1. Introduction
Glioma is a type of tumor that originates from neural stem cells or progenitor cells in the central nervous system, and it is a common form of primary central nervous system tumors, accounting for approximately 80% of all primary malignant brain tumors[
1]. According to the 2021 WHO classification update, gliomas are now categorized based on their histological and molecular biomarker characteristics, into adult diffuse gliomas, pediatric diffuse low-grade gliomas (DLGG), pediatric diffuse high-grade gliomas, anaplastic astrocytomas, and ependymal tumors, among others[
2]. In adult diffuse gliomas, grades 1 and 2 are classified as DLGG, while grades 3 and 4 are considered diffuse high-grade gliomas (DHGG)[
2], and DLGG constitutes about 5% of primary brain tumors and 15% of gliomas[
1,
3].
DLGG exhibit slow growth rates and long disease courses, with a wide range of median survival times from 5.6 to 13.3 years[
4]. Some patients can survive for over 10 years, but more than 70% of DLGG may progress to DHGG within a decade. Following malignant transformation, the median overall survival (mOS) of patients significantly decreases to approximately 2.4 years[
5]. With aggressive treatment, the median survival of DLGG can be extended to about 13 years[
6], and the current standard treatment regimen includes surgery, radiotherapy, and chemotherapy, among others[
7].
Radiotherapy plays a pivotal role in the treatment of DLGG, aiming to control postoperative residual tumor cells, delay tumor progression, and thereby improve patient survival rates[
8]. However, studies indicate that while radiotherapy is crucial, it may be associated with side effects such as cognitive decline[9-11]. For patients with a favorable prognosis, these side effects may impact their quality of life over the long term. Previous clinical trials have shown that early postoperative radiotherapy can extend progression-free survival (PFS), but it does not increase overall survival (OS)[
12]. Therefore, individualized decisions regarding the timing of postoperative radiotherapy, the cessation of radiotherapy, and the possibility of delayed radiotherapy for different DLGG patients remain important issues that require further research and clinical exploration.
This review systematically summarizes the potential risks and management measures of brain radiotherapy and reviews the research results related to the timing of postoperative radiotherapy in DLGG patients. By comparing the clinical outcomes of early versus delayed postoperative radiotherapy, this paper aims to provide a reference for future clinical practice, assisting neuro-oncologists in formulating the most appropriate radiotherapy plan based on the patient’s underlying disease, significant side effects of radiotherapy, and respect for the patient’s personal preferences, to achieve the best balance between survival and quality of life. Additionally, this paper questions previous research findings based on the 2021 WHO update of central nervous system tumor classification and explores the feasibility of using rapidly developing computer technologies to predict early cognitive decline following radiotherapy, in order to optimize treatment decisions.
2. The Treatment of Diffuse Low-Grade Gliomas
In terms of treatment strategies, adherence to the guidelines set forth by the National Comprehensive Cancer Network (NCCN) and the European Association of Neuro-Oncology (EANO) is paramount. The treatment regimen for gliomas encompasses surgical resection, radiotherapy, chemotherapy, targeted drug therapy, tumor treating fields, or a combination thereof[
7]. The standard treatment protocol begins with the safest possible tumor resection, followed by radiotherapy and chemotherapy aimed at eradicating residual cancer cells.
Radiotherapy and chemotherapy, as adjuvant therapies, have been demonstrated to further enhance the survival rates of patients with DLGG. Current clinical treatment recommendations suggest that for high-risk(over 40 years old, subtotal tumor resection, or biopsy only, tumor diameter greater than or equal to 6 cm) grade 2 oligodendroglioma patients over the age of 40 or those with incomplete tumor resection, the combination of radiotherapy with adjuvant PCV chemotherapy or TMZ chemotherapy is recommended. For high-risk(same as above) grade 2 astrocytomas, the suggestion is radiotherapy combined with PCV or TMZ adjuvant chemotherapy, or radiotherapy with concurrent and adjuvant TMZ chemotherapy. Factors such as tumor volume, astrocytic components, and Karnofsky Performance Status (KPS) score are crucial considerations in the formulation of clinical treatment plans.
For patients with a KPS score of 60 or above, if they are under the age of 40 and have had a complete tumor resection, observation with regular follow-up is an option. The decision to initiate adjuvant therapy should be made following thorough discussion with the patient and their family. For high-risk patients, postoperative adjuvant therapy should be actively considered.
In the realm of radiotherapy, given that DLGG patients generally have a favorable prognosis and radiotherapy may entail certain side effects, particularly long-term ones such as cognitive decline and cerebrovascular lesions, which can lead to prolonged suffering for the patient. Therefore, postoperative treatment strategies should be tailored to the individual patient’s condition, taking into account a variety of factors to achieve personalized treatment plan adjustments.
3. The Side Effects of Cranial Radiotherapy
Radiotherapy, while effective in damaging tumor cells, inevitably affects normal tissue, leading to adverse reactions associated with radiotherapy. These reactions encompass acute (short-term) adverse reactions and delayed (long-term) adverse reactions[13-15]. The subsequent section of this article outlines the common symptoms of these adverse reactions and discusses strategies for more effectively managing or preventing them. This information is intended to assist neurosurgeons and radiation oncologists in comprehensively evaluating the benefits and potential risks of radiotherapy for patients, thereby facilitating effective communication between healthcare providers and patients and collaboratively developing more optimized treatment plans.
3.1. Acute (Short-Term) Side Effects
3.1.1. Fatigue
It is widely recognized that during the course of radiotherapy, patients commonly experience significant fatigue or somnolence. In patients with primary brain tumors, particularly those with glioblastoma, approximately 80% to 90% of individuals report fatigue symptoms associated with radiotherapy. These fatigue symptoms are generally mild in most cases; however, it is noteworthy that as many as half of the patients may exhibit more severe symptoms that can limit daily activities, necessitate increased daytime sleep, or fail to show significant relief of fatigue after rest. Patients may begin to feel fatigued within the first two weeks of starting radiotherapy, and the level of fatigue may peak 2 to 4 weeks after the completion of treatment. These symptoms typically subside over several months, yet there are reports in the literature of some patients continuing to experience fatigue symptoms 6 to 12 months after the end of treatment. This persistence often indicates a need for further evaluation to explore other potential causes of fatigue, which may include sleep disorders, cardiopulmonary diseases, hematological issues, metabolic or endocrine disorders, nutritional deficiencies, depression or emotional disorders, and medication use (including antiepileptic drug therapy)[
16,
17].
3.1.2. Cerebral Edema
Cerebral edema typically occurs within days to weeks following radiotherapy to the brain, resulting from a temporary increase in vascular permeability due to radiation-induced vascular injury[
13,
18]. Patients may present with clinical manifestations of increased intracranial pressure, such as headache (which may also be caused by direct neural damage from radiotherapy), nausea, vomiting, nuchal rigidity, and exacerbation or onset of seizures. These symptoms severely impact the quality of life and treatment compliance of patients and, in severe cases, can even be life-threatening[
19,
20]. Many brain tumor patients already experience symptoms of mass effect due to the tumor and surrounding edema prior to treatment, such as motor and sensory deficits, memory impairment, and others. Radiotherapy may exacerbate swelling around the tumor, thereby aggravating symptoms of related neurological dysfunction, although these neurological impairments are typically temporary and not considered precursors to long-term cognitive damage. Physicians commonly use corticosteroids such as dexamethasone to alleviate cerebral edema, nonsteroidal anti-inflammatory drugs (e.g., acetaminophen, ibuprofen, loxoprofen) to control severe headaches, prophylactic antiepileptic drugs to manage the exacerbation or prevention of new-onset seizures, and central antiemetics (e.g., ondansetron) to relieve nausea and vomiting. Due to a lack of prospective clinical study data, the application of these medications often relies on the individual clinical experience of the physician and the severity of the symptoms[
21,
22].
3.1.3. Alopecia
Alopecia is a common side effect in patients undergoing whole brain radiotherapy (WBRT). This phenomenon may cause psychological distress for certain individuals. The degree of hair loss is closely related to the radiation dose: lower doses of radiotherapy typically result in reversible alopecia, with complete hair regrowth observed within 2 to 4 months after the completion of radiotherapy; however, higher doses of radiotherapy may lead to permanent hair loss[
23,
24]. The use of intensity-modulated radiotherapy (IMRT) with scalp-sparing techniques can mitigate the extent of hair loss to some degree.
3.1.4. Skin Damage
The skin is highly sensitive to radiation therapy, with more than 95% of patients undergoing radiotherapy experiencing skin reactions ranging from moderate to severe[
25]. In the early stages of radiotherapy, the skin may exhibit changes in color, erythema, and inflammatory responses. In more severe cases, the damaged skin may present with desquamation, atrophy, and/or the formation of ulcers[26-28]. Severe radiation dermatitis not only delays the execution of the tumor treatment plan but also significantly affects the patient’s quality of life, necessitating timely intervention for control. This includes physical therapy as well as oral and topical medication treatments[
29].
3.2. Delayed (Long-Term) Side Effects
3.2.1. Cognitive Decline
Studies have described the issue of late cognitive decline in brain tumor patients following radiotherapy, which is a significant concern for clinicians when considering early radiotherapy for patients with DLGG[
30]. Cognitive decline is particularly common in childhood acute lymphoblastic leukemia (ALL) survivors who have received cranial radiotherapy (CRT). With advancements in chemotherapy and a reduction in the frequency of CRT use, the incidence and severity of cognitive decline in pediatric patients have decreased[31-33].
In adults, both WBRT and partial brain radiotherapy are associated with neurocognitive impacts. A randomized study by the European Organisation for Research and Treatment of Cancer (EORTC) showed that patients reported more significant cognitive decline after one year of WBRT compared to stereotactic radiosurgery (SRS) alone[
34]. Corresponding imaging changes include nonspecific diffuse white matter alterations[35-37], which may occur in patients receiving WBRT doses above 20 Gy and could be related to the severity of symptoms after one year[
37]. Severe white matter brain disease, characterized by ataxia, confusion, memory loss, dementia, and even death in rare cases, occurs in less than 5% of patients and is typically associated with higher radiation dose fractions. Currently, WBRT rarely employs this fractionation method[
38]. Therefore, when counseling patients receiving WBRT, physicians should consider factors such as systemic treatment, concomitant medications, baseline neurocognitive function, comorbidities, and overall prognosis to better assess the impact of WBRT on neurocognition and quality of life. The impact of partial brain radiotherapy on neurocognition is not yet clear, and most studies have been conducted in patients with low-grade gliomas with favorable prognoses. An observational study in the Netherlands found that the negative impact of radiotherapy on cognition was observed only in patients receiving each fraction greater than 2 Gy, and the impact of radiotherapy on cognition was more significant than that of the brain tumor itself and the use of antiepileptic drugs[
39]. However, a subsequent study of the same cohort of 65 patients conducted formal neurocognitive testing an average of 12 years after diagnosis found that even patients receiving low fractionation radiotherapy doses (≤2 Gy/fraction) exhibited a gradual decline in some cognitive functions[
40]. Notably, this secondary analysis of cohort data excluded patients with tumor recurrence, thus overlooking the impact of tumor recurrence on cognitive function in patients not receiving radiotherapy. Currently, highly conformal and stereotactic radiotherapy techniques may mitigate the cognitive impact of radiotherapy to some extent. For instance, a study from India compared neurocognitive and neuroendocrine outcomes between patients receiving highly conformal radiotherapy (SCRT) and those receiving traditional radiotherapy over a 5-year period post-treatment, finding SCRT to be superior without affecting survival rates[
41]. For DLGG patients with a favorable prognosis, consideration may be given to delaying radiotherapy to avoid early cognitive decline, a topic that will be discussed in detail later in the text.
3.2.2. Radiation Necrosis
Radiation necrosis is a late complication of radiotherapy, typically appearing 1 to 3 years after treatment, but in some advanced cases, it has been reported even 10 years after radiotherapy has concluded[
42]. This necrosis often occurs near or around the treatment target area, and its clinical manifestations depend on the location of the necrosis, which may include focal neurological signs or symptoms, increased intracranial pressure, gait and balance abnormalities, and complex cranial nerve deficits. The incidence of radiation necrosis in patients undergoing postoperative SRS treatment is 4% to 18% in the 6 months to several years following treatment[43-46]. The risk is further increased by concurrent chemotherapy (17%), targeted therapy (25%), and immunotherapy (38%)[
41,
47,
48]. Differentiating radiation necrosis from tumor progression can be challenging. The imaging features seen on standard MRI are nonspecific and usually cannot be clearly distinguished from tumor progression, often necessitating serial follow-up to differentiate tumor growth from the evolution of radiation necrosis. Advanced imaging techniques may reveal lactate peaks or lipid peaks on MRS[
46], reduced cerebral blood volume (CBV) on tumor perfusion imaging[
44,
49], diffusion restriction on diffusion-weighted MRI[
50], and lack of uptake on PET/CT[
51].
Radiation necrosis is typically a self-limiting process, with symptoms resolving on their own within 5 to 7 months after onset. Asymptomatic patients can be observed without intervention. Symptomatic patients can be treated with medium doses of corticosteroids (e.g., dexamethasone 4-8 mg daily) until symptoms improve, followed by gradual tapering of the dose. Follow-up imaging after 1 to 2 months of intervention can help confirm the therapeutic response. For patients unable to tolerate corticosteroids or unable to taper the dose, treatment options may include bevacizumab or laser interstitial thermal therapy (LITT)[
52,
53]。
For refractory necrosis, LITT may be considered, and it can be combined with biopsy[
54,
55], although there is a lack of high-level clinical evidence to support this approach. In cases with significant mass effect or diagnostic uncertainty, surgical resection may be necessary.
3.2.3. Vascular Lesions
Radiation-induced vascular lesions are one of the late complications that may occur following cranial radiotherapy, including cavernous hemangiomas, ischemic stroke, intracranial hemorrhage, and moyamoya disease, which may be related to radiation damage to cerebral blood vessels. Literature reports suggest that the occurrence of this problem is associated with factors such as younger age at the time of treatment, radiation fields involving the internal carotid artery bed, the superior petrosal sinus, and the Willis circle, higher radiation doses, and chemotherapy[56-58].
Cavernous hemangiomas are the most common type of radiation-induced vascular lesion, and their volume may increase over time with a risk of hemorrhage. These lesions typically occur in patients 3 to 6 years after cranial irradiation, with reported incidence rates ranging from 3% to 43%, with a higher incidence in pediatric patients compared to adults, and increasing with the duration since the end of radiotherapy[59-62].
Ischemic stroke has been reported to occur in the late stages of radiotherapy. In a prospective, randomized study of meningioma patients with dose escalation, 20% of patients experienced ischemic stroke 5.6 years (median) after treatment[
63]. The total radiation dose to the Willis circle may be the most significant risk factor, especially when doses exceed 40Gy; however, even with lower total doses up to 10Gy, ischemic stroke may occur with longer follow-up[
64]. Although cranial irradiation rarely significantly affects the carotid arteries, it may still increase the risk of carotid artery disease and atherosclerosis, thereby increasing the risk of ischemic stroke[
65].
Vascular lesions similar to moyamoya disease have been reported to occur 40 months (median) after radiotherapy, with younger age (<5 years), type 1 neurofibromatosis, or low-grade glioma being independent risk factors[
66,
67].
Radiation-induced aneurysms are a rare but potentially fatal complication of radiotherapy. Nanney et al. reviewed 46 patients with brain tumors who received radiotherapy and found 69 intracranial aneurysms within the radiotherapy field over an average of 12 years. The pathogenesis is not fully understood, but it may be related to vascular endothelial cell damage, vascular wall degeneration, and atherosclerosis. Radiographically, these aneurysms are often cystic or multiple and are more easily detected on magnetic resonance weighted imaging[
68]. The diagnosis and treatment of radiation-induced aneurysms require a combination of medical history, clinical presentation, and imaging findings.
Currently, there are no clear guidelines for primary or secondary prevention of radiation-induced vascular lesions. Clinically, patients and their families should be informed about the risks, symptoms, and preventive measures associated with radiation-induced vascular lesions, emphasizing the importance of regular follow-up and monitoring to detect and manage complications in a timely manner. Information on lifestyle and health habits should also be provided to reduce the risk of complications. For patients with pre-existing vascular disease before radiotherapy, the decision to proceed with radiotherapy should be made based on the severity of the disease and the patient’s willingness to undergo treatment.
3.2.4. Post-radiotherapy Stroke-like Migraine Attacks (SMART Syndrome)
SMART syndrom is a rare delayed complication of cranial irradiation. Patients typically experience migraine-like headaches, seizures, and subacute stroke-like episodes several years after radiotherapy, with symptoms including hemiplegia, aphasia, and hemianopsia. These episodes usually present in a subacute manner and tend to resolve spontaneously within weeks. The exact mechanism of SMART syndrome is not fully understood, but it appears to be caused by radiation-induced excessive brain excitation, impaired self-regulation mechanisms, and vascular endothelial damage[
69].
Treatment for SMART syndrome primarily involves symptomatic management. Nonsteroidal anti-inflammatory drugs and opioids can alleviate headaches, while drugs like carbamazepine and topiramate can be used to control seizures. Additionally, patients need to understand and avoid triggers for their headaches, such as stress, sleep disorders, specific foods, or beverages[
70].
3.2.5. Endocrine Dysfunction
Late endocrine dysfunction is relatively common among patients following radiotherapy, with approximately 80% of patients experiencing hypothalamic and pituitary dysfunction after receiving treatment doses as low as 20Gy[71-73]. Endocrine-related issues may arise in patients within the first year following radiotherapy, and the likelihood of such issues increases over time. In non-pituitary tumor patients undergoing radiotherapy, 37% to 77% of patients are diagnosed with pituitary hypofunction between 3 to 13 years post-radiotherapy. Growth hormone deficiency (50%), gonadotropin deficiency (25%), hyperprolactinemia (24%), adrenocorticotropic hormone deficiency (19%), and central hypothyroidism (16%) are the most common pituitary dysfunctions[
73].
Patients undergoing cranial radiotherapy should undergo comprehensive endocrine function assessments prior to radiotherapy, including measurements of growth hormone, gonadotropins, prolactin, adrenocorticotropic hormone, and thyroid function. After radiotherapy, regular follow-up checks should be conducted to monitor changes in hormone levels and the function of related target organs. Hormone replacement therapy should be administered to patients with low or deficient hormone levels to maintain normal physiological function. For patients with hyperprolactinemia, treatment with dopamine agonists and other medications may be employed.
3.2.6. Impact on Vision
Radiation-induced optic neuropathy (RION) typically presents as painless visual impairment (unilateral or bilateral, depending on the location of the damage) between 6 to 24 months after treatment. Symptoms may progress gradually over 1 to 4 weeks following onset. The occurrence and severity of RION are positively correlated with the total radiation dose. It has been reported that for conventional fractionated radiotherapy, the incidence of optic neuropathy is extremely low when the total dose is below 55Gy; it ranges from 3% to 7% when the total dose is between 55 to 60Gy; and it increases to 7% to 20% when the dose exceeds 60 Gy[
74]. Single-dose radiation therapy exceeding 2.5Gy also appears to increase the risk of RION[
75]. In SRS treatment, the incidence of RION is extremely low when the single dose to the visual pathway is maintained below 10 Gy or 12Gy/fraction[
76,
77].
Official neuro-ophthalmic examination can be used to confirm visual loss and assess the health of the optic nerve and optic disc. MRI can reveal signal abnormalities in the optic nerve with optic nerve enhancement[
70,
78].
However, there is currently no definitive treatment that significantly improves the prognosis of patients with RION. Hyperbaric oxygen therapy, corticosteroids, and anticoagulant treatment may show some efficacy in specific cases, but there is a lack of high-level clinical evidence to support these treatment methods[
79]. For patients whose tumors have already affected optic nerve function, close monitoring of optic nerve function during radiotherapy is necessary to prevent further radiotherapy-induced severe and irreversible RION.
4. Postoperative Radiotherapy Decision-Making and Management in DLGG Patients
Despite the potential risks associated with radiotherapy, given its effectiveness in controlling tumor progression, it remains a treatment option that should be considered for every patient with DLGG. However, for neuro-oncologists, it is essential to comprehensively consider the individual characteristics of each patient and, based on thorough communication with the patient, develop the optimal treatment plan to achieve a balance between the benefits and potential risks of radiotherapy. For patients assessed to have a higher potential risk, such as those with severe cerebrovascular disease before radiotherapy, other treatment options such as chemotherapy can be considered first to avoid further exacerbation of cerebrovascular disease. For patients who experience severe side effects such as severe cerebral edema or visual dysfunction during radiotherapy, radiotherapy should be immediately discontinued and alternative treatment approaches should be adopted.
For patients with a favorable prognosis for DLGG, whether to delay postoperative radiotherapy should also be considered. Although patients who undergo postoperative radiotherapy have observed declines in neurocognitive function, there is individual variation in the occurrence of this phenomenon, but it can still be observed[
40,
80]. Early postoperative radiotherapy compared to delayed radiotherapy does not increase the patient’s OS, but it does provide a longer progression-free survival (PFS, approximately 1.5 years)[
12]. This means that if a DLGG patient undergoes cranial radiotherapy in the early postoperative period, they may have to endure the suffering of cognitive decline for the next decade. However, if patients undergo other treatments or observation first and receive radiotherapy when DLGG progresses to DHGG, they may avoid the suffering caused by cognitive decline but would need to experience tumor progression earlier. Therefore, for neuro-oncologists, more consideration and communication with patients are required to make the best decision.
4.1. Definitions of Early Radiotherapy and Delayed Radiotherapy
Currently, a broad consensus has not yet been reached regarding the definitions of early radiotherapy and delayed radiotherapy for patients with DLGG. In the study by Tiphaine Obara, early radiotherapy is defined as the administration of radiotherapy concurrent with first-line or second-line treatments (surgery, chemotherapy), whereas delayed radiotherapy is defined as the initiation of radiotherapy after at least a third-line treatment[
81]. Sanjay Dhawan’s research defines early radiotherapy as the initiation of radiotherapy within a few weeks post-surgery, and delayed radiotherapy as the administration of radiotherapy upon clinical symptoms or radiological evidence of progression[
82]. In Michela Buglione’s study, early postoperative radiotherapy is defined as within 3 months after surgery, while delayed postoperative radiotherapy is defined as the initiation of radiotherapy upon detection of tumor progression[
83]. Synthetically, early radiotherapy can be roughly defined as the administration of radiotherapy as soon as possible after surgery (usually within a few weeks to several months), whereas delayed radiotherapy can be defined as the option to forgo radiotherapy post-surgery until other treatment modalities have been exhausted and radiotherapy is initiated only when clinical symptoms and/or radiological evidence suggest tumor progression.
4.2. The Impact of Early Radiotherapy versus Delayed Radiotherapy on the Survival of DLGG Patients
As previously mentioned, for patients with DLGG, there is no significant difference in OS between early radiotherapy and delayed radiotherapy post-surgery; however, a difference exists in PFS. The most representative study demonstrating this is a randomized controlled trial published in 2005 by van den Bent, M.J. in The Lancet, which randomized 314 DLGG patients to either early radiotherapy or radiotherapy at the time of progression. The study revealed no statistically significant difference in median OS between the two groups, with median OS of 7.4 years for the early radiotherapy group and 7.2 years for the delayed radiotherapy group. The only difference observed was in median PFS, which was 5.3 years and 3.7 years, respectively. Additionally, the study reported better control of epileptic seizures in patients receiving early radiotherapy after one year[
84]. Johan A. F. Koekkoek also summarized 10 clinical studies on the effect of radiotherapy on seizure control in DLGG patients, all of which indicated that radiotherapy significantly reduced the frequency and severity of seizures[
85].
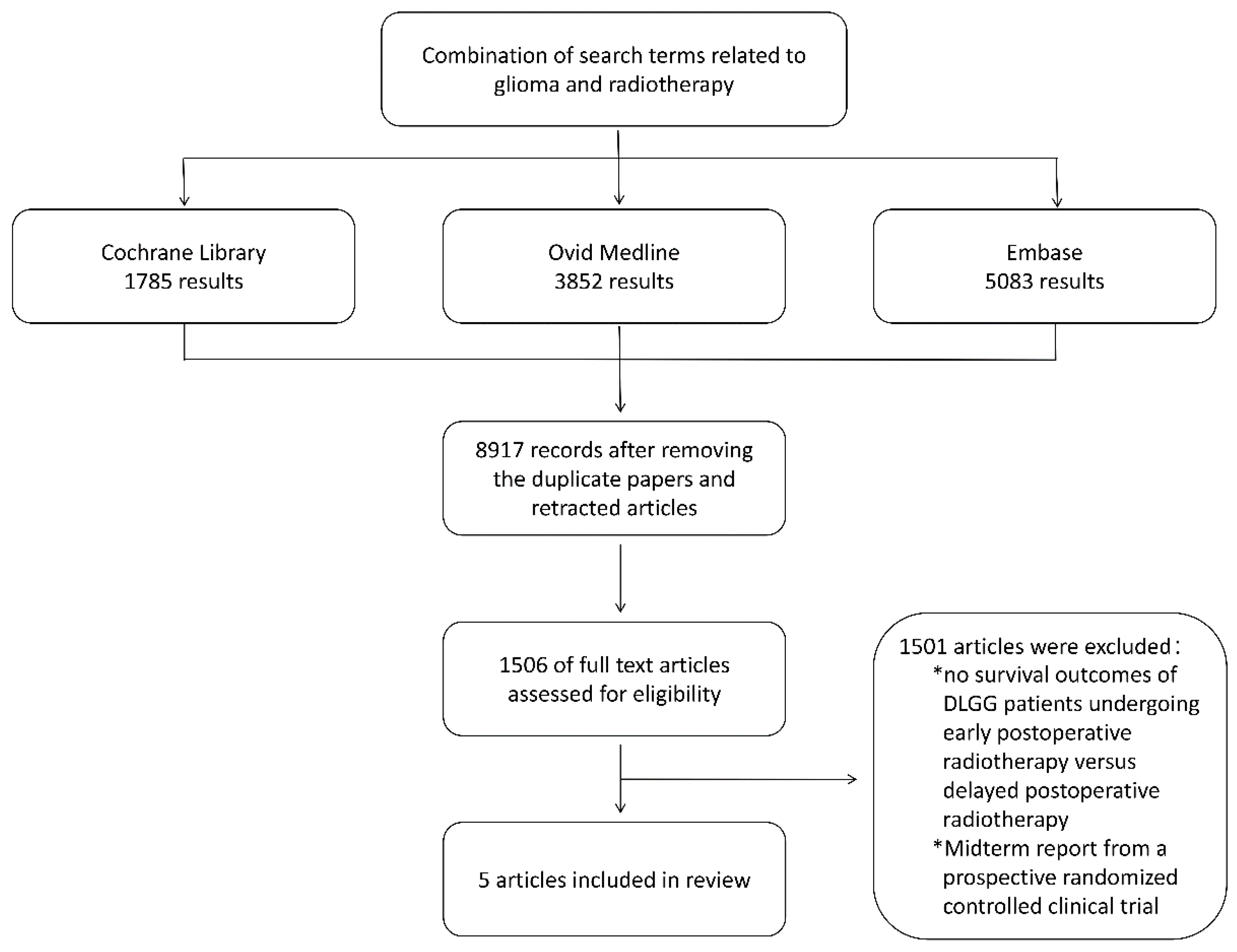
To further validate the findings of van den Bent, M.J., we conducted a literature search using electronic databases including the Cochrane Library, Ovid Medline, and Embase, with the search period ending in April 2024. The detailed search strategy is provided in Supplementary Table S1. The search included combinations of terms related to “glioma” and “radiotherapy,” restricted to English-language and human studies. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Two authors (Xin and Wenbo) determined the eligibility of articles for inclusion and were responsible for reviewing the full text of all selected articles. The inclusion criteria were: (i) adult patients with World Health Organization (WHO) grade II gliomas confirmed histologically, (ii) patients who had received radiotherapy (fractionated local radiation, stereotactic radiotherapy, or brachytherapy), (iii) a sample size of ≥5, (iv) biopsy or surgical resection performed, (v) histological types of astrocytoma, oligodendroglioma, or mixed oligodendroglioma, and (vi) WHO grade 2. Preliminary exclusion criteria were: (i) reviews and (ii) abstracts not published in full text. Additionally, we applied the following exclusion criteria to the remaining articles: (i) patients who had previously received cranial radiotherapy and (ii) patients who underwent craniotomy in addition to biopsy or resection of DLGG. We searched the reference lists of the selected full-text articles to identify other studies. From the selected articles, we extracted the following data: study design, sample size, clinical characteristics of the study population, and final survival outcomes (median OS/PFS, 5-year overall survival rate, and progression-free survival rate). In this literature search, a total of 8197 unique records were obtained. We assessed 1506 articles to ascertain their eligibility for further screening, ultimately incorporating 5 studies that met the inclusion criteria (detailed screening process is illustrated in
Figure 1).
In
Table 2, the primary findings from five studies regarding the survival-related outcomes of early postoperative radiotherapy versus delayed postoperative radiotherapy in patients with DLGG are presented.
The literature search suggests that, in addition to van den Bent, M.J.'s prospective randomized study specifically exploring the impact of early versus late postoperative radiotherapy on the survival of patients with DLGG, there are four other studies that have investigated this clinical issue retrospectively.
Michela Buglione’s study retrospectively analyzed 95 adult patients with low-grade gliomas who received early (within 3 months postoperatively) or late (after disease progression) radiotherapy. The study found that early radiotherapy significantly improved the PFS of patients, with 1-year, 5-year, and 10-year PFS rates of 79%, 49%, and 14%, respectively, compared to 74%, 39%, and 7% for late radiotherapy. However, early radiotherapy did not significantly impact OS, with 1-year, 5-year, and 10-year OS rates of 89%, 60%, and 51%, respectively. Additionally, the study found that younger patients (≤40 years) and those with a good functional status (high KPS score) had better PFS and OS, regardless of whether they received early or late radiotherapy. This study underscores the importance of radiotherapy in the treatment of low-grade gliomas and provides a reference for the timing of radiotherapy administration[
83].
Zoltán Hanzély’s research retrospectively analyzed long-term follow-up data on 97 patients with WHO grade II astrocytomas treated at the National Institute of Neurosurgery in Budapest, Hungary, between 1985 and 1997. The study divided patients into two groups: those who received early postoperative radiotherapy (37%) and those who received it later. The results showed that the 5-year and 10-year PFS rates for the early radiotherapy group were 52.2% and 30.7%, respectively, compared to 39.5% and 12.4% for the late radiotherapy group (p = 0.0388). However, there was no significant difference in the 5-year and 10-year disease-specific survival rates (DSS) between the two groups. Age and the extent of surgical resection were significant prognostic factors for DSS. Further analysis revealed that early radiotherapy significantly benefited patients with partial resection, with 5-year PFS and DSS rates of 60.0% and 66.7%, respectively, compared to 12.4% and 49.8% for patients who did not receive radiotherapy (p < 0.05). For patients with extensive resection, postoperative radiotherapy had no impact on PFS and DSS. The findings suggest that for patients with WHO grade II astrocytomas and partial resection, early postoperative radiotherapy can significantly improve PFS and DSS; for those with extensive resection, radiotherapy can be delayed until tumor progression[
86].
Christopher Leighton’s study retrospectively analyzed data from 167 adult patients with supratentorial low-grade gliomas treated at a regional cancer center in Canada between 1979 and 1995. All patients were histopathologically confirmed to have low-grade fibrillary astrocytomas, oligodendrogliomas, or mixed gliomas. The study aimed to investigate the impact of radiotherapy timing on prognosis. The results showed that the median overall survival for this group was 10.5 years, with 5-year and 10-year survival rates of 72% and 50%, respectively. The median tumor progression-free survival was 4.9 years, with 5-year and 10-year progression-free survival rates of 50% and 12%. Oligodendrogliomas or mixed gliomas had a better prognosis than astrocytomas. Multivariate analysis revealed that age less than 40 at diagnosis, presence of seizures, less postoperative residual tumor, a KPS score of 70 or higher, oligodendroglioma or mixed glioma pathology, and delayed radiotherapy were associated with longer overall survival. However, radiotherapy timing was not an independent prognostic factor, as patients who chose delayed radiotherapy often had other favorable prognostic factors. The study suggests that early postoperative radiotherapy may be an appropriate treatment strategy for patients with unfavorable prognostic factors, while delayed radiotherapy may be a viable option for those with favorable prognostic factors[
86].
Tiphaine Obara’s article included 339 patients with DLGG diagnosed at the Neuro-oncology Department in Nancy, France, between 1982 and 2017. The study analyzed their survival rates, natural prognostic factors, and treatment efficacy. The study found that the median survival for DLGG patients was 15.7 years, and for most of the follow-up period, they maintained their neurologic function and were able to live independently. The study confirmed the importance of the KPS score and tumor volume at diagnosis as independent prognostic factors. Additionally, the study found that surgical resection had a positive impact on survival, especially when the resection was complete. In terms of radiotherapy, the study found no significant difference in survival between early and late radiotherapy, but late radiotherapy (i.e., radiotherapy after malignant transformation of the tumor) was associated with a poorer prognosis. This study indicates that a personalized treatment strategy can achieve longer survival while maintaining the quality of life of the patient[
81].
In summary, these study results suggest that in the treatment of diffuse low-grade gliomas, early radiotherapy and delayed radiotherapy have little impact on OS, but early radiotherapy can significantly improve PFS. Younger patients and those with good neurologic function, regardless of whether they receive early or late radiotherapy, have better PFS and OS. These study results emphasize the importance of radiotherapy in the treatment of low-grade gliomas and provide a basis for choosing the timing of radiotherapy administration.
4.3. The Impact of DLGG Progression to DHGG on Patients
However, if a patient with a DLGG opts for a delayed postoperative radiotherapy, they may experience tumor progression at a shorter interval compared to those who receive early radiotherapy. Tumor progression can lead to various impacts, including the onset of more frequent and severe neurological symptoms such as epileptic seizures, cognitive decline, and motor dysfunction[87-89]. These factors should be considered by physicians when making treatment decisions. The extent to which these symptoms negatively affect the patient’s daily life and work is influenced by multiple factors, including the patient’s age, tumor location, molecular characteristics, extent of resection, and whether the patient had epileptic seizures before surgery and their severity. For patients who may suffer severe consequences due to tumor progression, close monitoring and the development of individualized treatment plans are particularly important.
5. Summary
The impact of radiotherapy on individual patients varies, partly due to factors such as tumor location and type, which lead to varying degrees of decline in neurofunction and status when tumor progression occurs. Therefore, the treatment needs of patients with DLGG are more specific, personalized, and diverse. According to the content presented in this article, for DLGG patients with favorable disease conditions, regular and meticulous follow-up monitoring may allow for radiotherapy to be initiated when clinical or radiological progression occurs. For patients with tumors in functional areas, larger residual tumor volumes, severe epileptic symptoms, or advanced age, the time to tumor progression may be shorter, and the impact on neurofunction and quality of life after tumor progression may be greater than the impact of radiotherapy. Therefore, for these patients, it may be necessary to decide whether to undergo early postoperative radiotherapy based on the specific circumstances. For patients who may have their underlying diseases or health status exacerbated by radiotherapy, the decision for postoperative radiotherapy requires extra caution. If severe cerebral edema occurs during radiotherapy, it should be discontinued as appropriate. Moreover, patients’ own needs should also be considered by physicians. For example, a pregnant patient with DLGG may prefer delayed postoperative radiotherapy over immediate postoperative radiotherapy, and a DLGG patient who requires intense mental labor may also choose not to undergo radiotherapy immediately after surgery to avoid the risk of cognitive decline. This requires adequate communication between clinical physicians and patients.
With the advancement of artificial intelligence (AI) technology, researchers are able to predict specific outcomes based on existing patient characteristics. AI can also be utilized to predict the risk of severe cognitive impairment and quality of life decline in DLGG patients undergoing early postoperative radiotherapy, aiding clinicians and patients in making joint decisions regarding the timing of radiotherapy. This requires extensive data training for AI.
In the context of the studies discussed in this article on early versus delayed postoperative radiotherapy for DLGG patients, due to the early publication dates, the pathological diagnoses of glioma patients were largely dependent on histology. However, the current diagnosis of diffuse gliomas now includes molecular diagnostics. In the past, WHO grade II astrocytomas may now be diagnosed as WHO grade IV astrocytomas or glioblastomas. Therefore, the results of these studies may need to be revisited, with some DLGG cases possibly being reclassified as DHGG, which could affect the study outcomes. It may be necessary to conduct retrospective studies under the new diagnostic criteria or new prospective studies to re-determine the accuracy of the impact of early versus delayed postoperative radiotherapy on the OS and PFS of DLGG patients.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
XZ and WW searched and assessed articles, and drafted the manuscript. YW, XL and WM supervised the review. All authors reviewed the manuscript.
Funding
This work was funded by the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-113), the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-019) for Yu Wang, the CAMS Innovation Fund for Medical Sciences (2021-12M-1-014), and by the National Natural Science Foundation of China (82151302)
Conflicts of Interest
All authors declared no conflict of interest.
Declarations
Consent for publication. All authors consent to the publication of this manuscript in the Journal of Neuro-oncology journal. Availability of data and materials. The complete search strategy is outlined in Supplementary Table S1.
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncology 2023, 25, iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Nunna, R.S.; Khalid, S.; Ryoo, J.S.; Sethi, A.; Byrne, R.W.; Mehta, A.I. Radiotherapy in adult low-grade glioma: nationwide trends in treatment and outcomes. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2021, 23, 628–637. [Google Scholar] [CrossRef]
- Lombardi, G.; Barresi, V.; Castellano, A.; Tabouret, E.; Pasqualetti, F.; Salvalaggio, A.; Cerretti, G.; Caccese, M.; Padovan, M.; Zagonel, V.; et al. Clinical Management of Diffuse Low-Grade Gliomas. Cancers 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Tom, M.C.; Park, D.Y.J.; Yang, K.; Leyrer, C.M.; Wei, W.; Jia, X.; Varra, V.; Yu, J.S.; Chao, S.T.; Balagamwala, E.H.; et al. Malignant Transformation of Molecularly Classified Adult Low-Grade Glioma. International journal of radiation oncology, biology, physics 2019, 105, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.A.O.; Chang, S. Treatment Strategies for Low-Grade Glioma in Adults. Journal of Oncology Practice 2016, 12. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nature Reviews Clinical Oncology 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Bouget, D.; Reinertsen, I.; Skjulsvik, A.J.; Sagberg, L.M.; Bø, H.K.; Gulati, S.; Sjåvik, K.; Solheim, O. Spatial distribution of malignant transformation in patients with low-grade glioma. Journal of Neuro-Oncology 2020, 146. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Voon, N.S.; Abdul Manan, H.; Yahya, N. Cognitive Decline following Radiotherapy of Head and Neck Cancer: Systematic Review and Meta-Analysis of MRI Correlates. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Palmer, J.D.; Klamer, B.G.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Association of Long-term Outcomes With Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial. JAMA Oncol 2022, 8, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Afra, D.; de Witte, O.; Ben Hassel, M.; Schraub, S.; Hoang-Xuan, K.; Malmstrom, P.O.; Collette, L.; Pierart, M.; Mirimanoff, R.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet (London, England) 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Sheline, G.E.; Wara, W.M.; Smith, V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 1980, 6, 1215–1228. [Google Scholar] [CrossRef]
- Wujanto, C.; Vellayappan, B.; Chang, E.L.; Chao, S.T.; Sahgal, A.; Lo, S.S. Radiotherapy to the brain: what are the consequences of this age-old treatment? Ann Palliat Med 2021, 10, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Tanguturi, S.K.; Alexander, B.M. Neurologic Complications of Radiation Therapy. Neurol Clin 2018, 36, 599–625. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Guerrero, D.; Sardell, S.; Cumins, S.; Wharram, B.; Traish, D.; Gonsalves, A.; Ashley, S.; Brada, M. Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: a prospective study. Radiother Oncol 2011, 100, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Faithfull, S.; Brada, M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol (R Coll Radiol) 1998, 10, 250–254. [Google Scholar] [CrossRef]
- Milano, M.T.; Sharma, M.; Soltys, S.G.; Sahgal, A.; Usuki, K.Y.; Saenz, J.M.; Grimm, J.; El Naqa, I. Radiation-Induced Edema After Single-Fraction or Multifraction Stereotactic Radiosurgery for Meningioma: A Critical Review. Int J Radiat Oncol Biol Phys 2018, 101, 344–357. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front Oncol 2012, 2, 73. [Google Scholar] [CrossRef]
- Young, D.F.; Posner, J.B.; Chu, F.; Nisce, L. Rapid-course radiation therapy of cerebral metastases: results and complications. Cancer 1974, 34, 1069–1076. [Google Scholar] [CrossRef]
- Arvold, N.D.; Pinnell, N.E.; Mahadevan, A.; Connelly, S.; Silverman, R.; Weiss, S.E.; Kelly, P.J.; Alexander, B.M. Steroid and anticonvulsant prophylaxis for stereotactic radiosurgery: Large variation in physician recommendations. Pract Radiat Oncol 2016, 6, e89–e96. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Graham, M.M.; Mahler, P.A.; Rasey, J.S. Use of steroids to suppress vascular response to radiation. Int J Radiat Oncol Biol Phys 1987, 13, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Severs, G.A.; Griffin, T.; Werner-Wasik, M. Cicatricial alopecia secondary to radiation therapy: case report and review of the literature. Cutis 2008, 81, 147–153. [Google Scholar] [PubMed]
- Ali, S.Y.; Singh, G. Radiation-induced Alopecia. Int J Trichology 2010, 2, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Porock, D.; Nikoletti, S.; Kristjanson, L. Management of radiation skin reactions: literature review and clinical application. Plast Surg Nurs 1999, 19, 185–192, 223; quiz 191-182. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- O'Sullivan, B.; Levin, W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol 2003, 13, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J.L. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol 2007, 17, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.J.; Webster, J.; Chung, B.; Marquart, L.; Ahmed, M.; Garantziotis, S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014, 14, 53. [Google Scholar] [CrossRef]
- Schaff, L.R.; Ioannou, M.; Geurts, M.; van den Bent, M.J.; Mellinghoff, I.K.; Schreck, K.C. State of the Art in Low-Grade Glioma Management: Insights From Isocitrate Dehydrogenase and Beyond. Am Soc Clin Oncol Educ Book 2024, 44, e431450. [Google Scholar] [CrossRef]
- Krull, K.R.; Zhang, N.; Santucci, A.; Srivastava, D.K.; Krasin, M.J.; Kun, L.E.; Pui, C.H.; Robison, L.L.; Hudson, M.M.; Armstrong, G.T. Long-term decline in intelligence among adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiation. Blood 2013, 122, 550–553. [Google Scholar] [CrossRef]
- Conklin, H.M.; Krull, K.R.; Reddick, W.E.; Pei, D.; Cheng, C.; Pui, C.H. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst 2012, 104, 1386–1395. [Google Scholar] [CrossRef]
- Iyer, N.S.; Balsamo, L.M.; Bracken, M.B.; Kadan-Lottick, N.S. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood 2015, 126, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013, 31, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.A., 3rd; Faraji, A.H.; Berkowitz, O.; Parry, P.V.; Hadelsberg, U.; Kano, H.; Niranjan, A.; Kondziolka, D.; Lunsford, L.D. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer 2013, 119, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Klein, J.P. Imaging of cancer therapy-induced central nervous system toxicity. Neurol Clin 2014, 32, 147–157. [Google Scholar] [CrossRef]
- Constine, L.S.; Konski, A.; Ekholm, S.; McDonald, S.; Rubin, P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys 1988, 15, 319–330. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Delattre, J.Y.; Posner, J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989, 39, 789–796. [Google Scholar] [CrossRef]
- Klein, M.; Heimans, J.J.; Aaronson, N.K.; van der Ploeg, H.M.; Grit, J.; Muller, M.; Postma, T.J.; Mooij, J.J.; Boerman, R.H.; Beute, G.N.; et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 2002, 360, 1361–1368. [Google Scholar] [CrossRef]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 2009, 8, 810–818. [Google Scholar] [CrossRef]
- Jalali, R.; Gupta, T.; Goda, J.S.; Goswami, S.; Shah, N.; Dutta, D.; Krishna, U.; Deodhar, J.; Menon, P.; Kannan, S.; et al. Efficacy of Stereotactic Conformal Radiotherapy vs Conventional Radiotherapy on Benign and Low-Grade Brain Tumors: A Randomized Clinical Trial. JAMA Oncol 2017, 3, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Strenger, V.; Lackner, H.; Mayer, R.; Sminia, P.; Sovinz, P.; Mokry, M.; Pilhatsch, A.; Benesch, M.; Schwinger, W.; Seidel, M.; et al. Incidence and clinical course of radionecrosis in children with brain tumors. A 20-year longitudinal observational study. Strahlenther Onkol 2013, 189, 759–764. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Lobato-Polo, J.; Zorro, O.; Flickinger, J.C.; Lunsford, L.D. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 2010, 66, 486–491; discussion 491-482. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Korogi, Y.; Tomiguchi, S.; Shigematsu, Y.; Ikushima, I.; Kira, T.; Liang, L.; Ushio, Y.; Takahashi, M. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000, 21, 901–909. [Google Scholar] [PubMed]
- Henry, R.G.; Vigneron, D.B.; Fischbein, N.J.; Grant, P.E.; Day, M.R.; Noworolski, S.M.; Star-Lack, J.M.; Wald, L.L.; Dillon, W.P.; Chang, S.M.; et al. Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 2000, 21, 357–366. [Google Scholar]
- Kimura, T.; Sako, K.; Tanaka, K.; Gotoh, T.; Yoshida, H.; Aburano, T.; Tanaka, T.; Arai, H.; Nakada, T. Evaluation of the response of metastatic brain tumors to stereotactic radiosurgery by proton magnetic resonance spectroscopy, 201TlCl single-photon emission computerized tomography, and gadolinium-enhanced magnetic resonance imaging. J Neurosurg 2004, 100, 835–841. [Google Scholar] [CrossRef]
- Correa, D.D.; DeAngelis, L.M.; Shi, W.; Thaler, H.T.; Lin, M.; Abrey, L.E. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol 2007, 81, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kiehna, E.N.; Mulhern, R.K.; Li, C.; Xiong, X.; Merchant, T.E. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. J Clin Oncol 2006, 24, 5283–5290. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, K.; Nakasu, Y.; Horiguchi, S.; Harada, H.; Nishimura, T.; Bando, E.; Okawa, H.; Furukawa, Y.; Hirai, T.; Endo, M. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol 2010, 99, 81–88. [Google Scholar] [CrossRef]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 2005, 26, 1455–1460. [Google Scholar]
- Schwartz, R.B.; Holman, B.L.; Polak, J.F.; Garada, B.M.; Schwartz, M.S.; Folkerth, R.; Carvalho, P.A.; Loeffler, J.S.; Shrieve, D.C.; Black, P.M.; et al. Dual-isotope single-photon emission computerized tomography scanning in patients with glioblastoma multiforme: association with patient survival and histopathological characteristics of tumor after high-dose radiotherapy. J Neurosurg 1998, 89, 60–68. [Google Scholar] [CrossRef]
- Miyatake, S.; Nonoguchi, N.; Furuse, M.; Yoritsune, E.; Miyata, T.; Kawabata, S.; Kuroiwa, T. Pathophysiology, Diagnosis, and Treatment of Radiation Necrosis in the Brain. Neurol Med Chir (Tokyo) 2015, 55 (Suppl 1), 50–59. [Google Scholar] [CrossRef]
- Bernhardt, D.; König, L.; Grosu, A.; Wiestler, B.; Rieken, S.; Wick, W.; Gempt, J.; Krieg, S.M.; Schmidt-Graf, F.; Sahm, F.; et al. DEGRO practical guideline for central nervous system radiation necrosis part 1: classification and a multistep approach for diagnosis. Strahlenther Onkol 2022, 198, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Hargreaves, E.L.; Khan, A.J.; Haffty, B.G.; Danish, S.F. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 2014, 74, 658–667; discussion 667. [Google Scholar] [CrossRef]
- Smith, C.J.; Myers, C.S.; Chapple, K.M.; Smith, K.A. Long-Term Follow-up of 25 Cases of Biopsy-Proven Radiation Necrosis or Post-Radiation Treatment Effect Treated With Magnetic Resonance-Guided Laser Interstitial Thermal Therapy. Neurosurgery 2016, 79 (Suppl 1), S59–s72. [Google Scholar] [CrossRef]
- Campen, C.J.; Kranick, S.M.; Kasner, S.E.; Kessler, S.K.; Zimmerman, R.A.; Lustig, R.; Phillips, P.C.; Storm, P.B.; Smith, S.E.; Ichord, R.; et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke 2012, 43, 3035–3040. [Google Scholar] [CrossRef] [PubMed]
- Bowers, D.C.; Liu, Y.; Leisenring, W.; McNeil, E.; Stovall, M.; Gurney, J.G.; Robison, L.L.; Packer, R.J.; Oeffinger, K.C. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2006, 24, 5277–5282. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.S.; Xie, H.; Merchant, T.E.; Yu, J.S.; Chao, S.T.; Suh, J.H. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol 2015, 122, 421–429. [Google Scholar] [CrossRef]
- Strenger, V.; Sovinz, P.; Lackner, H.; Dornbusch, H.J.; Lingitz, H.; Eder, H.G.; Moser, A.; Urban, C. Intracerebral cavernous hemangioma after cranial irradiation in childhood. Incidence and risk factors. Strahlenther Onkol 2008, 184, 276–280. [Google Scholar] [CrossRef]
- Burn, S.; Gunny, R.; Phipps, K.; Gaze, M.; Hayward, R. Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg 2007, 106, 379–383. [Google Scholar] [CrossRef]
- Lew, S.M.; Morgan, J.N.; Psaty, E.; Lefton, D.R.; Allen, J.C.; Abbott, R. Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. J Neurosurg 2006, 104, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Heckl, S.; Aschoff, A.; Kunze, S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer 2002, 94, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Sanford, N.N.; Yeap, B.Y.; Larvie, M.; Daartz, J.; Munzenrider, J.E.; Liebsch, N.J.; Fullerton, B.; Pan, E.; Loeffler, J.S.; Shih, H.A. Prospective, Randomized Study of Radiation Dose Escalation With Combined Proton-Photon Therapy for Benign Meningiomas. Int J Radiat Oncol Biol Phys 2017, 99, 787–796. [Google Scholar] [CrossRef] [PubMed]
- El-Fayech, C.; Haddy, N.; Allodji, R.S.; Veres, C.; Diop, F.; Kahlouche, A.; Llanas, D.; Jackson, A.; Rubino, C.; Guibout, C.; et al. Cerebrovascular Diseases in Childhood Cancer Survivors: Role of the Radiation Dose to Willis Circle Arteries. Int J Radiat Oncol Biol Phys 2017, 97, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, C.; Husmann, M.; Glanzmann, C.; Studer, G.; Amann-Vesti, B.R. Carotid artery disease after head and neck radiotherapy. Vasa 2015, 44, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.S.; Paulino, A.C.; Mai, W.Y.; Teh, B.S. Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys 2006, 65, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, N.J.; Robertson, R.; Kinnamon, D.D.; Scott, R.M.; Kieran, M.W.; Turner, C.D.; Chi, S.N.; Goumnerova, L.; Proctor, M.; Tarbell, N.J.; et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology 2007, 68, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Nanney, A.D., 3rd; El Tecle, N.E.; El Ahmadieh, T.Y.; Daou, M.R.; Bit Ivan, E.N.; Marymont, M.H.; Batjer, H.H.; Bendok, B.R. Intracranial aneurysms in previously irradiated fields: literature review and case report. World Neurosurg 2014, 81, 511–519. [Google Scholar] [CrossRef]
- Black, D.F.; Morris, J.M.; Lindell, E.P.; Krecke, K.N.; Worrell, G.A.; Bartleson, J.D.; Lachance, D.H. Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: a case series. AJNR Am J Neuroradiol 2013, 34, 2298–2303. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, L.; Tan, L.M.; Qin, L.X.; Wang, C.Y.; Zhang, H.N. Stroke-like Migraine Attacks after Radiation Therapy Syndrome. Chin Med J (Engl) 2015, 128, 2097–2101. [Google Scholar] [CrossRef]
- Pai, H.H.; Thornton, A.; Katznelson, L.; Finkelstein, D.M.; Adams, J.A.; Fullerton, B.C.; Loeffler, J.S.; Leibsch, N.J.; Klibanski, A.; Munzenrider, J.E. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys 2001, 49, 1079–1092. [Google Scholar] [CrossRef]
- Minniti, G.; Jaffrain-Rea, M.L.; Osti, M.; Esposito, V.; Santoro, A.; Solda, F.; Gargiulo, P.; Tamburrano, G.; Enrici, R.M. The long-term efficacy of conventional radiotherapy in patients with GH-secreting pituitary adenomas. Clin Endocrinol (Oxf) 2005, 62, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Kokshoorn, N.E.; Dekkers, O.M.; Neelis, K.J.; Biermasz, N.R.; Romijn, J.A.; Smit, J.W.; Pereira, A.M. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metab 2011, 96, 2330–2340. [Google Scholar] [CrossRef]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 2010, 76, S28–35. [Google Scholar] [CrossRef]
- Harris, J.R.; Levene, M.B. Visual complications following irradiation for pituitary adenomas and craniopharyngiomas. Radiology 1976, 120, 167–171. [Google Scholar] [CrossRef]
- Leavitt, J.A.; Stafford, S.L.; Link, M.J.; Pollock, B.E. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2013, 87, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Link, M.J.; Leavitt, J.A.; Stafford, S.L. Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery 2014, 75, 456–460; discussion 460. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.L.; Liao, E.A.; Trobe, J.D. Radiation-Induced Optic Neuropathy: Clinical and Imaging Profile of Twelve Patients. J Neuroophthalmol 2019, 39, 170–180. [Google Scholar] [CrossRef]
- Malik, A.; Golnik, K. Hyperbaric oxygen therapy in the treatment of radiation optic neuropathy. J Neuroophthalmol 2012, 32, 128–131. [Google Scholar] [CrossRef]
- Habets, E.J.; Taphoorn, M.J.; Nederend, S.; Klein, M.; Delgadillo, D.; Hoang-Xuan, K.; Bottomley, A.; Allgeier, A.; Seute, T.; Gijtenbeek, A.M.; et al. Health-related quality of life and cognitive functioning in long-term anaplastic oligodendroglioma and oligoastrocytoma survivors. J Neurooncol 2014, 116, 161–168. [Google Scholar] [CrossRef]
- Obara, T.; Blonski, M.; Brzenczek, C.; Mézières, S.; Gaudeau, Y.; Pouget, C.; Gauchotte, G.; Verger, A.; Vogin, G.; Moureaux, J.M.; et al. Adult Diffuse Low-Grade Gliomas: 35-Year Experience at the Nancy France Neurooncology Unit. Front Oncol 2020, 10, 574679. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Patil, C.G.; Chen, C.; Venteicher, A.S. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst Rev 2020, 1, Cd009229. [Google Scholar] [CrossRef]
- Buglione, M.; Pedretti, S.; Gipponi, S.; Todeschini, A.; Pegurri, L.; Costa, L.; Donadoni, L.; Grisanti, S.; Fontanella, M.; Liserre, R.; et al. Radiotherapy in low-grade glioma adult patients: a retrospective survival and neurocognitive toxicity analysis. Radiol Med 2014, 119, 432–439. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Afra, D.; de Witte, O.; Ben Hassel, M.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 2005, 366, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, J.A.; Kerkhof, M.; Dirven, L.; Heimans, J.J.; Reijneveld, J.C.; Taphoorn, M.J. Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro Oncol 2015, 17, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Leighton, C.; Fisher, B.; Bauman, G.; Depiero, S.; Stitt, L.; MacDonald, D.; Cairncross, G. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol 1997, 15, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Morshed, R.A.; Young, J.S.; Kroliczek, A.A.; Berger, M.S.; Brang, D.; Hervey-Jumper, S.L. A Neurosurgeon's Guide to Cognitive Dysfunction in Adult Glioma. Neurosurgery 2021, 89, 1–10. [Google Scholar] [CrossRef]
- Pallud, J.; McKhann, G.M. Diffuse Low-Grade Glioma-Related Epilepsy. Neurosurg Clin N Am 2019, 30, 43–54. [Google Scholar] [CrossRef]
- Jooma, R.; Waqas, M.; Khan, I. Diffuse Low-Grade Glioma - Changing Concepts in Diagnosis and Management: A Review. Asian J Neurosurg 2019, 14, 356–363. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).