Submitted:
07 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HPV and BLV Detection
2.3. Statistical Analysis
3. Results
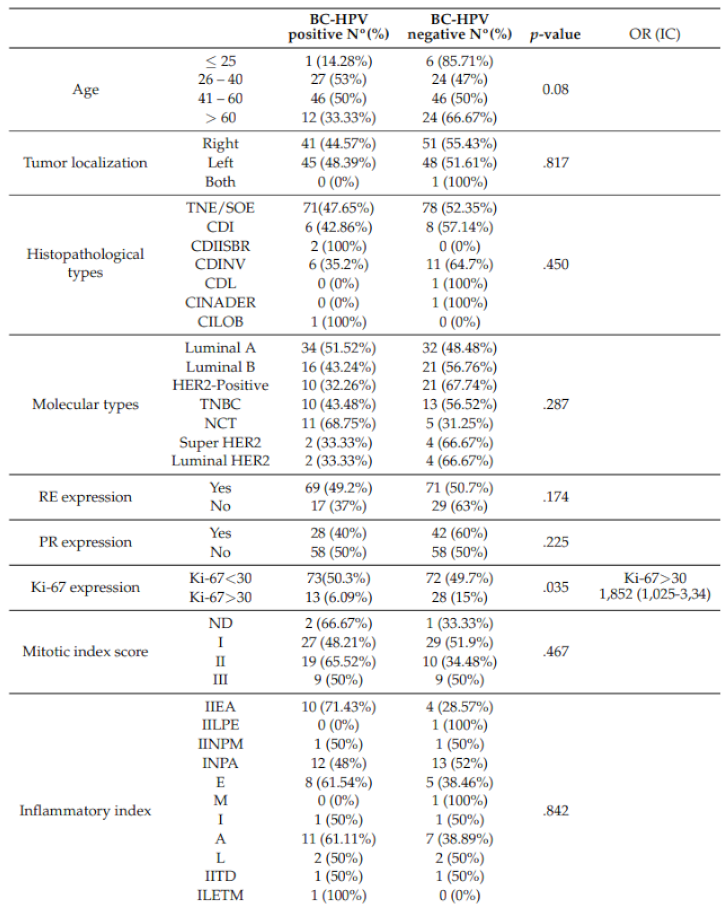
3.1. BLV and HPV DNA Detection in BC
3.2. HPV16 and 31 DNA Detection in BC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Soerjomataram, I.; Bray, F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nature Reviews Clinical Oncology 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- INCA, 2020.
- Buys, S.S.; Sandbach, J.F.; Gammon, A.; Patel, G.; Kidd, J.; Brown, K.L.; Sharma, L.; Saam, J.; Lancaster, J.; Daly, M.B. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 2017, 123, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Jabeen, A.; Al-Sarraf, R.; Farghaly, H.; Vranic, S.; Sultan, A.A.; Al Moustafa, A.E.; Al-Thawadi, H. The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Human Vaccines & Immunotherapeutics 2021, 17, 982–989. [Google Scholar] [CrossRef]
- Hsu, C.R.; Lu, T.M.; Chin, L.W.; Yang, C.C. Possible DNA viral factors of human breast cancer. Cancers 2010, 2, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Kudela, E.; Kudelova, E.; Kozubík, E.; Rokos, T.; Pribulova, T.; Holubekova, V.; Biringer, K. HPV-Associated Breast Cancer: Myth or Fact? Pathogens (Basel, Switzerland) 2022, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Malhone, C.; Longatto-Filho, A.; Filassi, J.R. Is Human Papilloma Virus Associated with Breast Cancer? A Review of the Molecular Evidence. Acta Cytologica 2018, 62, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Hameed, Y.; Ahmad, M.; Jalil Ur Rehman, n.; Ahmed, H.; Hussain, M.S.; Asif, R.; Murtaza, M.G.; Jawad, M.T.; Iqbal, M.J. Breast Cancer Risk and Human Papillomavirus Infection: A Bradford Hill Criteria Based Evaluation. Infectious Disorders Drug Targets 2022, 22, e200122200389. [Google Scholar] [CrossRef]
- Mareti, E.; Chatzakis, C.; Pratilas, G.C.; Liberis, A.; Vavoulidis, E.; Papanastasiou, A.; Dampali, R.; Daniilidis, A.; Zepiridis, L.; Dinas, K. Human papillomavirus in breast cancer of patients with cervical intraepithelial neoplasia or cervical cancer history. A systematic review and meta-analysis. Journal of B.U.ON.: official journal of the Balkan Union of Oncology 2021, 26, 707–713. [Google Scholar] [PubMed]
- zur Hausen, H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses in human cancers. Proc Assoc Am Physicians 1999, 111, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Badaracco, G.; Venuti, A.; Morello, R.; Muller, A.; Marcante, M.L. Human papillomavirus in head and neck carcinomas: prevalence, physical status and relationship with clinical/pathological parameters. Anticancer Res. 2000, 20, 1301–1305. [Google Scholar] [PubMed]
- Benson, E.; Li, R.; Eisele, D.; Fakhry, C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014, 50, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, F.; Castillo, A.; Koriyama, C.; Higashi, M.; Itoh, T.; Capetillo, M.; Shuyama, K.; Corvalan, A.; Eizuru, Y.; Akiba, S. Human papillomavirus-16 is integrated in lung carcinomas: a study in Chile. Br. J. Cancer 2007, 97, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, N.E.; Anwar, S.; Noreen, M.; Hashmi, S.N.; Murad, S. Detection of human papillomavirus-16 DNA in archived clinical samples of breast and lung cancer patients from North Pakistan. J. Cancer Res. Clin. Oncol. 2016, 142, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, A.; Venuti, A.; Marcante, M.L. Human papillomavirus in breast cancer. Breast Cancer Research and Treatment 1992, 21, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Spurr, T.P.; Chen, A.C.; Francis, G.D.; McMillan, N.A.J.; Saunders, N.A.; Law, M.; Bennett, I.C. High prevalence of human papillomaviruses in fresh frozen breast cancer samples. Journal of Medical Virology 2011, 83, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, F.; Khan, N.; Koriyama, C.; González, C.; Ampuero, S.; Padilla, O.; Solís, L.; Eizuru, Y.; Corvalán, A.; Akiba, S. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infectious Agents and Cancer 2011, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Balci, F.L.; Uras, C.; Feldman, S.M. Is human papillomavirus associated with breast cancer or papilloma presenting with pathologic nipple discharge? Cancer Treatment and Research Communications 2019, 19, 100122. [Google Scholar] [CrossRef] [PubMed]
- Baltzell, K.; Buehring, G.C.; Krishnamurthy, S.; Kuerer, H.; Shen, H.M.; Sison, J.D. Limited evidence of human papillomavirus in [corrected] breast tissue using molecular in situ methods. Cancer 2012, 118, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.R.; Pinheiro, L.G.P.; Almeida, P.R.C.d.; Ferreira, M.V.P.; Cruz, G.A.; Campelo, T.A.; Silva, C.S.; Lima, L.N.G.C.; Oliveira, B.M.K.d.; Lima, L.M.; et al. Association of breast cancer with human papillomavirus (HPV) infection in Northeast Brazil: molecular evidence. Clinics (Sao Paulo, Brazil) 2018, 73, e465. [Google Scholar] [CrossRef] [PubMed]
- De Carolis, S.; Storci, G.; Ceccarelli, C.; Savini, C.; Gallucci, L.; Sansone, P.; Santini, D.; Seracchioli, R.; Taffurelli, M.; Fabbri, F.; et al. HPV DNA Associates With Breast Cancer Malignancy and It Is Transferred to Breast Cancer Stromal Cells by Extracellular Vesicles. Frontiers in Oncology 2019, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Delgado-García, S.; Martínez-Escoriza, J.C.; Alba, A.; Martín-Bayón, T.A.; Ballester-Galiana, H.; Peiró, G.; Caballero, P.; PonceLorenzo, J. Presence of human papillomavirus DNA in breast cancer: a Spanish case-control study. BMC Cancer 2017, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Doosti, M.; Bakhshesh, M.; Zahir, S.T.; Shayestehpour, M.; Karimi-Zarchi, M. Lack of Evidence for a Relationship between High Risk Human Papillomaviruses and Breast Cancer in Iranian Patients. Asian Pacific journal of cancer prevention: APJCP 2016, 17, 4357–4361. [Google Scholar]
- Frega, A.; Lorenzon, L.; Bononi, M.; De Cesare, A.; Ciardi, A.; Lombardi, D.; Assorgi, C.; Gentile, M.; Moscarini, M.; Torrisi, M.R.; et al. Evaluation of E6 and E7 mRNA expression in HPV DNA positive breast cancer. European Journal of Gynaecological Oncology 2012, 33, 164–167. [Google Scholar]
- Gupta, I.; Ulamec, M.; Peric-Balja, M.; Ramic, S.; Al Moustafa, A.E.; Vranic, S.; Al-Farsi, H.F. Presence of high-risk HPVs, EBV, and MMTV in human triple-negative breast cancer. Human Vaccines & Immunotherapeutics 2021, 17, 4457–4466. [Google Scholar] [CrossRef]
- Habyarimana, T.; Attaleb, M.; Mazarati, J.B.; Bakri, Y.; El Mzibri, M. Detection of human papillomavirus DNA in tumors from Rwandese breast cancer patients. Breast Cancer (Tokyo, Japan) 2018, 25, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Dasgupta, H.; Roychowdhury, A.; Bhattacharya, R.; Mukherjee, N.; Roy, A.; Mandal, G.K.; Alam, N.; Biswas, J.; Mandal, S.; et al. Study of association and molecular analysis of human papillomavirus in breast cancer of Indian patients: Clinical and prognostic implication. PloS One 2017, 12, e0172760. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, C.; Lee, H.S.; Choi, Y.J.; Kim, H.Y.; Lee, J.; Chang, H.; Kim, A. Detection of Human Papillomavirus in Korean Breast Cancer Patients by Real-Time Polymerase Chain Reaction and Meta-Analysis of Human Papillomavirus and Breast Cancer. Journal of Pathology and Translational Medicine 2016, 50, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Castillo, A.; Koriyama, C.; Kijima, Y.; Umekita, Y.; Ohi, Y.; Higashi, M.; Sagara, Y.; Yoshinaka, H.; Tsuji, T.; et al. Human papillomavirus detected in female breast carcinomas in Japan. British Journal of Cancer 2008, 99, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chang, P.; Wang, L.; Yao, Q.; Guo, W.; Chen, J.; Yan, T.; Cao, C. The role of human papillomavirus infection in breast cancer. Medical Oncology (Northwood, London, England) 2012, 29, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Marawan, M.A.; Alouffi, A.; El Tokhy, S.; Badawy, S.; Shirani, I.; Dawood, A.; Guo, A.; Almutairi, M.M.; Alshammari, F.A.; Selim, A. Bovine Leukaemia Virus: Current Epidemiological Circumstance and Future Prospective. Viruses 2021, 13, 2167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, J.; Sun, W.; Zhang, J.; Huang, K.; Gu, X.; Yang, Y.; Xu, X.; Shi, Y.; Wang, C. Lack of association between bovine leukemia virus and breast cancer in Chinese patients. Breast cancer research: BCR 2016, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.P.; Saito, S.; Hara, Y.; Matsuura, R.; Takeshima, S.n.; Hosomichi, K.; Matsumoto, Y.; Furuta, R.A.; Takei, M.; Aida, Y. evidence of bovine leukemia virus proviral DNA and antibodies in human specimens from Japan. Retrovirology 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Romano, L.; Fernández-Tamayo, N.; Gómez-Conde, E.; Reyes-Cardoso, J.M.; Ortiz-Gutierrez, F.; Ceballos, G.; Valdivia,; Piña, P. ; Salcedo, M. Absence of human papillomavirus sequences in epithelial breast cancer in a Mexican female population. Medical Oncology (Northwood, London, England) 2012, 29, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyrizadeh, S.; Hosseini, S.Y.; Yaghobi, R.; Safaei, A.; Sarvari, J. Almost Complete Lack of Human Cytomegalovirus and Human papillomaviruses Genome in Benign and Malignant Breast Lesions in Shiraz, Southwest of Iran. Asian Pacific journal of cancer prevention: APJCP 2017, 18, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Goepfert, R.; Khan, N.A.; Koriyama, C.; Akiba, S.; Pérez-Sánchez, V.M. High-risk human papillomavirus in mammary gland carcinomas and non-neoplastic tissues of Mexican women: no evidence supporting a cause and effect relationship. Breast (Edinburgh, Scotland) 2011, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Goepfert, R.; Vela-Chávez, T.; Carrillo-García, A.; Lizano-Soberón, M.; Amador-Molina, A.; Oñate-Ocaña, L.F.; Hallmann, R.S.R. High-risk human papillomavirus (HPV) DNA sequences in metaplastic breast carcinomas of Mexican women. BMC Cancer 2013, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Eslamifar, A.; Ramezani, A.; Azadmanesh, K.; Bidari-Zerehpoosh, F.; Banifazl, M.; Aghakhani, A. Assessment of the Association between Human Papillomavirus Infection and Breast Carcinoma. Iranian Journal of Pathology 2015, 10, 41–46. [Google Scholar] [PubMed]
- Vernet-Tomas, M.; Mena, M.; Alemany, L.; Bravo, I.; De Sanjosé, S.; Nicolau, P.; Bergueiro, A.; Corominas, J.M.; Serrano, S.; Carreras, R.; et al. Human papillomavirus and breast cancer: no evidence of association in a Spanish set of cases. Anticancer Research 2015, 35, 851–856. [Google Scholar] [PubMed]
- Hedau, S.; Kumar, U.; Hussain, S.; Shukla, S.; Pande, S.; Jain, N.; Tyagi, A.; Deshpande, T.; Bhat, D.; Mir, M.M.; et al. Breast cancer and human papillomavirus infection: no evidence of HPV etiology of breast cancer in Indian women. BMC cancer 2011, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Ji, Y.; Ren, M.; Pang, B.; Chu, M.; Wei, L. Inconclusive role of human papillomavirus infection in breast cancer. Infectious Agents and Cancer 2015, 10, 36. [Google Scholar] [CrossRef]
- Gurgel, A.P.A.D.; Chagas, B.S.; do Amaral, C.M.M.; Albuquerque, E.M.B.; Serra, I.G.S.S.; Silva Neto, J.d.C.; Muniz, M.T.C.; de Freitas, A.C. Prevalence and genetic variability in capsid L1 gene of rare human papillomaviruses (HPV) found in cervical lesions of women from North-East Brazil. Biomed Res. Int. 2013, 2013, 546354. [Google Scholar] [CrossRef]
- Chagas, B.S.; Batista, M.V.d.A.; Crovella, S.; Gurgel, A.P.A.D.; Silva Neto, J.d.C.; Serra, I.G.S.S.; Amaral, C.M.M.; Balbino, V.Q.; Muniz, M.T.C.; Freitas, A.C. Novel E6 and E7 oncogenes variants of human papillomavirus type 31 in Brazilian women with abnormal cervical cytology. Infect. Genet. Evol. 2013, 16, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, A.P.A.D.; Chagas, B.S.; do Amaral, C.M.; Nascimento, K.C.G.; Leal, L.R.S.; Silva Neto, J.d.C.; Cartaxo Muniz, M.T.; de Freitas, A.C. Prevalence of human papillomavirus variants and genetic diversity in the L1 gene and long control region of HPV16, HPV31, and HPV58 found in North-East Brazil. Biomed Res. Int. 2015, 2015, 130828. [Google Scholar] [CrossRef] [PubMed]
- Conceição Gomes Nascimento, K.; Gonçalves Lima,; Mota Nunes, Z. ; Rêgo Barros Júnior, M.; de Aragão Batista, M.V.; Lucena Araujo, A.R.; da Costa Silva Neto, J.; Simas Chagas, B.; Almeida Diniz Gurgel, A.P.; de Freitas, A.C. Detection of Human Papillomavirus DNA in Paired Peripheral Blood and Cervix Samples in Patients with Cervical Lesions and Healthy Individuals. Journal of Clinical Medicine 2021, 10, 5209. [Google Scholar] [CrossRef]
- Shi, S.R.; Cote, R.J.; Wu, L.; Liu, C.; Datar, R.; Shi, Y.; Liu, D.; Lim, H.; Taylor, C.R. DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: heating under the influence of pH. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society 2002, 50, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Fuessel Haws, A.L.; He, Q.; Rady, P.L.; Zhang, L.; Grady, J.; Hughes, T.K.; Stisser, K.; Konig, R.; Tyring, S.K. Nested PCR with the PGMY09/11 and GP5(+)/6(+) primer sets improves detection of HPV DNA in cervical samples. J. Virol. Methods 2004, 122, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; DeLaney, A.; Shen, H.; Chu, D.L.; Razavian, N.; Schwartz, D.A.; Demkovich, Z.R.; Bates, M.N. Bovine leukemia virus discovered in human blood. BMC Infectious Diseases 2019, 19, 297. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; Shen, H.M.; Jensen, H.M.; Choi, K.Y.; Sun, D.; Nuovo, G. Bovine leukemia virus DNA in human breast tissue. Emerging Infectious Diseases 2014, 20, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Ngan, C.; Lawson, J.S.; Clay, R.; Delprado, W.; Whitaker, N.J.; Glenn, W.K. Early Human Papilloma Virus (HPV) Oncogenic Influences in Breast Cancer. Breast Cancer: Basic and Clinical Research 2015, 9, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Rassi, H.; Mansur, F.N. Investigation of Methylenetetrahydrofolate Reductase C677T Polymorphism and Human Papilloma Virus Genotypes in Iranian Breast Cancer. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy 2017, 36, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Salman, N.A.; Davies, G.; Majidy, F.; Shakir, F.; Akinrinade, H.; Perumal, D.; Ashrafi, G.H. Association of High Risk Human Papillomavirus and Breast cancer: A UK based Study. Scientific Reports 2017, 7, 43591. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehlou, N.; Mostafaei, S.; Etemadi, A.; Ghasemi, A.; Payandeh, M.; Hadifar, S.; Norooznezhad, A.H.; Kazemnejad, A.; Moghoofei, M. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC cancer 2019, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Charostad, J.; Azaran, A.; Nakhaei, M.; Astani, A.; Kaydani, G.A.; Motamedfar, A.; Makvandi, M. Upregulation of Interleukin-6 in HPV-Positive Breast Cancer Patients. Iranian journal of immunology: IJI 2021, 18, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.A.; Abo-Shadi, M.A.; Abdel Fattah, N.F.; Barakat, A.B.; Rabee, O.A.; Osman, A.M.; Helal, A.M.; Hashem, T.; Moneer, M.M.; Chehadeh, W.; et al. Presence of HPV, EBV and HMTV Viruses Among Egyptian Breast Cancer Women: Molecular Detection and Clinical Relevance. Infection and Drug Resistance 2021, 14, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Baldez da Silva, M.F.P.T.; Chagas, B.S.; Guimarães, V.; Katz, L.M.C.; Felix, P.M.; Miranda, P.M.; Lima, A.A.; Arraes, L.C.; Martins, D.B.G.; Lima Filho, J.L.; et al. HPV31 and HPV33 incidence in cervical samples from women in Recife, Brazil. Genet. Mol. Res. 2009, 8, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Chagas, B.S.; Comar, M.; Gurgel, A.P.A.D.; Paiva, S.; Seraceni, S.; de Freitas, A.C.; Crovella, S. Association study between cervical lesions and single or multiple vaccine-target and non-vaccine target Human Papillomavirus (HPV) types in women from northeastern Brazil. PLoS One 2015, 10, e0132570. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M.; Sandstrom, R.E.; zur Hausen, H.; Buck, C.E. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005, 7, R1–11. [Google Scholar] [CrossRef] [PubMed]
- Piana, A.F.; Sotgiu, G.; Muroni, M.R.; Cossu-Rocca, P.; Castiglia, P.; De Miglio, M.R. HPV infection and triple-negative breast cancers: an Italian case-control study. Virology Journal 2014, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, J.; Sun, W.; Zhang, J.; Huang, K.; Gu, X.; Yang, Y.; Xu, X.; Shi, Y.; Wang, C. Lack of association between bovine leukemia virus and breast cancer in Chinese patients. Breast Cancer Research 2016, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Canova, R.; Weber, M.N.; Budaszewski, R.F.; da Silva, M.S.; Schwingel, D.; Canal, C.W.; Kreutz, L.C. Bovine leukemia viral DNA found on human breast tissue is genetically related to the cattle virus. One Health 2021, 13, 100252. [Google Scholar] [CrossRef] [PubMed]
- Schwingel, D.; Andreolla, A.P.; Erpen, L.M.S.; Frandoloso, R.; Kreutz, L.C. Bovine leukemia virus DNA associated with breast cancer in women from South Brazil. Scientific Reports 2019, 9, 2949. [Google Scholar] [CrossRef] [PubMed]
- Baltzell, K.A.; Shen, H.M.; Krishnamurthy, S.; Sison, J.D.; Nuovo, G.J.; Buehring, G.C. Bovine leukemia virus linked to breast cancer but not coinfection with human papillomavirus: Case-control study of women in Texas. Cancer 2018, 124, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; Shen, H.; Schwartz, D.A.; Lawson, J.S. Bovine leukemia virus linked to breast cancer in Australian women and identified before breast cancer development. PLoS ONE 2017, 12, e0179367. [Google Scholar] [CrossRef] [PubMed]
- Buehring, G.C.; Shen, H.M.; Jensen, H.M.; Jin, D.L.; Hudes, M.; Block, G. Exposure to Bovine Leukemia Virus Is Associated with Breast Cancer: A Case-Control Study. PloS One 2015, 10, e0134304. [Google Scholar] [CrossRef] [PubMed]
- Delarmelina, E.; Buzelin, M.A.; Souza, B.S.d.; Souto, F.M.; Bicalho, J.M.; Câmara, R.J.F.; Resende, C.F.; Bueno, B.L.; Victor, R.M.; Galinari, G.C.F.; et al. High positivity values for bovine leukemia virus in human breast cancer cases from Minas Gerais, Brazil. PloS One 2020, 15, e0239745. [Google Scholar] [CrossRef] [PubMed]
| Sequence (5’3’) | Size (bp) | |
|---|---|---|
| β-globina | 110 bp | |
| PC04 | ACACAACTGTGTTCACTAGC | |
| GH20 | CAACTTCATCCACGTTCACC | |
| HPV DNA | ||
| *MY09 | CGTCCMARRGGAWACTGATC | 450 bp |
| *MY11 | GCMCAGGGWCATAAYAATGG | |
| HPV DNA | ||
| GP5 | TTTGTTACTGTGGTAGATAC | 110 bp |
| GP6 | GAAAAATAAACTGTAAATCA | |
| E6 HPV16 | GAGAAACTGCAATGTTTCAGGACC | 81 bp |
| TGTATAGTTGTTTGCAGCTCTGTGC | ||
| E6 HPV31 | CGTTTTCGGTTACAGTTTTACAAGC | 76 bp |
| AGCTGGACTGTCTATGACAT | ||
| TAX1 | CTTCGGGATCCATTACCTG | 373 bp |
| GCTCGAAGGGGGAAAGTGAA | ||
| TAX2 | ATGTCACCATCGATGCCTGG | 113 bp |
| AGCTGGACTGTCTATGACAT | ||
| GAG | ACCCTACTCCGGCTGACCTA | 272 bp |
| CTTGGACGATGGTGGACCAA |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





