Submitted:
12 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
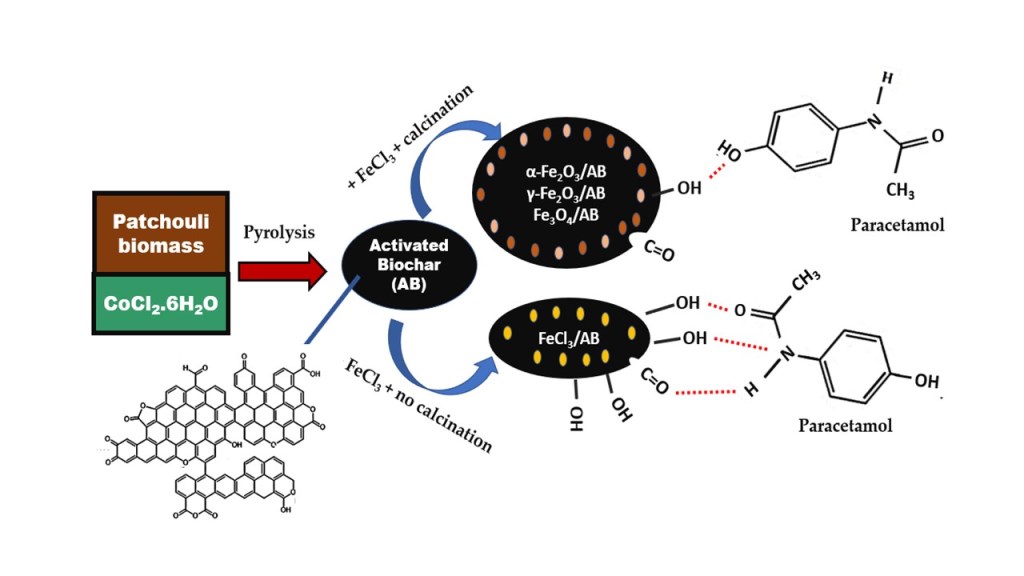
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials of Research
2.2. Preparation of Activated Biochar and Composite
- Activated biochar by pyrolysis using CoCl2 activator: Biiochar Aktivator CoCl2 100-120
- Composite by calcination of activated biochar-FeCl3 at 400oC: BIFe4
- Composite by calcination of activated biochar-FeCl3 at 600oC: BIFe6
- Composite by calcination of activated biochar-FeCl3 at 800oC: BIFe8
- Activated biochar by pyrolysis using CoCl2 activator: Biochar Co 52_39
- Activated biochar – FeCl3 before calcination: B.IFe
- Composite by calcination of activated biochar-FeCl3 at 400oC: B.IFe 400
- Composite by calcination of activated biochar-FeCl3 at 600oC: B.IFe 600
- Composite by calcination of activated biochar-FeCl3 at 800oC: B.IFe 800
2.3. Characterization of Activated Biochar and Composites
2.4. Adsorption Test
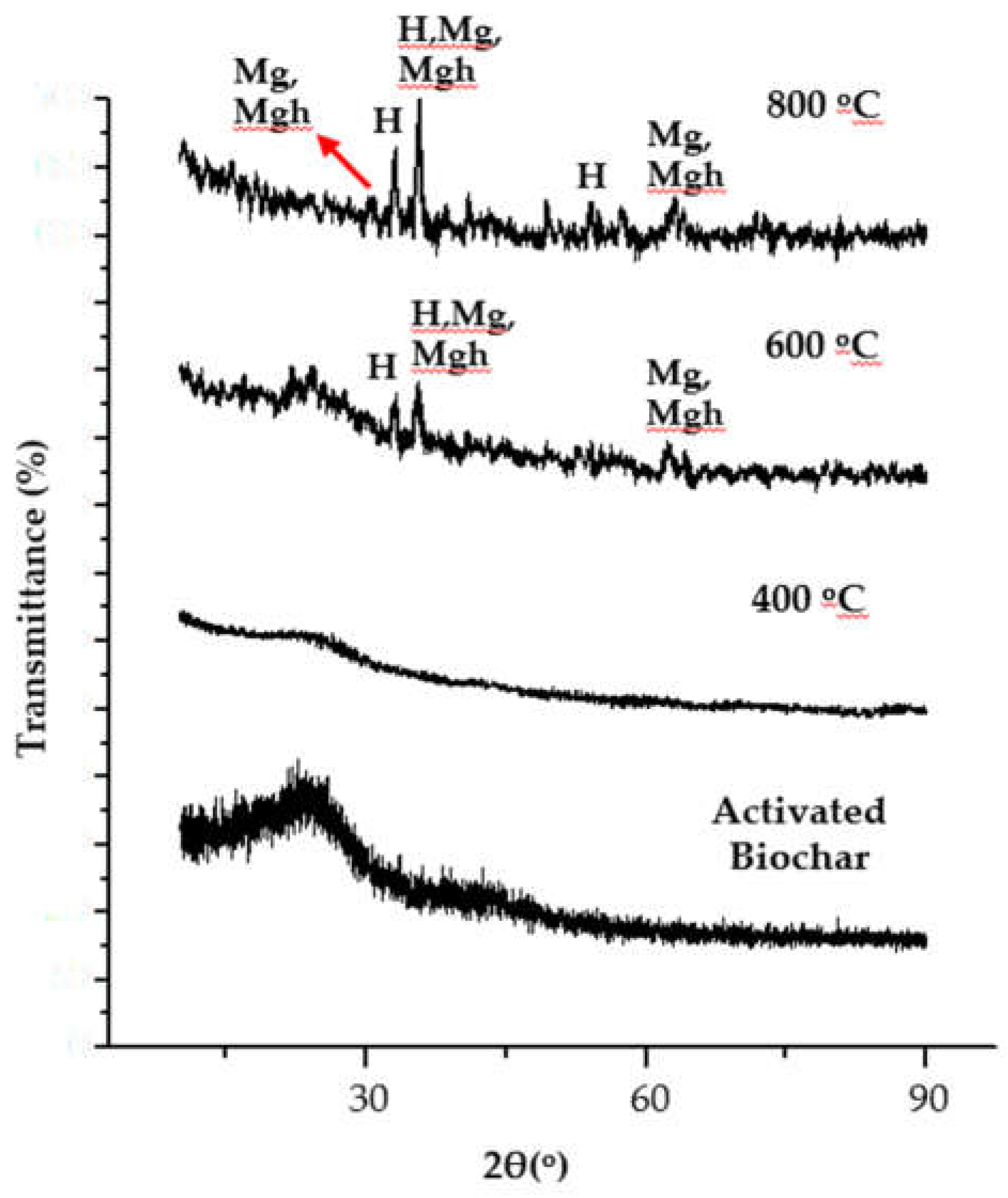
3. Results and Discussions
3.1. Crystal Structure of the Composites
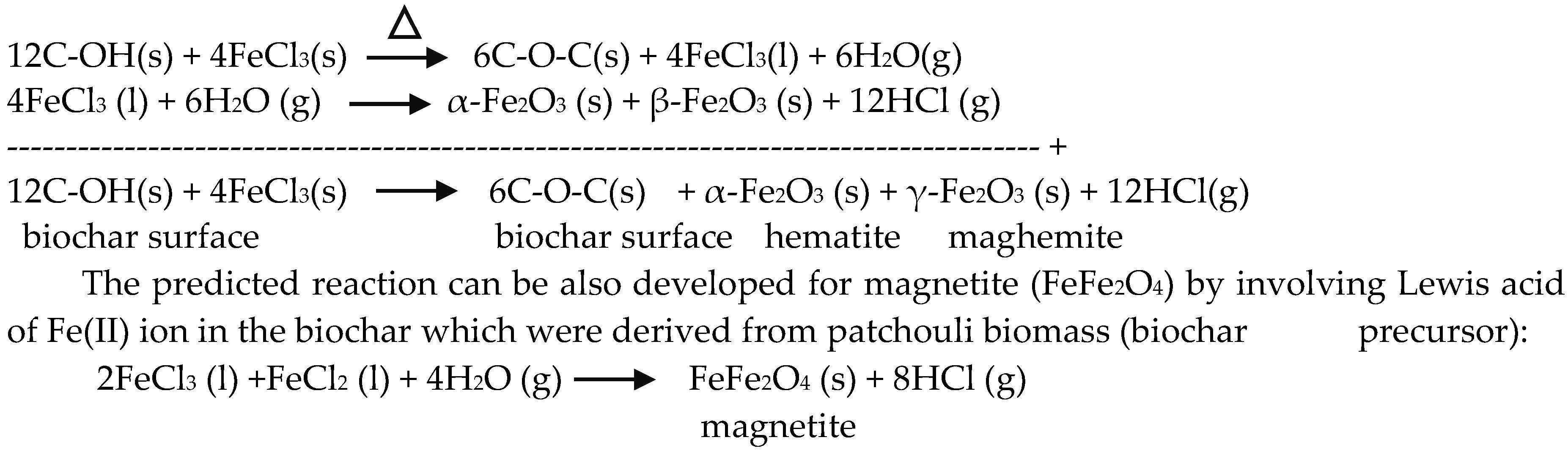
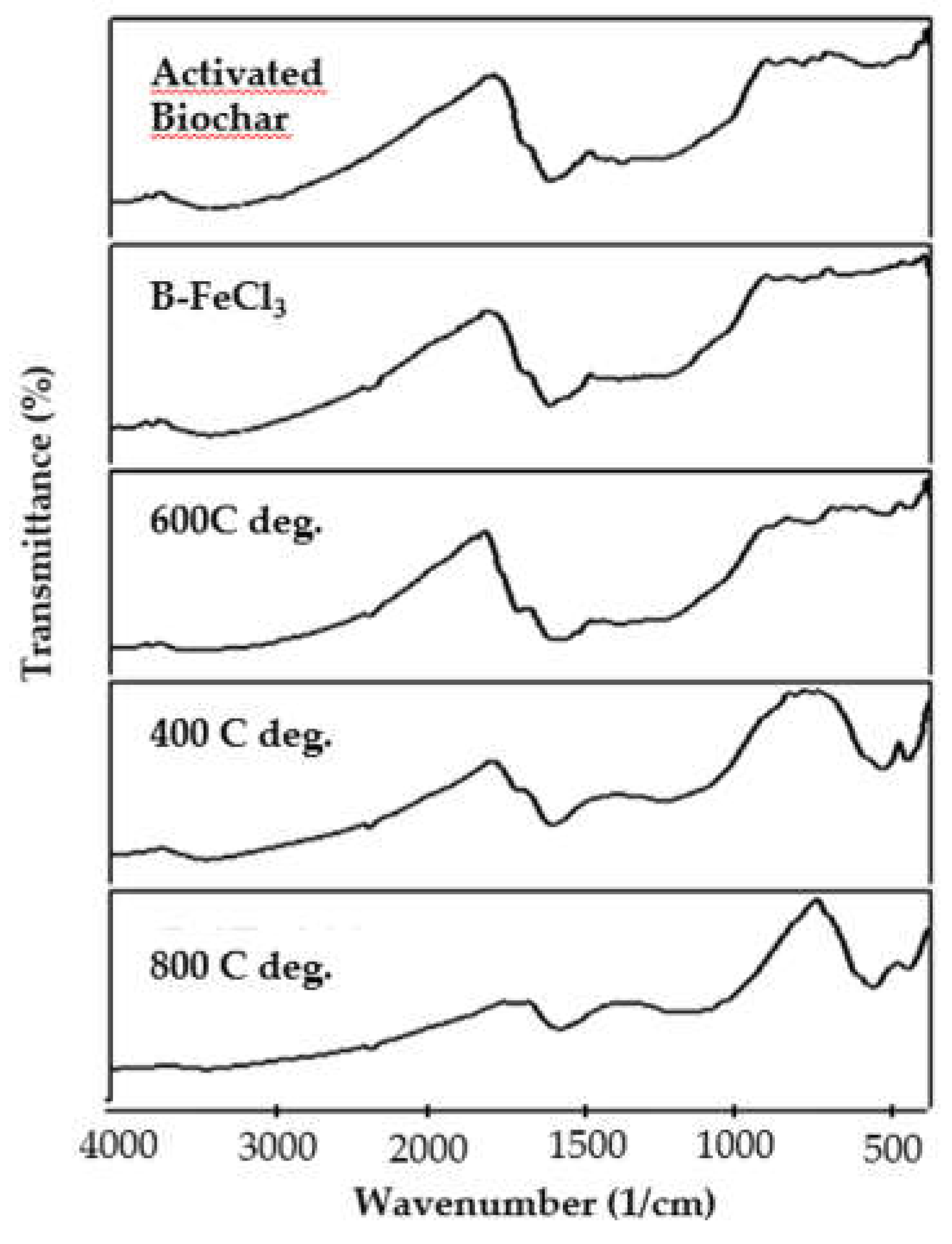
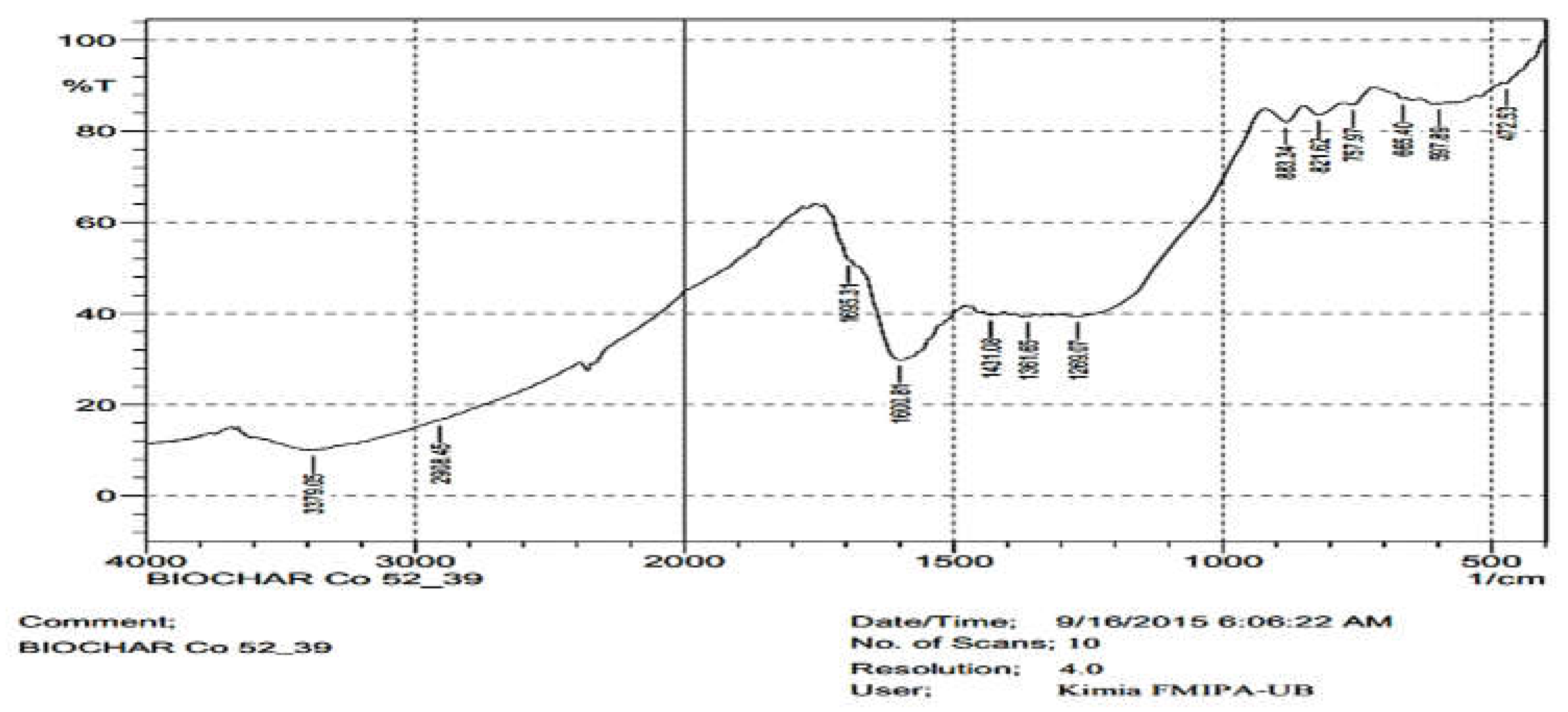
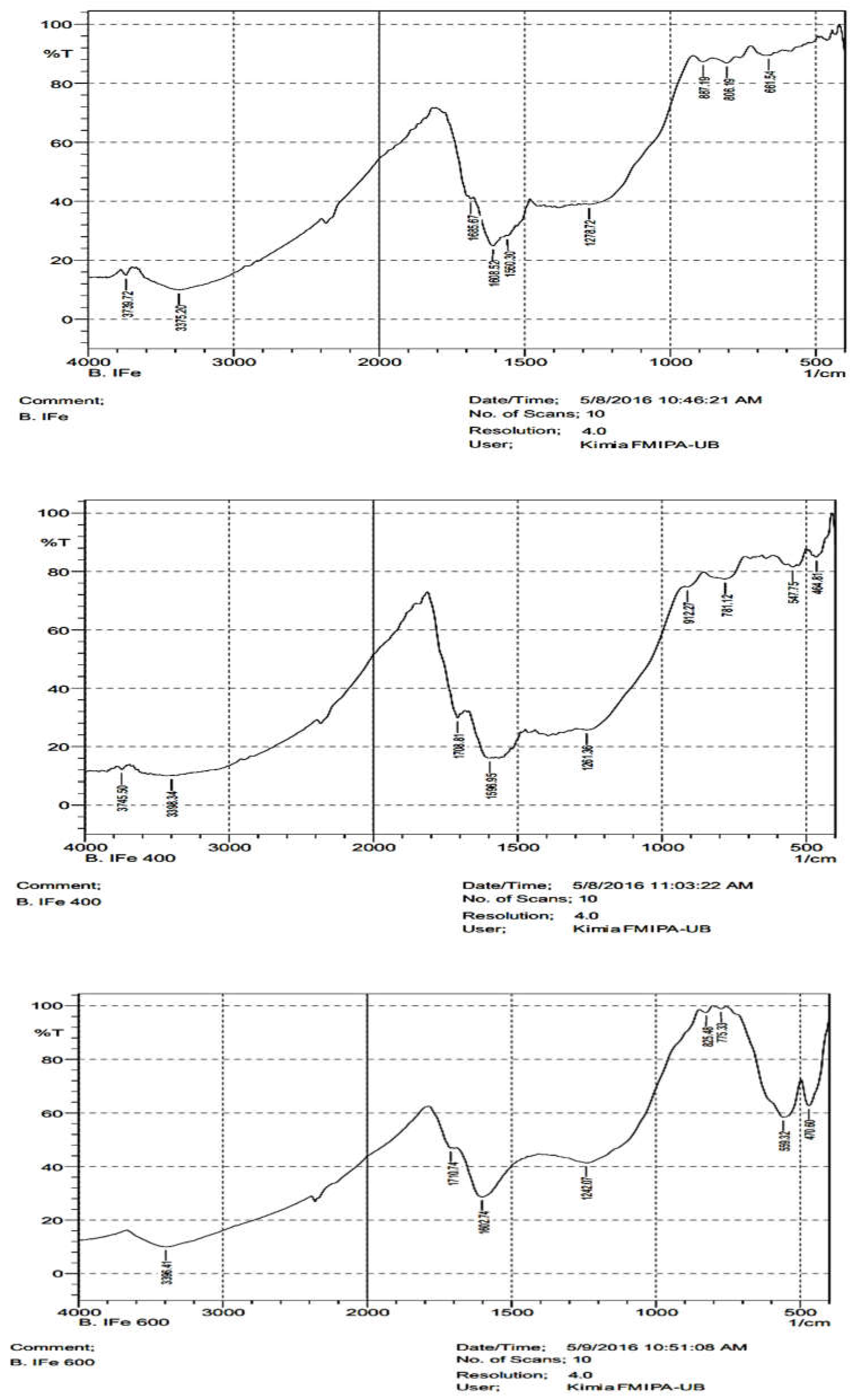
3.2. Surface Functional Group of the Composites
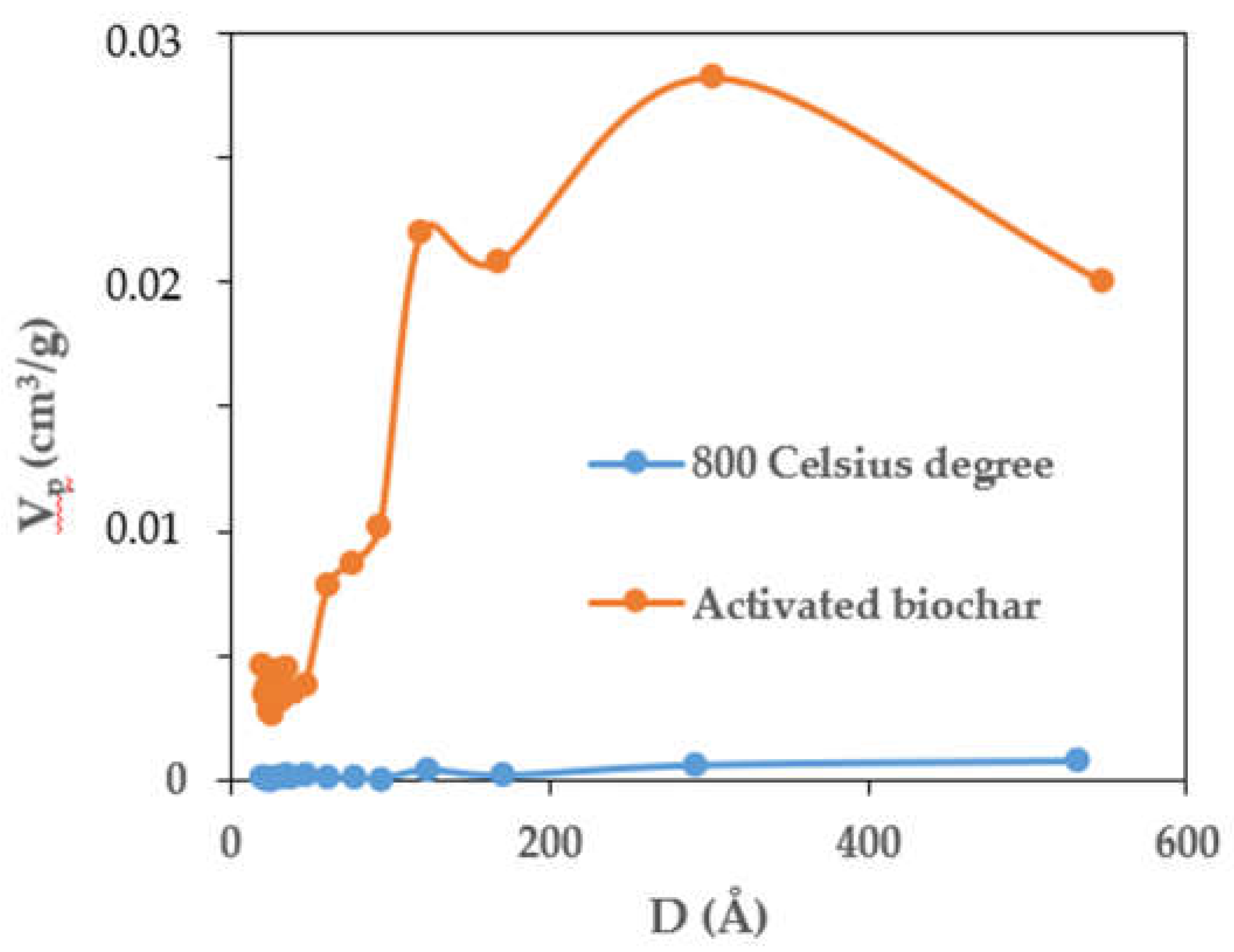
3.3. Porosity of the Composite
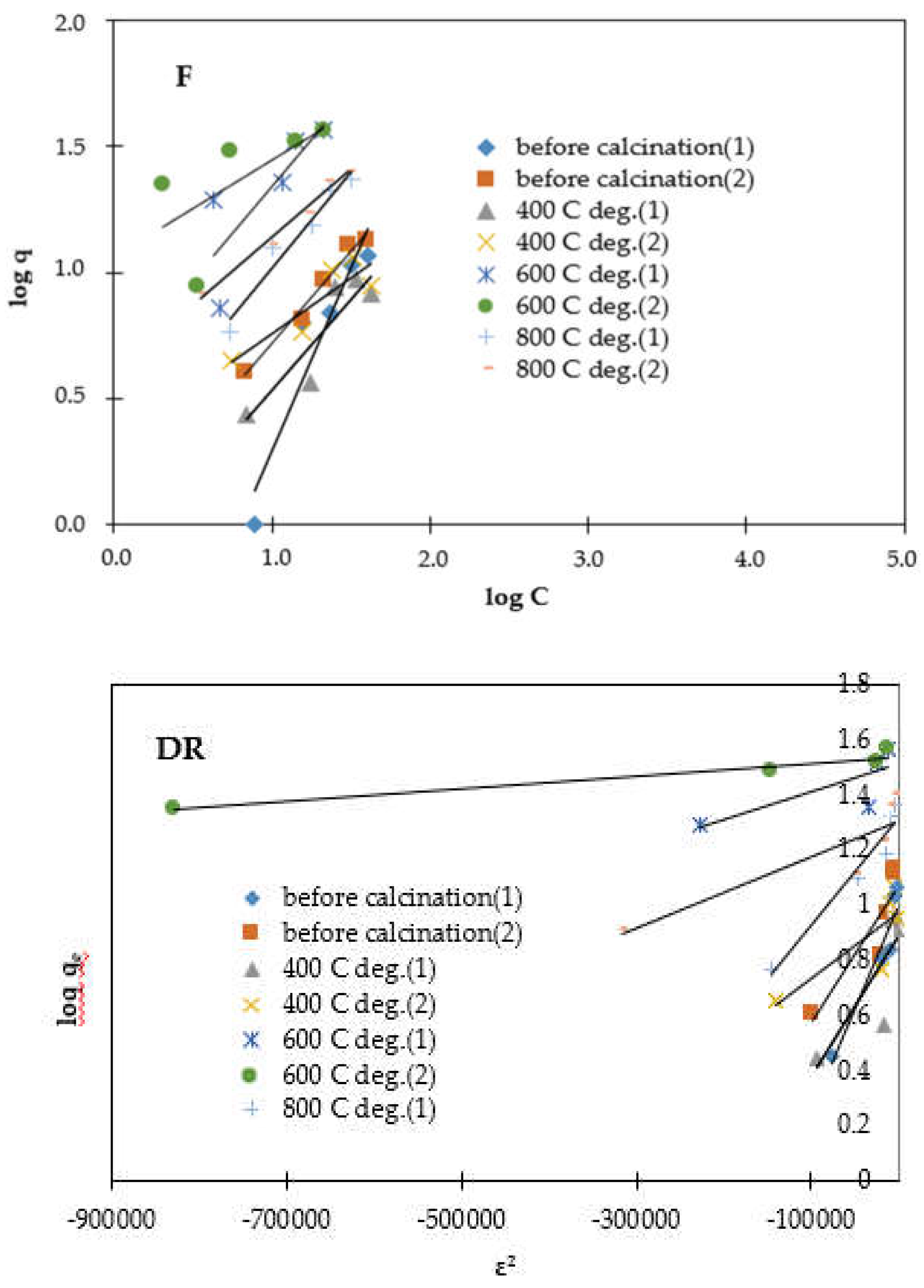
3.4. Adsorption
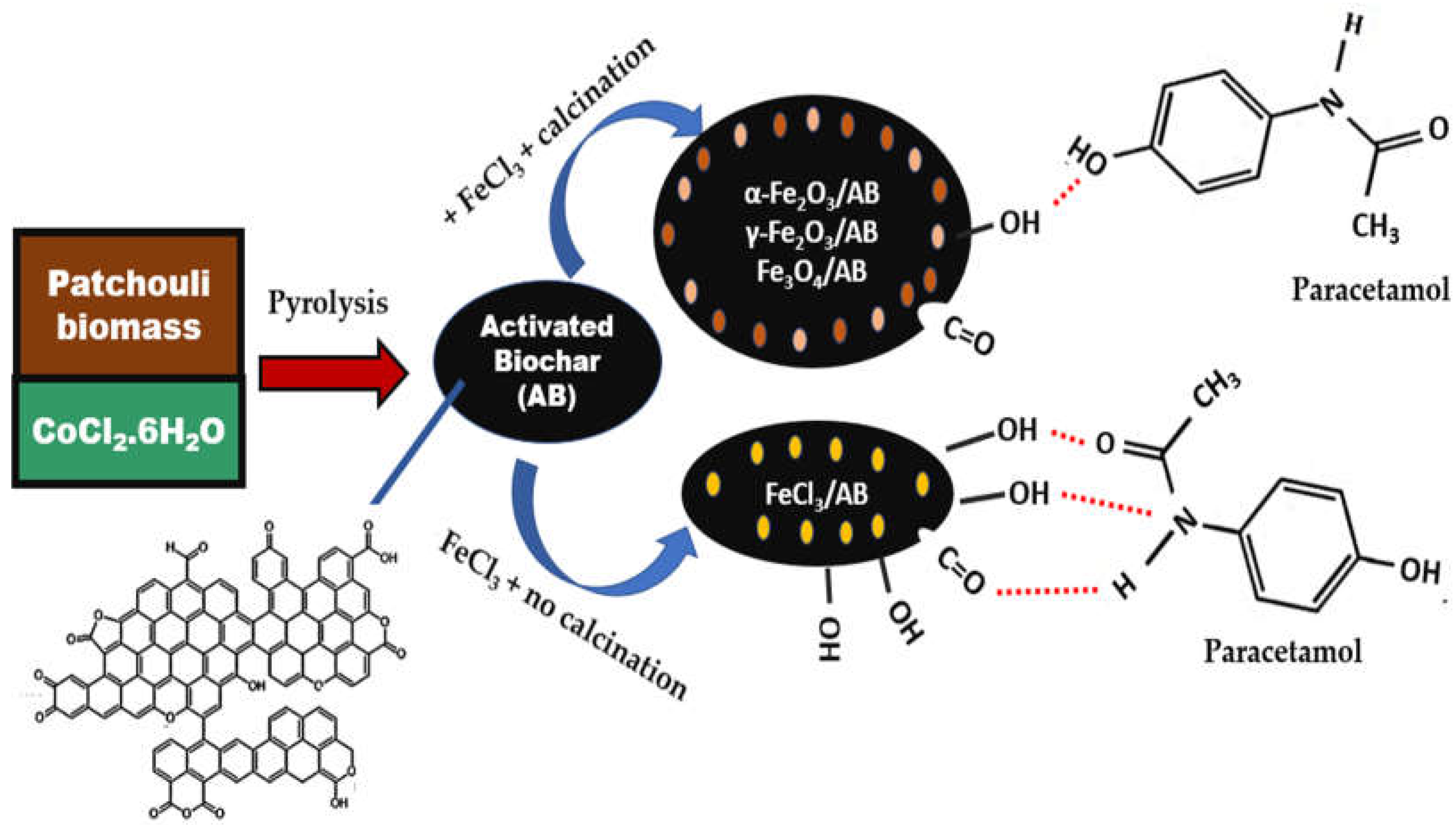
5. Conclusions
Availability Statement
Author Contributions
Funding
Acknowledgement
Conflicts of Interest
References
- Ukoba, K.; Jen, T.C. Biochar and Application of Machine Learning: A Review; Intechopen, UK, 2022; pp. 1-20. [CrossRef]
- Ernsting, A. Biochar – A Climate Smart Solution? Climate Change and Agriculture. Report 2011, 1, 1-20. http://www.cidse.org/publications/just-food/food-and-climate/download/92_e8375ee2b26c274a112f516b61278168.html.
- Agarwal, M.; Tardio, J.; Mohan, S.V. Pyrolysis Biochar from Cellulosic Municipal Solid Waste as Adsorbent for Azo Dye Removal: Equilibrium Isotherms and Kinetics Analysis. International Journal of Environmental Science and Development 2015, 6(1), 67–72. [Google Scholar] [CrossRef]
- Xiao., J.; Bi., E.; Du., B.; Zhao., X.; Xing, C. Xiao. J.; Bi. E.; Du. B.; Zhao. X.; Xing, C. Surface Characterization of Maize-Straw-derived Biochar and Their Sorption Performance for MTBE and Benzene. Environmental Earth Sciences, 2014; 71, 5195–5205. [Google Scholar] [CrossRef]
- Krismawati, A. Nilam dan Potens Pengembangannya: Kalteng Jadikan Komoditas Rintisan. Tabloid Sinar Tani 2005, 26 http://www.litbang.pertanian.go.id/artikel/one/91/pdf/Nilam%20dan%20Potensi%20Pengembangannya.pdf.
- Setianingsih, T.; Masruri; Ismuyanto, B. (UB, Malang, Jawa Timur, Indonesia). Laporan Akhir PUPT 2015: Pembuatan Komposit Biochar-Metal Berbasis Limbah Tanaman Nilam Untuk Meminimasi Kontaminan Air Dalam Menunjang Ketersediaan Air Berkelanjutam, 2015. https://www.google.co.id/books/edition/Sintesis_Fasa_Padat_Komposit_Nano_Kaolin/euPpEAAAQBAJ?hl=en&gbpv=1&dq=tutik+setianingsih+darjito&pg=PP1&printsec=frontcover.
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.-P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent Developments in Understanding Biochar’s Physical–Chemistry. Agronomy 2021, 11(615), 1–42. [Google Scholar] [CrossRef]
- Setyawan, H. Y; Sunyoto,N.M.S.; Sugiarto, Y.; Dewanti, B.S.D.; Widayanti, V.T.; Hakim, L.; Kurniawan, S.; Nugroho, G.A.; Ulandari, D.; Choirun, A.; Hanindipto,F.A.; Sundari,S.A.; Pamungkas, I.A.; Pratama,A.P.A, Wan, Z. Characterisation of biochar from various carbon sources, BIO Web of Conferences 90, 06003 (2024), ICGAB 2023, Malang, Indonesia, 24 of 23. 20 October. [CrossRef]
- Yaashikaaa,P.R., Kumara, P.S.; Varjanic, S.; Saravanan, A. Review A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnology Reports, 2020; 28, 1–15. [CrossRef]
- Fenta, A.A. State of the art of biochar in Ethiopia. A review, Heliyon 2024, 10, 1–9. [Google Scholar] [CrossRef]
- Elangovan, R.; Rangasami, S.R.S.; Murugaragavan, R.; Sekaran, N.C. Characteristics of biochar: A review, The Pharma Innovation Journal 2022; 11(12), 243-246. https://www.thepharmajournal.com/archives/2022/vol11issue12/PartC/11-10-244-971.
- Nkoh, J.N.; Baquy, M.A.-A.; Mia, S.; Shi, R.; Kamran, M.A.; Mehmood, K.; Xu, R. A Critical-Systematic Review of the Interactions of Biochar with Soils and the Observable Outcomes. Sustainability 2021, 13, 1–22. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Review Physico-chemical Properties and Microbial Responses in Biochar-amended Soils: Mechanisms and Future Directions Agriculture. Ecosystems and Environment. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Saha, P.; Bera, A,; Barman, A. A Review on Biochar and Its Application in Agriculture. Chem Sci Rev Lett 2022, 11, 184–188. [Google Scholar] [CrossRef]
- Paz-ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; and Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid Earth. 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Domingues, M.T.; Buenoa, C.C.; Watanabea, C.H.; Fracetoa, L.F.; Loyola-liceac, J.C.; Crowleyb, D.; Rosaa, A.H. Polymeric Alginate Microspheres Containing Biochar to Immobilize Phosphate Ions. Chemical EngineeringTransactions. 2014, 37, 109–111. [Google Scholar]
- Ajema, L. Effects of Biochar Application on Beneficial Soil Organism Review. International Journal of Research Studies in Science, Engineering and Technology 2018, 5, pp.9–18. [Google Scholar]
- Saputra, E.; Putu, S.; Susilowati, L.E.; Dewi, R.A.S. Populasi bakteri dan respirasi mikroba tanah pada rhizosfer tanaman jagung ( Zea mays L.) yang diberi pupuk terpadu dan biochar sekam padi pada masa vegetatif maksimum. Agroteksos, 2023; 33, 680–689. [Google Scholar] [CrossRef]
- Kandel, A. , Dahal, S. , & Mahatara, S. A review on biochar as a potential soil fertility enhancer to agriculture. Archives of Agriculture and Environmental Science 2021, 6, 108–113. [Google Scholar] [CrossRef]
- Saleh, M.E.; El-refaey, A.E.; and Mahmoud, A.H. Effectiveness of Sunflower Seed Husk Biochar for Removing Copper Ions from Wastewater: A Comparative Study. Soil & Water Research 2016, 11, 53–63. [Google Scholar] [CrossRef]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and Characterization of A Novel MnOx-loaded Biochar and Its Adsorption Properties for Cu2+ in Aqueous Solution. Chemical Engineering Journal 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Yu, X.; Qin, A.; Liao, L. ; Du,R.; Tian,N.; Huang,S.; and Chunwe. Removal of Organic Dyes by Anostructure ZnO-Bamboo Charcoal Composites with Photocatalysis Function. Advances in Materials Science and Engineering, 2015; 1–6. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Gu, Y.; Xu, Y.; Zeng, G.; Hu, X.; Liu, S.; Wang, X.; Liu, S.; Li, J. Biochar-based nano - composites for the decontamination of waste. water: A review, Bioresource Technology 2016, 212, 318–333. [Google Scholar]
- Moosavi, E.; Dastgheib, S.; and Karimzadeh, R. Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. Energies 2012, 5, 4233–4250. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, B.S.; Pallavkar, S.; Ho, T.C.; Hopper, J.; Guo, G. Magnetic Nanocomposites for Environmental Remediation. Advanced Powder Technology 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Ahuja, Kalia, R. A.; Sikka, A.R.; Chaitra, P. Nano Modifications of Biochar to Enhance Heavy Metal Adsorption from Wastewaters: A Review, ACS Omega 2022, 7, 45825–45836. [CrossRef]
- Wang, M.C.; Sheng, G.D.; Qiu, Y.P.A. Novel Manganese-oxide/Biochar Composite for Efficient Removal of Lead(II) from Aqueous Solutions. International Journal of Environmental Science Technology 2015, 12, 1719–1726. [Google Scholar] [CrossRef]
- Venkatesh, R.; Sekaran, P.R. ; Udayakumar,K.; Jagadeesh, D.; Raju, K.; Bay, M.B. Adsorption and Photocatalytic Degradation Properties of Bimetallic Ag/MgO/Biochar Nanocomposites, Hindawi Adsorption Science & Technology 2022, 2022, 1-14. [CrossRef]
- Han, Z.; Sani, B.; Mrozik, W.; Obst, M.; Beckingham, B.; Karapanagioti, H.K. , Werner D. Magnetite Impregnation Effects on the Sorbent Properties of Activated Carbons and Biochars. Water Research 2015, 70(1), 394–403. [Google Scholar] [CrossRef] [PubMed]
- Weidner, E.; Karbassiyazdi, E.; Altaee, A.; Jesionowski, T. Ciesielczyk, F. Hybrid Metal Oxide/Biochar Materials for Wastewater Treatment Technology: A Review. ACS Omega 2022, 7, 27062–27078. [Google Scholar] [CrossRef]
- Liang, H.; Zhu, C.; Wang, A.; Chen, F. Facile preparation of NiFe2O4/biochar composite adsorbent for efcient adsorption removal of antibiotics in water, Carbon Research 2024, 3(2), 1-13. [CrossRef]
- Wang, L.; Ok, Y.S. ; Tsang, DCW; Alessi, D. S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes, and sustainability implications, Soil Use and Management 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Halász, L.; Vincze, A.; Solymosi, J. Use of The Microwave Impregnation of Active Carbon. Arms Technology 2008, 7, 533–550. [Google Scholar]
- Fang, C.; Zhang, T.; Jiang, L. R.; and Wang, Y. Application of Magnesium Modified Corn Biochar for Phosphorus Removal and Recovery from Swine Wastewate. International Journal of Environmental Research and Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer Nanocomposites Based on unctionalized Carbon Nanotubes, Progress in Polymer Science 2010, 35, 837-867. https://www.researchgate.net/publication /221950822_PolymerNanocomposites_ Based_on_Functionalized_Carbon_Nanotubes.
- Ilomuanya, M.; Ohere, A.F.; Zubair, S.A.; and Ukoma, U.U. Evaluation of adsorption capacity of acetaminophen on activated charcoal dosage forms available in Nigeria by in vitro adsorption studies and scanning electron microscopy. Tropical Journal of Pharmaceutical Research 2017, 16, 1105–1112. [Google Scholar] [CrossRef]
- Mohd, N.; Sudirman, M.F.A.E.; and Draman, S.F.S. Isotherm and Thermodynamic Study of Paracetamol Removal in Aqueous Solution by Activated Carbon. Journal of Engineering and Applied Sciences 2015, 10(20), 1819-6608. https://www.arpnjournals.org/jeas/research_papers/rp_2015/jeas_1115_2903.pdf.
- Ferreira, R.C.; Junior, O.M.C.; Carvalho, K.Q.; Arroyo, P.A.; and Barrosa, M.A.S.D. Effect of Solution pH on the Removal of Paracetamol by Activated Carbon of Dende Coconut Mesocarp, Chem. Biochem. Eng. Q. 2015, 29, 47–53. [Google Scholar] [CrossRef]
- Ferreira, R.C.; De Lima, H.H.C.; Cândido, A.A.; Junior, O.M.C.; Arroyo, P.A.; De Carvalho, K.Q.; Gauze, G.F.; Barros, M.A.S.D. Adsorption of Paracetamol Using Activated Carbon of Dende and Babassu Coconut Mesocarp. World Academy of Science, Engineering and Technology International Journal of Biotechnology and Bioengineering 2015, 9, 717–722. [Google Scholar]
- Jedynak, K.; Charmas, B. Adsorption properties of biochars obtained by KOH activation. Adsorption 2024, 30, 167–183. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, L.; Guo, J.; Wang, Y.; Ji, l.; Song, W. Preparation of Bi2MoO6/kelp biochar nanocomposite for enhancing degradability of methylene blue. Applied Ecology and Environmental Research 2018, 16(5), 5837–5847. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Miao,J.; Liu,Q.; Cai, J.; An,X.; Chen, F.; Cheng,L.; Chen, W.; Luo, H.; Zhang, X.; Zhang, K.; Ma, D. Na4P2O7-Modified Biochar Derived from Sewage Sludge: Effective Cu(II)-Adsorption Removal from Aqueous Solution. Adsorption Science & Technology 2023, Article ID 8217910, 1-15. [CrossRef]
- Khalil, M.M.; Afifi, A.A.; Mohamed, R.F. Synthesis, Characterization and Analytical Applications of Biochar Nanocomposites for Decontamination of Kohafawastewater Treatment Plants, Fayoum, Egypt. Plant Archives 2019, 19(2), 4559-4564. https://cabidigitallibrary.org by 203.78.117.254.
- Alwar, A.; Ahdiaty, R.; Doong, R. magnetic Fe3O4-CuO/biochar nanocomposite for adsorption of inorganic anions from aqueous solution. Rasayan J. Chem. 2022, 15(4), 2466–2476. [Google Scholar] [CrossRef]
- Wang, K.; Remón, J.; Jiang, Z.; Ding, W. Recent Advances in the Preparation and Application of Biochar Derived from Lignocellulosic Biomass: A Mini Review. Polymers 2024, 16, 851. [Google Scholar] [CrossRef]
- Díaz, B.; Sommer-Márquez, A.; Ordoñez, P.E.; Bastardo-González, E.; Ricaurte, M.; Navas-Cárdenas, C. Synthesis Methods, Properties, and Modifications of Biochar-Based Materials for Wastewater Treatment: A Review. Resources 2024, 13, 8. [Google Scholar] [CrossRef]
- Setianingsih, T. ; Masruri; Ismuyanto, B. Effect of Calcination Temperature on Structural Properties of Biochar-MCln Composite from Patchouli Biomass and Its Application for Drug Adsorption, International Journal of ChemTech Research 2016, 9, 610–621. [Google Scholar]
- Setianingsih, T., Masruri; Ismuyanto, B. Influence of Impregnation Ratio on physicochemistry of patchouli biochar using CoCl2 chemical activator for adsorption of drug pollutants, International Journal of Civil Engineering and Technology (IJCIET) 2017, 8(5), 709–716. https://iaeme.com/MasterAdmin/Journal_uploads/IJCIET/VOLUME_8_ISSUE_5/IJCIET_08_05_079.pdf.
- Setianingsih, T. ; Masruri; Ismuyanto, B. Study of Pyrolysis Temperature Influence on Physicochemistry of Patchouli Biochar and Patchouli pyrolysis Reaction Using CoCl2 Chemical Activator. J. Mater. Environ. Sci. 2021, 12, 787–797. [Google Scholar]
- Setianingsih, T. ; Masruri; Ismuyanto, B. Study of Chemical Activator in Preparation of Biochar Adsorbent from Patchouli Biomass for Removing Drug Contaminan. International Journal of ChemTech Research 2017, 10, 10–19. [Google Scholar]
- Setianingsih, T. ; Masruri; Ismuyanto, B. Synthesis of Patchouli Biochar Cr2O3 Composite Using Double Acid Oxidators for Paracetamol Adsorption. J. Pure App. Chem. Res. 2018, 7, 60–69. [Google Scholar] [CrossRef]
- Setianingsih, T. ; Masruri; Ismuyanto, B. Study of salt oxidator type influence on physichochemistry of patchouli biochar–Cr2O3 composite and organic contaminant adsorption. International Journal of Civil Engineering and Technology (IJCIET) 2021, 12, 17–28. [Google Scholar] [CrossRef]
- Setianingsih, T.; Masruri; Ismuyanto, B. Biochar dan Fungsionalisasi Biochar, UB Press, Malang, Indonesia, 2018; 93-111. https://books.google.co.id/books/about/Biochar_dan_Fungsionalisasi_Biochar.html?id=snLcDwAAQBAJ&redir_esc=y.
- Setianingsih, T. ; Masruri; Ismuyanto, B. (UB, Malang, Jawa Timur, Indonesia). Laporan Akhir PUPT 2016: Pembuatan Komposit Biochar-Metal Berbasis Limbah Tanaman Nilam Untuk Meminimasi Kontaminan Air Dalam Menunjang Ketersediaan Air Berkelanjutam, 2016. [Google Scholar]
- Li, D. , Tian, Y., Qiao, Y. Forming Active Carbon Monoliths from H3PO4 – loaded Sawdust with Addition of Peanut Shell Char. BioResources 2014, 9, 4981–4992. [Google Scholar]
- Manoj, K. and Kunjomana, A. G. Study of Stacking Structure of Amorphous Carbon by X-ray Diffraction Technique, International Journal of Electrochemical Science 2012, 7, 3127–3134. [Google Scholar]
- Setianingsih, T.; Darjito; Kamulyan, B. Sintesis Senyawa Anorganik dengan Metode Fasa Padat in Sintesis fasa Padat Komposit Nano Kaolin CNS Termodifikasi Fe(III) dan Zn(II) dengan Tanur Microwave; MNC Publishing: Malang, Indonesia, 2023; volume 1, pp. 20-22.
- Zhu, W.; Winterstein, J.; Maimon, I.; Yin, Q.; Yuan, L.; Kolmogorov, A.N.; Sharma, R.; Zhou, G. Atomic Structural Evolution during the Reduction of α-Fe2O3 Nanowires, J Phys Chem C Nanomater Interfaces 2016, 120(27), 14854–14862. [CrossRef]
- Setianingsih, T.; Kartini, K.; Arryanto, Y. Sintesis Karbon Mesopori Dari Fruktosa Dengan Menggunakan Aktivator Seng Borosilikat. Disertasi. Program Studi S3 Ilmu Kimia, FMIPA, UGM, Jogjakarta. https://etd.repository.ugm.ac.id/penelitian/detail/94598.
- Zhang, C. Porous biochar material for carbon capture and wastewater treatment. PhD Thesis, School of Chemistry and Chemical Engineering of Queen’s University Belfast, Belfast, United Kingdom. https://pureadmin.qub.ac.uk/ws/portalfiles/portal/491036656/PhD_Thesis_Chen_Zhang_Pure.pdf.
- Chayande. SP. and Yenkie, M.K.N. Chayande. SP. and Yenkie, M.K.N. Characterization of Activated Carbon Prepared From Almond Shells For Scavenging Phenolic Pollutants, Chemical Science Transaction 2013, 2(3): 835-840 http://www.e-journals.in/pdf/V2N3/835-840.
- Sahira, Mandira, A. Prasad, P.B. and Ram, P.R. Effects of Activating Agents on the Activated Carbons Prepared from Lapsi Seed Stone. Research Journal of Chemical and Environmental Sciences 2013, 3, 19–24. [Google Scholar]
- Lakshmi, P.K. Jayashree, M. Shakila, B.K., Gino, A.K. Green and Chemically Synthesized Copper Oxide Nanoparticles-A Preliminary Research Towards Its Toxic Behavior. International Journal of Pharmacy and Pharmaceutical Sciences 2015. 17(1), 156-150 http://innovareacademics.in/journals/index.php/ijpps/article/view/3868.
- Lowell, S.; Shields, J.E. Powder Surface Area and Porosity. 3nd ed. Chapman and Hall Ltd, New York, 1991 https://www.google.co.id/books/edition/Powder_Surface_Area_and_Porosity/mCz4CAAAQBAJ?hl=engbpv=1.
- Hamid, S.B.A. Chowdhury, Z.Z. and Zain, S.M. Base Catalytic Approach: A Promising Technique for the Activation of Biochar for Equilibrium Sorption Studies of Copper, Cu(II) Ions in Single Solute System. Materials 2014, 7: 2815-2832 https://www.mdpi.com/1996-1944/7/4/2815/pdf.
- Kyzioł-Komosińska, J. Rosik-Dulewska, C. Franus, M. Antoszczyszyn-Szpicka, P. Czupioł, J. Krzyżewska, I. Sorption Capacities of Natural and Synthetic Zeolites for Cu(II) Ions. Polish Journal of Environmental Studies 2015, 24, 1111–1123. [Google Scholar] [CrossRef]
- Mohammed, R.R. Decolorisation of Biologically Treated Palm Oil Mill Effluent (POME) Using Adsorption Technique, International Refereed Journal of Engineering and Science 2013, 2(2): 01-11 http://www.irjes.com/Papers/vol2-issue10/Version%20%201/A02100111.
- Ismadji, S., Tong, D.S., Edi, F., Soetaredjo, Ayucitra, A., Yu, W.H., Zhou, C.H. Bentonite-hydrochar Composite for Removal of Ammonium from Koi Fish Tank. Applied Clay Science 2015, 114, 467–475 http://ac.els-cdn.com/S0169131715300077/1-s2.0-S0169131715300077-main.pdf?_tid=ad641c86-9b31-11e6-820700000aacb361&acdnat=1477455014_c7b912891ee5da0008e5962 cc3c15c22.
- Olalekan, A.P. Dada A. O. Okewale A.O. Comparative Adsorption Isotherm Study of The Removal of Pb2+ and Zn2+ Onto Agricultural Waste. Research Journal of Chemical and Environmental Sciences 2013, 1, 22–27. [Google Scholar]
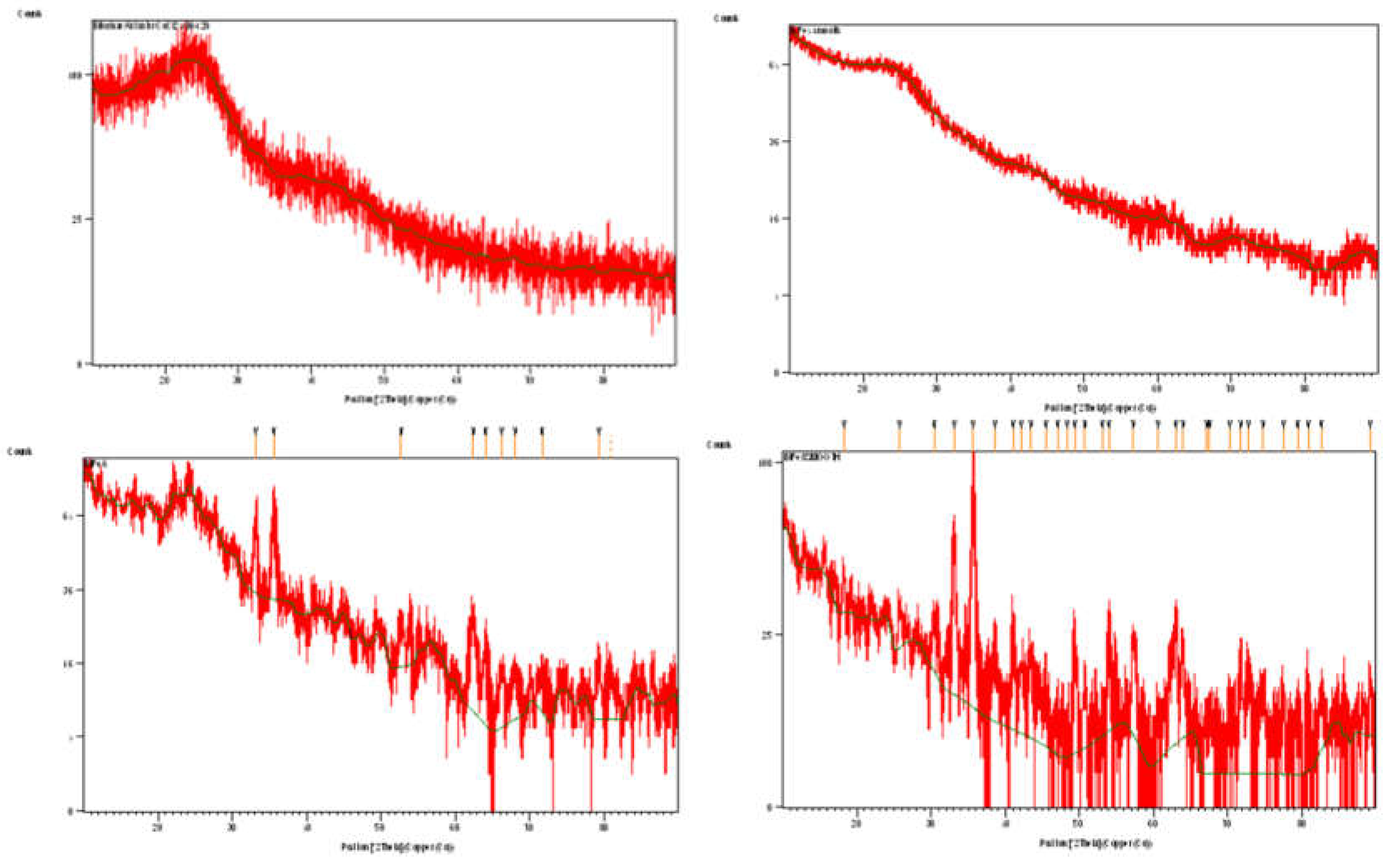
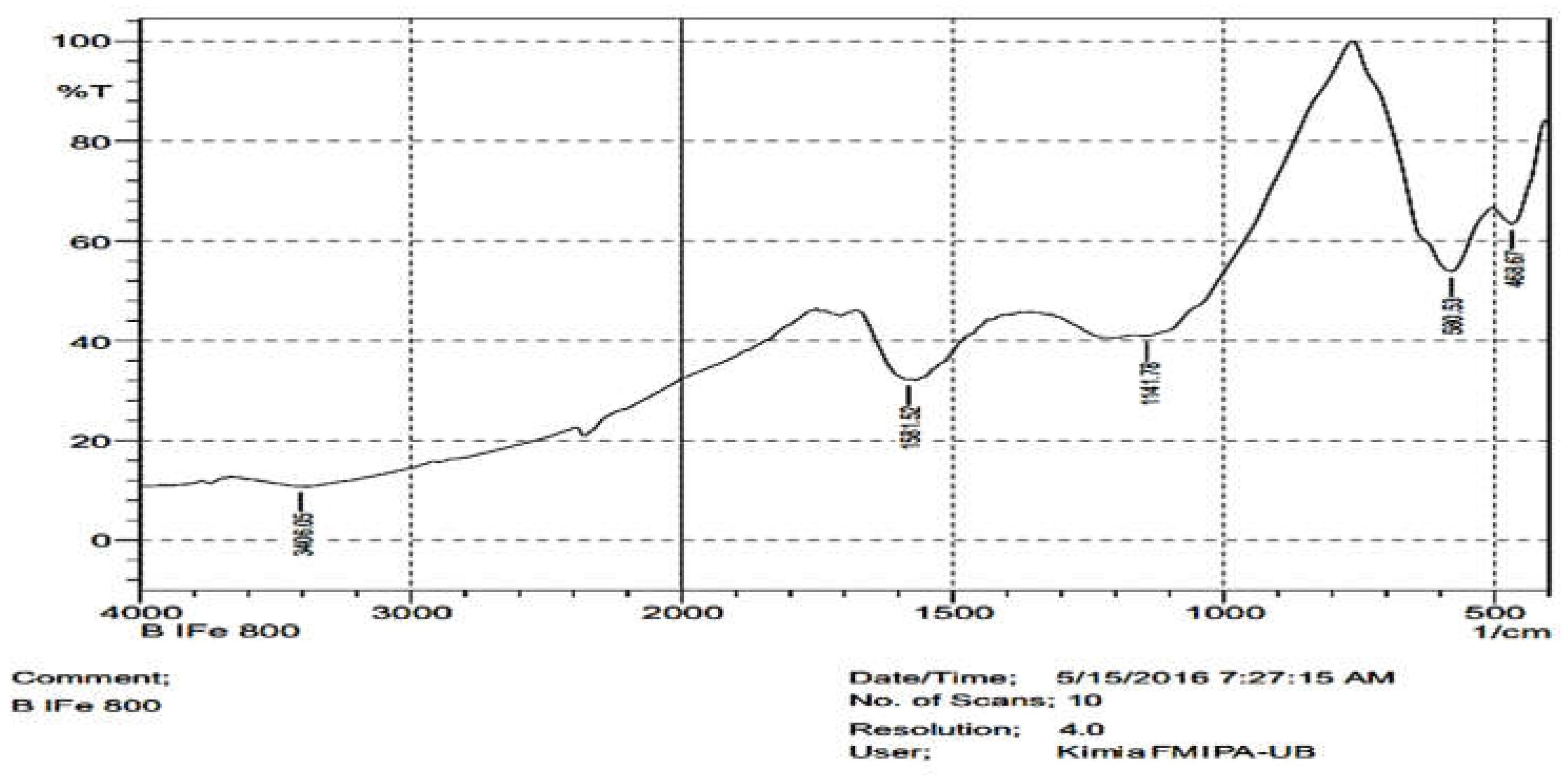
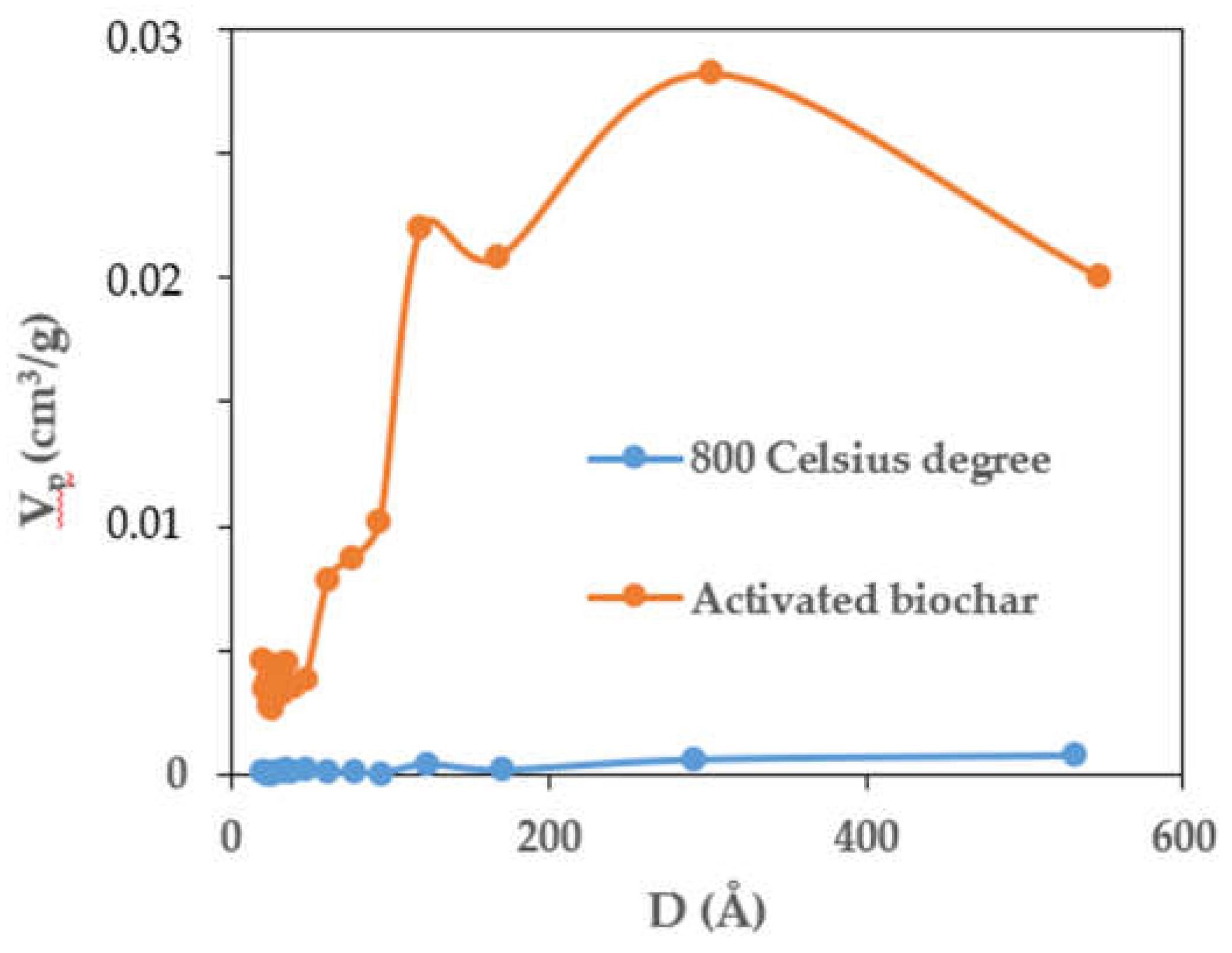

| 600 oC | 800 oC | AMCSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (α - Fe2O3/ hematite)……….. | ||||||||||
| 2Ɵ | d(Å) | I(cts) | 2Ɵ | d(Å) | I(cts) | d(Å) | IR | hkl | ||
| 33.11 | 2.71 | 27.34 | 33.11 | 2.71 | 49.97 | 2.70 | 100.00* | 104 | ||
| 35.51 | 2.53 | 34.03 | 35.60 | 2.52 | 77.34 | 2.52 | 74.38* | 110 | ||
| 40.98 | 2.20 | 18.64 | 2.21 | 22.00 | 113 | |||||
| 43.36 | 2.09 | 16.22 | 2.08 | 2.02 | 202 | |||||
| 45.36 | 2.00 | 8.49 | 2.08 | 2.02 | 202 | |||||
| 47.13 | 1.93 | 3.56 | 1.84 | 38.07 | 024 | |||||
| 49.45 | 1.84 | 20.78 | 1.84 | 39.00 | 24 | |||||
| 52.79 | 1.73 | 8.91 | 54.08 | 1.70 | 23.99 | 1.70 | 46.28* | 116 | ||
| 57.33 | 1.61 | 20.08 | 1.60 | 10.00 | 122 | |||||
| 62.44 | 1.49 | 19.70 | 63.08 | 1.47 | 27.96 | 1.49 | 31.84 | 214 | ||
| 64.08 | 1.45 | 14.51 | 63.98 | 1.47 | 17.68 | 1.45 | 30.00 | 300 | ||
| 67.15 | 1.39 | 5.09 | 1.35 | 3.18 | 208 | |||||
| 70.31 | 1.34 | 6.94 | 1.35 | 3.18 | 208 | |||||
| 71.66 | 1.32 | 5.94 | 71.78 | 1.31 | 15.08 | 1.31 | 11.48 | 1010 | ||
| 72.79 | 1.30 | 13.55 | 1.31 | 1.73 | 119 | |||||
| 74.74 | 1.27 | 10.36 | 1.26 | 7.50 | 220 | |||||
| 77.51 | 1.23 | 8.37 | 1.23 | 1.26 | 306 | |||||
| 79.28 | 1.21 | 9.20 | 79.42 | 1.21 | 4.51 | 1.21 | 1.01 | 223 | ||
| 80.90 | 1.19 | 8.10 | 80.85 | 1.19 | 8.82 | 1.19 | 1.68 | 312 | ||
| 82.60 | 1.17 | 7.45 | 1.16 | 6.13 | 0210 | |||||
| 89.15 | 1.10 | 10.95 | 1.10 | 8.61 | 226 | |||||
| 600 oC | 800 oC | AMCSD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (γ - Fe2O3/maghemite) | |||||||||
| 2Ɵ | d (Å) | I(cts) | 2Ɵ | d (Å) | I(cts) | d(Å) | IR | hkl | |
| 30.36 | 2.94 | 14.99 | 2.94 | 33.71* | 220 | ||||
| 35.51 | 2.53 | 34.03 | 35.60 | 2.52 | 77.34 | 2.51 | 100* | 311 | |
| 43.36 | 2.09 | 16.22 | 2.08 | 16.89 | 400 | ||||
| 52.79 | 1.73 | 8.91 | 1.70 | 11.16 | 422 | ||||
| 54.08 | 1.70 | 23.99 | 1.70 | 11.16 | 422 | ||||
| 57.33 | 1.61 | 20.08 | 1.60 | 21.09 | 511 | ||||
| 62.44 | 1.49 | 19.70 | 63.08 | 1.47 | 27.96 | 1.47 | 40.17* | 440 | |
| 71.66 | 1.32 | 5.94 | 71.78 | 1.31 | 13.55 | 1.32 | 4.00 | 620 | |
| 74.74 | 1.27 | 10.36 | 1.27 | 8.18 | 533 | ||||
| 80.90 | 1.19 | 8.10 | 79.42 | 1.21 | 4.51 | 1.20 | 2.02 | 444 | |
| 600 oC | 800 oC | AMCSD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (α – Fe3O4/magnetite) | |||||||||
| 2Ɵ | d (Å) | I(cts) | d (Å) | IR | hkl | ||||
| 30.36 | 2.94 | 14.99 | 2.97 | 28.10* | 220 | ||||
| 35.51 | 2.53 | 34.03 | 35.60 | 2.52 | 77.34 | 2.53 | 100.00* | 311 | |
| 43.36 | 2.09 | 16.22 | 2.10 | 20.13 | 400 | ||||
| 52.79 | 1.73 | 8.91 | 54.08 | 1.70 | 23.99 | 1.71 | 9.59 | 422 | |
| 57.33 | 1.61 | 20.08 | 1.62 | 6.40 | 333 | ||||
| 62.44 | 1.49 | 19.70 | 63.08 | 1.47 | 27.96 | 1.48 | 41.80* | 440 | |
| 71.66 | 1.32 | 5.94 | 71.78 | 1.31 | 15.08 | 1.33 | 3.54 | 620 | |
| 74.74 | 1.27 | 10.36 | 1.28 | 8.82 | 533 | ||||
| 79.28 | 1.21 | 9.20 | 79.42 | 1.21 | 4.51 | 1.21 | 2.64 | 444 | |
| 89.15 | 1.10 | 10.95 | 1.09 | 5.54 | 553 | ||||
| 600 oC | 800 oC | AMCSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (FeCl3 /molysite) | ||||||||||
| 2Ɵ | d(Å) | I(cts) | 2Ɵ | d(Å) | I(cts) | d(Å) | I(cts) | hkl | ||
| 5.81 | 58.45* | 003 | ||||||||
| 5.03 | 36.21* | 101 | ||||||||
| 30.36 | 2.94 | 14.99 | 2.90 | 4.25 | 006 | |||||
| 33.11 | 2.71 | 27.34 | 33.11 | 2.71 | 49.97 | 2.69 | 94.03* | 213 | ||
| 42.07 | 2.15 | 11.98 | 2.10 | 4.01 | 205 | |||||
| 50.81 | 1.80 | 9.18 | 1.81 | 2.91 | 314 | |||||
| Sample | Vp | SBET | D | Vmeso* | Smeso* | Vmicro** | Smicro** | Vmacro*** | Smacro*** |
|---|---|---|---|---|---|---|---|---|---|
| cm3/g | m2/g | nm | cm3/g | m2/g | cm3/g | m2/g | cm3/g | m2/g | |
| Composite | 0.140 | 7.113 | 78.598 | 0.002 | 1.602 | 0.138 | 5.510 | 0.004 | 0.140 |
| Activated Biochar | 0.330 | 471.672 | 27.980 | 0.137 | 87.096 | 0.193 | 384.580 | 0.065 | 2.730 |
| Model |
Parameter | No calcination | 400 oC |
600 oC |
800 oC |
|---|---|---|---|---|---|
| (FeCl3/AB) | (FexOy/AB) | (FexOy/AB) | (FexOy/AB) | ||
| Freundlich | R2 (F) * | 0.952 | 0.794 | 0.903 | 0.970 |
| n | 4.32 | 1.83 | 1.99 | 1.58 | |
| Langmuir | R2 (L) * | 0.975 | 0.827 | 0.867 | 0.964 |
| qm (mg/g) | 39.97 | 13.27 | 39.23 | 56.37 | |
| RL | 0.953 | 0.957 | 0.939 | 0.861 | |
| Dubinin R. | R2 (DR) * | 0.953 | 0.957 | 0.939 | 0.861 |
| E (KJ/mol) | 0.283 | 0.348 | 0.427 | 0.530 | |
| qs (mg/g) | 11.97 | 9.91 | 39.22 | 20.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




