Submitted:
14 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
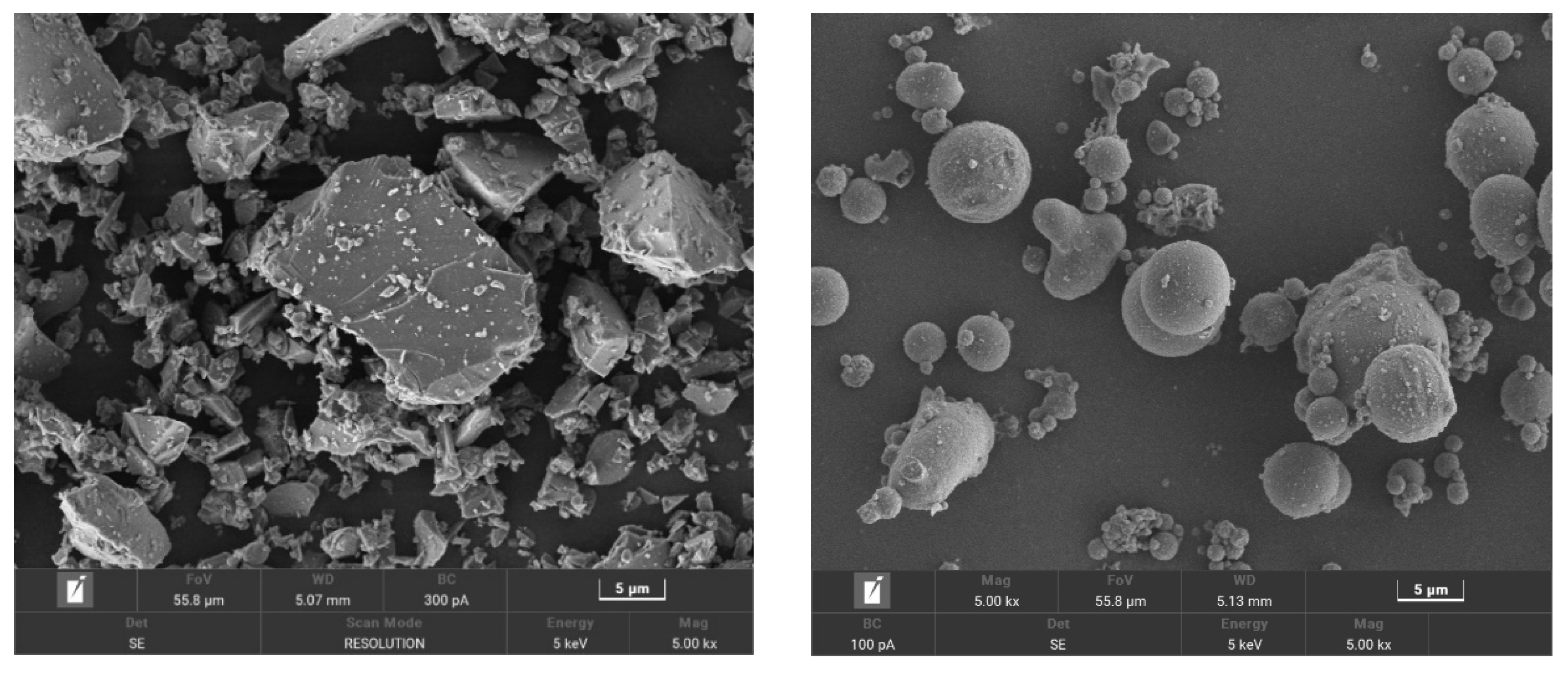
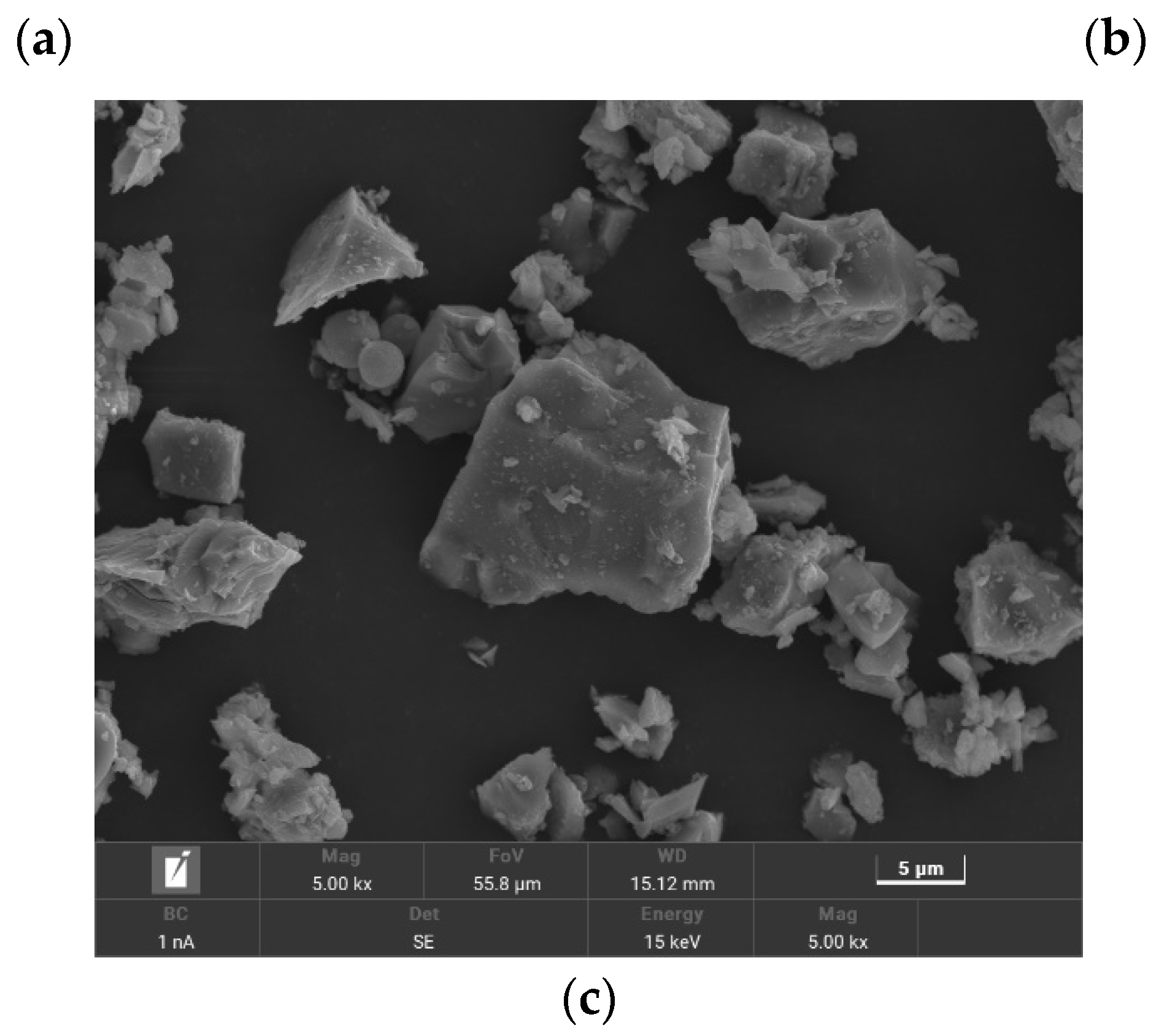
2.2. Mixture Proportions and Specimen Preparation
2.3. Methods
2.3.1. Compressive Strength and Flexural Strength
2.3.2. Isothermal Calorimetry Analysis
2.3.3. Chemically Bound Water and PH values
2.3.4. Scanning Electron Microscopy (SEM) and Mercury Intrusion Porosimetry (MIP) Analysis
2.3.5. Characterization
3. Results
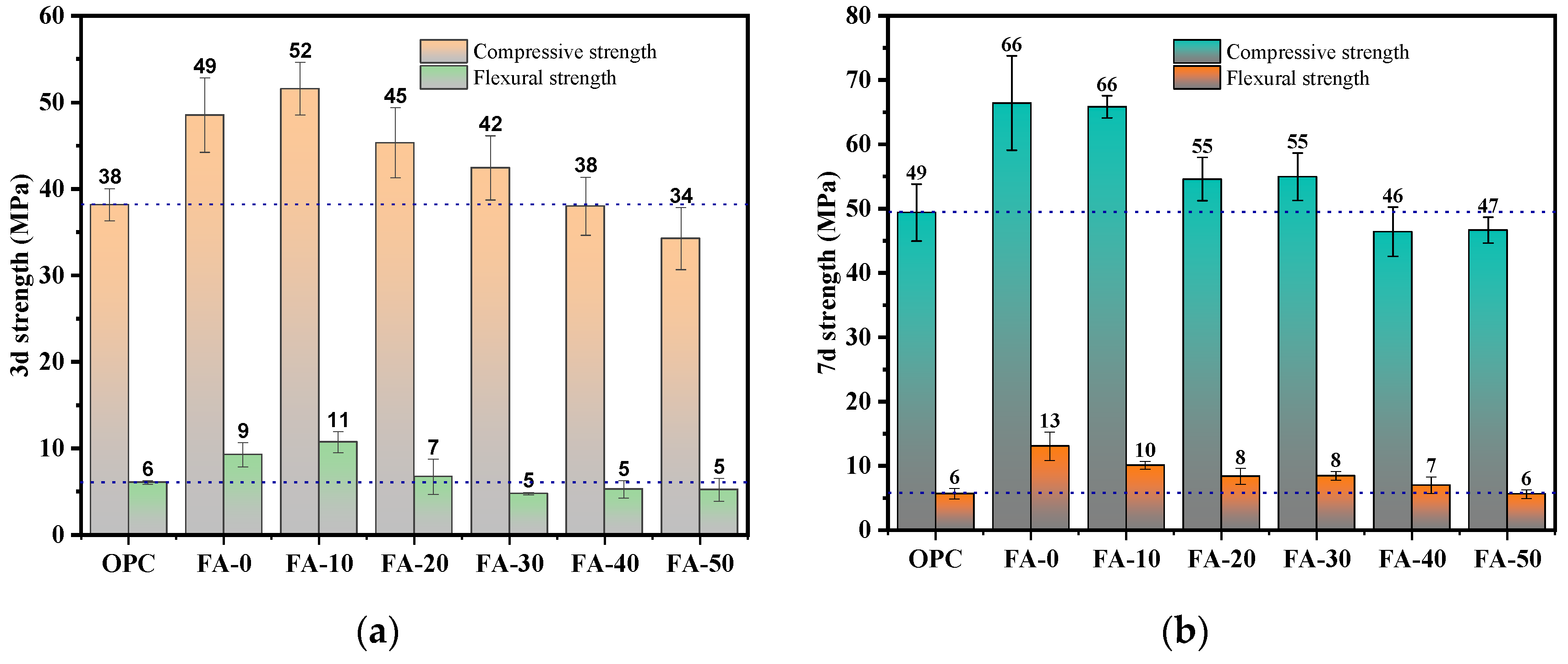
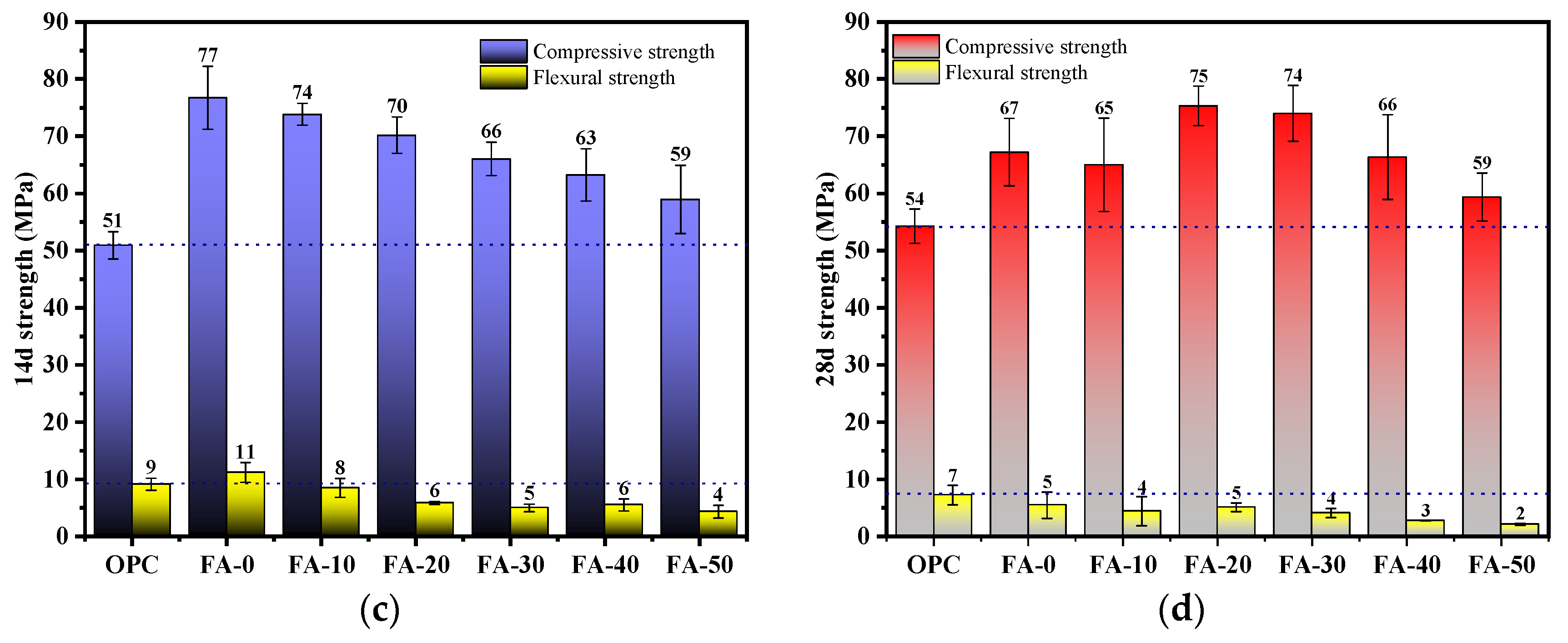
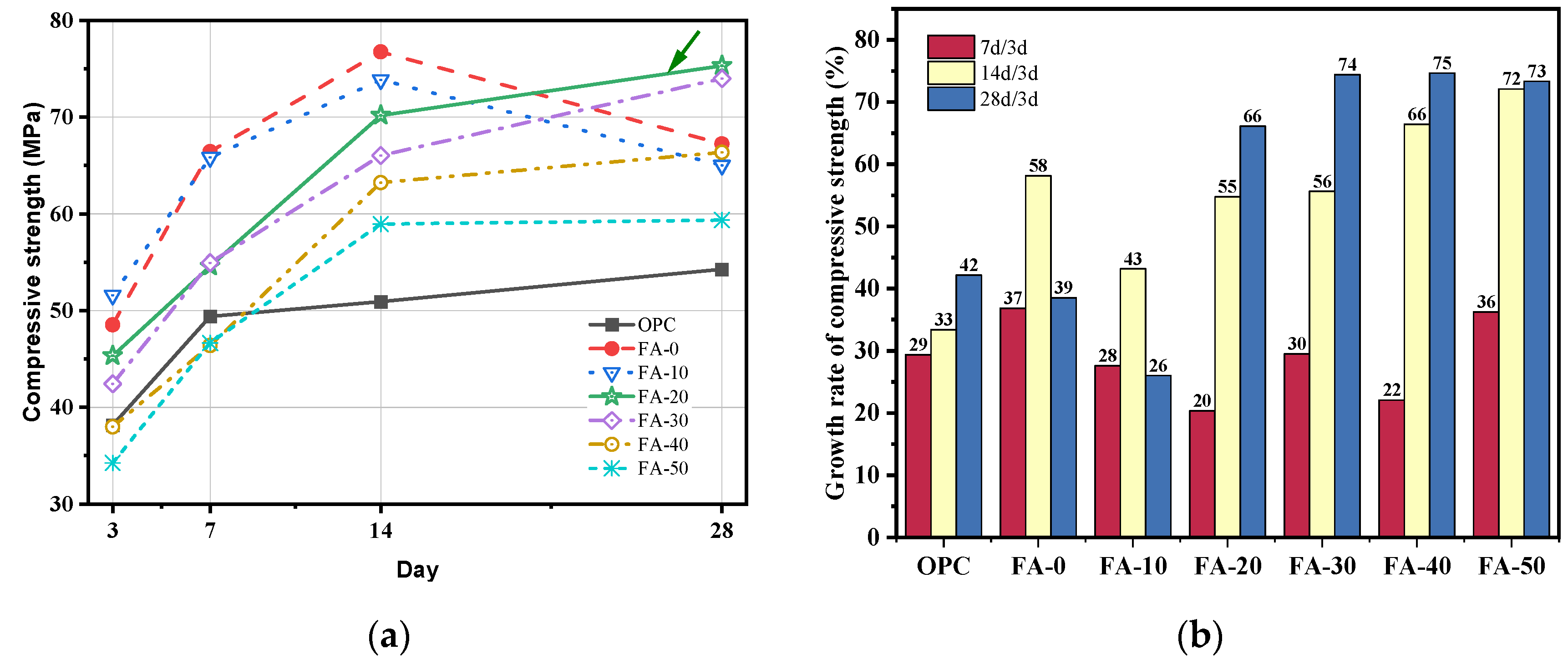
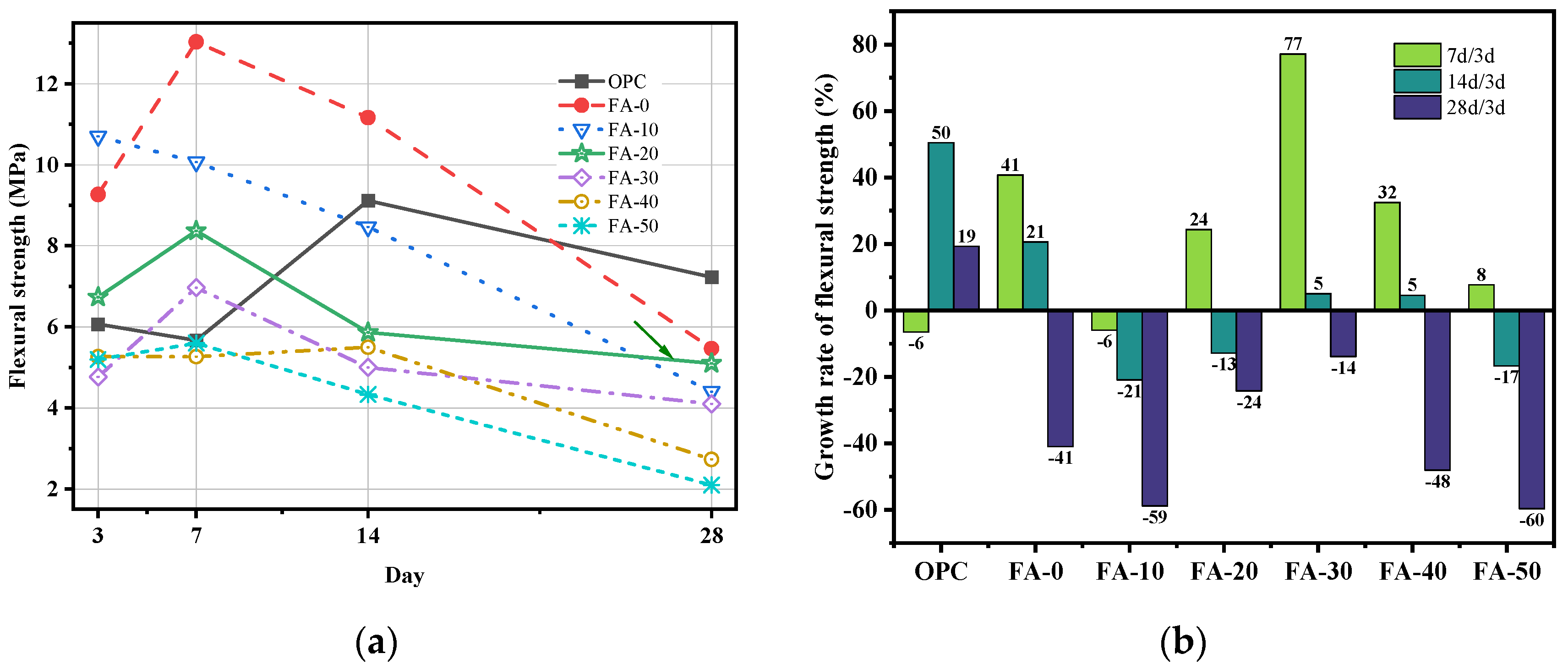
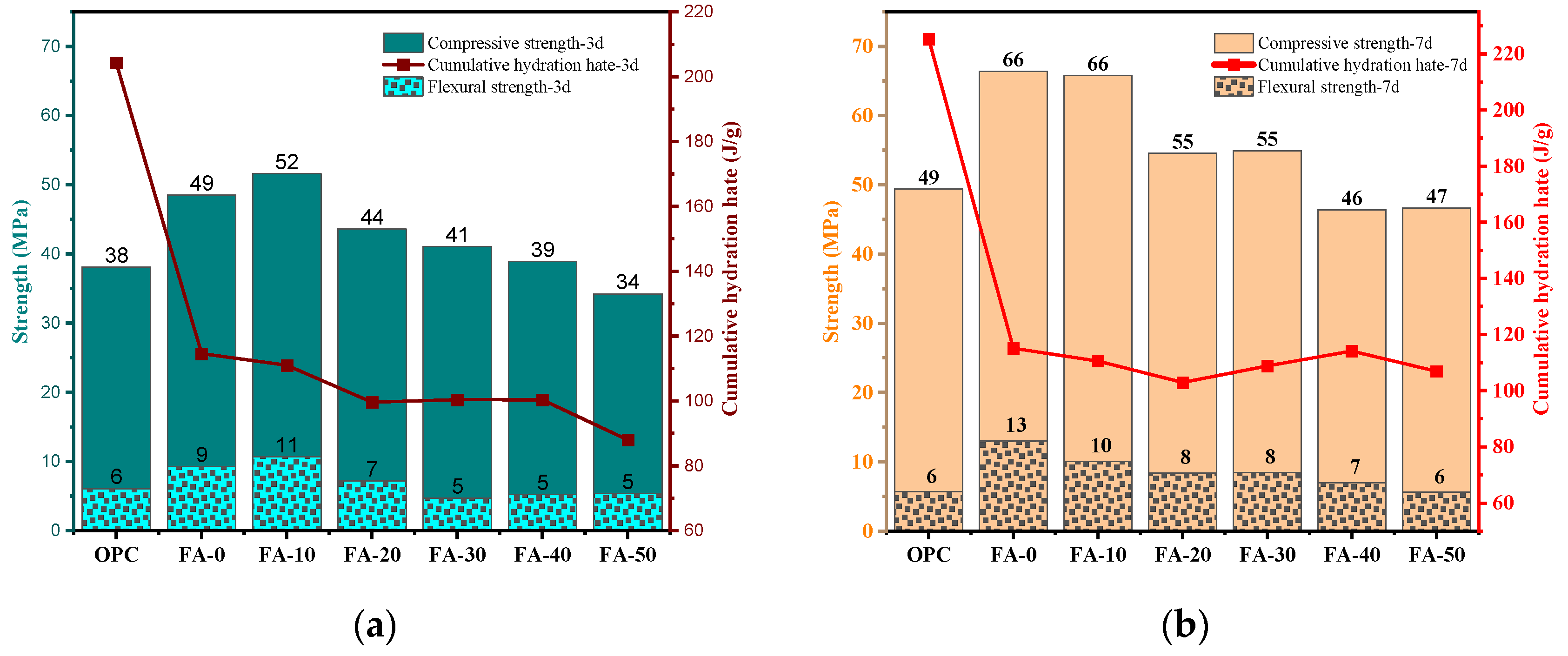
3.1. Compressive Strength and Flexural Strength
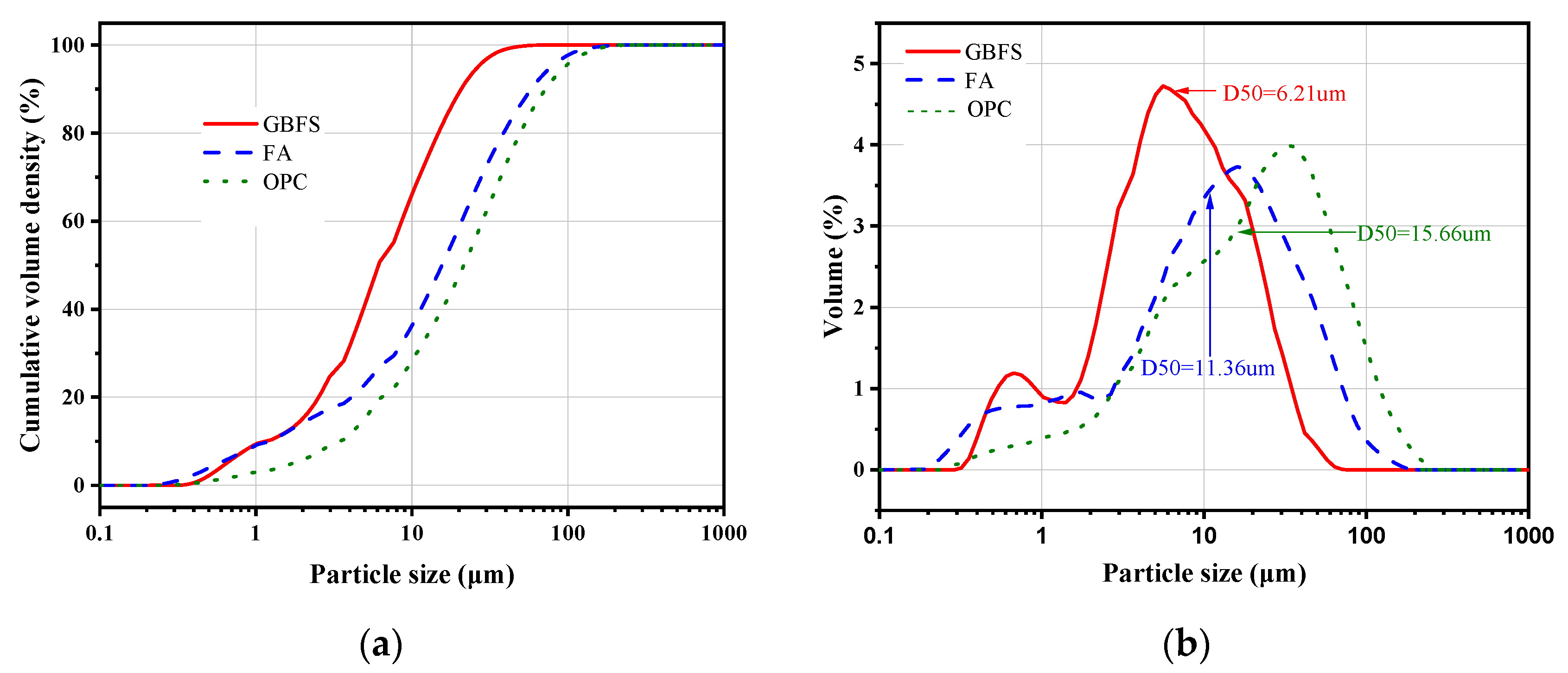
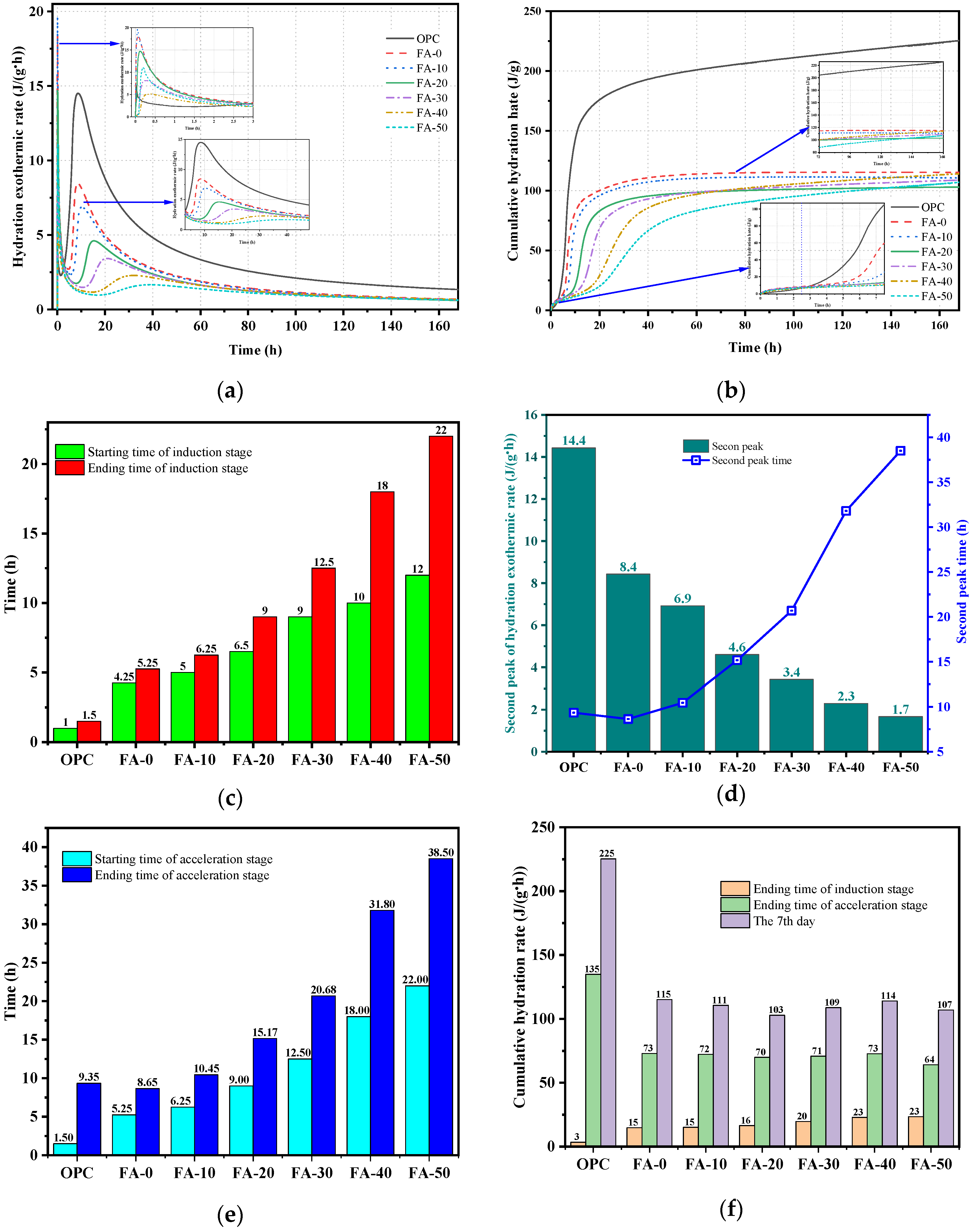
3.2. Isothermal Calorimetry Analysis
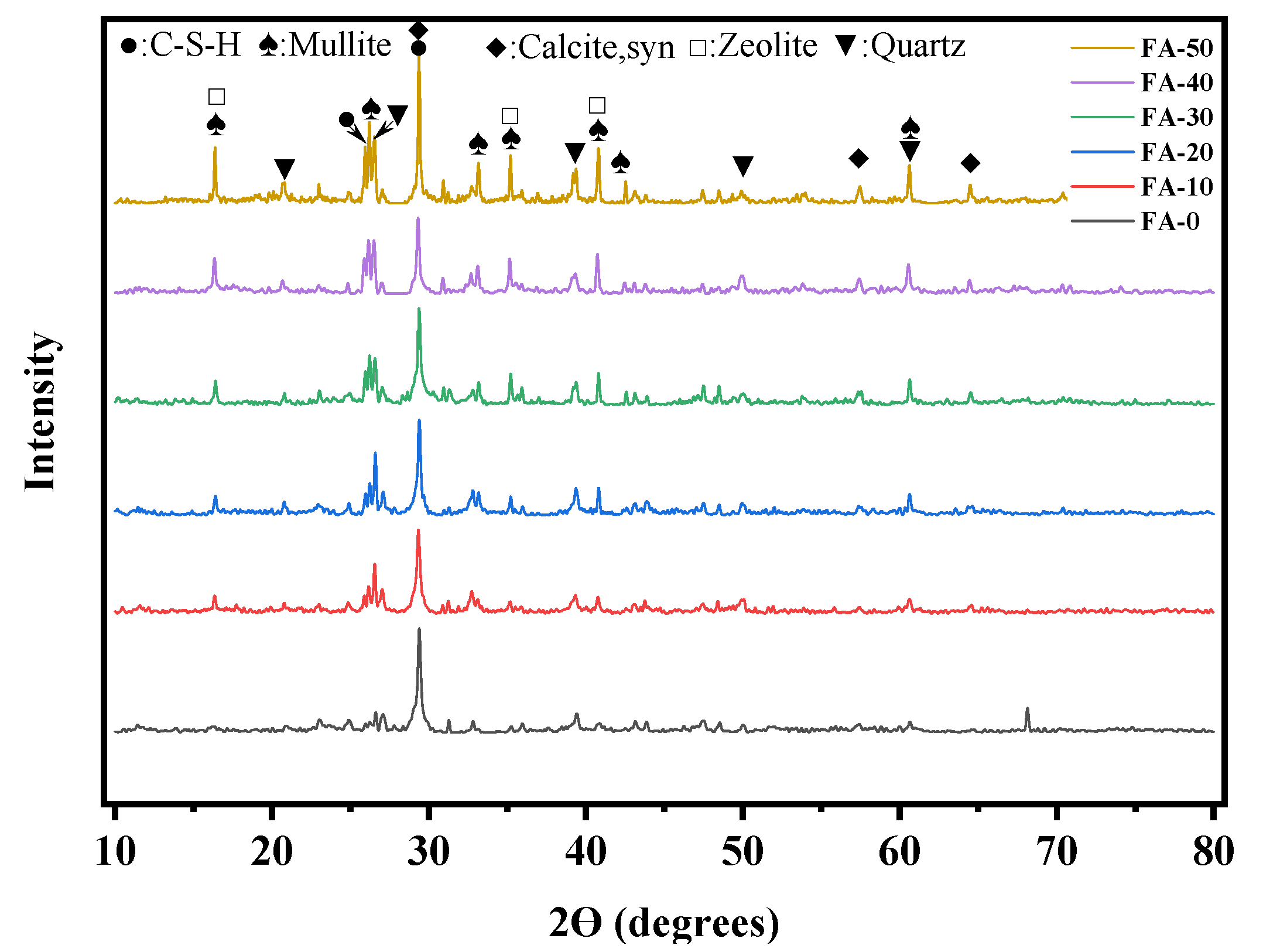
3.3. X-ray diffraction (XRD) analysis
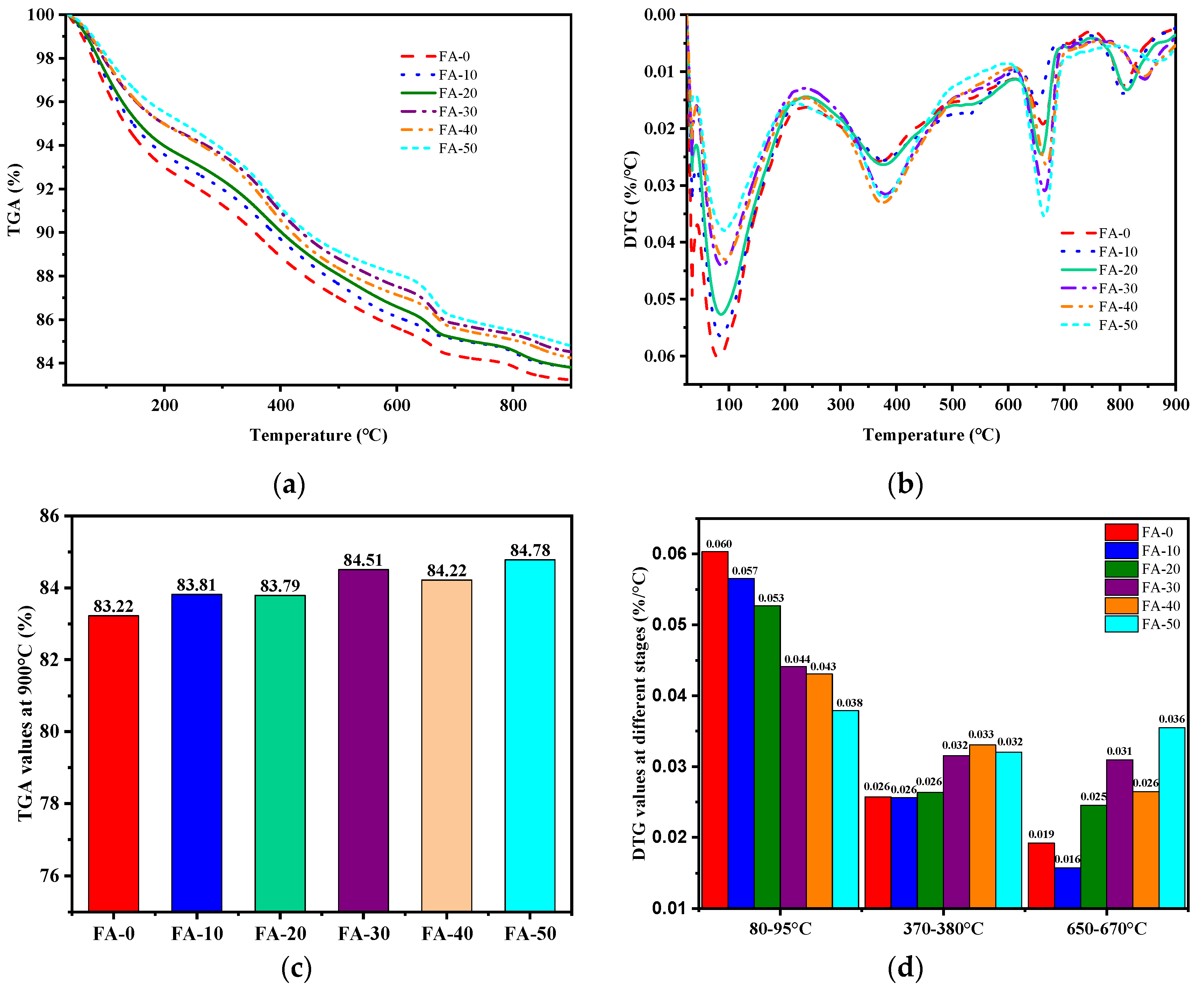
3.4. Thermal Analysis
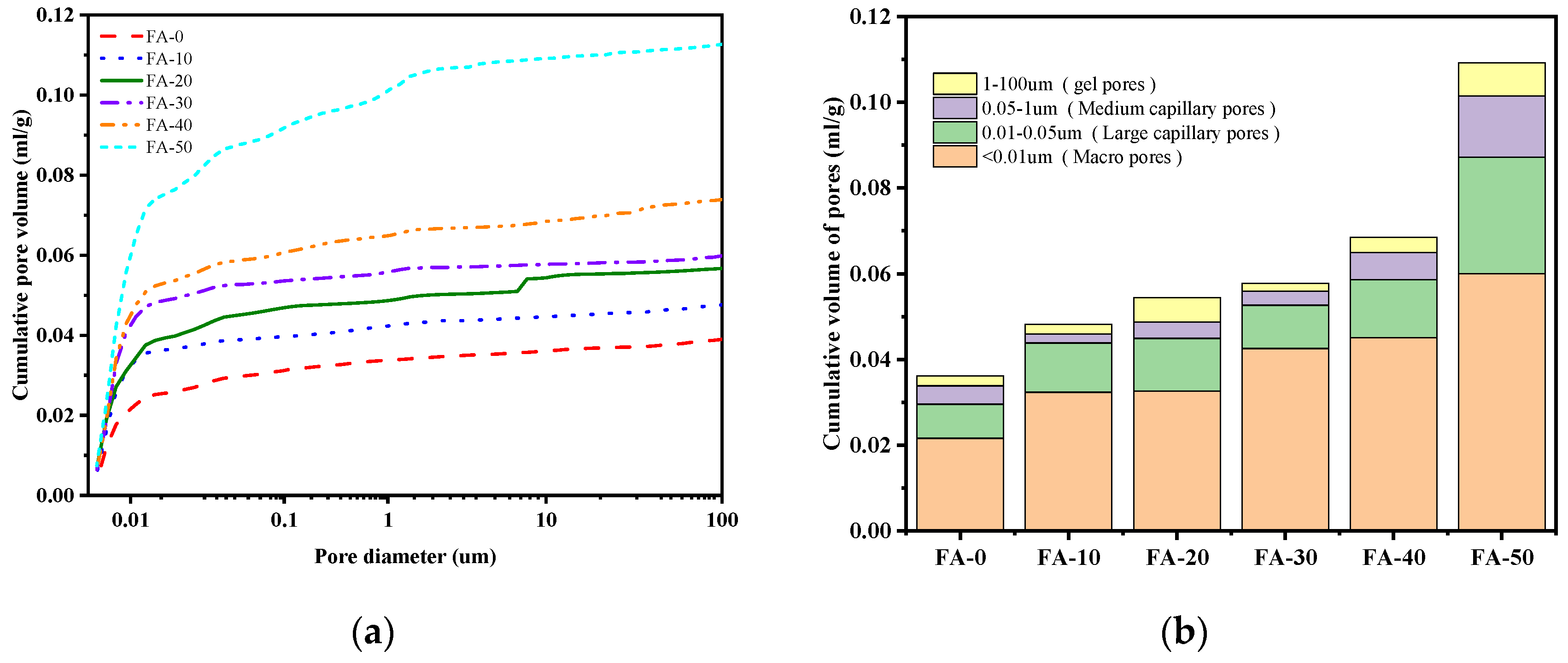
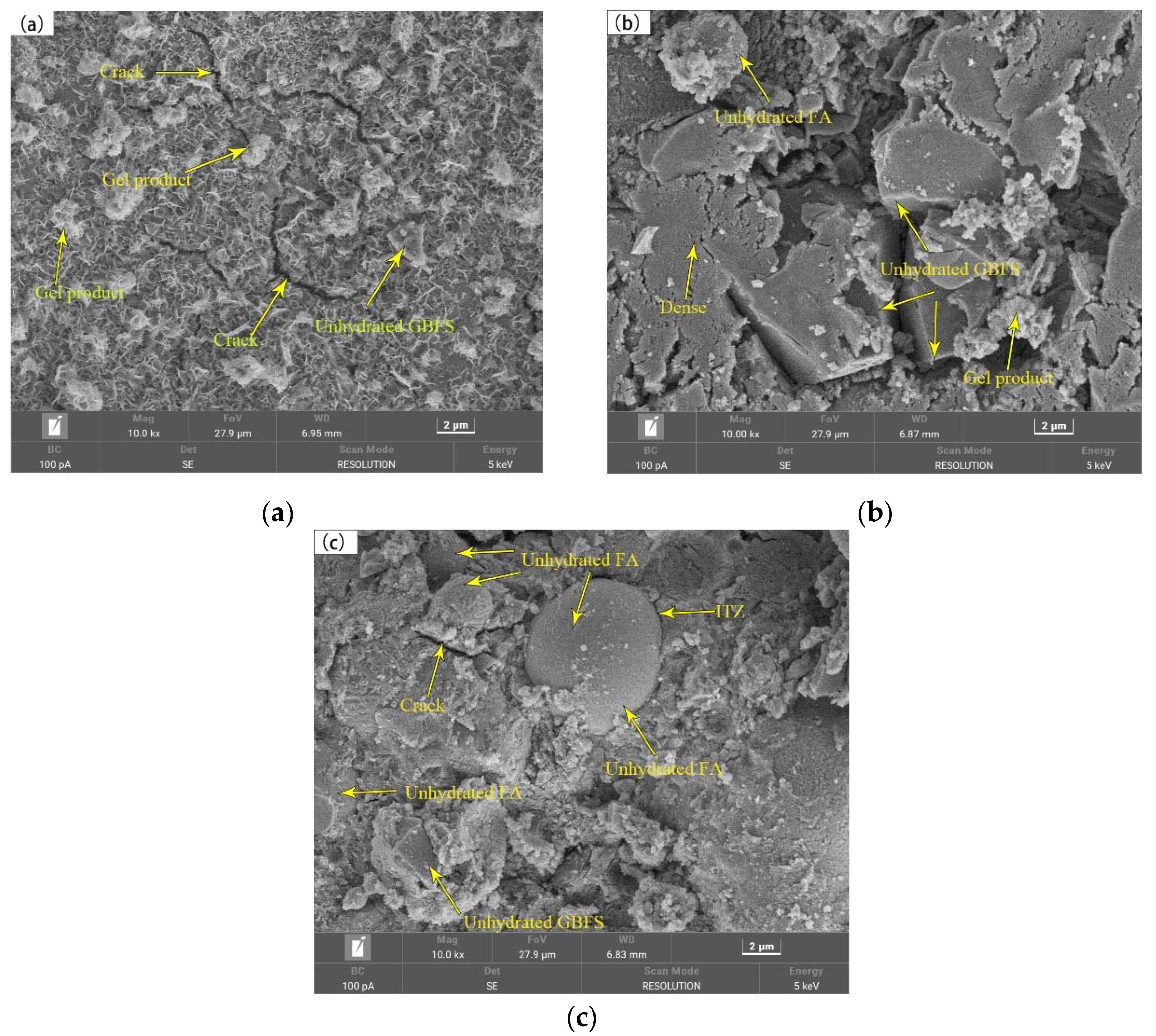
3.5. Pore Structure and Scanning Electron Microscopy
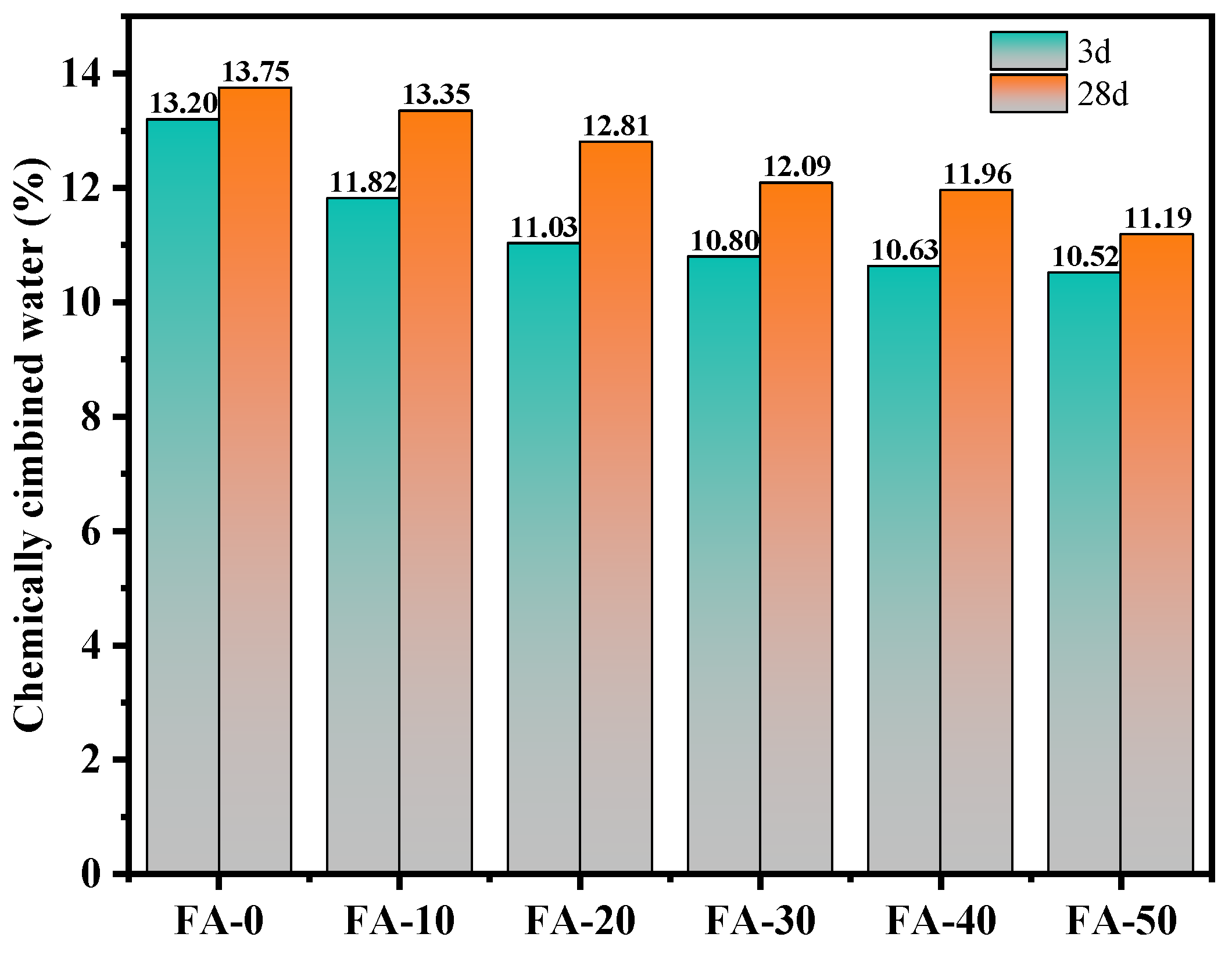
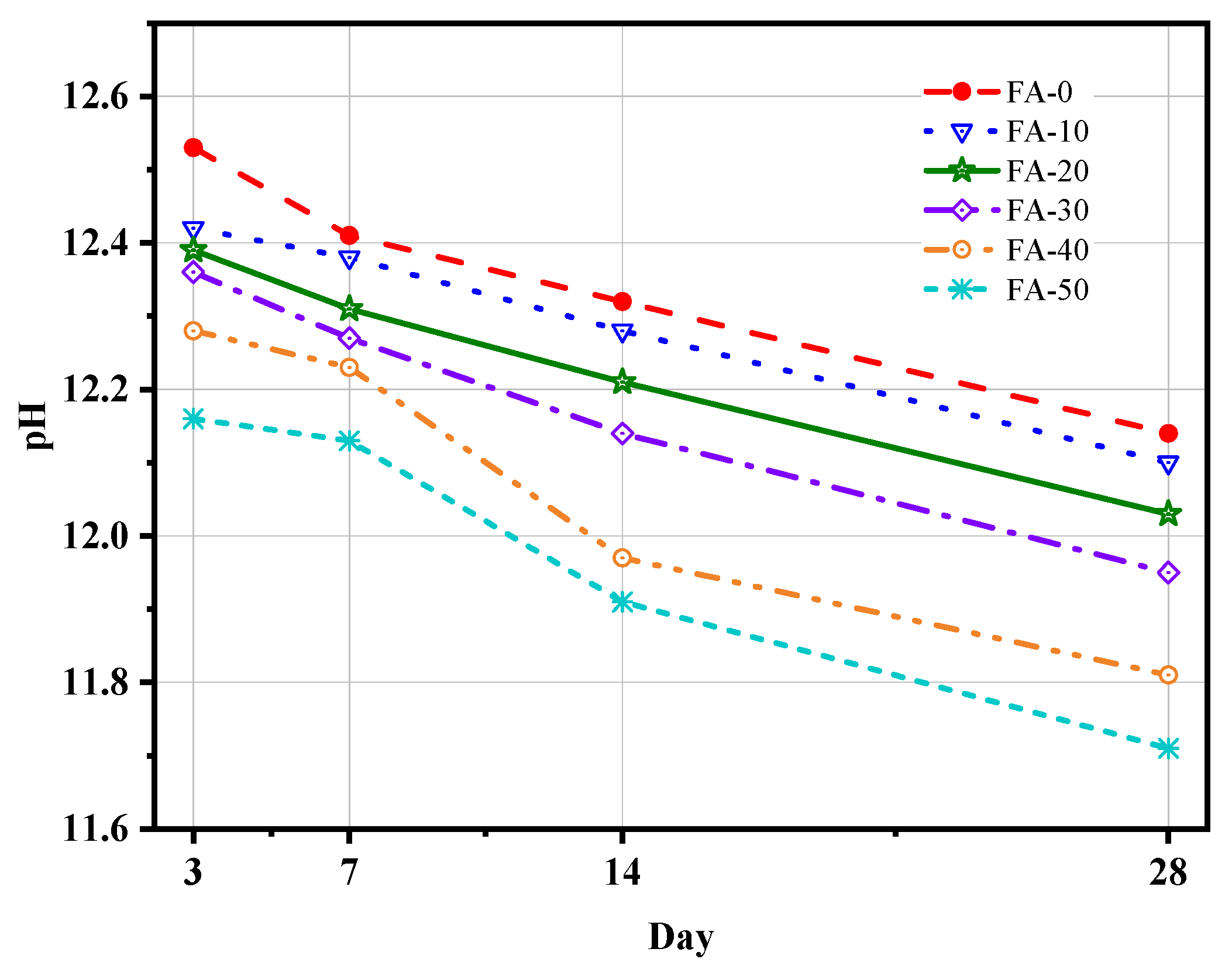
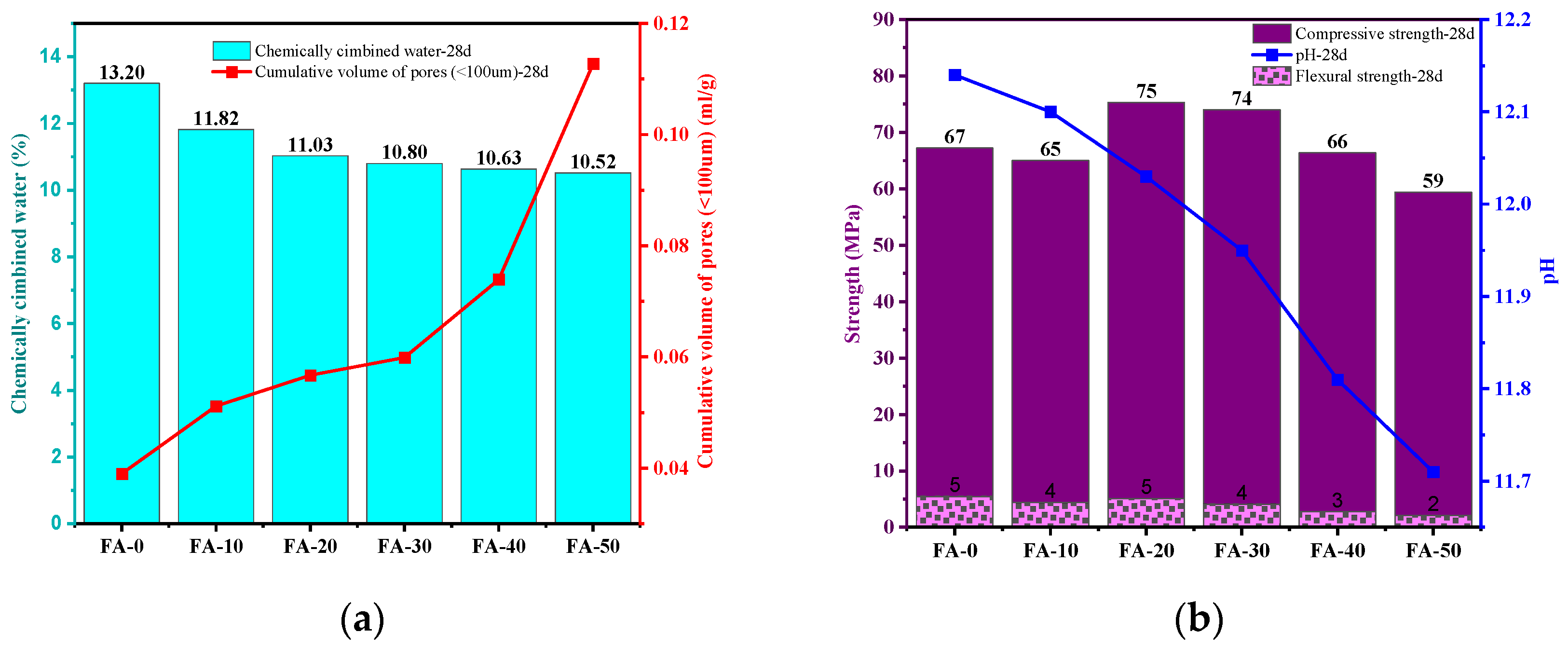
3.6. Chemically Combined Water and pH Values
4. Discussion
5. Conclusions
- Compared to OPC, AAGC exhibited higher early compressive and flexural strengths. After 7 days, the compressive strength of OPC grew slowly. Excluding FA-0 and FA-10, the increase in compressive strength of AAGC from 7 to 28 days was significantly greater than that of OPC, with the 28-day compressive strength of AAGC exceeding that of OPC. However, the 28-day flexural strength of AAGC was lower than that of OPC. As the gelation activity of FA is low, an increase in FA content resulted in a general decline in the compressive strength of AAGC at the same age. When the FA content was 20%, AAGC exhibited no strength regression, and the 28-day compressive strength was highest (75 MPa), with a relatively low degree of flexural strength regression.
- The hydration heat release rate curves of OPC and AAGC similarly included pre-induction, induction, acceleration, deceleration, and steady periods. During the induction period, the hydration heat release rate and cumulative hydration heat of OPC were lower than those of AAGC, leading to higher compressive strength of AAGC with 0-30% FA content compared to OPC. In the pre-induction period, the cumulative hydration heat of OPC gradually surpassed that of AAGC. During the steady period, the cumulative hydration heat of FA-40, FA-50, and OPC increased slowly, with FA continuing to participate in the hydration reaction, resulting in the 14-28 day compressive strength of FA-40 and FA-50 being higher than that of OPC.
- The primary hydration products of AAGC included mullite, C-S-H (PDF), calcite, zeolite, and quartz. At the same temperature, as the FA content increased, the mass loss of AAGC decreased.
- With the increase in FA content, the 28-day pore volume (<100 µm) increased, leading to lower compressive and flexural strengths of AAGC with high FA content. The early hydration heat release rate of AAGC with low FA content was high, resulting in the formation of more microcracks within the AAGC paste and causing strength regression in FA-0 and FA-10.
- As the FA content increased, the chemically bound water and pH of AAGC decreased, which was roughly consistent with the strength development trend of AAGC. The chemically bound water and pH to some extent reflected the degree of hydration reaction of AAGC.
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunant, C.F.; Joseph, S.; Prajapati, R.; Allwood, J.M. Electric recycling of Portland cement at scale. Nature 2024, 629, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Materials and Structures 2014, 47. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environmental Research Letters 2016, 11, 074029. [Google Scholar] [CrossRef]
- Zunino, F.; Dhandapani, Y.; Ben Haha, M.; Skibsted, J.; Joseph, S.; Krishnan, S.; Parashar, A.; Juenger, M.C.G.; Hanein, T.; Bernal, S.A.; et al. Hydration and mixture design of calcined clay blended cements: review by the RILEM TC 282-CCL. Materials and Structures 2022, 55. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Tuyan, M.; Nehdi, M. Alkali-activated and geopolymer materials developed using innovative manufacturing techniques: A critical review. Construction and Building Materials 2021, 303, 124483. [Google Scholar] [CrossRef]
- Sun, B.; Ye, G.; De Schutter, G. A review: Reaction mechanism and strength of slag and fly ash-based alkali-activated materials. Construction and Building Materials 2022, 326, 126843. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zheng, X.; Niu, X.; Fang, Y. Effect of active MgO on the hydration kinetics characteristics and microstructures of alkali-activated fly ash-slag materials. Construction and Building Materials 2022, 361, 129677. [Google Scholar] [CrossRef]
- Montoya, A.; Rodríguez-Barboza, L.; Colmenero, F.; Cárcel-Carrasco, J.; Gómez-Zamorano, L. Composite Cements Using Ground Granulated Blast Furnace Slag, Fly Ash, and Geothermal Silica with Alkali Activation. Buildings 2023, 13, 1854. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Yang, T.; Zhang, Z. The degradation mechanisms of alkali-activated fly ash/slag blend cements exposed to sulphuric acid. Construction and Building Materials 2018, 186, 1177–1187. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Z.; Wang, H. Early hydration kinetics and microstructure development of hybrid alkali activated cements (HAACs) at room temperature. Cement and Concrete Composites 2021, 123, 104200. [Google Scholar] [CrossRef]
- Srinivasamurthy, L.; Chevali, V.; Zhang, Z.; Longhi, M.; Loh, T.; Wang, H. Mechanical property and microstructure development in alkali activated fly ash slag blends due to efflorescence. Construction and Building Materials 2022, 332, 127273. [Google Scholar] [CrossRef]
- Feng, Y.; Xue, Z.; Baifa, Z.; Xie, J.h.; Chen, C.; Tan, J.; Zhao, C. Effects of phosphogypsum substitution on the performance of ground granulated blast furnace slag/fly ash-based alkali-activated binders. Journal of Building Engineering 2023, 70, 106387. [Google Scholar] [CrossRef]
- Soltan, M.; Abo-El-Enein, S.; Zaky Sayed, A.; El-sokkary, T.M.; Hammad, H. Incorporation of Cement Bypass Flue Dust in Fly Ash and Blast Furnace Slag-based Geopolymer. Case Studies in Construction Materials 2018, 8. [Google Scholar] [CrossRef]
- Htut, T.; Keong, L.; Parnham, G.; Rowles, M.; Ng, T. Investigation of geopolymers containing fly ash and ground-granulated blast-furnace slag blended by amorphous ratios. Construction and Building Materials 2019, 222, 731–737. [Google Scholar]
- Wang, M.; Xu, J.; Zhang, X.; Tan, L.; Mei, Y. Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials. Buildings 2024, 14. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Sun, N.; Wang, J.; Xue, K.; Xu, L.; Ren, Y.; Yan, Z.; Sima, T. Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China. Buildings 2024, 14. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, C.; Jia, Y.; Guo, W.; Zhang, Y.; Qiu, Y.; Zhao, Q. Preparation and curing method of red mud-calcium carbide slag synergistically activated fly ash-ground granulated blast furnace slag based eco-friendly geopolymer. Cement and Concrete Composites 2023, 139, 104999. [Google Scholar] [CrossRef]
- Zhonghu, W.; Zhang, H.; Pu, S.; Cai, G.; Duan, W.; Song, H.; Zeng, C.; Yang, Y. Synergistic preparation of geopolymer using electrolytic manganese residue, coal slag and granulated blast furnace slag. Journal of Building Engineering 2024, 91, 109609. [Google Scholar]
- Chandrasekaran, R.; Saha, S. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Construction and Building Materials 2017, 145, 615–620. [Google Scholar]
- Zhao, Y.; Yang, C.; Li, K.; Yang, J.; Wu, Z.; Yan, C. Mechanical Performances and Frost Resistance of Alkali-Activated Coal Gangue Cementitious Materials. Buildings 2022, 12, 2243. [Google Scholar] [CrossRef]
- Pu, S.; Zhu, Z.; Wang, W.; Duan, W.; Wu, Z.; Li, N. Water resistance of fly ash phosphoric acid-based geopolymer. Developments in the Built Environment 2022, 12. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Lv, J.; Yang, J.; He, B.; Han, P.; Bai, X.-H. Mechanical properties and hydration behaviour of circulating fluidised bed fly ash- ground granulated blast furnace slag-lime ecofriendly cementitious material. Construction and Building Materials 2023, 409, 133964. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, X.; Liu, M.; Fang, B.; Wang, C.; Mi, H. Compatibility of sodium hydroxide, sodium silicate and calcium-enriched additives in alkali-activated materials: From the perspectives of flowability, strength and microstructure. Construction and Building Materials 2023, 403, 133102. [Google Scholar] [CrossRef]
- Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Monitoring early age elastic and viscoelastic properties of alkali-activated slag mortar by means of repeated minute-long loadings. Developments in the Built Environment 2023, 16, 100275. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Guo, M.-Z.; Wang, J.; Chen, Y.-T. Impact of heat curing regime on the compressive strength and drying shrinkage of alkali-activated slag mortar. Developments in the Built Environment 2023, 14, 100123. [Google Scholar] [CrossRef]
- Guan, X.; Luo, W.; Liu, S.; García, A.; Do, H.; Li, B. Ultra-high early strength fly ash-based geopolymer paste cured by microwave radiation. Developments in the Built Environment 2023, 14, 100139. [Google Scholar] [CrossRef]
- Kamath, M.; Prashant, S.; Ralegaonkar, R. Microstructure Properties of Popular Alkali-Activated PastesCured in Ambient Temperature. Buildings 2023, 13. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I.; Fuentes, A.; Fraire-Luna, P.; Gorokhovsky, A.; Mendoza-Suarez, G. Hydration Products and Reactivity of Blast-Furnace Slag Activated by Various Alkalis. Journal of the American Ceramic Society 2003, 86, 2148–2153. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Yang, T.; Zhou, S.; Wei, H.; Zhang, Z. Calcium carbide residue as auxiliary activator for one-part sodium carbonate-activated slag cements: compressive strength, phase assemblage and environmental benefits. Construction and Building Materials 2021, 308, 125015. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, M.; Wang, A.; Zhang, Z.; Dai, J.-G. Alkali-activated materials partially activated using flue gas residues: An insight into reaction products. 2023, 371, 130760.
- Ahmad, M.; Lanping, Q.; Fang, Y.; Wang, A.; Dai, J.-G. A multiscale study on gel composition of hybrid alkali-activated materials partially utilizing air pollution control residue as an activator. Cement and Concrete Composites 2022, 136, 104856. [Google Scholar] [CrossRef]
- Kovtun, M.; Kearsley, E.; Shekhovtsova, J. Chemical acceleration of a neutral granulated blast-furnace slag activated by sodium carbonate. Cement and Concrete Research 2015, 72. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Carbonation induced phase evolution in alkali-activated slag/fly ash cements: The effect of silicate modulus of activators. Construction and Building Materials 2019, 223, 566–582. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Yang, E.-H. Alkali-activated ground granulated blast-furnace slag incorporating incinerator fly ash as a potential binder. Construction and Building Materials 2016, 112, 1005–1012. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lei, Z.; Gao, M.; Sun, J.; Tong, L.; Shunman, C.; Wang, Y. Designing low-carbon fly ash based geopolymer with red mud and blast furnace slag wastes: Performance, microstructure and mechanism. Journal of environmental management 2024, 354, 120362. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Falliano, D.; Vecchio, F.; Marano, G. Investigation on the compressive strength and durability properties of alkali-activated slag mortar: Effect of superabsorbent polymer dosage and water content. Developments in the Built Environment 2024, 17, 100322. [Google Scholar] [CrossRef]
- Nedunuri, A.; Salman, M. Fundamental understanding of the setting behaviour of the alkali activated binders based on ground granulated blast furnace slag and fly ash. Construction and Building Materials 2021, 291C, 123243. [Google Scholar] [CrossRef]
- Li, G.; Tan, H.; Zhang, J.; Deng, X.; Liu, X.; Zhongtao, L. Ground granulated blast-furnace slag/fly ash blends activated by sodium carbonate at ambient temperature. Construction and Building Materials 2021, 291, 123378. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cement and Concrete Research 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Zhu, H.; Zhang, Y.; Provis, J.; Wang, H. Effect of drying procedures on pore structure and phase evolution of alkali-activated cements. Cement and Concrete Composites 2018, 96. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, J.; Ma, T.; Wang, S.; Li, C. Modification mechanism of flue gas desulfurization gypsum on fly ash and ground granulated blast-furnace slag alkali-activated materials: Promoting green cementitious material. Construction and Building Materials 2023, 396, 132400. [Google Scholar] [CrossRef]
- Kabay, N.; Miyan, N.; Özkan, H. Basic oxygen furnace and ground granulated blast furnace slag based alkali-activated pastes: Characterization and optimization. Journal of Cleaner Production 2021, 327, 129483. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Ming, F.; Yang, C.; Chen, L.; Liu, Y. Influence of low-temperature curing on the mechanical strength, hydration process, and microstructure of alkali-activated fly ash and ground granulated blast furnace slag mortar. Construction and Building Materials 2021, 269, 121811. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient use of steel slag in alkali-activated fly ash-steel slag-ground granulated blast furnace slag ternary blends. Construction and Building Materials 2020, 259, 119814. [Google Scholar] [CrossRef]
- Li, B.; Sun, Q.; Chen, X.; Xu, Z.; Yang, L. Preparation and microstructure analysis of alkali-activated ground granulated blast furnace slag-steel slag grouting materials. Case Studies in Construction Materials 2024, 20, e03235. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Cao, X.; Liu, Q.; Zang, H. Influence of the combination of calcium oxide and sodium carbonate on the hydration reactivity of alkali-activated slag binders. Journal of Cleaner Production 2017, 171. [Google Scholar] [CrossRef]
- Eldidamony, H.; Amer, A.; El-sokkary, T.M.; Abdel-Gawwad, H. Effect of substitution of granulated slag by air-cooled slag on the properties of alkali activated slag. Ceramics International 2013, 39, 171–181. [Google Scholar] [CrossRef]
- Qing-Hua, C.; Tagnit-Hamou, A.; Sarkar, S.L. Strength and Microstructural Properties of Water Glass Activated Slag. MRS Online Proceedings Library 1991, 245, 49–54. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of combined activator of Ca(OH)2 and Na2CO3 on workability and compressive strength of alkali-activated ferronickel slag system. Cement and Concrete Composites 2021, 123, 104179. [Google Scholar] [CrossRef]
- Nedeljković, M.; Ghiassi, B.; Van der Laan, S.; li, Z.; Ye, G. Effect of curing conditions on the pore solution and carbonation resistance of alkali-activated fly ash and slag pastes. Cement and Concrete Research 2018, 116, 146–158. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore Solution Chemistry of Alkali-Activated Ground Granulated Blast Furnace Slag. Cement and Concrete Research 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Huang, G.; Wang, M.; Liu, Q.; Zhao, S.; Liu, H.; Liu, F.; Feng, L.; Song, J. Simultaneous utilization of mine tailings and steel slag for producing geopolymers: Alkali-hydrothermal activation, workability, strength, and hydration mechanism. Construction and Building Materials 2024, 414, 135029. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Yang, T.; Liu, C.; Zhang, Z. Increasing mechanical strength and acid resistance of geopolymers by incorporating different siliceous materials. Construction and Building Materials 2018, 175, 411–421. [Google Scholar] [CrossRef]
- Kohani Khoshkbijari, R.; Farahmandfar, A.; Zehni, N.; Samimi, M. Properties of ground granulated blast-furnace slag-based geopolymer mortars containing glass powder, feldspar, and metakaolin under different curing conditions. Construction and Building Materials 2024, 435, 136753. [Google Scholar] [CrossRef]
- Ates, F.; Park, K.; Kim, K.; Woo, B.-H.; Kim, H.G. Effects of treated biomass wood fly ash as a partial substitute for fly ash in a geopolymer mortar system. Construction and Building Materials 2023, 376, 1–11. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cement and Concrete Research 2017, 103. [Google Scholar] [CrossRef]
- Yuan, R. Cementitious material science. Wuhan University of Technology Press. Wuhan 1996. [Google Scholar]
- Bernal, S.; Provis, J.; Rose, V.; Mejia, R. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cement and Concrete Composites 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Shi, C. Alkali-Activated Cements and Concretes; 2003.
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Effects of slag substitution on physical and mechanical properties of fly ash- based alkali activated binders (AABs). Cement and Concrete Research 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal Reaction Kinetics and Temperature Dependence of Alkali Activation of Slag, Fly Ash and Their Blends. Construction and Building Materials 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Mahmood, A.; Babaee, M.; Foster, S.; Castel, A. Capturing the early-age physicochemical transformations of alkali-activated fly ash and slag using ultrasonic pulse velocity technique. Cement and Concrete Composites 2022, 130, 104529. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative Kinetic and Structural Analysis of Geopolymers. Part 1. The Activation of Metakaolin With Sodium Hydroxide. Thermochimica Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.; Wang, H.; Bullen, F.; Reid, A. Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin. Thermochimica Acta 2013, 565, 163–171. [Google Scholar] [CrossRef]
- G, I.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of sodium carbonate/sodium silicate activator on the rheology, geopolymerization and strength of fly ash/slag geopolymer pastes. Cement and Concrete Composites 2018, 97. [Google Scholar] [CrossRef]
- Nath, S.K.; Mukherjee, S.; Maitra, S.; Kumar, S. Kinetics study of geopolymerization of fly ash using isothermal conduction calorimetry. Journal of Thermal Analysis and Calorimetry 2017, 127, 1953–1961. [Google Scholar] [CrossRef]
- Liang, G.; Yao, W. Effect of diatomite on the reaction kinetics, early-age chemical shrinkage and microstructure of alkali-activated slag cements. Construction and Building Materials 2023, 376, 131026. [Google Scholar] [CrossRef]
- Huang, G.; lv, Y.; Ren, S.; Liao, Y.; Wang, X.-Y.; Guo, R.; Lin, R.-S. Production of low-CO2 ternary binder using red sandstone, cement, and granulated blast furnace slag: A comprehensive performance analysis. Construction and Building Materials 2024, 431, 136576. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Wu, K. Shrinkage mitigation, strength enhancement and microstructure improvement of alkali-activated slag/fly ash binders by ultrafine waste concrete powder. Composites Part B Engineering 2022, 231, 109570. [Google Scholar] [CrossRef]
- Lin, R.-S.; Han, Y.; Wang, X.-Y. Macro–meso–micro experimental studies of calcined clay limestone cement (LC3) paste subjected to elevated temperature. Cement and Concrete Composites 2021, 116, 103871. [Google Scholar] [CrossRef]
- Rivera, O.G.; Long, W.R.; Weiss, C.; Moser, R.D.; Williams, B.; Torres Cancel, K.; Gore, E.R.; Allison, P. Effect of elevated temperature on alkali-activated geopolymeric binders compared to portland cement-based binders. Cement and Concrete Research 2016, 90, 43–51. [Google Scholar] [CrossRef]
- Wang, K.; Shah, S.; Mishulovich, A. Effects of Curing Temperature and NaOH Addition on Hydration and Strength Development of Clinker-Free CKD-Fly Ash Binders. Cement and Concrete Research 2004, 34, 299–309. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; 2015.
- Silva, D.; John, V.; Ribeiro, J.L.; Roman, H.R. Pore size distribution of hydrated cement pastes modified with polymers. Cement and Concrete Research - CEM CONCR RES 2001, 31, 1177–1184. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G.; Wang, Z.; Huang, T.; Wang, P. Evolutions in the properties and microstructure of cement mortars containing hydroxyethyl methyl cellulose after controlling the air content. Cement and Concrete Composites 2022, 129, 104487. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Ning, Y.; Casas, J. Utilization of ultra-fine dredged sand from the Yangtze River in alkali-activated slag/fly ash mortars: Mechanical properties, drying shrinkage and microstructure. Case Studies in Construction Materials 2023, 19, e02264. [Google Scholar] [CrossRef]
- Luo, X.; Huang, L.; Wei, L.; Chen, M.; Zhou, Z.; Zhang, T. A technique for preparing one-part geopolymers by activating alkali-fused lithium slag with solid sodium silicate. Construction and Building Materials 2024, 435, 136817. [Google Scholar] [CrossRef]
- Zhang, M.; He, M.; Zhang, J. Mitigation of efflorescence for multi-componential geopolymer: Influence of steel slag, flue gas desulfurization gypsum and pre-curing periods. Journal of Cleaner Production 2023, 403, 136835. [Google Scholar] [CrossRef]
- Lu, X.; Tian, Y.; Jiskani, I.M.; Zhou, W.; Zhao, B.; Ding, X.; Ao, Z. Innovate geopolymer synthesis for green mine road construction: Analysis of efflorescence behavior and strength analysis. Construction and Building Materials 2023, 401, 132963. [Google Scholar] [CrossRef]
| SiO₂ | Al₂O₃ | Fe₂O₃ | CaO | TiO₂ | K₂O | SO₃ | MgO | P₂O₃ | Na₂O | |
|---|---|---|---|---|---|---|---|---|---|---|
| GGBFS | 29.43 | 14.38 | 0.90 | 44.50 | 1.61 | 0.35 | 2.34 | 5.49 | 0.03 | 0.28 |
| FA | 48.70 | 35.90 | 5.06 | 3.88 | 0.70 | 1.36 | 0.80 | 0.61 | 0.50 | 0.38 |
| OPC | 16.20 | 5.05 | 2.61 | 65.85 | 0.78 | 1.25 | 3.07 | 3.42 | 1.19 | 0.24 |
| Bulk density(kg/m3) | Apparent density (kg/m3) |
Specific surface area(m2/kg) | Porosity (%) |
|
|---|---|---|---|---|
| GGBFS | 843.13 | 2832.00 | 623.45 | 70.23 |
| FA | 863.44 | 2381.60 | 551.50 | 63.75 |
| OPC | 1163.34 | 3090.12 | 376.40 | 62.35 |
| NO. | OPC (%) |
GGBFS (%) |
FA (%) |
Na2SiO3·9H2O(%) | Water (%) |
|---|---|---|---|---|---|
| OPC | 100 | 0 | 0 | 0 | 40 |
| FA-0 | 0 | 100 | 0 | 13.75 | 32.16 |
| FA-10 | 0 | 90 | 10 | ||
| FA-20 | 0 | 80 | 20 | ||
| FA-30 | 0 | 70 | 30 | ||
| FA-40 | 0 | 60 | 40 | ||
| FA-50 | 0 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





