Submitted:
13 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables
2.2.1. Lipid Parameters
2.2.2. Immunological Parameters
2.2.3. Other Parameters
2.3. Statistical Analysis
3. Results
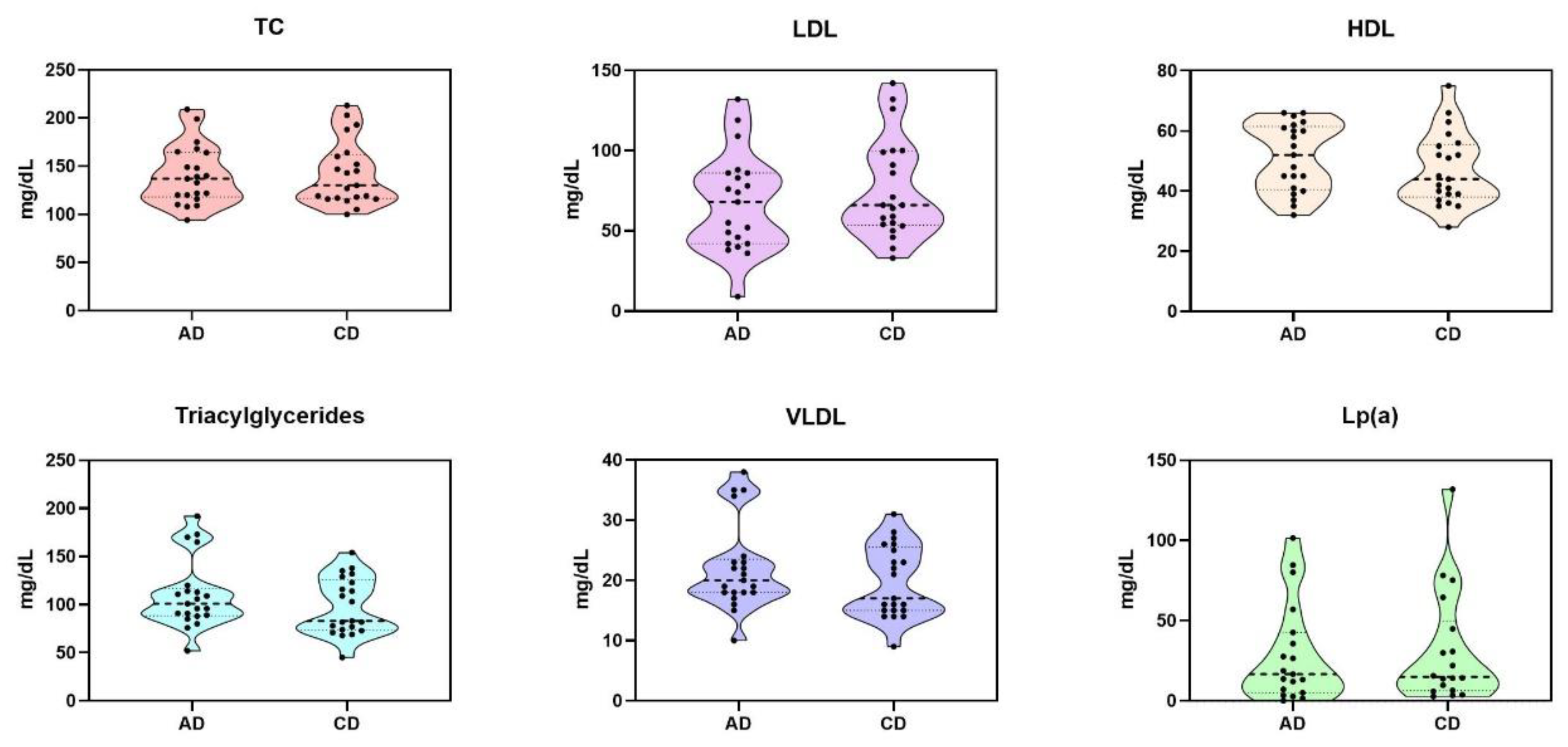
3.1. Lipidic Profile Results
3.2. Complement Levels
3.3. Leucocyte Count and Subpopulations
3.4. Inflammatory and Nutritional Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul;139(2). [CrossRef]
- Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–14. [CrossRef]
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Oct;108(17):2154–69.
- Briasoulis A, Bakri GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013 Mar;15(3). [CrossRef]
- Ahmed M, Alalawi F, Alnour H, Gulzar K, Alhadari A. Five-Year Mortality Analysis in Hemodialysis Patients in a Single-Center in Dubai. Saudi Journal of Kidney Diseases and Transplantation. 2020 Sep;31(5):1062–8. [CrossRef]
- de Arriba G, Avila GG, Guinea MT, Alia IM, Herruzo JA, Ruiz BR, et al. Mortality of hemodialysis patients is associated with their clinical situation at the start of treatment. Nefrología (English Edition). 2021 Jul;41(4):461–6. [CrossRef]
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. American Journal of Kidney Diseases. 2019 Mar;73(3):A7–8.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun;139(25):E1082–143. [CrossRef]
- Mach F, Baigent C, Catapano AL, Koskina KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019 Nov;290:140–205.
- Alloubani A, Nimer R, Samara R. Relationship between Hyperlipidemia, Cardiovascular Disease and Stroke: A Systematic Review. Curr Cardiol Rev. 2021 Dec;17(6):051121189015. [CrossRef]
- Official Journal of the international Society of nephrology KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. 2013 [cited 2024 Jul 3]. Available from: www.publicationethics.org.
- Sarnak MJ, Bloom R, Muntner P, Rahman M, Saland JM, Wilson PWF, et al. KDOQI US commentary on the 2013 KDIGO Clinical Practice Guideline for Lipid Management in CKD. Am J Kidney Dis. 2015 Mar;65(3):354–66. [CrossRef]
- Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. 2018 Dec;14(12):727–49. [CrossRef]
- Soohoo M, Moradi H, Obi Y, Kovesdy CP, Kalantar-Zadeh K, Streja E. Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million U.S. veterans. J Clin Lipidol. 2019 Sep;13(5):744-753.e15. [CrossRef]
- Huang Y, Zhong Q, Chen J, Qin X, Yang Y, He Y, et al. Relationship of serum total cholesterol and triglyceride with risk of mortality in maintenance hemodialysis patients: a multicenter prospective cohort study. Ren Fail. 2024;46(1). [CrossRef]
- Kopecky C, Ebtehaj S, Genser B, Drechsler C, Krane V, Antlanger M, et al. HDL Cholesterol efflux does not predict cardiovascular risk in hemodialysis patients. Journal of the American Society of Nephrology. 2017 Mar;28(3):769–75. [CrossRef]
- Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, et al. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol. 2014 May;25(5):1073–82. [CrossRef]
- Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. 2014;29(8):1554–62. [CrossRef]
- Yu J, Xia X, Lin T, Huang N, Qiu Y, Yang X, et al. Non-high-density lipoprotein cholesterol and mortality among peritoneal dialysis patients. J Clin Lipidol. 2021 Sep;15(5):732–42. [CrossRef]
- Xie X, Zhang X, Xiang S, Yan X, Huang H, Tian Y, et al. Association of very Low-density Lipoprotein Cholesterol with All-cause and Cardiovascular Mortality in Peritoneal Dialysis. Kidney Blood Press Res. 2017 May;42(1):52–61. [CrossRef]
- Webb AT, Reaveley DA, O’Donnell M, O’Connor B, Seed M, Brown EA. Lipoprotein (a) in patients on maintenance haemodialysis and continuous ambulatory peritoneal dialysis. Nephrology Dialysis Transplantation. 1993 Jan;8(7):609–13. [CrossRef]
- Goldwasser P, Michel MA, Collier J, Mittman N, Fein PA, Gusik SA, et al. Prealbumin and Lipoprotein(a) in Hemodialysis: Relationships With Patient and Vascular Access Survival. American Journal of Kidney Diseases. 1993 Jul;22(1):215–25. [CrossRef]
- Cressman MD, Heyka RJ, Paganini EP, O’Neil J, Skibinski CI, Hoff HF. Lipoprotein(a) is an independent risk factor for cardiovascular disease in hemodialysis patients. Circulation. 1992;86(2):475–82. [CrossRef]
- Gonzáles-Rubianes DZ, Figueroa-Osorio LK, Benites-Zapata VA, Pacheco-Mendoza J, Herrera-Añazco P. Utility of TG/HDL-c ratio as a predictor of mortality and cardiovascular disease in patients with chronic kidney disease undergoing hemodialysis: A systematic review. Hemodial Int. 2022 Apr;26(2):137–46. [CrossRef]
- Wanner C, Krane V, März W, Olschewski M, Mann JFE, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005 Jul;353(3):238–48. [CrossRef]
- Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009 Apr;360(14):1395–407.
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. 2013;2013(9). [CrossRef]
- Kim JE, Park S, Kim M seok, Kang SJ, Lee JW, Kim KS, et al. Statin initiation and all-cause mortality in incident statin-naïve dialysis patients. Atherosclerosis. 2021 Nov; 337:59–65. [CrossRef]
- Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. [CrossRef]
- Nowak KL, Chonchol M. Does Inflammation Affect Outcomes in Dialysis Patients? Semin Dial. 2018 Jul;31(4):388.
- Yang Y, Xu Y, Liu S, Lu P, Zhou H, Yang M. The systemic inflammation indexes predict all-cause mortality in peritoneal dialysis patients. Ren Fail. 2023;45(1). [CrossRef]
- Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrology Dialysis Transplantation. 2008 Jul;23(7):2337–43. [CrossRef]
- Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004 Jan;291(4):451–9.
- Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002;61(5):1887–93. [CrossRef]
- Gao P, Rong HH, Lu T, Tang G, Si LY, Lederer JA, et al. The CD4/CD8 ratio is associated with coronary artery disease (CAD) in elderly Chinese patients. Int Immunopharmacol. 2017 Jan;42:39–43.
- Selathurai A, Deswaerte V, Kanellakis P, Tipping P, Toh BH, Bobik A, et al. Natural killer (NK) cells augment atherosclerosis by cytotoxic-dependent mechanisms. Cardiovasc Res. 2014;102(1):128–37. [CrossRef]
- Hertle E, Van Greevenbroek MMJ, Stehouwer CDA. Complement C3: an emerging risk factor in cardiometabolic disease. Diabetologia. 2012 Apr;55(4):881. [CrossRef]
- Palikhe A, Sinisalo J, Seppänen M, Haario H, Meri S, Valtonen V, et al. Serum complement C3/C4 ratio, a novel marker for recurrent cardiovascular events. Am J Cardiol. 2007 Apr;99(7):890–5. [CrossRef]
- Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013 Feb;25(1):47–53. [CrossRef]
- de Sequera Ortiz P, Pérez García R, Molina Nuñez M, et al. Prospective randomised multicentre study to demonstrate the benefits of haemodialysis without acetate (with citrate): ABC-treat Study. Acute effect of citrate. Estudio prospectivo aleatorizado multicéntrico para demostrar los beneficios de la hemodiálisis sin acetato (con citrato): Estudio ABC-treat. Efecto agudo del citrato. Nefrologia (Engl Ed). 2019;39(4):424-433. [CrossRef]
- Molina Nuñez M, De Alarcón R, Roca S, Álvarez G, Ros MS, Jimeno C, et al. Citrate versus Acetate-Based Dialysate in On-Line Haemodiafiltration. A Prospective Cross-Over Study. Blood Purif. 2015 May;39(1–3):181–7.
- Broseta JJ, López-Romero LC, Cerveró A, Devesa-Such R, Soldevila A, Bea-Granell S, et al. Improvements in Inflammation and Calcium Balance of Citrate versus Acetate as Dialysate Buffer in Maintenance Hemodialysis: A Unicentric, Cross-Over, Prospective Study. Blood Purif. 2021 Sep;50(6):914–20. [CrossRef]
- Mahmood D, Stegmayr BG. Haemodialysis with Tinzaparin Versus Dialysate Citrate as Anticoagulation. Blood Purif. 2018 Jul;46(3):257–63. [CrossRef]
- Morin RJ, Srikantaiah M v., Woodley Z, Davidson WD. Effect of hemodialysis with acetate vs bicarbonate on plasma lipid and lipoprotein levels in uremic patients. J Dial. 1980;4(1):9–20. [CrossRef]
- de Sequera P, Pérez-García R, Molina M, et al. Advantages of the use of citrate over acetate as a stabilizer in hemodialysis fluid: A randomized ABC-treat study. Nefrologia (Engl Ed). 2022;42(3):327-337. [CrossRef]
- Wu X, Zhou L, Zhan X, Wen Y, Wang X, Feng X, et al. Low-Density Lipoprotein Cholesterol and Mortality in Peritoneal Dialysis. Front Nutr. 2022 Jul;9. [CrossRef]
- Shen Y, Schmaderer C, Ossadnik A, Hammitzsch A, Carbajo-Lozoya J, Bachmann Q, et al. Immunophenotypic Characterization of Citrate-Containing A Concentrates in Maintenance Hemodialysis: A Pre-Post Study. Int J Nephrol. 2023;2023. [CrossRef]
- Ahbap E, Sakaci T, Kara E, Sahutoglu T, Koc Y, Basturk T, et al. Neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin Nephrol. 2016 Apr;85(4):199–208. [CrossRef]
- Castilho JL, Shepherd BE, Koethe J, Turner M, Bebawy S, Logan J, et al. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS. 2016 Mar;30(6):899–907. [CrossRef]
- Morell EB, Cabeza JS, Muñoz Á, Marín I, Masiá M, Gutiérrez F, et al. The CD4/CD8 Ratio is Inversely Associated with Carotid Intima-Media Thickness Progression in Human Immunodeficiency Virus-Infected Patients on Antiretroviral Treatment. AIDS Res Hum Retroviruses. 2016 Jul;32(7):648–53.
- Bernal E, Serrano J, Perez A, Valero S, Garcia E, Marín I, et al. The CD4:CD8 ratio is associated with IMT progression in HIV-infected patients on antiretroviral treatment. J Int AIDS Soc. 2014 Nov;17(4 Suppl 3). [CrossRef]
- Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010 Jan;24(2):243. [CrossRef]
- van Dijk RA, Duinisveld AJF, Schaapherder AF, Mulder-Stapel A, Hamming JF, Kuiper J, et al. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc. 2015;4(4). [CrossRef]
- Lisowska KA, Dębska-Ślizień A, Jasiulewicz A, Heleniak Z, Bryl E, Witkowski JM. Hemodialysis Affects Phenotype and Proliferation of CD4-Positive T Lymphocytes. J Clin Immunol. 2012;32(1):189. [CrossRef]
- Bonacina F, Moregola A, Svecla M, Coe D, Uboldi P, Fraire S, et al. The low-density lipoprotein receptor–mTORC1 axis coordinates CD8+ T cell activation. J Cell Biol. 2022 Nov;221(11). [CrossRef]
- Hedrick CC. Lymphocytes in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):253–7. [CrossRef]
- Clerc G, Roux PM. Lymphocyte subsets in severe atherosclerosis before revascularization. Ann Intern Med. 1997;126(12):1004–5. [CrossRef]
- Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol. 2001;37(1):127–36. [CrossRef]
- Kotfis K, Biernawska J, Zegan-Barańska M, Żukowski M. Peripheral Blood Lymphocyte Subsets (CD4+, CD8+ T Cells, NK Cells) in Patients with Cardiovascular and Neurological Complications after Carotid Endarterectomy. Int J Mol Sci. 2015 May;16(5):10077. [CrossRef]
- Li Y, Sha Y, Wang H, He L, Li L, Wen S, et al. Intracellular C3 prevents hepatic steatosis by promoting autophagy and very-low-density lipoprotein secretion. FASEB J. 2021 Dec;35(12). [CrossRef]
- Murray I, Sniderman AD, Havel PJ, Cianflone K. Acylation stimulating protein (ASP) deficiency alters postprandial and adipose tissue metabolism in male mice. J Biol Chem. 1999 Dec;274(51):36219–25. [CrossRef]
- Paglialunga S, Fisette A, Yan Y, Deshaies Y, Brouillette JF, Pekna M, et al. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. 2008 Mar;294(3). [CrossRef]
- Kossmann RJ, Gonzales A, Callan R, Ahmad S. Increased efficiency of hemodialysis with citrate dialysate: A prospective controlled study. Clinical Journal of the American Society of Nephrology. 2009;4(9):1459–64.
- Grundström G, Christensson A, Alquist M, Nilsson LG, Segelmark M. Replacement of acetate with citrate in dialysis fluid: a randomized clinical trial of short term safety and fluid biocompatibility. BMC Nephrol. 2013;14(1). [CrossRef]
- Pérez-García R, Jaldo MT, Puerta M, et al. Hypomagnesaemia in haemodialysis is associated with increased mortality risk: its relationship with dialysis fluid. La hipomagnesemia en hemodiálisis se asocia a mayor riesgo de mortalidad: su relación con el líquido de diálisis. Nefrologia (Engl Ed). 2020;40(5):552-562. [CrossRef]
| Components | Fresenius ACF 3A5 |
Fresenius Smartbag CA 211.5 |
|---|---|---|
| Sodium (mmol/L) | 140 | 138 |
| Potassium (mmol/L) | 2 | 2 |
| Calcium (mmol/mL) | 1,5 | 1,5 |
| Magnesium (mmol/mL) | 0,5 | 0,5 |
| Chloride (mmol/mL) | 106 | 109 |
| Acetate (mmol/L) | 4 | - |
| Citrate (mmol/L) | - | 1 |
| Glucose (g/L) | 1 | 1 |
| Variable | Acetate | Citrate | p-value |
|---|---|---|---|
| Total Leucocytes x 103/mm3, mean ± SD | 7.1± 3.9 | 6.3 ± 2.2 | 0.03 |
| Neutrophils x 103/mm3, mean ± SD | 4.72 ± 3.64 | 4.09 ± 1.75 | 0.412 |
| NLR, mean ± SD | 3.69 ± 3.01 | 3.85 ± 1.93 | 0.776 |
| Lymphocytes x 103/mm3, mean ± SD | 1.37 ± 0.46 | 1.21 ± 0.47 | 0.037 |
| CD19 %, mean ± SD | 121.48 ± 103.26 | 133.95 ± 88.31 | 0.423 |
| CD3+ %, mean ± SD | 72.9 ± 12.69 | 68.48 ± 11.86 | 0.005 |
| CD8+ %, mean ± SD | 33.43 ± 14.56 | 30.67 ± 12.85 | 0.05 |
| CD4+ %, mean ± SD | 517.67 244.83 | 479.05 193.74 | 0.272 |
| CD4/CD8, mean ± SD | 1.53 ± 1.05 | 1.57 ± 1.08 | 0.393 |
| CD56+ CD16+ NK cells %, mean ± SD | 16.95 ± 7.85 | 19.24 ± 8.84 | 0.035 |
| C3 x 103/mm3, mean ± SD | 107.81 ± 19.71 | 115.14 ± 21.30 | 0.009 |
| C4 x 103/mm3, mean ± SD | 25.48 ± 7.05 | 26.57 ± 6.78 | 0.097 |
| Variable | Acetate | Citrate | p-value |
|---|---|---|---|
| Glucose (mg/d), mean ± SD | 119.15 ± 35.70 | 126.7 ± 59.52 | 0.439 |
| Uric acid (mg/d), mean ± SD | 5.36 ± 1.43 | 5.38 ± 1.29 | 0.943 |
| Amylase (mg/d), mean ± SD | 134.05 ± 85.72 | 125.10 ± 88.86 | 0.457 |
| CK (mg/d), mean ± SD | 82.24 ± 95.51 | 135.67 ± 204.27 | 0.129 |
| Total Protein (mg/d), mean ± SD | 6.79 ± 0.75 | 6.77 ± 0.80 | 0.805 |
| Albumin (mg/d), mean ± SD | 4.01 ± 0.40 | 3.92 ± 0.46 | 0.119 |
| TSAT (mg/d), mean ± SD | 24.90 ± 9.47 | 29.14 ± 17.19 | 0.132 |
| Transferrin (mg/d), mean ± SD | 177.29 ± 29.93 | 169.62 ± 32.03 | 0.066 |
| Magnesium (mg/d), mean ± SD | 2.11 ± 0.19 | 2.05 ± 0.26 | 0.192 |
| Haptoglobin (mg/d), mean ± SD | 154.67 ± 67.57 | 153.64 ± 75.90 | 0.912 |
| Vitamin B12 (mg/d), mean ± SD | 745 ± 478.39 | 753.62 ± 435.85 | 0.865 |
| Folic acid (mg/d), mean ± SD | 16.26 ± 8.23 | 15.90 ± 7.26 | 0.844 |
| ESR (mg/d), mean ± SD | 41.19 ± 22.92 | 53.62 ± 35.24 | 0.03 |
| D-dimer (mg/d), mean ± SD | 1497.5 ± 1614.27 | 1490.56 ± 1344.63 | 0.978 |
| hs-CRP (mg/d), mean ± SD | 19.58 ± 59.26 | 16.46 ± 29.75 | 0.832 |
| Ferritin (mg/d), mean ± SD | 334.67 ± 231.94 | 398.71 ± 246.55 | 0.108 |
| Prealbumin (mg/d), mean ± SD | 26.21 ± 7.59 | 24.89 ± 8.089 | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





