Submitted:
13 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Filed Detected Physiological Traits
2.4. Grain Filling Rate and Duration
2.5. Grain Yield and Weight
2.6. Data Analysis
3. Results
3.1. Physiological Traits: CCI, gs and NDVI
3.2. Chlorophyll Fluorescence Parameters
3.3. Grain Yield and Components
4. Discussion
4.1. Physiological Parameters: CCI, NDVI, and gs
4.2. Chlorophyll Fluorescence Parameters
4.3. Grain Yield and Component
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Buckley, D.H.; Drinkwater, L.E. Agricultural management and labile carbon additions affect soil microbial community structure and interact with carbon and nitrogen cycling. Micro. Ecol. 2013, 66, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends in Ecol. & Evol. 2003, 18, 182–188. [Google Scholar]
- Shennan, C.; Krupnik, T.J.; Baird, G.; Cohen, H.; Forbush, K.; Lovell, R.J.; Olimpi, E.M. Organic and conventional agriculture: A useful framing? Ann. Rev. Environ. and Reso. 2017, 42, 317–346. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. App. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Hammad, H.M.; Khaliq, A.; Abbas, F.; Farhad, W.; Fahad, S.; Aslam, M.; Shah, G.M.; Nasim, W.; Mubeen, M.; Bakhat, H.F. Comparative effects of organic and inorganic fertilizers on soil organic carbon and wheat productivity under the arid region. Comm. in Soil Sci. & Plant Anal. 2020, 51, 406–422. [Google Scholar]
- Xu, X.; Pang, D.; Chen, J.; Luo, Y.; Zheng, M.; Yin, Y.; Yi, L.; Wang, Z. Straw return accompanies by low nitrogen moderately promoted deep root. Field Crops Res. 2018, 221: 71–80.
- Chen, J.; Gong, Y.; Wang, S.; Guan, B.; Balkovic, J.; Kraxner, F. To burn or retain crop residues on croplands? An integrated analysis of crop residue management in China. Sci. Total Environ. 2019, 662, 141–150. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, M.; Feng, G.; Zhang, W.; Lu, C. Crop yield and soil organic matter after long–term straw return to soil in China. Nutr. Cycl. Agroecosys. 2015, 102, 371–381. [Google Scholar] [CrossRef]
- Xiaobing, L.; Zhi, W.; Linjie, M.; Nan, C.; Yali, M.; Zhiguo, Z. Crop residue incorporation combined with potassium fertilizer increased cotton canopy apparent photosynthesis and seed cotton yield in a barley-cotton rotation system, Archi. Agron. & Soil Sci. 2021. 67, 300-312.
- Petrescu-Mag, R.M.; Banatean-Dunea, I.; Vesa, S.C.; Copacinschi, S.; Petrescu, D.C. What do Romanian farmers think about the effects of pesticides? Perceptions and willingness to pay for bio-pesticides. Sustain. 2019, 11, 3628. [Google Scholar]
- Agegnehu, G.; Tsigie, A.; Tesfaye, A. Evaluation of crop residue retention, compost, and inorganic fertilizer application on barley productivity and soil chemical properties in the central Ethiopian highlands. Ethiop. J. Agric. Sci. 2012, 22, 45–61. [Google Scholar]
- Sarwar, G.; Schmeisky, H.; Hussain, N.; Muhammad, S.; Tahir, M.A.; Saleem, U. Variations in nutrient concentrations of wheat and paddy as affected by different levels of compost and chemical fertilizer in normal soil. Pak. J. Bot. 2009, 41, 2403–2410. [Google Scholar]
- Delgado-Baquerizo, M.; Eldridge, D.; Maestre, F.; Karunaratne, S.; Trivedi, P.; Reich, P.; Singh, B. 2017. Climate legacies drive global soil carbon stocks in terrestrial ecosystems. Sci. Adv. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Comino, J. ; Lopez-Vicente, ´ M. ; Kumar, V.; Rodríguez-Seijo, A.; Valko, ´ O.; Rojas, C.; Pourghasemi, H.R.; Salvati, L.; Bakr, N.; Vaudour, E. 2020. Soil science challenges in a new era: a transdisciplinary overview of relevant topics. Air, Soil & Water Res. 2020, 13, 1178622120977491. [Google Scholar]
- Nawaz, A.; Lal, R.; Shrestha, R.K.; Farooq, M. Mulching affects soil properties and greenhouse gas emissions under long-term no-till and plough-till systems in Alfisol of Central Ohio. Land Degrad. & Develop. 2017, 28 (2), 673–681.
- Dong, W.; Liu, E.; Wang, J.; Yan, C.; Li, J.; Zhang, Y. Impact of tillage management on the short-and long-term soil carbon dioxide emissions in the dryland of Loess Plateau in China. Geoder. 2017, 307, 38–44. [Google Scholar] [CrossRef]
- Toosi, E.R.; Kravchenko, A.N.; Guber, A.K.; Rivers, M.L. 2017. Pore characteristics regulate the priming and fate of carbon from plant residue. Soil Biol. Biochem. 113, 219–230.
- Iqbal, A.; He, L.; Ali, I.; Ullah, S.; Khan, A.; Khan, A.; Akhtar, K.; Wei, S.; Zhao, Q.; Zhang, J. Manure combined with chemical fertilizer increases rice productivity by improving soil health, post-anthesis biomass yield, and nitrogen metabolism. PLoS ONE 2020, 15, e0238934. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zi, Y.; Li, C.; Peng, Y.; Zhu, X.; Guo, W. Dry matter accumulation, partitioning, and remobilization in high-yielding wheat under rice–wheat rotation in China. Agron. J. 2016, 108, 604–614. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Lotfi R, Ghassemi-Golezani K, Najafi N. Grain filling and yield of mung bean affected by salicylic acid and silicon under salt stress. J. Plant Nutr. 2018, 41 (14): 1778-1785.
- Gorooei, A.; Gaiser, T.; Aynehband, A.; Rahnama, A.; Kamali, B. The Effect of Farming Management and Crop Rotation Systems on Chlorophyll Content, Dry Matter Translocation, and Grain Quantity and Quality of Wheat (Triticum aestivum L.) Grown in a Semi-Arid Region of Iran. Agron. 2023, 13, 1007. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco J., M. Regulation and function of root exudates. Plant Cell and Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, X.; Tian, Y.; Liu, X.; Cao, W. Analysis of common canopy vegetation indices for indicating leaf nitrogen accumulations in wheat and rice. Inter. J. Appl. Earth Observ. 2008, 10, 1–10. [Google Scholar] [CrossRef]
- Dalal, R.C.; Strong, W.M.; Doughton, J.A.; Weston, E.J.; Copper, J.E.; Wildermuth, G.B.; Lehane, K.J.; King, A.J.; Holmes, J.E. Sustaining Productivity of a Vertisol at Warra, Queensland, with Fertilizer, No-tillage, or Legumes. Aust. J. Agric. Res. 1998, 38, 489–501. [Google Scholar] [CrossRef]
- Lotfi, R.; Pessarakli, M. Effects of Crop Rotation and Tillage on Winter Wheat Growth and Yield under Cold Dryland Conditions. Crops 2023, 3, 88–100. [Google Scholar] [CrossRef]
- Yin, X.; McClure, M.A. Relationship of Corn Yield, Biomass, and Leaf Nitrogen with Normalized Difference Vegetation Index and Plant Height. Agron. J. 2013, 105, 1005–1016. [Google Scholar] [CrossRef]
- Chu, X.; Guo, Y.; He, J.; Yao, X.; Zhu, Y.; Cao, W.; Cheng, T.; Tian, Y. Comparison of Different Hyperspectral Vegetation Indices for Estimating Canopy Leaf Nitrogen Accumulation in Rice. Agron. J. 2014, 106, 1911–1920. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient, pp. 321–362. In G.C. Papageorgiou and Govindjee (eds.). Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer. 2004, Dordrecht, the Netherlands.
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, R.; Abbasi, A.; Kalaji, H.M.; Eskandari, I.; Sedghieh, V.; Khorsandi, H.; Sadeghian, N.; Yadav, S.; Rastogi, A. The role of potassium on drought resistance of winter wheat cultivars under cold dryland conditions: Probed by chlorophyll a fluorescence. Plant Phys. & Biochem., 2022, 182(3), 45-54.
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Center, M.D.; Allakhverdiev, S.I.; Goltsev. V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Phys. & Biochem., 2014, 81, 16–25. [Google Scholar]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source-sink ratios during grain filling in wheat and barley under contrasting environments. Field Crop Res. 2013, 150, 42–51. [Google Scholar] [CrossRef]
- Giunta, F.; Motzo, R.; Pruneddu, G. Has long-term selection for yield in durum wheat also induced changes in leaf and canopy traits? Field Crops Res. 2008, 106, 68–76. [Google Scholar] [CrossRef]
- Wu, X.L.; Tang, Y.L.; Li, C.S.; Wu, C.; Huang, G.; Rong, M.A. (2014). Characteristics of grain filling in wheat growing in Sichuan Basin. Acta Agron. Scien. 2014, 40(2), 337−345.
- Cossani, C.M.; Slafe, G.A.; Savin, R. Do barley and wheat (bread and durum) differ in grain weight stability through seasons and water–nitrogen treatments in a Mediterranean location? Field Crops Res. 2011, 121, 240–247. [Google Scholar] [CrossRef]
- Motzo, R.; Giunta, F.; Pruneddu, G. The response of rate and duration of grain filling to long-term selection for yield in Italian durum wheat. Crop & Past. Sci. 2010, 61, 162–169. [Google Scholar]
- Hazratkulova, S.; Sharma, R.; Alikulov, S.; Islomov, S.; Yuldashev, T.; Ziyaev, Z.; Khalikulov, Z.; Ziyadullaev, Z.; Turok, J. Analysis of genotypic variation for normalized difference vegetation index and its relationship with grain yield in winter wheat under terminal heat stress. Plant Breed. 2012, 131, 716–721. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, V.K.; Chand, R.; Sharma, S.; Kumar, U.; Jaiswal, J.P.; Choudhary, M.; Mahato, A.; Ashutosh. ; Singh, P.; Joshi, A. NDVI and grain fill duration are important to be considered in breeding for terminal heat stress tolerance in wheat. J. Agron. & Crop Sci. 2023, 209, 489–501. [Google Scholar]
- Brennan, J.; Hackett, R.; McCabe, T.; Grant, J.; Fortune, R.A.; Forristal, P.D. The effect of tillage system and residue management on grain yield and nitrogen use efficiency in winter wheat in a cool Atlantic climate. Euro. J. Agron. 2014, 54:61–69.
- Malhi, S.S.; Nyborg, M.; Solberg, E.D.; Dyck, M.F.; Puurveen, D. Improving crop yield and N uptake with long-term straw retention in two contrasting soil types. Field Crops Res. 2011, 124, 378–391. [Google Scholar] [CrossRef]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop residue contributions to phosphorus pools in agricultural soils: a review. Soil Biol. & Biochem. 2014, 74, 127–137. [Google Scholar]
- Evans, J.; Fettell, N.A.; Coventry, D.R.; O’Connor, G.E.; Walsgott, D.N.; Mahoney, J.; Armstrong, E.L. Wheat response after temperate crop legumes in south-eastern Australia. Aust. J. Agric. Res. 1991, 42, 31–43. [Google Scholar] [CrossRef]
- Geng, S.; Tan, J.; Li, L.; Miao, Y.; Wang, Y. Legumes can increase the yield of subsequent wheat with or without grain harvesting compared to Gramineae crops: A meta-analysis. Euro. J. Agron. 142, 126643.
- Cernay, C.; Makowski, D.; Pelzer, E. Preceding cultivation of grain legumes increases cereal yields under low nitrogen input conditions. Environ. Chem. Let. 2017, 16, 631–636. [Google Scholar] [CrossRef]
- Aslam, F.; Khaliq, A.; Matloo, A.; Tanveer, A.; Hussain, S.; Zahir, Z.H. 2017. Allelopathy in agro-ecosystems: a critical review of wheat allelopathy-concepts and implications. Chemoecol. 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Mohammadi, R.; Amri, A. Genotype x environment interaction for durum wheat grain yield and selection for drought tolerance in irrigated and drought environments in Iran. J. Crop Sci. & Biotech. 2011, 14(4), 265–274.
| Month | 2017-18 | 2018-19 | ||
| Precipitation (mm) |
Average temperature (˚C) | Precipitation (mm) |
Average temperature (˚C) |
|
| October | 0.2 | 11.4 | 9.7 | 13.7 |
| November | 36 | 8.5 | 47 | 5.5 |
| December | 48 | -0.8 | 91 | 2.3 |
| January | 29 | 1.4 | 41 | -2.5 |
| February | 85 | -0.9 | 86 | -1.2 |
| March | 80 | 4.3 | 56 | 0.2 |
| April | 55 | 8.6 | 116 | 5.2 |
| May | 67 | 10.4 | 43 | 9.6 |
| June | 23 | 16.7 | 4.2 | 18.1 |
| July | 0 | 24.6 | 0 | 22.7 |
| August | 0 | 24.8 | 0.5 | 23.6 |
| September | 1.8 | 20.0 | 0 | 19.2 |
| Soil Depth (cm) | Soil Texture (%) | pH | K | P | TN | OC | CaCO3 | SP | ||
| Sand | Silt | Clay | mg kg-1 | % | ||||||
| 0-30 | 39 | 42 | 19 | 7.6 | 453 | 7.33 | 0.13 | 0.66 | 7.1 | 51 |
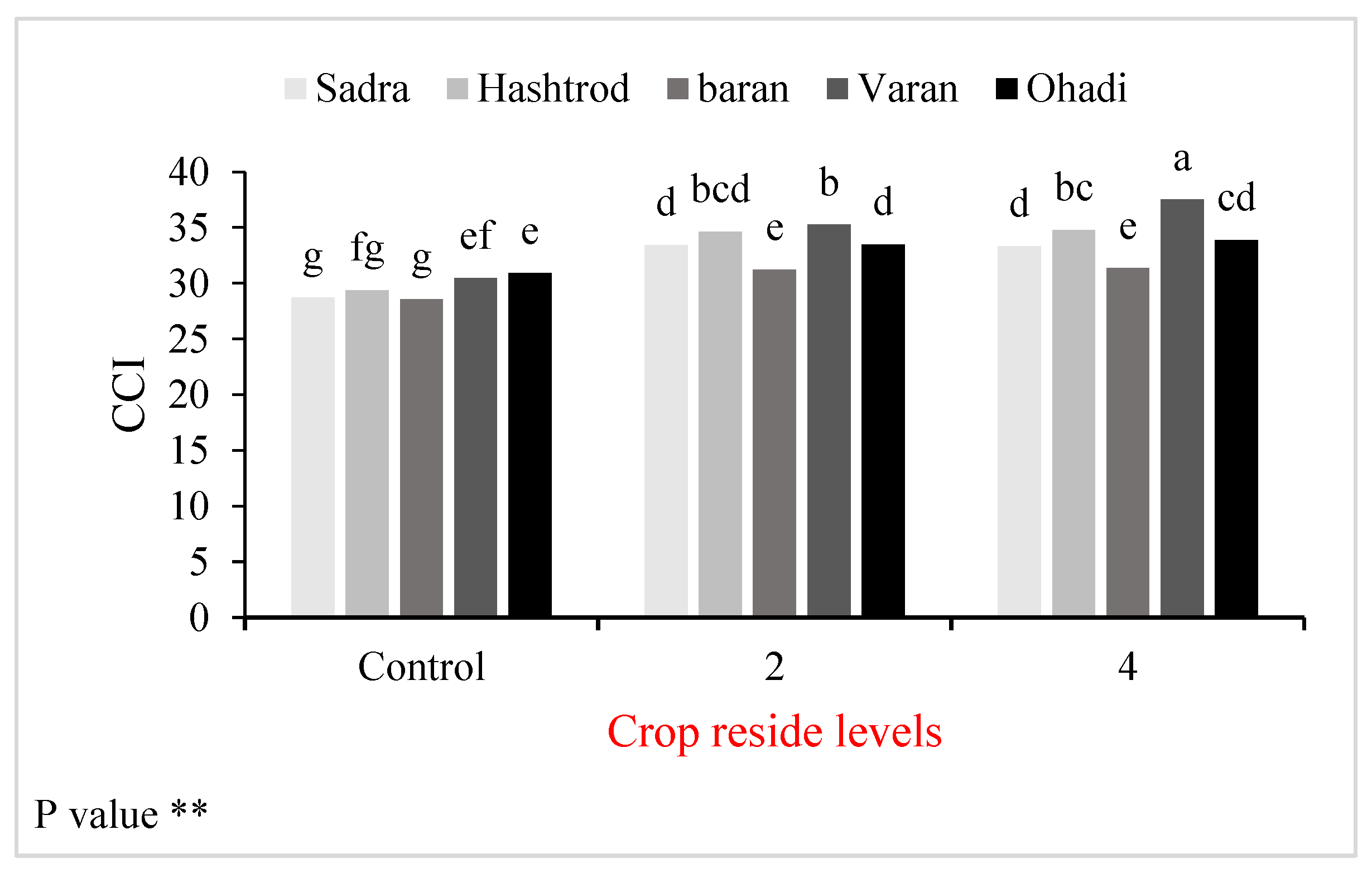
| CCI | 2017-18 | 2018-19 | ||
| P value ** | wheat-wheat | vetch-wheat | wheat-wheat | vetch-wheat |
| Sadra | 31.67 EFG | 32.74 DEF | 31.24 FG | 31.59 EFG |
| Hashtrod | 33.83 CD | 34.64 BC | 30.74 GH | 32.48 DEF |
| Baran | 29.61 HI | 32.38 DEF | 29.26 I | 30.27 GHI |
| Varan | 37.16 A | 35.99 AB | 31.44 EFG | 33.01 DE |
| Ohadi | 34.99 BC | 33.03 DE | 31.16 FG | 31.84 EFG |
| gs (mmol m-2 S-1) | ||||
| P value ** | ||||
| Sadra | 12.55 H | 13.92 E | 12.60 H | 14.51 D |
| Hashtrod | 13.34 FG | 14.34 D | 13.34 FG | 14.61 D |
| Baran | 13.77 E | 15.44 AB | 13.77 E | 15.22 BC |
| Varan | 13.72 EF | 15.66 A | 13.57 EFG | 15.79 A |
| Ohadi | 13.24 G | 15.15 BC | 13.84 E | 15.00 C |
| NDVI | wheat-wheat | vetch-wheat | ||||||
| P value * | control | 2 T ha-1 | 4 T ha-1 | control | 2 T ha-1 | 4 T ha-1 | ||
| Sadra | 0.47 FG | 0.51 BC | 0.49 DE | 0.45 HI | 0.47 FG | 0.49 DE | ||
| Hashtrod | 0.45 HI | 0.48 EF | 0.45 HI | 0.45 HI | 0.48 EF | 0.50 CD | ||
| Baran | 0.46 GH | 0.51 BC | 0.48 EF | 0.46 GH | 0.52 AB | 0.52 AB | ||
| Varan | 0.48 EF | 0.52 AB | 0.48 EF | 0.49 DE | 0.53 A | 0.53 A | ||
| Ohadi | 0.43 J | 0.48 EF | 0.44 IJ | 0.44 IJ | 0.49 DE | 0.51 BC | ||
| gs | ||||||||
| P value ** | ||||||||
| Sadra | 11.23 N | 13.45 H-K | 13.06 KL | 12.16 M | 14.97 E | 15.53 D | ||
| Hashtrod | 12.85 L | 13.75 G-J | 13.43 IJK | 13.14 KL | 14.27 F | 16.03 C | ||
| Baran | 13.21 KL | 14.23 FG | 13.87 F-I | 13.33 JKL | 15.41 DE | 17.26 A | ||
| Varan | 12.98 KL | 14.02 FG | 13.95 FGH | 12.88 L | 16.78 B | 17.52 A | ||
| Ohadi | 13.22 KL | 13.95 FGH | 13.45 H-K | 13.37 I-L | 15.12 DE | 16.74 B | ||
| Treatments | ABS/RC | VJ | VI | PI | ||
| P value | ** | ** | ** | ** | ||
| 2017-18 | wheat-wheat | control | 2.206 GH | 0.45 G | 0.53 F | 0.34 F |
| 2 T ha-1 | 3.314 B | 0.66 D | 0.77 B | 0.61 B | ||
| 4 T ha-1 | 3.558 A | 0.50 F | 0.61 E | 0.48 E | ||
| vetch-wheat | control | 2.250 FG | 0.42 H | 0.51 F | 0.35 F | |
| 2 T ha-1 | 2.924 D | 0.73 C | 0.74 BC | 0.62 B | ||
| 4 T ha-1 | 2.236 G | 0.82 A | 0.92 A | 0.67 A | ||
| 2018-19 | wheat-wheat | control | 2.1673 HI | 0.42 H | 0.46 G | 0.32 F |
| 2 T ha-1 | 3.219 C | 0.62 E | 0.66 D | 0.51 DE | ||
| 4 T ha-1 | 3.333 B | 0.50 F | 0.53 F | 0.51 DE | ||
| vetch-wheat | control | 2.280 F | 0.43 H | 0.43 G | 0.32 F | |
| 2 T ha-1 | 2.7493 E | 0.66 D | 0.66 D | 0.54 CD | ||
| 4 T ha-1 | 2.129 I | 0.75 B | 0.72 C | 0.56 C | ||
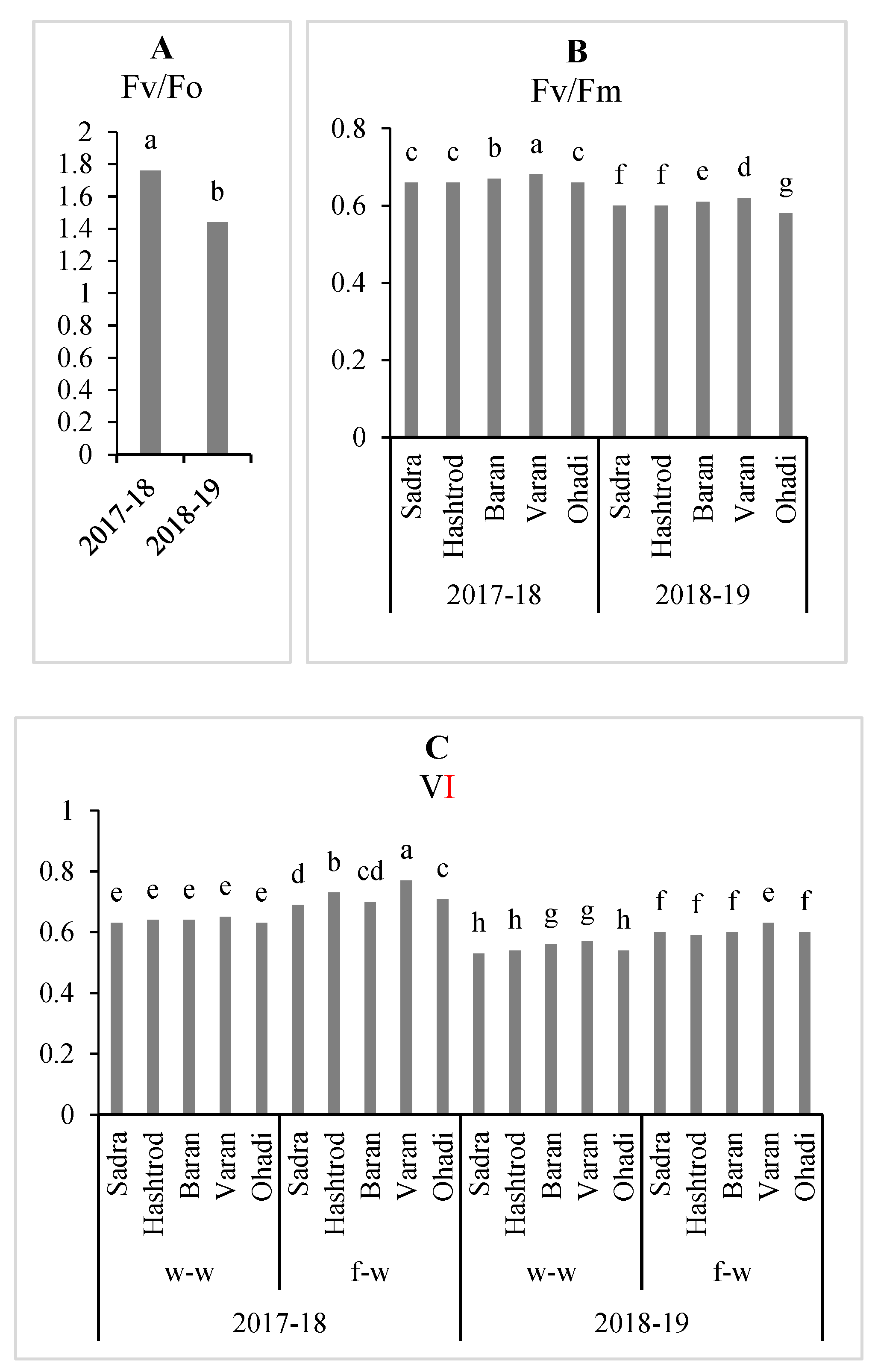
| Treatments | Fv/Fo | Fv/Fm | VJ | VI | PI | ||
| P value | ** | ** | ** | ** | ** | ||
| wheat-wheat | control | Sadra | 1.38 IJ | 0.59 KLM | 0.42 IJ | 0.49 KL | 0.34 L |
| Hashtrod | 1.46 HI | 0.60 JKL | 0.44 HI | 0.49 KL | 0.34 L | ||
| Baran | 1.28 J-M | 0.61 IJK | 0.44 HI | 0.50 K | 0.33 L | ||
| Varan | 1.29 JKL | 0.60 JKL | 0.43 HIJ | 0.5 K | 0.33 L | ||
| Ohadi | 1.19 LM | 0.58 LM | 0.43 HIJ | 0.48 KLM | 0.33 L | ||
| 2 T ha-1 | Sadra | 1.67 FG | 0.63 GHI | 0.63 F | 0.70 HI | 0.56 EF | |
| Hashtrod | 1.65 G | 0.64 FGH | 0.63 F | 0.71 H | 0.55 FG | ||
| Baran | 1.46 HI | 0.65 EFG | 0.65 E | 0.72 GH | 0.56 EF | ||
| Varan | 1.52 H | 0.67 CDE | 0.66 E | 0.75 EF | 0.60 BCD | ||
| Ohadi | 1.45 HI | 0.64 FGH | 0.63 F | 0.70 HI | 0.54 FG | ||
| 4 T ha-1 | Sadra | 1.18 M | 0.63 GHI | 0.51 G | 0.56 J | 0.53 GH | |
| Hashtrod | 1.18 M | 0.63 GHI | 0.51 G | 0.58 J | 0.49 IJ | ||
| Baran | 1.22 LM | 0.62 HIJ | 0.50 G | 0.57 J | 0.48 JK | ||
| Varan | 1.20 LM | 0.61 IJK | 0.51 G | 0.58 J | 0.51 HI | ||
| Ohadi | 1.26 LKM | 0.60 JKL | 0.49 G | 0.56 J | 0.46 K | ||
| vetch-wheat | control | Sadra | 1.53 H | 0.59 KLM | 0.45 H | 0.47 LM | 0.34 L |
| Hashtrod | 1.51 H | 0.58 LM | 0.41 J | 0.47 LM | 0.34 L | ||
| Baran | 1.37 IJ | 0.59 KLM | 0.42 IJ | 0.47 LM | 0.34 L | ||
| Varan | 1.26 KLM | 0.6 JKL | 0.42 IJ | 0.48 KLM | 0.33 L | ||
| Ohadi | 1.33 JK | 0.57 M | 0.42 IJ | 0.46 M | 0.33 L | ||
| 2 T ha-1 | Sadra | 1.83 E | 0.67 CDE | 0.70 CD | 0.68 I | 0.59 CD | |
| Hashtrod | 1.94 D | 0.67 CDE | 0.71 CD | 0.70 HI | 0.58 DE | ||
| Baran | 1.76 EF | 0.68 BCD | 0.69 D | 0.71 H | 0.58 DE | ||
| Varan | 1.97 D | 0.70 AB | 0.72 C | 0.74 FG | 0.59 CD | ||
| Ohadi | 1.66 G | 0.66 DEF | 0.66 E | 0.68 I | 0.58 DE | ||
| 4 T ha-1 | Sadra | 2.30 B | 0.68 BCD | 0.79 A | 0.79 CD | 0.61 BC | |
| Hashtrod | 2.25 B | 0.68 BCD | 0.77 B | 0.81 BC | 0.61 BC | ||
| Baran | 2.33 AB | 0.69 BC | 0.80 A | 0.77 DE | 0.62 B | ||
| Varan | 2.40 A | 0.72 A | 0.81 A | 0.89 A | 0.66 A | ||
| Ohadi | 2.13 C | 0.67 CDE | 0.77 B | 0.82 B | 0.61 BC | ||
| Treatments | 1000 GW (g) |
GFR (mg grain-1 day-1) |
GFD (day) |
GY (t ha-1) |
||
| P value | ** | ** | ** | * | ||
| wheat-wheat | control | Sadra | 35.26 S | 0.77 DE | 37.52 FG | 2.2941 K |
| Hashtrod | 35.78 R | 0.77 DE | 37.49 FG | 2.5258 J | ||
| Baran | 36.86 NO | 0.79 BC | 37.28 FGH | 2.5910 IJ | ||
| Varan | 37.07 L-N | 0.80 B | 37.27 FGH | 2.7381 GH | ||
| Ohadi | 36.51 PQ | 0.77 DE | 37.58 F | 1.8849 M | ||
| 2 T ha-1 | Sadra | 36.33 Q | 0.70 HI | 36.59 I | 2.5832 IJ | |
| Hashtrod | 36.43 Q | 0.71 GH | 36.92 F-I | 2.6409 HIJ | ||
| Baran | 37.74 HI | 0.77 DE | 36.64 HI | 2.9173 EF | ||
| Varan | 38.04 FG | 0.78 CD | 37.22 F-I | 3.0400 DE | ||
| Ohadi | 36.97 MNO | 0.75 F | 38.15 E | 2.1031 L | ||
| 4 T ha-1 | Sadra | 37.30 JKL | 0.61 L | 36.62 HI | 2.5365 J | |
| Hashtrod | 37.57 IJ | 0.64 K | 37.1 F-I | 2.6107 HIJ | ||
| Baran | 38.62 CD | 0.72 G | 37.11 F-I | 2.8448 FG | ||
| Varan | 38.86 C | 0.71 GH | 37.16 F-I | 3.0761 CD | ||
| Ohadi | 37.26 KLM | 0.71 GH | 36.89 GHI | 2.0758 L | ||
| vetch-wheat | control | Sadra | 36.73 OP | 0.75 F | 37.22 F-I | 2.6216 HIJ |
| Hashtrod | 37.247 LM | 0.76 EF | 37.21 F-I | 2.6599 HIJ | ||
| Baran | 37.61 HIJ | 0.82 A | 35.63 J | 2.7254 GHI | ||
| Varan | 37.95 GH | 0.83 A | 35.45 J | 2.6518 HIJ | ||
| Ohadi | 37.75 HI | 0.79 BC | 36.7 HI | 1.9110 M | ||
| 2 T ha-1 | Sadra | 37.73 HI | 0.69 I | 38.64 DE | 3.0793 CD | |
| Hashtrod | 37.66 HIJ | 0.69 I | 38.63 DE | 3.1949 C | ||
| Baran | 38.81 GHI | 0.75 F | 39.11 CD | 3.3286 B | ||
| Varan | 38.67 CD | 0.76 EF | 39.35 BC | 3.4491 B | ||
| Ohadi | 37.507 IJK | 0.72 G | 39.83 AB | 2.7091 G-I | ||
| 4 T ha-1 | Sadra | 38.243 EF | 0.65 K | 38.76 CD | 3.3456 B | |
| Hashtrod | 38.443 DE | 0.67 J | 38.89 CD | 3.3667 B | ||
| Baran | 40.107 A | 0.69 I | 39.85 AB | 3.7032 A | ||
| Varan | 40.387 A | 0.71 GH | 39.82 AB | 3.8099 A | ||
| Ohadi | 39.353 B | 0.69 I | 40.09 A | 2.8307 FG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





