Introduction
Deep brain stimulation (DBS) is an effective surgical treatment performed in numerous treatment-resistant psychiatric diseases such as obsessive–compulsive disorder (OCD) [
1] and addictions [
2]. However, in addiction, stimulation was shown to be effective when tested with multiple classes of abused drugs or substances like alcohol and tobacco [
3], but few data are available regarding results in reducing Behavioral Addiction (BA). The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [
4] explicitly included behaviors in the addictions category though pathological gambling (PG) or gambling disorder is the only officially recognized behavioral addiction [
5]. It is defined as an inability to control gambling behavior itself, leading to serious health consequences, and financial and legal problems. Suggestions have been made to classify disorders like Hypersexual Disorder (HD) and Compulsive Shopping (CS) as impulse control disorders (ICD). HD is characterized by a “persistent pattern of failure to control intense, repetitive sexual impulses or urges” [
6,
7] and CS by an urge for shopping and spending that leads to subjective distress and/or impairs quality of life. DBS is considered a promising intervention for BA as it simultaneously falls within the scope of addiction and in the wider-ranging scope of OCD. However, the use of this invasive neuromodulation to investigate these indications is debatable with respect to its risks and benefits compared to traditional treatment methods (including non-invasive neurostimulation like transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) [
8,
9,
10]. This has already been mentioned for cases of “classical” addiction with substance use disorder [
11].
With regard to Parkinson’s disease (PD), studies have identified that this pathology is frequently associated with various ICDs including BA [
12]. A recent review [
13] revealed that strikingly similar (a) deficits in dopaminergic receptor expression, (b) connectivity changes in corticostriatal circuitry and (c) neural responses to cue exposure are observed in both ICDs in PD and addictive disorders including BA. These findings point to the value of adopting a transdiagnostic approach when studying potential treatment of addiction. And these ICD PD patients could represent a good model to explore BA. The profile of psychological characteristics in ICD PD patients resembles that of patients without PD [
14] and regardless of dopamine replacement therapy, even if certain specific features for sociodemographic or impulsivity can be distinguished [
15].
Pathophysiological models suggest that OCD might be associated with dysfunctions in cortico-striato-pallido-thalamo-cortical neuronal circuits. Subthalamic nucleus (STN) can be one of the key nodes of this neuronal circuitry [
16,
17]. Mallet et al. [
18] reported a significant improvement of symptoms after none-motor-STN-DBS to treat patients with severe OCD. Moreover, DBS to the STN is a surgical procedure that for many years has been known to improve the motor symptoms of PD [
19] and some articles have reported the possible positive effects of the STN-DBS procedure in PD on ICD [
20], but clinical trials are needed to provide more evidence.
The aim of the present study was to investigate the effects of STN-DBS on ICD in PD beyond the established indications for STN-DBS as recommended by recent Witt and al. paper [
21]. We monitor ICD (PG, HD, CS) and psychiatric evolution in PD STN-DBS procedures regardless of PD scores; each patient being their own control. A secondary aim was to investigate the effect of DBS treatment on depression, anxiety, and mania symptoms.
Materials & Methods
Patients
We prospectively reported the follow-up of ICD (9 PG, 2 HD, 1 CS) and the motor effect of STN-DBS in 12 PD patients with a pre-existing ICD, consecutively included in a multicenter study. All the patients met the requirements of the United Kingdom Parkinson Disease Society Brain Bank criteria [
22]. They all suffered from severe motor fluctuations and levodopa-induced dyskinesias that were not improved by changes in their antiparkinsonian treatment. The selection criteria for STN-DBS were: an excellent response to Levodopa tested during an acute Levodopa challenge (>50%), no postural instability during the best on period (postural instability = 0) from item 29 of the Unified Parkinson’s Disease Rating Scale motor examination (UPDRS3) [
23], absence of dementia (Mini Mental Status > 24) [
24]. All patients who are deemed good candidates for DBS surgery should undergo magnetic resonance imaging of the brain prior to surgery in order to rule out any secondary diagnosis or structural concerns within the brain. The surgical procedure was based on the direct location of STN using stereotactic nuclear magnetic resonance and electrophysiological mapping (recording and stimulation of the STN area), as reported elsewhere [
25]. Stimulation was adjusted postoperatively during DBS programming in a symptom-specific manner [
26].
BA and psychiatric data
Patients who met PG DSM 5 criteria [
4] or HD criteria according to the Sexual Addiction Screening Test (SAST) [
27] or the CS criteria of McElroy [
28] were included in this study. An evaluation of the severity of the pathology was also performed with the Clinical Global Impressions Scale - Severity (CGI) [
29] and a self-report scale inspired by the OCD Scale [
30] rates the dimension of craving behaviors (ECCA score).
All the patients completed the survey at 3-months pre-surgery (baseline), 6-months post-surgery and 12-months post-surgery.
Psychiatric evaluations included mood disorders, especially anxiety, depression and mania since hypomania and depression are the most common adverse effects associated with STN-DBS and emotional feeling measures. No history of psychiatric disorders was recorded from the preoperative evaluation using DSM 5 criteria [
4]. The psychiatric evaluations were the Hospital Anxiety and Depression scale (HAD) [
31], the Montgomery-Asberg Depression Scale (MADRS) [
32], the Bech-Rafaelsen Mania Scale (MAS) [
33] and the Positive and Negative Emotionality Scale (EPN-31) [
34]. HAD is valued because it is relatively free of physical and cognitive symptoms that could be associated with STN-DBS. Depression rating scales have been recommended by the Movement Disorder Society Task Force on Rating Scales in Movement Disorders [
35]. Two subscores for anxiety (HADA) and depression (HADD) were used. MAS is a validated scale and is usually used for the longitudinal monitoring of manic symptoms or for the evaluation of mixed states. Emotional dysregulation is thought to be linked to PD-STN-DBS [
36] so it was also interesting to consider its relation with other psychiatric dimensions and BA. EPN-31 is a scale that measures emotional process and produces both a positive and a negative emotionality score. A reference French healthy adult population had a mean positive emotion score of 70.1 ± 16.0 and a mean negative emotion score of 32.0 ± 14.30 [
37].
Assessments
Three months before surgery (baseline time), demographic data (age, gender), clinical characteristics (disease duration, PD and psychiatric medication, UPDRS3, classification by Hoehn and Yahr stage), and Schwab and England Activities of Daily Living were collected. UPDRS3 scores were assessed in the “ON” and “OFF” medication states six and twelve-months post-surgery to remove the confounding effects of dopaminergic medication in the follow-up. Response to L-dopa was also evaluated using a standardized method [
38]. All the scores were established post-operatively on stimulation and on drug therapy at least one hour after intake. The calculation of the cumulative daily L-dopa equivalent daily dose (LEDD) was based on Levodopa correspondences adapted from Thobois et al. [
39].
Ethical statement
All the participants gave their informed consent and were free to withdraw from the study at any time. The general procedures were approved by the Institutional Ethics Committee (2009-A00409-48) and performed in accordance with the ethical standards set out in the Declaration of Helsinki of 1964.
Statistical methods
The statistical analysis was performed using Stata software (version 15; StataCorp, College Station, TX, USA). All the tests were two-sided, with an alpha level set at 5%. Categorical data are presented as numbers and percentages, and quantitative data as mean ± standard deviation. Longitudinal analyses were carried out by linear mixed models considering time (baseline, M6, and M12) as a fixed effect, and the patient as a random effect. UPDRS3 ON and UPDRS3 OFF were compared at M6 and M12 with the Wilcoxon signed-rank test.
Results
Preoperative characteristics
Twelve right-handed patients with PD treated with bilateral STN-DBS were included (8 males) with an average age of 60.6 ± 5.6 years, a mean duration of PD of 10.3 ± 3.7 years and a cumulative mean LEDD of 1006 ± 269 mg/day. The Hoehn and Yahr stage, Schwab and England stage and levodopa medication indicated a severe disease requiring PD surgery. Motor examination using UPDRS3 described a low severity of motor symptoms of PD (11.8 ± 8.3). Clinical details are summarized in
Table 1. A supplementary file describes all the individual data of the subjects included in this study.
Nine patients met the PG DSM-5 criteria (6 male), two HD criteria using the Sexual Addiction Screening Test (all males) and one female the CS criteria of McElroy were included. Overall, the group of 12 subjects showed a mean severity score (CGI) of BA symptoms at markedly impaired (4.5 ± 0.8): the HS patient was mildly impaired; the PG and CS patients were markedly impaired. The patients had a moderate intensity of craving (17.9 ± 8.7) regardless of their BA.
Concerning medication, agonist dopaminergic adjunction was found in 10 patients. One PG patient was taking antipsychotic, anxiolytic and antidepressant treatments at the same time, and one PG patient was taking benzodiazepine treatment. No HD or CS patients were taking psychiatric medications.
Overall, at baseline, no depressive or anxiety illness was observed. HD patients had no depressive or anxious symptoms (HADA, HADD and MADRS scores) but four PG patients presented a mild to moderate anxiety (HADA) and one a mild depression (HADD and MADRS score). An MAS score at baseline reflects that only one PG patient had a moderate mania state. The CS case showed severe anxious and mild depressive symptoms at baseline.
The PG group reported a lower mean level of positive emotions compared to the reference, but the CS case demonstrated a considerable disturbance of positive and negative emotions.
Safety of STN-DBS
No serious adverse events occurred during surgery or in the perioperative phase. During the 1-year follow-up, there was none device-related adverse event and among the stimulation-related adverse events, dysarthria was the only motor side-effect, occurring in one case. None other side-effect such as cognitive impairment, dementia or sleep disorders were observed.
Evolution at 6 and 12-mounths post-surgery
BA evolution
No patient showed a BA during the follow-up other than that initially detected at baseline. None of the HD and CS patients showed any signs of BA at M6 or M12. PG disappeared at M6 for all the patients but 33% (n=3) exhibited PG again at M12. For these three patients who still presented a BA at M12, two showed improved addiction severity scores (patient1: CGI-baseline=4 to CGI-M12=3; patient2: CGI-baseline=5 to CGI-M12=5) and craving scores (patient1: ECCA-baseline=5 to ECCA-M12=5; patient2: ECCA-baseline=22 to ECCA-M12=11). However, the clinical severity and craving dimension of one male PG patient worsened (CGI-baseline=5 to CGI-M12=6; ECCA-baseline=16 to ECCA-M12=27). Overall, 75% of STN-DBS PD patients were cured of their BA within 12-months post-surgery.
PD evolution
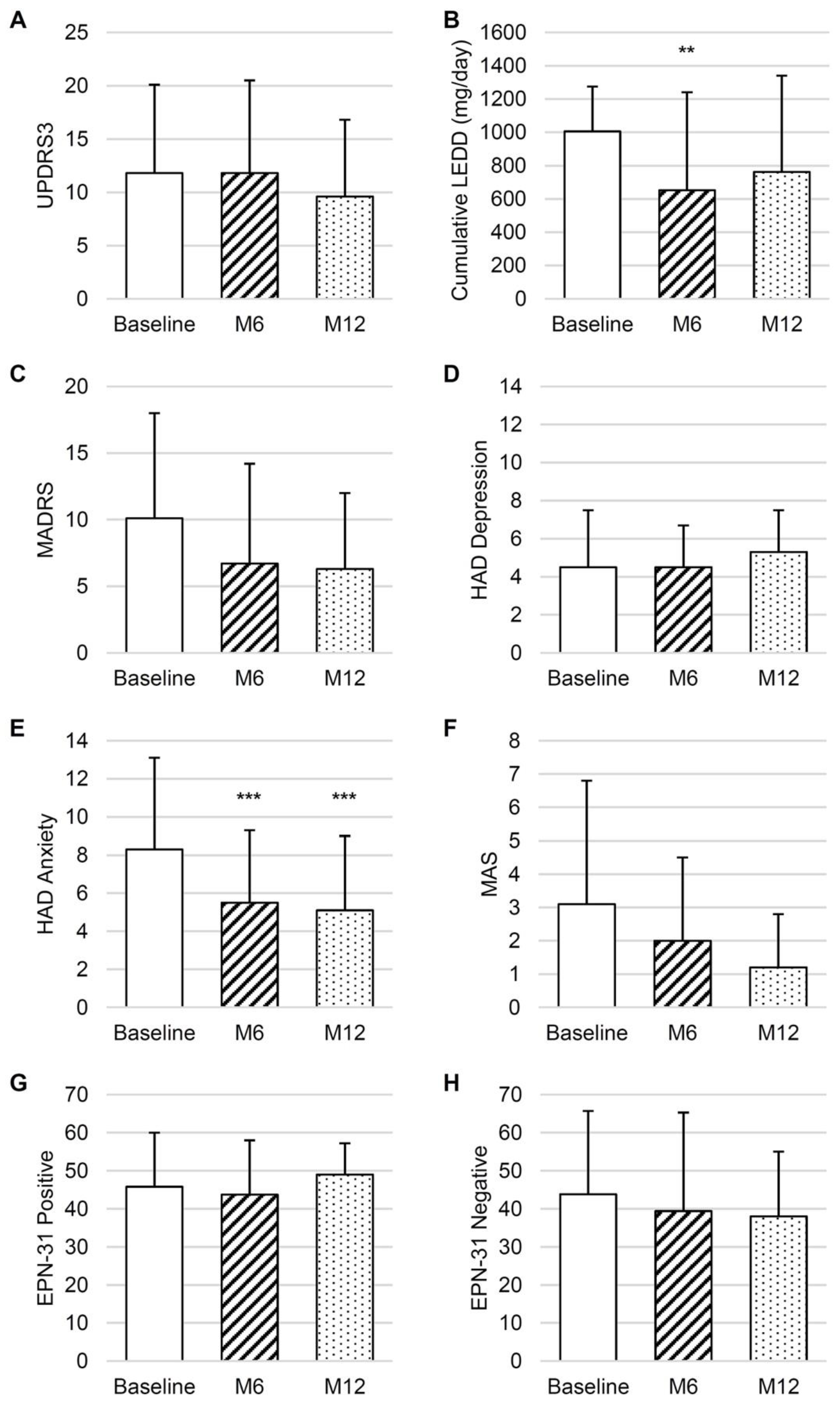
Longitudinal analyses revealed that the UPDRS3 ON scores were not significantly decreased at M6 and M12 post-surgery (
Figure 1A). UPDRS3 ON was significantly lower than UPDRS3 OFF at M6 (respectively 11.8 ± 8.7 and 18.9 ± 13.0, p=0.02) and at M12 (respectively 9.6 ± 7.2 and 13.5 ± 10.3, p=0.02). When analyzing the minimal clinically significant difference [
40] of changes in the UPDRS3 score at M6, compared to baseline, no modification was observed for 4/12 patients, an improvement was observed for 4/12 patients while a deterioration was observed for 4/12 patients. At M12 compared to baseline, no modification was observed for 6/12 patients, an improvement was observed for 4/12 patients and a deterioration was observed for 2/12 patients.
All the addictive behaviors showed a mild to marked illness (CGI-S) but the dimension of craving was mainly observed in the PG group (56% had a score >20 ECCA and a mean score at 19).
A significant decrease in cumulative LEDD was observed between baseline and M6 (
Figure 1B). The number of dopamine agonist users was stable (10 at baseline, 7 at M6, and 7 at M12), as was the number of patients with psychiatric treatments (2 at baseline, 4 at M6, and 5 at M12).
Psychiatric evolution
There was no significant difference regarding the depression score (MADRS and HADD) between baseline and M6 or M12 (
Figure 1C,D). For anxiety (HADA), a significant decrease was observed between baseline and the follow-up-point (
Figure 1E). Only one patient out of the 12 presented an anxiety disorder (score above 11) at 1 year of follow-up versus 3 initially.
There was no significant difference in the mania score compared to baseline (
Figure 1F). Individually, no subject suffered from manic state during the follow-up. The PG subject who initially had moderate mania at baseline did not demonstrate symptoms post-surgery. HD patients had no onset of depressive symptoms during the follow-up (HAD and MADRS scores). At M12, two of the four anxious PG patients did not present anxiety disorder symptoms, one improved his anxiety symptoms to minor anxiety disorder and one stayed at moderate anxiety state. The CS case improved his anxiety and depression symptoms to minor anxiety and a non-depressive symptoms state at M12.
The PG group mainly improved their level of positive emotions and only two patients had low scores under 40 at M12. The CS cases mainly improved their positive and negative emotion scores to standard level. Emotional balance observed through the EPN-31 score did not differ significantly during the annual assessment (
Figure 1G,H).
Stimulation parameters
There was no modification in the CS and HS cases regarding the DBS dose of electrical stimulation at M6 or M12. However, in the PG group, the left contact of only one patient underwent modifications (stimulation of a deeper contact) although 66% (n=8) had modifications of amplitude and/or frequency stimulations on the right and/or left contacts (5 had their amplitude stimulation increased). There was no change in pulse width at any other time (all at 60µsec).
Discussion
STN-DBS impact on ICD in PD
In 12 BA cases, 100% recovered at M6 although this figure dropped to 75% at M12. This implied that bilateral STN-DBS has a significant impact on ICD without any major side effect of stimulation for most patients. Indeed, there was n
o de novo ICD (differing from those initially assessed) or mania state post-surgery even after one year. HS, PG, and CS have been included in dopamine dysregulation syndrome and hyperdopaminergic behaviors when described in PD [
41]. The prospective PD cohort study results showed an improvement of hyperdopaminergic behaviors after DBS but related to the reduction of dopaminergic treatment rather than the direct effect of stimulation [
42,
43,
44,
45,
46] and dopamine agonist (DA) which have been suggested to influence their development in PD [
47,
48,
49]. However, this is mostly because it concerns high, specific doses of DA. The hypothesis suggesting beneficial effects on ICD due to the reduction of dopaminergic drug load does not seems to sufficiently explain their improvement in our population and goes in the direction of another publication [
14]. At baseline, 2/12 of our patients did not take this therapy and 8/12 had very low doses. Moreover, none of our patients had been administered with pramipexole (dopamine agonist) [
50] or aripiprazole (atypical antipsychotic drug) [
51], both assumed to be drugs strongly associated with the development of PG, HS and CS. On the other hand, in addition to dopaminergic mechanisms, nondopaminergic mechanisms are also considered to be involved in the pathophysiological mechanisms underlying DBS efficacy [
52]. In addition, in PD, the degeneration of cholinergic neurons occurs in the pedunculopontine nucleus (PPN) which plays a major role in the coordination of gait and posture but may also be involved in ICD development [
53]. It has been hypothesized that STN-DBS has an effect through the retrograde activation of cholinergic PPN projection neurons [
52]. A recent article [
21] confirms worth considering the possibility of a specific stimulation effect and the importance to assess individual evolution of ICD regarding STN-DBS. Our results support the strength of the recommendation (100% of consensus level) given in this guideline: STN-DBS can be recommended to treat signs and symptoms of an ICD in patients eligible for deep brain stimulation.
We must also note that the addictive symptoms of one case (PG) were worse at M12 despite recovering at M6 (
severity and craving score). There was also a transitory effect on motor signs that were improved at M6 but not at M12 compared to baseline. However, there was no modification of electrical parameters or drug treatment between M6 and M12 and no significant modification of anxiety or depression score either. Previous works have shown PG to develop exclusively after STN DBS surgery in PD [
54,
55,
56] but none, to our knowledge, have described a transitory stage of resolved symptoms and resurgence post-stimulation without modifying stimulation or dopamine therapy [
57].
STN-DBS impact on motor signs in PD
Surprisingly, there was no significant improvement of mean motor signs after STN-DBS in our group although the literature is abundant on the positive effects of this treatment in PD [
19,
58]. However, there was an effect on motor signs since, although the score was almost the same between pre and post-surgery, there was a significant difference between the ON and OFF stimulation motor scores. This can be explained by the level of motor signs at baseline which was lower in our group than that generally observed in the series of STN-DBS PD patients with a score around twice as high [
46]. The effectiveness of STN-DBS on motor symptoms in our group was more complex to observe when the baseline scores were low. In parallel, the STN-DBS effect allowed a significant reduction of dopaminergic treatments to achieve the same level of motor experiences in daily living. We observed the classical effect on reducing dopamine drug use significantly after stimulation [
59].
STN-DBS impact on psychiatric evaluation in PD
Our sample of patients seems different from those observed in other studies, in particular there were no even moderately depressive patients whereas the literature suggests that pre-operative PD patients are affected up to 40% and often associated with anxiety [
60,
61]. However, a work that compared PD patients with BA compared to PD-free patients with BA showed no significant difference regarding anxiety disorder [
15]. It cannot be excluded that emotions related to positive patient expectations from an upcoming intervention positively influenced the pre-DBS anxiety score in our population. Regarding psychiatric states, STN-DBS seems to improve anxiety scores significantly but not depression scores. A previous study has already associated STN-DBS with beneficial effects on various non-motor signs in PD including anxiety [
62], even for a longer stimulation period [
63] and excluding depression [
64]. Anxiety has been linked to emotion regulation [
28] but in our study we did not find a significant difference when using the EPN-31 between baseline and follow-up. This tool seems to be more linked with depression state than anxiety [
65], which could explain why it did not change significantly since the depression score was quite stable.
Limitations of the study
The findings of this study have certain limitations. Firstly, this was a pilot investigation of STN-DBS BA PD with a small sample size due to the scarce resources available and the various ethical concerns taken into considerations in the process of conducting neuromodulation research in this domain, given the vulnerability of the patients. The result cannot be generalized to all kinds of BA observed in PD. Furthermore, life events during the study follow-up were not available and could have potentially influenced mood state during the study. The role of contact position was not explored within the sample. It may have provided information on structural and pathophysiological mechanisms because our knowledge about the STN sub-structure involved in specific functions and the action of DBS on neurotransmission is still limited. Lastly, the relationship with stimulation parameters (frequency and plot used) was not studied due to sample size. Indeed, and apart from contact position, debate is ongoing about the therapeutic potential of STN-DBS in the treatment of addiction and the frequency of stimulation used [
66].
Conclusions
In conclusion, our findings add new data to the existing literature by demonstrating evidence regarding the benefit of STN-DBS therapy on BA in our case series of PD patients. In addition, we found that the impact of STN-DBS stimulation on motor signs was lower than expected. Future studies to explore and optimize stimulation parameters and targets for STN-DBS may provide substantial clinical contributions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This research was funded by a national clinical research hospital program carried out under the aegis of the French Ministry of Health, grant number “PHRC N 2008 MALLET”.
Acknowledgments
In memoriam of Dr. Laurent Mallet. His contribution to research on addiction while alive has provided much to our current knowledge.
Authorship
All the authors have approved the manuscript and agree with its submission to Brain Sciences. They agree to be personally accountable for the authors’ contributions listed below: de Chazeron I: Original Draft Preparation. Lambert C: Formal Analysis. Brousse G: Investigation Supervision. Houeto J-L: Investigation. Jaafari N: Investigation. Rascol O: Investigation. Ory-Magne F: Investigation. Krack P: Investigation. Barroche P: Investigation. Schwan R: Investigation. Doe de Maindreville A: Investigation. Vidailhet M: Investigation. Welter M L: Investigation. Llorca P.M: Writing – Review & Editing.
Conflicts of Interest
We confirm that neither the manuscript nor any parts of its content are currently under consideration for publication with or published in another journal.
References
- Wu:, H.; Hariz, M.; Visser-Vandewalle, V.; Zrinzo, L.; Coenen, V.A.; Sheth, S.A.; Bervoets, C.; Naesström, M.; Blomstedt, P.; Coyne, T.; et al. Deep Brain Stimulation for Refractory Obsessive-Compulsive Disorder (OCD): Emerging or Established Therapy? Mol Psychiatry 2021, 26, 60–65. [Google Scholar] [CrossRef]
- Luigjes, J.; van den Brink, W.; Feenstra, M.; van den Munckhof, P.; Schuurman, P.R.; Schippers, R.; Mazaheri, A.; De Vries, T.J.; Denys, D. Deep Brain Stimulation in Addiction: A Review of Potential Brain Targets. Mol Psychiatry 2012, 17, 572–583. [Google Scholar] [CrossRef]
- Wang, T.R.; Moosa, S.; Dallapiazza, R.F.; Elias, W.J.; Lynch, W.J. Deep Brain Stimulation for the Treatment of Drug Addiction. Neurosurg Focus 2018, 45, E11. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (5th Ed.); 2013.
- Petry, N.M.; Zajac, K.; Ginley, M.K. Behavioral Addictions as Mental Disorders: To Be or Not To Be? Annual Review of Clinical Psychology 2018, 14, 399–423. [Google Scholar] [CrossRef]
- World Health Organisation International Statistical Classification of Diseases and Related Health Problems (11th Ed.). 2019.
- Kraus, S.W.; Krueger, R.B.; Briken, P.; First, M.B.; Stein, D.J.; Kaplan, M.S.; Voon, V.; Abdo, C.H.N.; Grant, J.E.; Atalla, E.; et al. Compulsive Sexual Behaviour Disorder in the ICD-11. World Psychiatry 2018, 17, 109–110. [Google Scholar] [CrossRef]
- Protasio, M.I.B.; da Silva, J.P.L.; Arias-Carrión, O.; Nardi, A.E.; Machado, S.; Cruz, M.S. Repetitive Transcranial Magnetic Stimulation to Treat Substance Use Disorders and Compulsive Behavior. CNS Neurol Disord Drug Targets 2015, 14, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zucchella, C.; Mantovani, E.; Federico, A.; Lugoboni, F.; Tamburin, S. Non-Invasive Brain Stimulation for Gambling Disorder: A Systematic Review. Front Neurosci 2020, 14, 729. [Google Scholar] [CrossRef]
- Sakreida, K.; Köhler, M.E.; Langguth, B.; Schecklmann, M.; Poeppl, T.B. Effect of Prefrontal Transcranial Direct Current Stimulation on Sexual Arousal: A Proof of Concept Study. Neurophysiol Clin 2023, 53, 102847. [Google Scholar] [CrossRef]
- Lo, C.; Mane, M.; Kim, J.H.; Berk, M.; Sharp, R.R.; Lee, K.H.; Yuen, J. Treating Addiction with Deep Brain Stimulation: Ethical and Legal Considerations. Int J Drug Policy 2023, 113, 103964. [Google Scholar] [CrossRef]
- Weintraub, D.; David, A.S.; Evans, A.H.; Grant, J.E.; Stacy, M. Clinical Spectrum of Impulse Control Disorders in Parkinson’s Disease. Mov Disord 2015, 30, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ramdave, S.; Dawson, A.; Carter, A.; Dissanayaka, N.N.W. Unmasking Neurobiological Commonalities between Addictive Disorders and Impulse Control Disorders in Parkinson’s Disease. Brain Imaging Behav 2020, 14, 2785–2798. [Google Scholar] [CrossRef]
- Leplow, B.; Renftle, D.; Thomas, M.; Michaelis, K.; Solbrig, S.; Maetzler, W.; Berg, D.; Liepelt-Scarfone, I. Characteristics of Behavioural Addiction in Parkinson’s Disease Patients with Self-Reported Impulse Control Disorder and Controls Matched for Levodopa Equivalent Dose: A Matched Case-Control Study. J Neural Transm (Vienna) 2023, 130, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, A.; Jiménez-Murcia, S.; Fernández-Aranda, F.; Granero, R.; Grall-Bronnec, M.; Victorri-Vigneau, C.; Bulteau, S.; Derkinderen, P.; Vanelle, J.M.; Hakansson, A.; et al. A Comparison of Treatment-Seeking Behavioral Addiction Patients with and without Parkinson’s Disease. Front Psychiatry 2017, 8, 214. [Google Scholar] [CrossRef]
- Albin, R.L.; Young, A.B.; Penney, J.B. The Functional Anatomy of Basal Ganglia Disorders. Trends Neurosci 1989, 12, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, R.; Castrioto, A.; Krack, P. Prefrontal--STN Projections, the Highway for Emotion and Cognition Control. Mov Disord 2014, 29, 305. [Google Scholar] [CrossRef] [PubMed]
- Mallet, L.; Polosan, M.; Jaafari, N.; Baup, N.; Welter, M.-L.; Fontaine, D.; du Montcel, S.T.; Yelnik, J.; Chéreau, I.; Arbus, C.; et al. Subthalamic Nucleus Stimulation in Severe Obsessive-Compulsive Disorder. N Engl J Med 2008, 359, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W.; et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N Engl J Med 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Broen, M.; Duits, A.; Visser-Vandewalle, V.; Temel, Y.; Winogrodzka, A. Impulse Control and Related Disorders in Parkinson’s Disease Patients Treated with Bilateral Subthalamic Nucleus Stimulation: A Review. Parkinsonism Relat Disord 2011, 17, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.; Levin, J.; van Eimeren, T.; Hasan, A.; Ebersbach, G. ; German Parkinson’s Guideline Group Diagnostics and Treatment of Impulse Control Disorders, Psychosis and Delirium: Systemic Review-Based Recommendations - Guideline “Parkinson’s Disease” of the German Society of Neurology. J Neurol 2024. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson’s Disease: A Clinico-Pathological Study of 100 Cases. J Neurol Neurosurg Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Fahn, S.; Elton, R.; Members of the UPDRS Development Committe The Unified Parkinson’s Disease Rating Scale. 1987, 153–163.
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiatr Res 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, J.-J.; Coste, J.; Ouchchane, L.; Caire, F.; Nuti, C.; Derost, P.; Cristini, V.; Gabrillargues, J.; Hemm, S.; Durif, F.; et al. Brain Mapping in Stereotactic Surgery: A Brief Overview from the Probabilistic Targeting to the Patient-Based Anatomic Mapping. Neuroimage 2007, 37 Suppl 1, S109–115. [Google Scholar] [CrossRef]
- Picillo, M.; Lozano, A.M.; Kou, N.; Puppi Munhoz, R.; Fasano, A. Programming Deep Brain Stimulation for Parkinson’s Disease: The Toronto Western Hospital Algorithms. Brain Stimul 2016, 9, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Carnes, P. Sexual Addiction Screening Test. Tenn Nurse 1991, 54, 29. [Google Scholar] [PubMed]
- McElroy, S.L.; Keck, P.E.J.; Pope, H.G.J.; Smith, J.M.; Strakowski, S.M. Compulsive Buying: A Report of 20 Cases. J Clin Psychiatry 1994, 55, 242–248. [Google Scholar] [PubMed]
- Busner, J.; Targum, S.D. The Clinical Global Impressions Scale: Applying a Research Tool in Clinical Practice. Psychiatry (Edgmont) 2007, 4, 28–37. [Google Scholar] [PubMed]
- Anton, R.F.; Moak, D.H.; Latham, P. The Obsessive Compulsive Drinking Scale: A Self-Rated Instrument for the Quantification of Thoughts about Alcohol and Drinking Behavior. Alcohol Clin Exp Res 1995, 19, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Montgomery, S.M. Depressive Symptoms in Acute Schizophrenia. Prog Neuropsychopharmacol 1979, 3, 429–433. [Google Scholar] [CrossRef]
- Bech, P.; Bolwig, T.G.; Kramp, P.; Rafaelsen, O.J. The Bech-Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatr Scand 1979, 59, 420–430. [Google Scholar] [CrossRef]
- Pélissolo, A.; Rolland, J.-P.; Perez-Diaz, F.; Jouvent, R.; Allilaire, J.-F. [Dimensional approach of emotion in psychiatry: validation of the Positive and Negative Emotionality scale (EPN-31)]. Encephale 2007, 33, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Barone, P.; Brown, R.G.; Leentjens, A.F.G.; McDonald, W.M.; Starkstein, S.; Weintraub, D.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Depression Rating Scales in Parkinson’s Disease: Critique and Recommendations. Mov Disord 2007, 22, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Tonello, D.; Rizzi, L.; Zibetti, M.; Lanotte, M.; Lopiano, L. Alexithymia in Patients with Parkinson’s Disease Treated with DBS of the Subthalamic Nucleus: A Case-Control Study. Front Psychol 2014, 5, 1168. [Google Scholar] [CrossRef] [PubMed]
- Diener, E.; Smith, H.; Fujita, F. The Personality Structure of Affect. J Pers Soc Psychol. 1995, 69, 130–141. [Google Scholar] [CrossRef]
- Ulla, M.; Thobois, S.; Llorca, P.-M.; Derost, P.; Lemaire, J.-J.; Chereau-Boudet, I.; de Chazeron, I.; Schmitt, A.; Ballanger, B.; Broussolle, E.; et al. Contact Dependent Reproducible Hypomania Induced by Deep Brain Stimulation in Parkinson’s Disease: Clinical, Anatomical and Functional Imaging Study. J Neurol Neurosurg Psychiatry 2011, 82, 607–614. [Google Scholar] [CrossRef]
- Thobois, S. Proposed Dose Equivalence for Rapid Switch between Dopamine Receptor Agonists in Parkinson’s Disease: A Review of the Literature. Clin Ther 2006, 28, 1–12. [Google Scholar] [CrossRef]
- Horváth, K.; Aschermann, Z.; Ács, P.; Deli, G.; Janszky, J.; Komoly, S.; Balázs, É.; Takács, K.; Karádi, K.; Kovács, N. Minimal Clinically Important Difference on the Motor Examination Part of MDS-UPDRS. Parkinsonism & Related Disorders 2015, 21, 1421–1426. [Google Scholar] [CrossRef]
- Ardouin, C.; Chéreau, I.; Llorca, P.-M.; Lhommée, E.; Durif, F.; Pollak, P.; Krack, P. ; groupe évaluation comportementale de la maladie de Parkinson [Assessment of hyper- and hypodopaminergic behaviors in Parkinson’s disease]. Rev Neurol (Paris) 2009, 165, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, M.M.; Rajah, T.; Delgado, L.F.; Dafsari, H.S. The Effect of Deep Brain Stimulation on the Non-Motor Symptoms of Parkinson’s Disease: A Critical Review of the Current Evidence. NPJ Parkinsons Dis 2017, 3, 16024. [Google Scholar] [CrossRef]
- Ardouin, C.; Voon, V.; Worbe, Y.; Abouazar, N.; Czernecki, V.; Hosseini, H.; Pelissolo, A.; Moro, E.; Lhommée, E.; Lang, A.E.; et al. Pathological Gambling in Parkinson’s Disease Improves on Chronic Subthalamic Nucleus Stimulation. Mov Disord 2006, 21, 1941–1946. [Google Scholar] [CrossRef]
- Bandini, F.; Primavera, A.; Pizzorno, M.; Cocito, L. Using STN DBS and Medication Reduction as a Strategy to Treat Pathological Gambling in Parkinson’s Disease. Parkinsonism Relat Disord 2007, 13, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Castrioto, A.; Funkiewiez, A.; Debû, B.; Cools, R.; Lhommée, E.; Ardouin, C.; Fraix, V.; Chabardès, S.; Robbins, T.W.; Pollak, P.; et al. Iowa Gambling Task Impairment in Parkinson’s Disease Can Be Normalised by Reduction of Dopaminergic Medication after Subthalamic Stimulation. J Neurol Neurosurg Psychiatry 2015, 86, 186–190. [Google Scholar] [CrossRef]
- Onder, H.; Comoglu, S. Investigation of the Nonmotor Symptoms in Patients with STN-DBS Therapy in Comparison with Those without STN-DBS. J Neural Transm (Vienna) 2024. [Google Scholar] [CrossRef]
- Voon, V.; Kubu, C.; Krack, P.; Houeto, J.-L.; Tröster, A.I. Deep Brain Stimulation: Neuropsychological and Neuropsychiatric Issues. Mov Disord 2006, 21 Suppl 14, S305–327. [Google Scholar] [CrossRef]
- Weintraub, D.; Siderowf, A.D.; Potenza, M.N.; Goveas, J.; Morales, K.H.; Duda, J.E.; Moberg, P.J.; Stern, M.B. Association of Dopamine Agonist Use with Impulse Control Disorders in Parkinson Disease. Arch Neurol 2006, 63, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.A.; O’Sullivan, S.S.; Evans, A.H.; Lees, A.J.; Schrag, A. Pathological Gambling in Parkinson’s Disease: Risk Factors and Differences from Dopamine Dysregulation. An Analysis of Published Case Series. Mov Disord 2007, 22, 1757–1763. [Google Scholar] [CrossRef]
- Dodd, M.L.; Klos, K.J.; Bower, J.H.; Geda, Y.E.; Josephs, K.A.; Ahlskog, J.E. Pathological Gambling Caused by Drugs Used to Treat Parkinson Disease. Arch Neurol 2005, 62, 1377–1381. [Google Scholar] [CrossRef]
- Grall-Bronnec, M.; Sauvaget, A.; Perrouin, F.; Leboucher, J.; Etcheverrigaray, F.; Challet-Bouju, G.; Gaboriau, L.; Derkinderen, P.; Jolliet, P.; Victorri-Vigneau, C. Pathological Gambling Associated With Aripiprazole or Dopamine Replacement Therapy: Do Patients Share the Same Features? A Review. J Clin Psychopharmacol 2016, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Patel, S.; Gavine, B.; Buchanan, T.; Bogdanovic, M.; Sarangmat, N.; Green, A.L.; Bloem, B.R.; FitzGerald, J.J.; Antoniades, C.A. Deep Brain Stimulation and Levodopa Affect Gait Variability in Parkinson Disease Differently. Neuromodulation 2023, 26, 382–393. [Google Scholar] [CrossRef]
- Stefani, A.; Galati, S.; Brusa, L.; Pierantozzi, M.; Peppe, A.; Stanzione, P. Pathological Gambling from Dopamine Agonist and Deep Brain Stimulation of the Nucleus Tegmenti Pedunculopontine. BMJ Case Rep 2010, 2010, bcr0220102774. [Google Scholar] [CrossRef]
- Smeding, H.M.M.; Goudriaan, A.E.; Foncke, E.M.J.; Schuurman, P.R.; Speelman, J.D.; Schmand, B. Pathological Gambling after Bilateral Subthalamic Nucleus Stimulation in Parkinson Disease. J Neurol Neurosurg Psychiatry 2007, 78, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Hälbig, T.D.; Tse, W.; Frisina, P.G.; Baker, B.R.; Hollander, E.; Shapiro, H.; Tagliati, M.; Koller, W.C.; Olanow, C.W. Subthalamic Deep Brain Stimulation and Impulse Control in Parkinson’s Disease. Eur J Neurol 2009, 16, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, P.; Rickards, H.; Cavanna, A.E. Impulse Control Disorders Following Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease: Clinical Aspects. Parkinsons Dis 2011, 2011, 658415. [Google Scholar] [CrossRef]
- Heiden, P.; Heinz, A.; Romanczuk-Seiferth, N. Pathological Gambling in Parkinson’s Disease: What Are the Risk Factors and What Is the Role of Impulsivity? Eur J Neurosci 2017, 45, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Pak, K. Comparison of UPDRS III Score between Young and Late Onset Parkinson Disease after Deep Brain Stimulation: A Meta-Analysis. Medicine (Baltimore) 2023, 102, e35861. [Google Scholar] [CrossRef] [PubMed]
- Hvingelby, V.S.; Pavese, N. Surgical Advances in Parkinson’s Disease. Curr Neuropharmacol 2024, 22, 1033–1046. [Google Scholar] [CrossRef]
- Lieberman, A. Depression in Parkinson’s Disease -- a Review. Acta Neurol Scand 2006, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Schinwelski, M.; Konkel, A.; Grabowski, K.; Libionka, W.; Wąż, P.; Sitek, E.J.; Sławek, J. The Impact of Subthalamic Deep Brain Stimulation on Sleep and Other Non-Motor Symptoms in Parkinson’s Disease. Parkinsonism Relat Disord 2019, 64, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hogg, E.; Wertheimer, J.; Graner, S.; Tagliati, M. Deep Brain Stimulation and Nonmotor Symptoms. Int Rev Neurobiol 2017, 134, 1045–1089. [Google Scholar] [CrossRef]
- Bucur, M.; Papagno, C. Deep Brain Stimulation in Parkinson Disease: A Meta-Analysis of the Long-Term Neuropsychological Outcomes. Neuropsychol Rev 2023, 33, 307–346. [Google Scholar] [CrossRef]
- Aviles-Olmos, I.; Kefalopoulou, Z.; Tripoliti, E.; Candelario, J.; Akram, H.; Martinez-Torres, I.; Jahanshahi, M.; Foltynie, T.; Hariz, M.; Zrinzo, L.; et al. Long-Term Outcome of Subthalamic Nucleus Deep Brain Stimulation for Parkinson’s Disease Using an MRI-Guided and MRI-Verified Approach. J Neurol Neurosurg Psychiatry 2014, 85, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Pelissolo, A. [Balance Émotionnelle Dans Les Troubles Anxieux et Dépressifs]. 2011, 169, 124.
- Degoulet, M.; Tiran-Cappello, A.; Combrisson, E.; Baunez, C.; Pelloux, Y. Subthalamic Low-Frequency Oscillations Predict Vulnerability to Cocaine Addiction. Proc Natl Acad Sci U S A 2021, 118, e2024121118. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).