1. Introduction
The management of newly diagnosed brain metastases (BMs) encompasses a spectrum of therapeutic modalities, including surgical interventions, radiotherapies, and other adjunctive treatments [
1,
2,
3]. Among radiotherapies, stereotactic radiotherapy (SRT) is commonly delivered in a single fraction (radiosurgery), which achieves excellent local control (LC) with low toxicity. However, this approach is not suitable for patients with tumors larger than 2-3 cm at the maximum diameter because radiosurgery is associated with a higher incidence of radiation-induced brain necrosis (BN) than fractionated SRT [
4,
5,
6]. Fractionated SRT for large BMs aims to deliver highly conformal treatments while improving LC and decreasing neurotoxicity through potential dose escalations and the inter-fraction repair of normal tissues [
7,
8].
BN is a well-characterized adverse effect of SRT and is occasionally associated with serious neurological sequelae. BN encompasses a broad clinical spectrum; patients may present with an incidental imaging finding in the absence of symptoms or with symptoms including neurological deficits, headaches, and seizures [
9]. While many risk factors have been implicated in the development of BN, including the prescribed radiation dose, treated volume, histology, and the use of concurrent systemic therapies, they have almost exclusively been validated in patients treated with radiosurgery for small lesions [
6]. Few studies have investigated the effects of the fractionated SRT dose, fractionation, and target volume on the risk of BN [
6]. Dose-volume predictors of the normal brain have not yet been established in fractionated SRT [
6,
10]. Therefore, the purpose of the present study was to evaluate potentially modifiable dose-volume metrics that predict BN after fractionated SRT.
2. Materials and Methods
2.1. Study Design and Patients
Patients treated with SRT for BMs at a single institution between 2012 and 2021 were reviewed. SRT for BMs was performed according to prospective protocols [
11]. Patients who fulfilled the following inclusion criteria were treated with SRT: (1) World Health Organization performance status of 0–2, (2) patient conditions allowing the same body position in an immobilizing device for more than 20 min, and (3) BM number ≤10. Exclusion criteria were as follows: (1) previous surgery or history of radiotherapy for BM, (2) meningitis carcinomatosa, (3) pregnancy or potential pregnancy, (4) psychiatric disorders, and (5) contraindication to iodine or gadolinium. Informed consent was obtained from all patients or their guardians.
2.2. Radiotherapy Details
Patients were placed in a supine position and a thermoplastic mask was molded to the head and attached to the head support. The planning target volume (PTV) denoted a visible tumor on computed tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI) plus 0-2 mm depending on nearby critical organs.
In the first protocol employed between 2012 and 2016, the basic prescribed dose was 35 Gy/5 fractions (fr). A dose of 30 Gy/3 fr was permitted for small lesions (maximum diameter <1.5 cm), while 37.5 Gy/5 fr was used for large lesions (≥3 cm). Since SRT for large BMs (≥15 cc) was associated with a risk of neurotoxicity, we employed different fractionation protocols for these tumors [
11]. Between 2017 and 2021, the protocol for large BMs was revised to reduce the incidence of BN. Treatment for large lesions (≥15 cc) with 35 Gy or 37.5 Gy/5 fr was superseded by 40 Gy/10 fr. In the new protocol, 30 Gy/3 fr for small lesions and 35 Gy/ 5 fr for other lesions remained.
Dose constraints were applied to adjacent structures; the maximum doses to the brain stem, optic nerve, and optic chiasm were limited to <36 Gy/10 fr, 25 Gy/5 fr, or 18 Gy/3 fr [
7]. To satisfy these limitations, the intensity-modulated irradiation technique was applied. SRT was delivered with tomotherapy. Treatment was performed three times a week to efficiently utilize reoxygenation phenomena [
12,
13]. Any systematic agent was not allowed around the time of these treatments [
3].
2.3. Patient Follow-Ups, Endpoints, and Dose-Volume Parameters
Patients were regularly followed up with physical examinations and contrast-enhanced MRI after SRT. Local recurrence was defined as a ≥20% increase in the maximum diameter of the contrast-enhanced tumor on MRI or CT. BN was pathologically verified or diagnosed by perfusion and functional MRI (fMRI) or C-11 methionine positron emission tomography (MetPET) [
14,
15]. Toxicities were recorded according to the Common Terminology Criteria for AEs v.4.0, the Japanese Clinical Oncology Group version.
To examine dose-volume predictors of BN, the physical dose to the targets and the normal brain (VxGy) were extracted for every 1 Gy. The conformity index (CI) and uniformity index (UI) were calculated according to the following formulae [
16].
Abbreviations in these formulae are as follows: VPTV = PTV (cc), TVPV = lesion volume (cc) covered by the prescribed isodose, VTV = prescribed isodose volume (cc), D5% = minimum dose delivered to 5% of PTV. Lower CI indicates higher conformity, while lower UI indicates better homogeneity. Ideal CI and UI are both 1.
Biological equivalent doses (BED) to the brain with an α/β ratio of 2 Gy were calculated using the linear-quadratic model to assess different fractionation schedules [
12,
13]. A conversion table after rounding figures in each schedule is shown in
Table 1. In this analysis, the normal brain (the brain minus visible tumors) volume receiving xx Gy BED in 2 Gy fractions was described as VxxGyE.
2.4. Statistical Analysis
Fisher’s exact test or a one-way analysis of variance was applied to compare categorical or continuous variables. Overall survival (OS), LC, and symptomatic BN (grade 2) probabilities were evaluated using the Kaplan–Meier method from the start of SRT. The cumulative incidence of local recurrence was calculated, accounting for death as a competing risk. Death and local recurrence were assumed as risks of BN.
A logistic regression analysis (LRA) was conducted to identify BN dosimetric predictors. Multicollinearity in dose-volume parameters introduces errors in a multivariate regression analysis. Collinearity was tested using Pearson’s correlation coefficient (PCC) between each dose-volume parameter, assuming collinearity as >0.5 absolute PCC in this study [
17,
18]. The sample size was calculated with Smeden’s formula [
19,
20]. Given 2-4 candidate predictors and an outcome proportion of 0.08, a sample size of at least 56-105 participants was required to target a mean absolute error of 0.05 between observed and true outcome probabilities.
With deviations from the linear-quadratic model in a high dose per fraction schedule [
12,
13], VxxGyE in the 3-fraction group may not correspond biologically to that in the 5- or 10-fraction group. Therefore, LRA was also conducted after excluding the 3-fraction group.
All analyses were performed in EZR, which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [
21].
3. Results
3.1. Patient Characteristics, Treatment Details, and Outcomes
Patient and treatment details are summarized in
Table 2. In total, 112 patients with 215 BMs were treated with these protocols. Among them, 76 patients (68%) had lung cancers and 106 had extracranial lesions. Three-fraction SRT was delivered to 34 patients with 74 BMs, 5-fraction SRT to 58 with 106 BMs, and 10-fraction SRT to 20 with 35 BMs. Mean target volumes in the 3-, 5-, and 10-fraction groups were 4.3±4.7, 15.4±14.9, and 25.9±13 cc, respectively (
p <0.00001). Of 215 BMs, 32 were ≥15 cc. Five BMs are located in the brainstem, 4 in the thalamus, and 2 in the basal ganglia. Seventy BMs (33%) were in the frontal cortex or subcortex, 38 (18%) in the parietal lobe, 36 (17%) in the occipital lobe, 35 (16%) in the temporal lobe, and 25 (12%) in the cerebrum. Age, sex, extracranial disease, primary cancer, CI and UI did not significantly differ between these 3 groups (
p >0.08) (
Table 2).
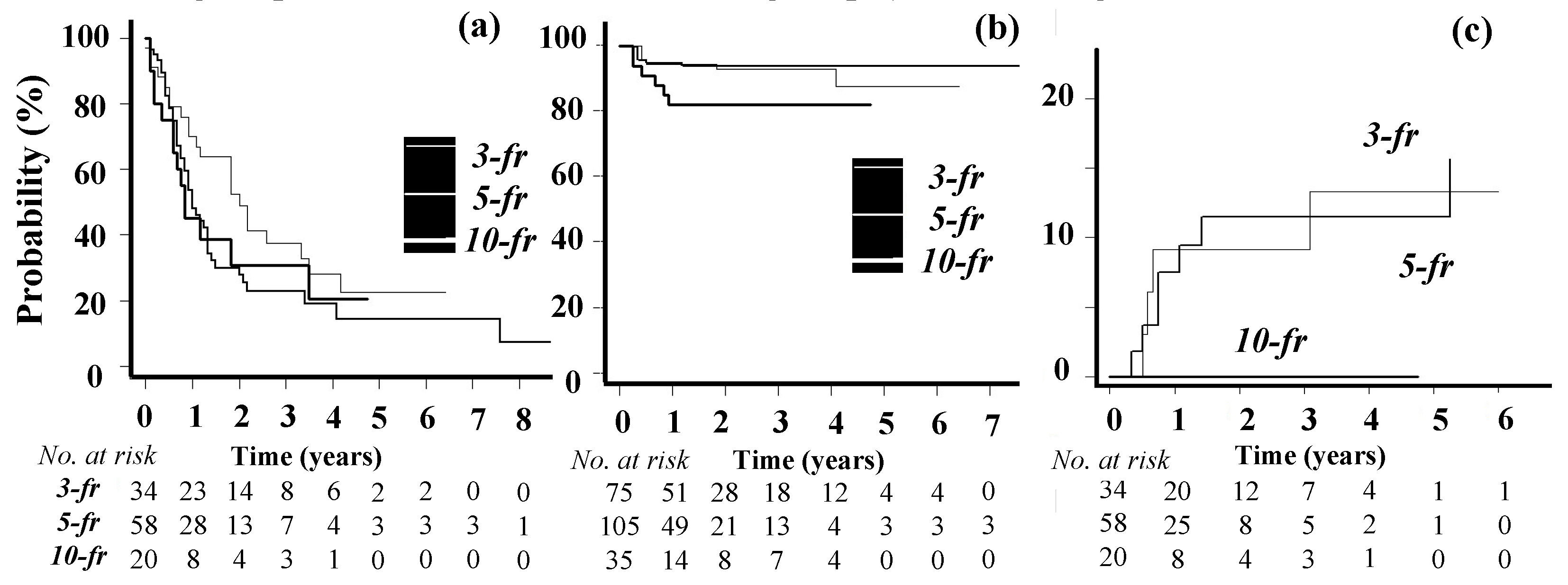
OS and LC curves are shown in
Figure 1a and
Figure 1b. The 1-year OS rate was 54% (median, 13 months). During the follow-up period, new BMs developed in 36 patients. One-year LC rates were 92% in the 3-fraction group, 94% in the 5-fraction group, and 82% in the 10-fraction group (Gray’s test,
p = 0.11).
3.2. Toxicities
Grade 2 seizure was observed in 8 patients and grade 2 headache in 1. Grade 5 and 1 intratumor bleeding occurred in 1 patient each. BN (≥grade 2) developed in 4 patients in the 3-fraction group, 7 in the 5-fraction group, and 0 in the 10-fraction group. Among them, 5 cases were pathologically confirmed following craniotomy. Two cases were diagnosed using MetPET, and the other 4 by fMRI and were verified after the follow-up. Grade 1 BN was observed in 3 patients in the 3-fraction group, 2 in the 5-fraction group, and 1 in the 10-fraction group. Grade 1 BN was asymptomatic and diagnosed with fMRI and local recurrence was not suspected in any case after careful follow-ups. The incidence of BN (≥grade 2) at 1 year in the Kaplan-Meir method was 9% in the 3-fraction group, 8% in the 5-fraction group, and 0% in the 10-fraction group (
p = 0.29) (
Figure 1c).
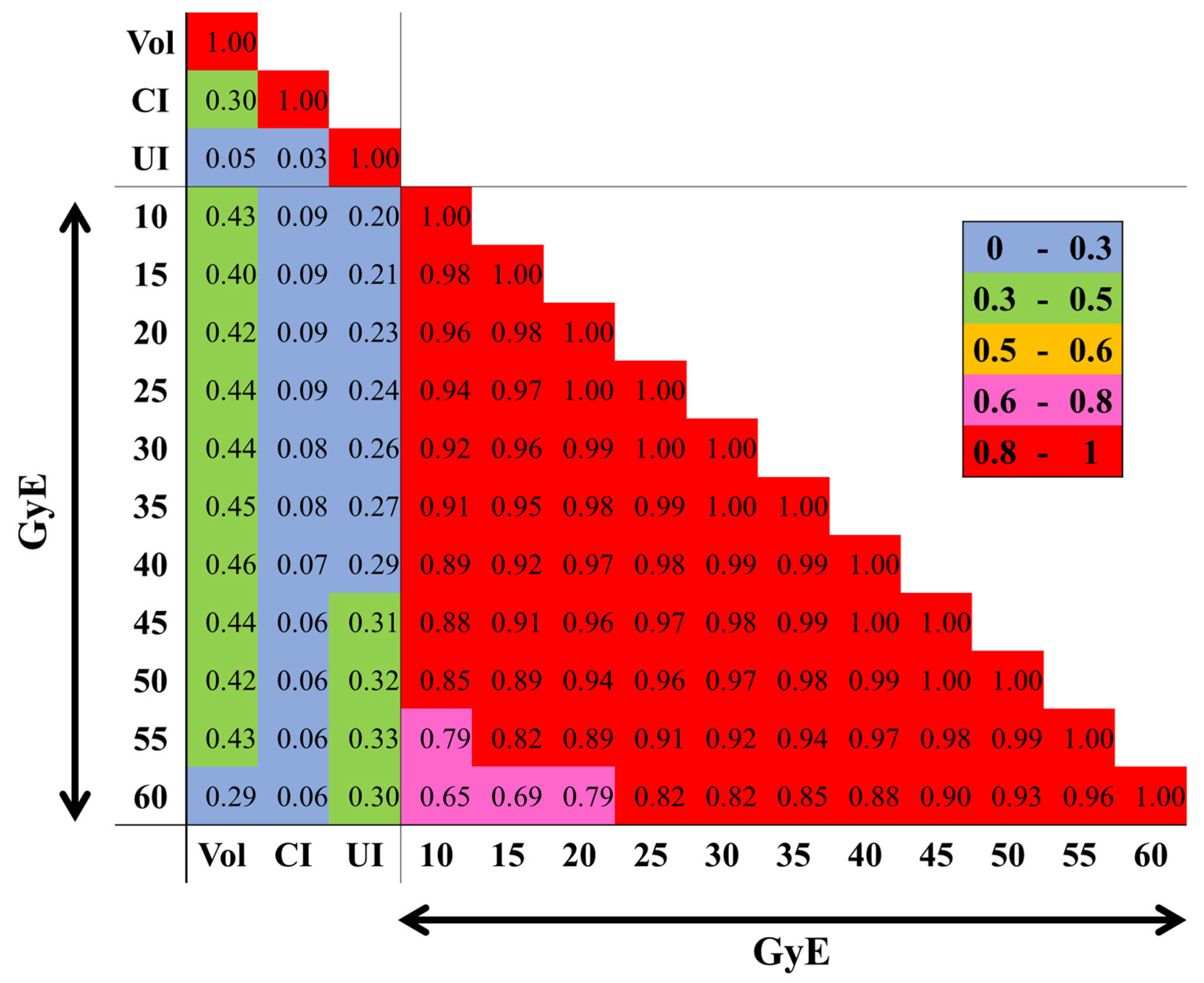
3.3. Multivariate Analyses of BN
Figure 2 shows how each dosimetric variable is correlated to one another. Strong multicollinearity (absolute PCC ≥0.65) appeared between each dose. Therefore, PTV (cc), CI, UI, and each VxxGyE (cc) were included as continuous variables in LRA to examine the models [
22,
23]. In analyses of 112 patients, no significant parameter was identified (
Figure 3a and
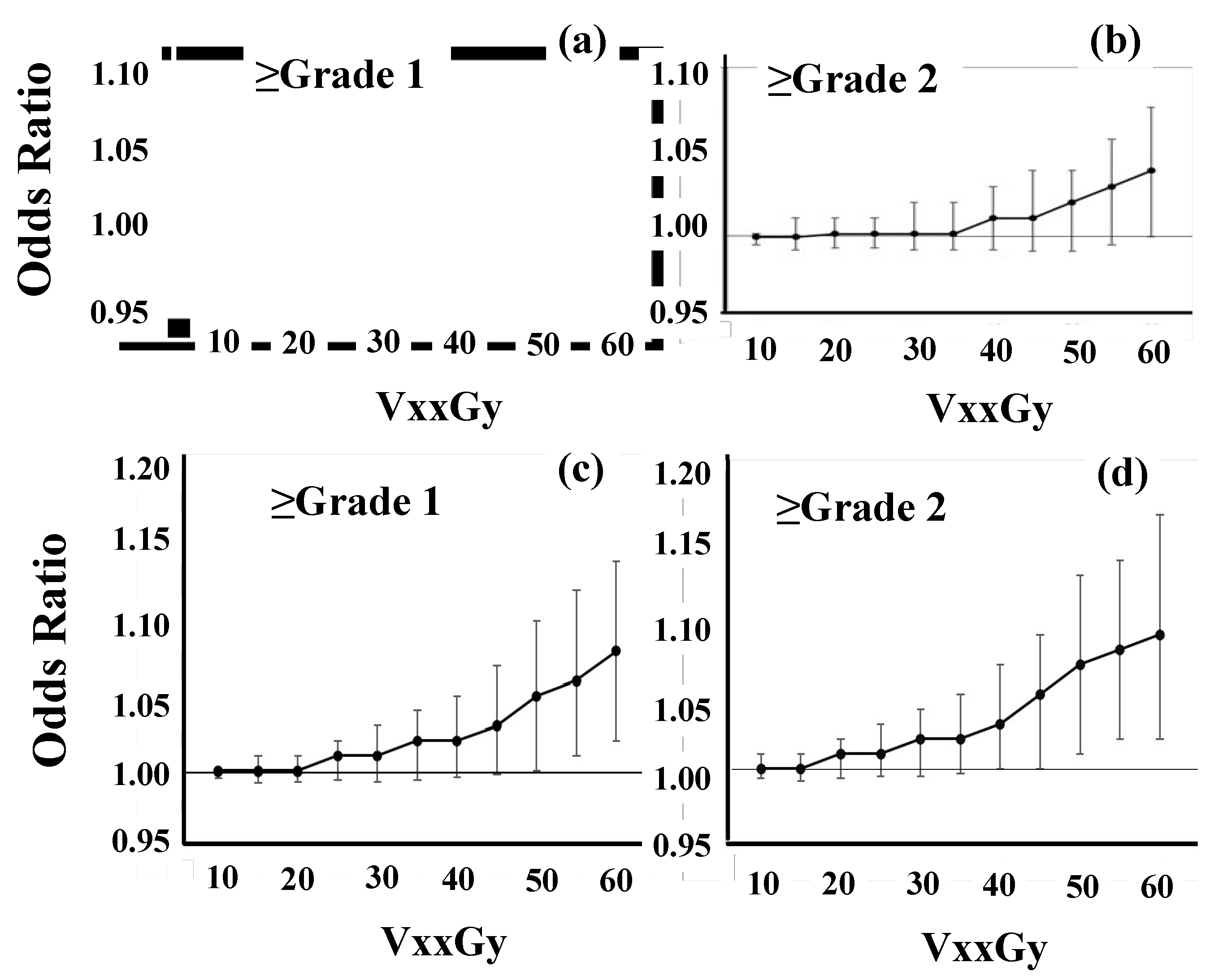
Figure 3b).
After excluding the 3-fraction group, the incidence of BN was higher in patients with larger V50GyE, V55GyE, and V60GyE (
Figure 3c and
Figure 3d). Odds ratios for ≥grade 1 and ≥grade 2 BN were 1.06 (95% confidence interval: 1.01-1.12,
p <0.03) and 1.06 (1.01-1.12,
p <0.02) for V55GyE (cc), respectively (
Table S1). Similar results were observed in cases with larger V50GyE and V60GyE. In consideration of previously reported sample sizes and risks [
4], PTV (cc) and each VxxGyE (cc) were included in the final LRA (
Table 3). V50-60GyE appeared to be a significant predictor of grades 1 and 2 BN.
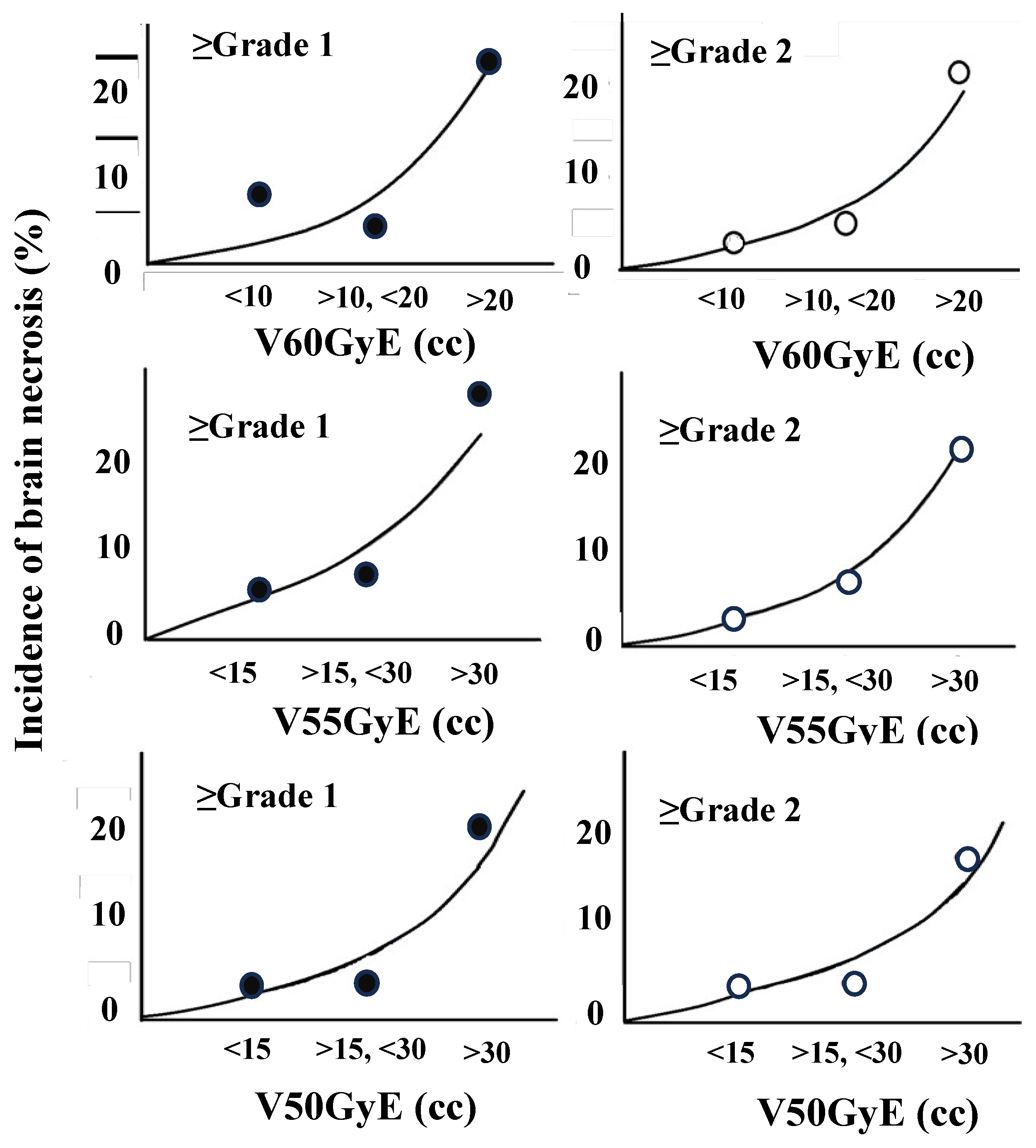
The incidences of BN (≥grade 2) were 3% (1/35) (V60GyE ≤10 cc; mean ± standard deviation, 4.4 ± 2.8cc), 5% (1/21) (>10 cc, ≤20 cc; 13.5 ± 2.2 cc), and 21% (5/29) (>20 cc; 35.4 ± 15.3 cc) (
Figure 4, V60GyE (cc)). BN (≥grade 2) occurred in 3% (1/35) (V55GyE ≤15 cc; 7.6 ± 4.1 cc), 8% (2/28) (>15 cc, ≤30 cc; 21.3 ± 4.9 cc), and 22% (5/23) (> 30 cc; 48.1 ± 17.7 cc) and also in 4% (V50GyE ≤15 cc; 8.1 ± 4.0 cc), 4% (>15 cc, ≤30 cc; 20.4 ± 3.8 cc), and 18% (>30 cc; 48.4 ± 21.6 cc) (
Figure 4, V55GyE (cc) and V50GyE (cc)).
These 2 variables were categorized into 3 groups on an ad hoc basis. The odds ratios of BN (≥grade 2) were significantly higher with V60GyE >20 cc (
p = 0.04) or V55GyE >30 cc (
p = 0.04) (
Table 4 and
Table S2).
4. Discussion
With improvements in the prognosis of patients with advanced-stage cancer, the incidence of BN is recognized as the dose-limiting toxicity of SRT for BMs. In single-fraction SRT, V12Gy of the normal brain is canonically regarded as a dose-limiting indicator based on clinical data for small BMs [
24]. While large lesions are often treated with fractionated SRT in actual clinical settings [
4,
8,
25,
26], dose-volume predictors of the normal brain have not yet been established. The present study conducted comprehensive dose-volume analyses of BM patients receiving fractionated SRT. In the 5- and 10-fraction groups, normal brain volume receiving high BED was correlated with higher BN incidence. The incidence of BN was less than 8% for V60GyE ≤20 cc or V55GyE ≤30 cc. These doses correspond to 28 or 30 Gy/5 fr and 37 or 40 Gy/10 fr, respectively. Therefore, these results suggest that the following dose constraints should be at least maintained: V28Gy <30 cc or V30 <20 cc in 5-fraction SRT and V37Gy <30 cc or V40 <20 cc in 10-fraction SRT. In addition, this result implies that PTV margin should be cut off as much as possible to reduce high BED volume.
The latest guidelines [
1,
5,
6,
10] suggest provisional dose constraints of the normal brain in fractionated SRT for BMs. In the consensus statement, V25Gy, V28.8Gy, and V30 of the normal brain in 5-fraction SRT cannot exceed 16, 7, or 10.5-30 cc, respectively. This recommendation is based on 2 clinical studies. Inoue et al. [
27] examined 85 BMs in 78 patients. There were 16 lesions with V28.8Gy ≥7.0 cc, and two developed extensive brain edema due to BN. None of the patients with V28.8Gy <7.0 cc developed edema that required surgical intervention. Andreaska et al. [
28] conducted a multi-institutional retrospective review of 117 BMs in 83 patients treated with 5-fraction SRT. In lesions without prior SRT, V25Gy >16 cc and V30Gy >10 cc were associated with a significantly higher risk of BN. Although these findings provide insights into dose-volume predictors, these reports do not mention collinearity between parameters and the reason why these parameters are included. Multicollinearity is a statistical phenomenon characterized by strong correlations or dependencies among predictor variables in a regression model [
18]. It occurs when 2 or more variables strongly correlate with each other, making it difficult for the model to differentiate the individual effects of each variable on the dependent variable. Errors stemming from violations of the multicollinearity assumption are relevant to radiation dose-volume research. Due to strong correlations among variables derived from points along individual organ dose-volume histogram curves, dose-volume analyses are susceptible to multicollinearity errors. The present study analyzed dose-volume parameters comprehensively in consideration of the multicollinearity of each parameter, suggesting that higher BED rather than lower affected the incidence of BN in 5- or 10-fraction SRT. The result partially supports the guidelines’ recommendations [
1,
5,
6,
10].
In the context of pathophysiology of BN, there are 2 main theories: i) glial cell damage and ii) vascular injury [
9]. In the first scenario, radiation may also damage glial cells. Radiation-induced cell damage leads to the accumulation of double-strand deoxyribonucleic acid (dsDNA) in the cytosol of tumor, stromal, endothelial, and immune cells, activating the cGAS-STING pathway [
29,
30]. In this pathway, cGAS, an enzyme that recognizes cytosolic dsDNA, induces the up-regulation of type 1 interferons and dendritic cell activation, ultimately triggering various inflammatory effector responses. A higher radiation dose, to a certain degree, induced the greater accumulation of dsDNA in the cytosol. Therefore, it is reasonable that higher BED has potential as a dose-volume predictor.
In contrast to 5- and 10-fraction SRT outcomes, BN predictors were not clarified in 3-fraction SRT in the present study. These results are partially explained by linear-quadratic model limitations. The model fits well if a single-fraction dose was less than 2-fold of the organ α/β ratio. With a higher dose per fraction, the quadratic cell-killing component dominates in the model, and the deviation becomes evident [
12,
13]. Therefore, VxxGyE in the 3-fraction group may not correspond biologically to that in the 5- or 10-fraction group. In addition, radiation disrupts the blood-brain barrier, resulting in increased capillary leakiness and vascular permeability in the second scenario of the BN pathophysiology [
9,
29]. Radiation, particularly in large fraction sizes >8 Gy, activates acid sphingomyelinase and induces the up-regulation of ceramide, which causes anarchic vessel sprouting resulting in ischemia and cell death. These pathologies of BN in the 3-fraction group may be different from those in the 5- and 10-fraction groups.
There are several limitations in the present study. Since the study cohort was mainly treated for large BMs, the incidence of BN may have been higher than with small lesions. Furthermore, SRT was delivered with tomotherapy co-planer irradiation. In addition, potential biases cannot be excluded from the case-control design. Therefore, a larger prospective registry cohort is needed to address these limitations.
5. Conclusions
This comprehensive analysis suggests that at least V55GyE ≤30 cc or V60GyE ≤20 cc should be maintained to lower the risk of BN in 5- or 10-fraction SRT. The dose constraints are; V28Gy <15 cc or V30Gy <10 cc in 5-fraction SRT and V37Gy <15 cc or V40Gy <10 cc in 10-fraction SRT. In addition, PTV margin should be cut off as much as possible.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Logistic regression analyses of grade 1 or 2 brain necrosis in 5- and 10-fraction groups, and Table S2: Logistic regression analyses of grade 1 brain necrosis in 5- and 10-fraction groups after categorizing variables.
Author Contributions
TM devised the concept for this manuscript and created the manuscript’s original draft. YS and AH critically reviewed the manuscript’s content, provided essential revisions, and offered expert commentary regarding the reference articles that shaped the manuscript’s content. TM, YK, and YE collected dosimetry data and TM, ST, NK, TT, NT, and YS collected pathological and clinical data. All listed authors reviewed the manuscript and provided meaningful contributions to its creation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI Grant Number 23K07197.
Institutional Review Board Statement
The present study was approved by the Institutional Review Board of Nagoya City University (No. 60-19-0207).
Informed Consent Statement
Patient consent was waived due to the need for informed consent as part of the study approval in line with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. Therefore, research content was disclosed in the form of opt-out on the website.
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author, Taro Murai. These data are not publicly accessible as they contain information that could compromise the privacy of the research participants.
Conflicts of Interest
Taro Murai has received research support from Telix Pharmaceuticals and honorariums for educational seminars from Novartis. All other authors have no conflicts of interest to declare.
References
- Schiff, D.; Messersmith, H.; Brastianos, P.K.; Brown, P.D.; Burri, S.; Dunn, I.F.; Gaspar, L.E.; Gondi, V.; Jordan, J.T.; Maues, J.; et al. Radiation Therapy for Brain Metastases: ASCO Guideline Endorsement of ASTRO Guideline. J Clin Oncol 2022, 40, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Benkhaled, S.; Schiappacasse, L.; Awde, A.; Kinj, R. Stereotactic Radiosurgery and Stereotactic Fractionated Radiotherapy in the Management of Brain Metastases. Cancers (Basel) 2024, 16, 1093. [Google Scholar] [CrossRef] [PubMed]
- Burney, I.A.; Aal Hamad, A.H.; Hashmi, S.F.A.; Ahmad, N.; Pervez, N. Evolution of the Management of Brain Metastases: A Bibliometric Analysis. Cancers (Basel) 2023, 15, 5570. [Google Scholar] [CrossRef] [PubMed]
- Crouzen, J.A.; Petoukhova, A.L.; Broekman, M.L.D.; Fiocco, M.; Fisscher, U.J.; Franssen, J.H.; Gadellaa-van Hooijdonk, C.G.M.; Kerkhof, M.; Kiderlen, M.; Mast, M.E.; et al. SAFESTEREO: Phase II Randomized Trial to Compare Stereotactic Radiosurgery with Fractionated Stereotactic Radiosurgery for Brain Metastases. BMC Cancer 2023, 23, 273. [Google Scholar] [CrossRef]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I.; et al. Tumor Control Probability of Radiosurgery and Fractionated Stereotactic Radiosurgery for Brain Metastases. Int J Radiat Oncol Biol Phys 2021, 110, 53–67. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int J Radiat Oncol Biol Phys 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Murai, T.; Ogino, H.; Manabe, Y.; Iwabuchi, M.; Okumura, T.; Matsushita, Y.; Tsuji, Y.; Suzuki, H.; Shibamoto, Y. Fractionated Stereotactic Radiotherapy Using CyberKnife for the Treatment of Large Brain Metastases: A Dose Escalation Study. Clin Oncol (R Coll Radiol) 2014, 26, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys 2019, 103, 618–630. [Google Scholar] [CrossRef]
- Vaios, E.J.; Winter, S.F.; Shih, H.A.; Dietrich, J.; Peters, K.B.; Floyd, S.R.; Kirkpatrick, J.P.; Reitman, Z.J. Novel Mechanisms and Future Opportunities for the Management of Radiation Necrosis in Patients Treated for Brain Metastases in the Era of Immunotherapy. Cancers (Basel) 2023, 15, 2432. [Google Scholar] [CrossRef]
- Ladbury, C.; Pennock, M.; Yilmaz, T.; Ankrah, N.K.; Andraos, T.; Gogineni, E.; Kim, G.G.; Gibbs, I.; Shih, H.A.; Hattangadi-Gluth, J.; et al. : Stereotactic Radiosurgery in the Management of Brain Metastases: A Case-Based Radiosurgery Society Practice Guideline. Adv Radiat Oncol 2024, 9, 101402. [Google Scholar] [CrossRef]
- Murai, T.; Hayashi, A.; Manabe, Y.; Sugie, C.; Takaoka, T.; Yanagi, T.; Oguri, T.; Matsuo, M.; Mori, Y.; Shibamoto, Y. Efficacy of Stereotactic Radiotherapy for Brain Metastases Using Dynamic Jaws Technology in the Helical Tomotherapy System. Br J Radiol 2016, 89, 20160374. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Miyakawa, A.; Otsuka, S.; Iwata, H. Radiobiology of Hypofractionated Stereotactic Radiotherapy: What Are the Optimal Fractionation Schedules? J Radiat Res 2016, 57, i76–i82. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Shibamoto, Y.; Iwata, H.; Murata, R.; Sugie, C.; Ito, M.; Ogino, H. Compatibility of the Linear-Quadratic Formalism and Biologically Effective Dose Concept to High-Dose-Per-Fraction Irradiation in a Murine Tumor. Int J Radiat Oncol Biol Phys 2011, 81, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Sato, J.; Akahane, M.; Furuta, T.; Mori, H.; Abe, O. Recognizing Radiation-induced Changes in the Central Nervous System: Where to Look and What to Look For. Radiographics 2021, 41, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Mayo, Z.S.; Halima, A.; Broughman, J.R.; Smile, T.D.; Tom, M.C.; Murphy, E.S.; Suh, J.H.; Lo, S.S.; Barnett, G.H.; Wu, G.; et al. Radiation Necrosis or Tumor Progression? A Review of the Radiographic Modalities Used in the Diagnosis of Cerebral Radiation Necrosis. J Neurooncol 2023, 161, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Shibamoto, Y.; Manabe, Y.; Murata, R.; Sugie, C.; Hayashi, A.; Ito, H.; Miyoshi, Y. Intensity-Modulated Radiation Therapy Using Static Ports of Tomotherapy (TomoDirect): Comparison with the TomoHelical Mode. Radiat Oncol 2013, 8, 68. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Ellsworth, S.G.; van Rossum, P.S.N.; Mohan, R.; Lin, S.H.; Grassberger, C.; Hobbs, B. Declarations of Independence: How Embedded Multicollinearity Errors Affect Dosimetric and Other Complex Analyses in Radiation Oncology. Int J Radiat Oncol Biol Phys 2023, 117, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E., Jr.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- van Smeden, M.; Moons, K.G.; de Groot, J.A.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. Sample Size for Binary Logistic Prediction Models: Beyond Events Per Variable Criteria. Stat Methods Med Res 2019, 28, 2455–2474. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Ogundimu, E.O.; Cook, J.A.; Manach, Y.L.; Altman, D.G. Quantifying the Impact of Different Approaches for Handling Continuous Predictors on the Performance of a Prognostic Model. Stat Med 2016, 35, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dhiman, P.; Qi, C.; Bullock, G.; van Smeden, M.; Riley, R.D.; Collins, G.S. Poor Handling of Continuous Predictors in Clinical Prediction Models Using Logistic Regression: A Systematic Review. J Clin Epidemiol 2023, 161, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Korytko, T.; Radivoyevitch, T.; Colussi, V.; Wessels, B.W.; Pillai, K.; Maciunas, R.J.; Einstein, D.B. 12 Gy Gamma Knife Radiosurgical Volume Is a Predictor for Radiation Necrosis in Non-AVM Intracranial Tumors. Int J Radiat Oncol Biol Phys 2006, 64, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Gutschenritter, T.; Venur, V.A.; Combs, S.E.; Vellayappan, B.; Patel, A.P.; Foote, M.; Redmond, K.J.; Wang, T.J.C.; Sahgal, A.; Chao, S.T.; et al. The Judicious Use of Stereotactic Radiosurgery and Hypofractionated Stereotactic Radiotherapy in the Management of Large Brain Metastases. Cancers (Basel) 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 x 9 gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.K.; Sato, H.; Seto, K.; Torikai, K.; Suzuki, Y.; Saitoh, J.; Noda, S.E.; Nakano, T. Five-Fraction CyberKnife Radiotherapy for Large Brain Metastases in Critical Areas: Impact on the Surrounding Brain Volumes Circumscribed with a Single Dose Equivalent of 14 Gy (V14) to Avoid Radiation Necrosis. J Radiat Res 2014, 55, 334–342. [Google Scholar] [CrossRef]
- Andruska, N.; Kennedy, W.R.; Bonestroo, L.; Anderson, R.; Huang, Y.; Robinson, C.G.; Abraham, C.; Tsien, C.; Knutson, N.; Rich, K.M.; et al. Dosimetric Predictors of Symptomatic Radiation Necrosis after Five-Fraction Radiosurgery for Brain Metastases. Radiother Oncol 2021, 156, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Constanzo, J.; Faget, J.; Ursino, C.; Badie, C.; Pouget, J.P. Radiation-Induced Immunity and Toxicities: The Versatility of the cGAS-STING Pathway. Front Immunol 2021, 12, 680503. [Google Scholar] [CrossRef]
- Loganadane, G.; Dhermain, F.; Louvel, G.; Kauv, P.; Deutsch, E.; Le Pechoux, C.; Levy, A. Brain Radiation Necrosis: Current Management with a Focus on Non-Small Cell Lung Cancer Patients. Front Oncol 2018, 8, 336. [Google Scholar] [CrossRef]
Figure 1.
(a) Overall survival Kaplan-Maier curves in 3-, 5-, and 10-fraction groups, (b) local control curves of BMs treated with 3-, 5-, and 10-fraction SRT, and (c) the incidence of grade 2 or higher brain necrosis. -fr; –fraction groups.
Figure 1.
(a) Overall survival Kaplan-Maier curves in 3-, 5-, and 10-fraction groups, (b) local control curves of BMs treated with 3-, 5-, and 10-fraction SRT, and (c) the incidence of grade 2 or higher brain necrosis. -fr; –fraction groups.
Figure 2.
Correlation heatmap of each dosimetric parameter. Absolute PCCs between CI, Vol, UI and each VxxGyE are shown. Colors depicted in the vertical bar on the right side reflect positive (absolute PCC >0.5) and negative (≤0.5) correlations. CI; conformity index, PCC; Pearson’s correlation coefficient, UI; uniformity index, Vol; planning target volume (cc), VxxGyE; the normal brain volume (cc) receiving a xx Gy biological equivalent dose in 2 Gy fractions.
Figure 2.
Correlation heatmap of each dosimetric parameter. Absolute PCCs between CI, Vol, UI and each VxxGyE are shown. Colors depicted in the vertical bar on the right side reflect positive (absolute PCC >0.5) and negative (≤0.5) correlations. CI; conformity index, PCC; Pearson’s correlation coefficient, UI; uniformity index, Vol; planning target volume (cc), VxxGyE; the normal brain volume (cc) receiving a xx Gy biological equivalent dose in 2 Gy fractions.
Figure 3.
Odds ratios in logistic regression analyses. In model development, variables included VxxGy (cc), CI, UI, and PTV (cc). Dots indicate the odds ratio of VxxGy and bars are 95% confidence intervals. (a, b) The risk of grade 1 or 2 brain necrosis in all 112 patients. (c, d) After excluding the 3-fraction group.
Figure 3.
Odds ratios in logistic regression analyses. In model development, variables included VxxGy (cc), CI, UI, and PTV (cc). Dots indicate the odds ratio of VxxGy and bars are 95% confidence intervals. (a, b) The risk of grade 1 or 2 brain necrosis in all 112 patients. (c, d) After excluding the 3-fraction group.
Figure 4.
Incidence of brain necrosis (%) in V50GyE, V55GyE, and V60GyE. The incidences of brain necrosis (≥grade 2) were 3% (V60GyE ≤10 cc), 5% (>10 cc, ≤20 cc), and 21% (>20 cc)(V60GyE). Brain necrosis (≥grade 2) occurred in 3% (V55GyE ≤10 cc), 8% (>15 cc, ≤30 cc), and 22% (>30 cc) and also in 4% (V50GyE ≤15 cc), 4% (>15 cc, ≤30 cc), and 18% (>0 cc).
Figure 4.
Incidence of brain necrosis (%) in V50GyE, V55GyE, and V60GyE. The incidences of brain necrosis (≥grade 2) were 3% (V60GyE ≤10 cc), 5% (>10 cc, ≤20 cc), and 21% (>20 cc)(V60GyE). Brain necrosis (≥grade 2) occurred in 3% (V55GyE ≤10 cc), 8% (>15 cc, ≤30 cc), and 22% (>30 cc) and also in 4% (V50GyE ≤15 cc), 4% (>15 cc, ≤30 cc), and 18% (>0 cc).
Table 1.
Conversion table in each fraction schedule.
Table 1.
Conversion table in each fraction schedule.
| |
|
Biological equivalent dose in 2 Gy/fr |
fr No. |
10 |
15 |
20 |
25 |
30 |
35 |
40 |
45 |
50 |
55 |
60 |
| Dose (Gy) |
3 |
8 |
10 |
12 |
14 |
16 |
17 |
19 |
20 |
21 |
23 |
24 |
| 5 |
10 |
14 |
15 |
17 |
20 |
21 |
23 |
25 |
27 |
28 |
30 |
| 10 |
12 |
16 |
20 |
23 |
26 |
28 |
31 |
33 |
35 |
37 |
40 |
Table 2.
Patient characteristics and treatment details.
Table 2.
Patient characteristics and treatment details.
| |
|
|
3 -fraction |
5-fraction |
10-fraction |
| Patient number |
34 |
58 |
20 |
| Age |
(mean ± SD) |
67.1 ± 10.0 |
65.9 ± 9.9 |
65.2 ± 16.2 |
| Sex (female, male) |
8, 26 |
27, 31 |
8, 12 |
| Extracranial disease (+, -) |
31, 2 |
56, 2 |
19, 1 |
| Performance status (0, 1, 2) |
6, 25, 3 |
9, 41, 8 |
6, 7, 7 |
| Primary cancer (patient No) |
|
|
|
| |
Lung cancer |
29 |
37 |
10 |
| |
GI cancer |
2 |
5 |
3 |
| |
Breast cancer |
1 |
4 |
2 |
| |
Renal cancer |
0 |
4 |
0 |
| |
Sarcoma |
0 |
2 |
1 |
| |
Urothelial cancer |
1 |
2 |
1 |
| |
Others |
1 |
4 |
3 |
| Total BM No. |
75 |
105 |
35 |
| median (range)/patient |
1.5 (1-9) |
1 (1-8) |
1 (1-6) |
| |
single, multiple |
17, 17 |
38, 20 |
13, 7 |
| Total PTV (cc) (mean ± SD) |
4.3 ± 4.7 |
15.4 ± 14.9 |
25.9 ± 13.0 |
| Prescribed dose (Gy) * |
30 (18-30) |
35 (30-37.5) |
40 (36-40) |
| D95%(Gy) (mean ± SD) |
29.2 ± 1.9 |
34.2 ± 2.7 |
37.7 ± 1.7 |
| D98%(Gy) (mean ± SD) |
28.7 ±1.9 |
33.6 ± 2.8 |
37.0 ± 1.9 |
| D2%(Gy) (mean ± SD) |
32.1 ± 2.1 |
37.8 ± 3.1 |
41.5 ± 2.2 |
| CI (mean ± SD) |
3.05 ± 7.13 |
1.85 ± 3.18 |
1.16 ± 0.63 |
| UI (mean ± SD) |
1.09 ± 0.05 |
1.10 ± 0.08 |
1.09 ± 0.06 |
Table 3.
Logistic regression analyses of grade 1 or 2 brain necrosis in 5- and 10-fraction groups.
Table 3.
Logistic regression analyses of grade 1 or 2 brain necrosis in 5- and 10-fraction groups.
| Grade 1 brain necrosis |
| |
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
0.98 |
(0.93-1.04) |
0.52 |
| V60GyE (cc) |
1.07 |
(1.02-1.12) |
0.01 |
| |
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
0.98 |
(0.93-1.04) |
0.48 |
| V55GyE (cc) |
1.05 |
(1.01-1.1) |
0.02 |
| |
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
0.98 |
(0.93-1.04) |
0.49 |
| V50GyE (cc) |
1.04 |
(1-1.08) |
0.04 |
| Grade 2 brain necrosis |
| |
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
0.99 |
(0.93-1.05) |
0.68 |
| V60GyE (cc) |
1.09 |
(1.03-1.15) |
0.005 |
| |
Odds ratio |
(95% confidence interval) |
p-value |
| PTV(cc). |
0.99 |
(0.93-1.05) |
0.63 |
| V55GyE(cc) |
1.07 |
(1.00-1.12) |
0.01 |
| |
Odds ratio |
(95% confidence interval) |
p-value |
| PTV(cc). |
0.99 |
(0.93-1.05) |
0.63 |
| V50GyE(cc) |
1.06 |
(1.01-1.11) |
0.01 |
Table 4.
Logistic regression analyses of grade 2 brain necrosis in 5- and 10-fraction groups after categorizing variables.
Table 4.
Logistic regression analyses of grade 2 brain necrosis in 5- and 10-fraction groups after categorizing variables.
| |
|
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
(<8) |
1.00 |
|
|
| |
(≥8, <15) |
0.15 |
(0.01-3.18) |
0.22 |
| |
(≥15) |
0.26 |
(0.02-3.52) |
0.31 |
| V60GyE (cc) |
(<10) |
1.00 |
|
|
| |
(≥10, <20) |
1.81 |
(0.10-32.1) |
0.69 |
| |
(≥20) |
18.1 |
(1.14-290) |
0.04 |
| |
|
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
(<8) |
1.00 |
|
|
| |
(≥8, <15) |
0.17 |
(0.01-3.33) |
0.24 |
| |
(≥15) |
0.19 |
(0.01-2.94) |
0.23 |
| V55GyE (cc) |
(<15) |
1.00 |
|
|
| |
(≥15, <30) |
4.45 |
(0.33-59.8) |
0.26 |
| |
(≥30) |
28.7 |
(1.19-691) |
0.04 |
| |
|
Odds ratio |
(95% confidence interval) |
p-value |
| PTV (cc) |
(<8) |
1.00 |
|
|
| |
(≥8, <15) |
0.26 |
(0.02-4.09) |
0.34 |
| |
(≥15) |
0.39 |
(0.04-3.61) |
0.40 |
| V50GyE (cc) |
(<15) |
1.00 |
|
|
| |
(≥15, <30) |
1.43 |
(0.08-25.3) |
0.81 |
| |
(≥30) |
9.28 |
(0.69-126) |
0.09 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).