+4.879 | 0.80 | 0.55 | 0.43 | 0 | 0.45 | 0.87 | 0.42 | 0.32 | 0.04 | 0.34 | |
|
3 (n = 26) |
x = (B/R) | -0.77 | ln(Chl-a) = -3.315*x+8.383 | 0.58 | 0.88 | 0.63 | 0 | 0.73 | 0.61 | 0.62 | 0.59 | 0.05 | 0.46 |
| x = (B/G) | -0.75 | ln(Chl-a) = -4.749*x+8.377 | 0.52 | 0.90 | 0.67 | 0 | 0.64 | 0.61 | 0.70 | 0.59 | 0.02 | 0.64 | |
| x = (B-R)/G | -0.78 | ln(Chl-a) = -4.685*x+5.016 | 0.58 | 0.85 | 0.63 | 0 | 0.60 | 0.68 | 0.63 | 0.53 | 0.06 | 0.58 | |
| B. Full atmospheric correction (Landsat 8 Level 2 reflectance) | |||||||||||||
|
Calibration set (Sample Size) |
Predictor | Correlation (r) | Chl-a Models | Train | Test | ||||||||
| R2 | RMSE | NRMSE | Bias | MAE | R2 | RMSE | NRMSE | Bias | MAE | ||||
|
1 (n = 9) |
x = (B/R) | -0.84 | ln(Chl-a) = -2.033*x+4.637 | 0.71 | 0.65 | 0.49 | 0 | 0.61 | 0.71 | 0.54 | 0.44 | 0.01 | 0.51 |
| x = (B/G) | -0.86 | ln(Chl-a) = -3.75*x+4.695 | 0.72 | 0.64 | 0.48 | 0 | 0.54 | 0.79 | 0.45 | 0.37 | -0.05 | 0.39 | |
| x = (B-R)/G | -0.88 | ln(Chl-a) = -4.331*x+2.501 | 0.8 | 0.47 | 0.41 | 0 | 0.41 | 0.74 | 0.65 | 0.41 | 0.00 | 0.60 | |
|
2 (n = 17) |
x = (B/R) | -0.67 | ln(Chl-a) = -2.157*x+5.121 | 0.37 | 0.95 | 0.76 | 0 | 0.76 | 0.64 | 0.74 | 0.53 | 0.00 | 0.56 |
| x = (B/G) | -0.66 | ln(Chl-a) = -2.981*x+4.787 | 0.38 | 0.87 | 0.75 | 0 | 0.74 | 0.46 | 1.03 | 0.66 | -0.10 | 0.85 | |
| x = (B-R)/G | -0.69 | ln(Chl-a) = -3.515*x+2.932 | 0.42 | 0.93 | 0.73 | 0 | 0.75 | 0.63 | 0.69 | 0.54 | -0.06 | 0.56 | |
|
3 (n = 26) |
x = (B/R) | -0.63 | ln(Chl-a) = -1.923*x+5.064 | 0.32 | 1.08 | 0.8 | 0 | 0.80 | 0.53 | 0.78 | 0.65 | 0.09 | 0.68 |
| x = (B/G) | -0.63 | ln(Chl-a) = -4.003*x+5.325 | 0.37 | 1.13 | 0.77 | 0 | 0.90 | 0.53 | 0.57 | 0.65 | -0.06 | 0.50 | |
| x = (B-R)/G | -0.63 | ln(Chl-a) = -3.867*x+3.018 | 0.35 | 1.06 | 0.78 | 0 | 0.81 | 0.52 | 0.79 | 0.66 | 0.09 | 0.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





