Submitted:
13 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
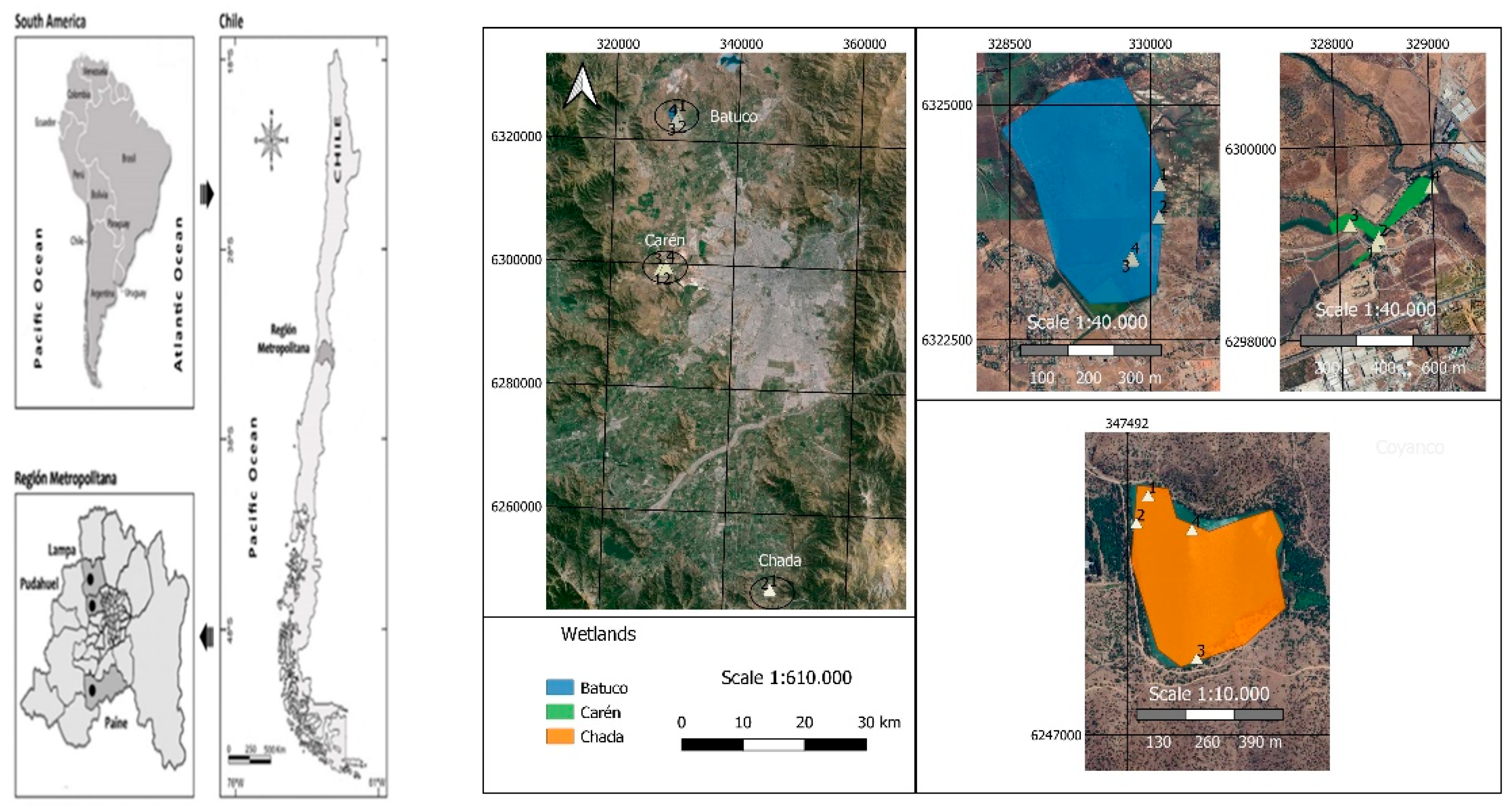
2.1. Study Areas
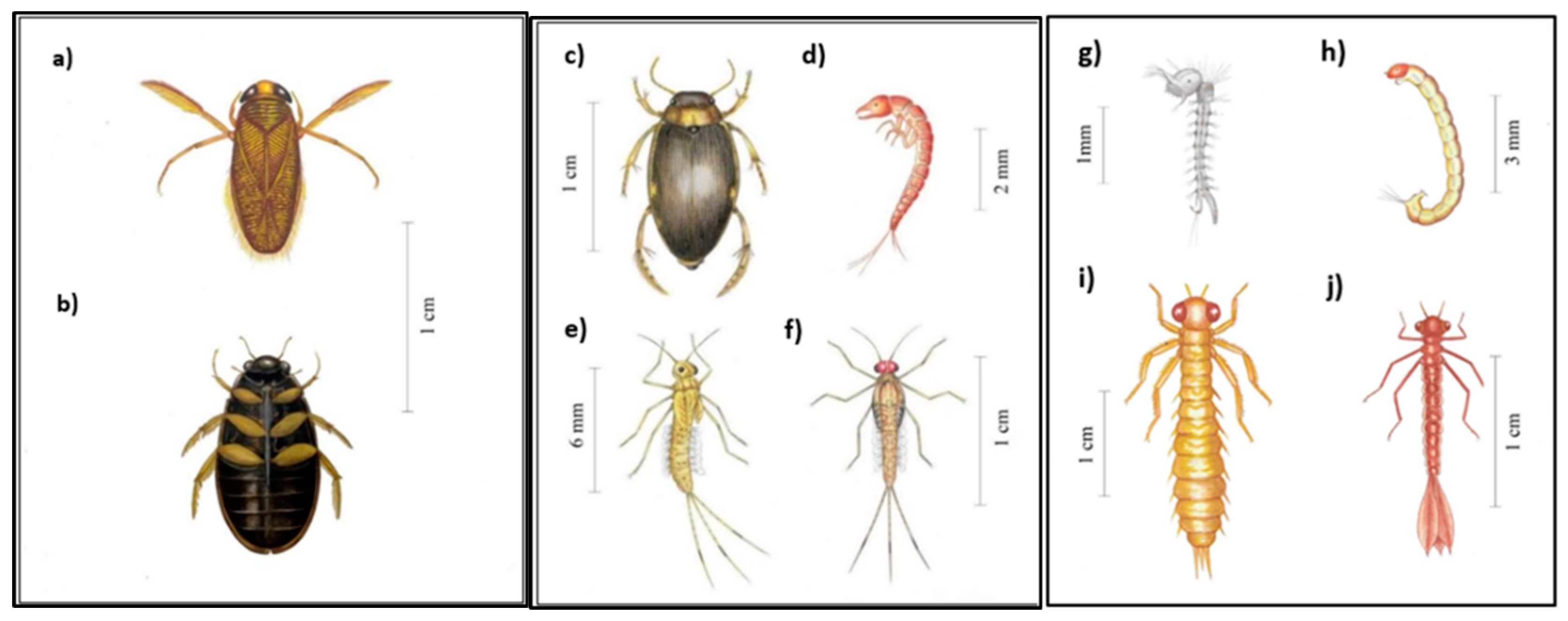
2.2. Sampling and Identification of Aquatic Insects
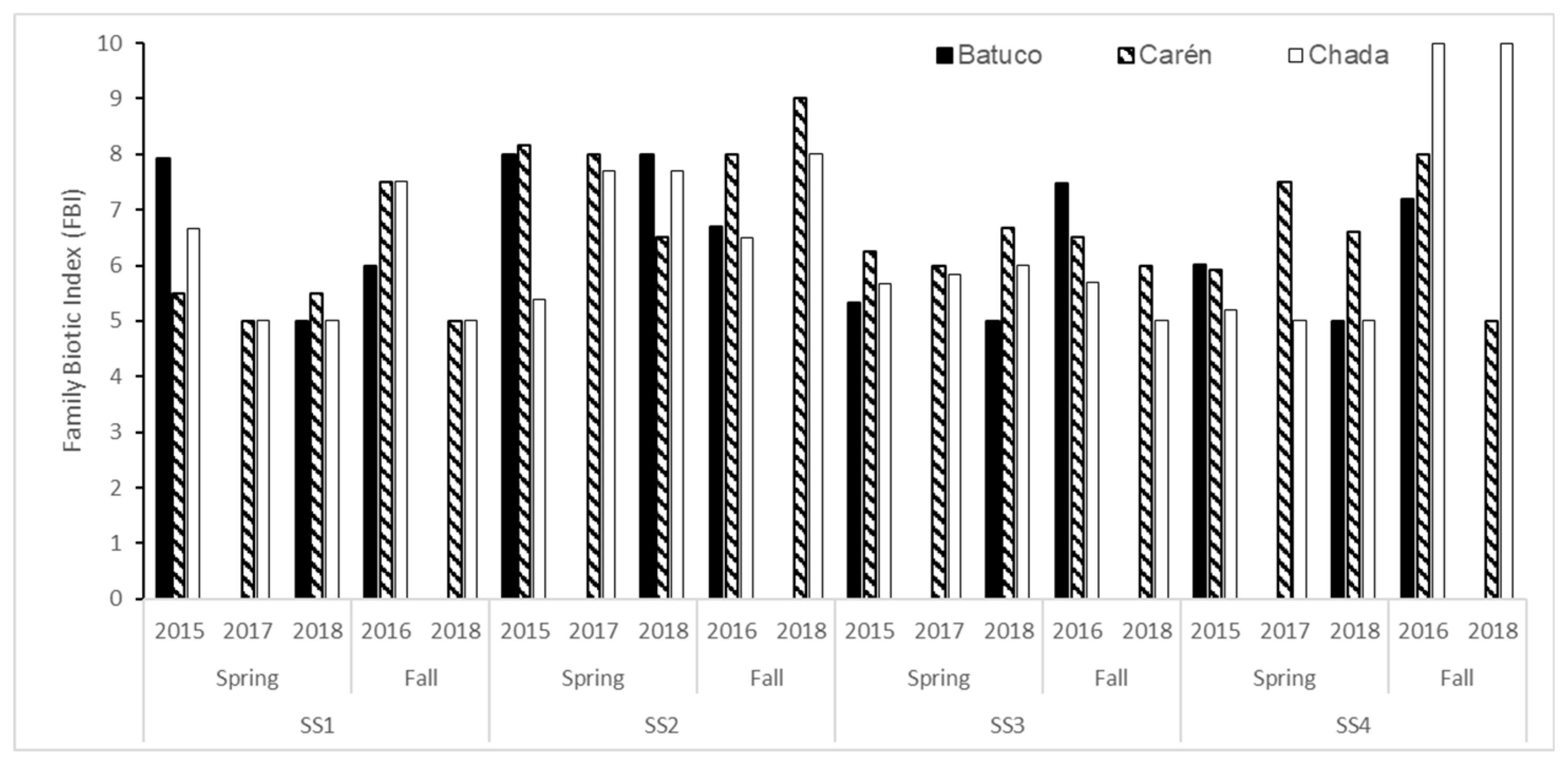
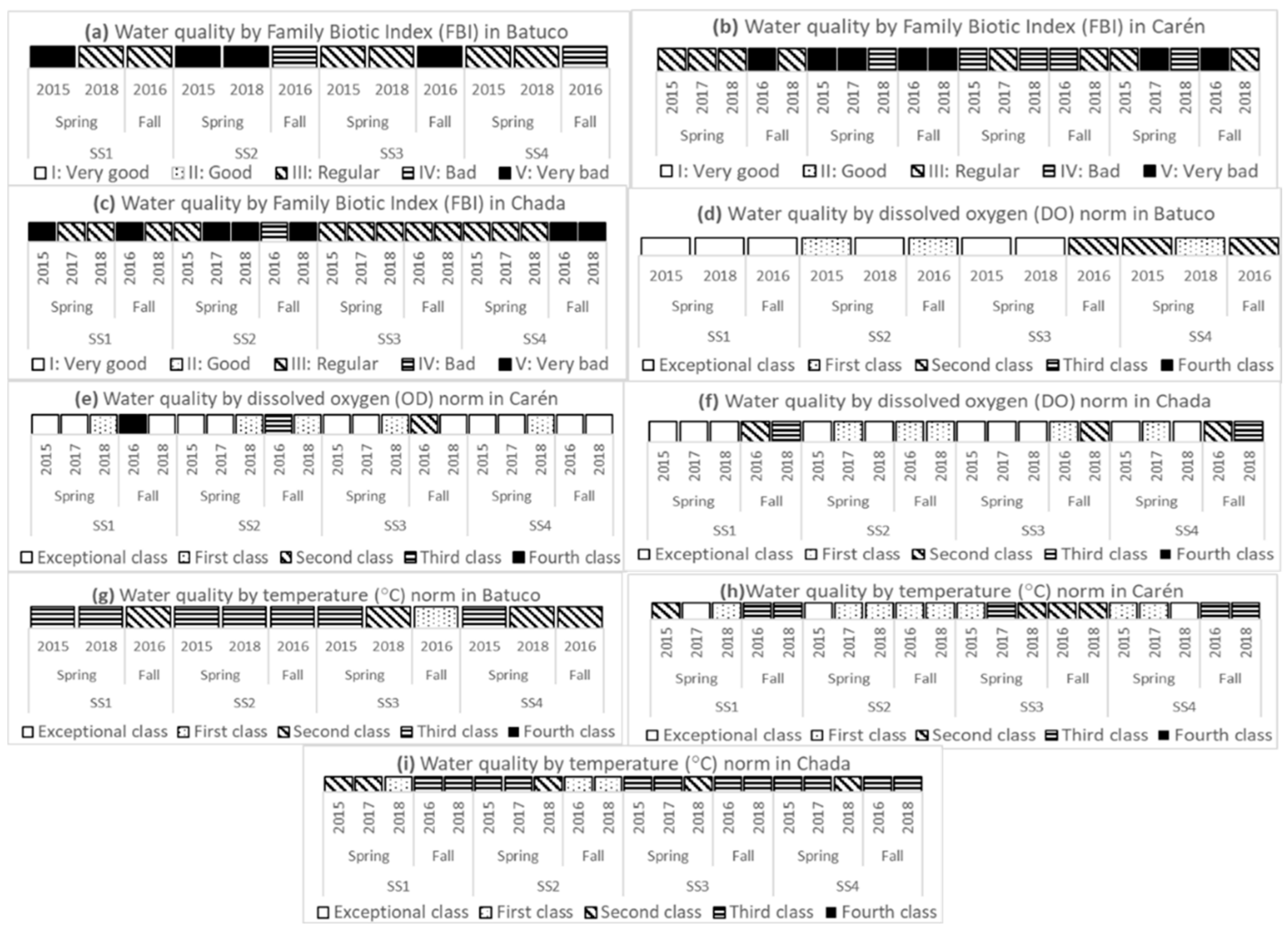
2.3. Determination of Water Quality Classes by Family Biotic Index (FBI)
2.4. Measurement and Determination of Water Quality Classes Using Physicochemical Variables
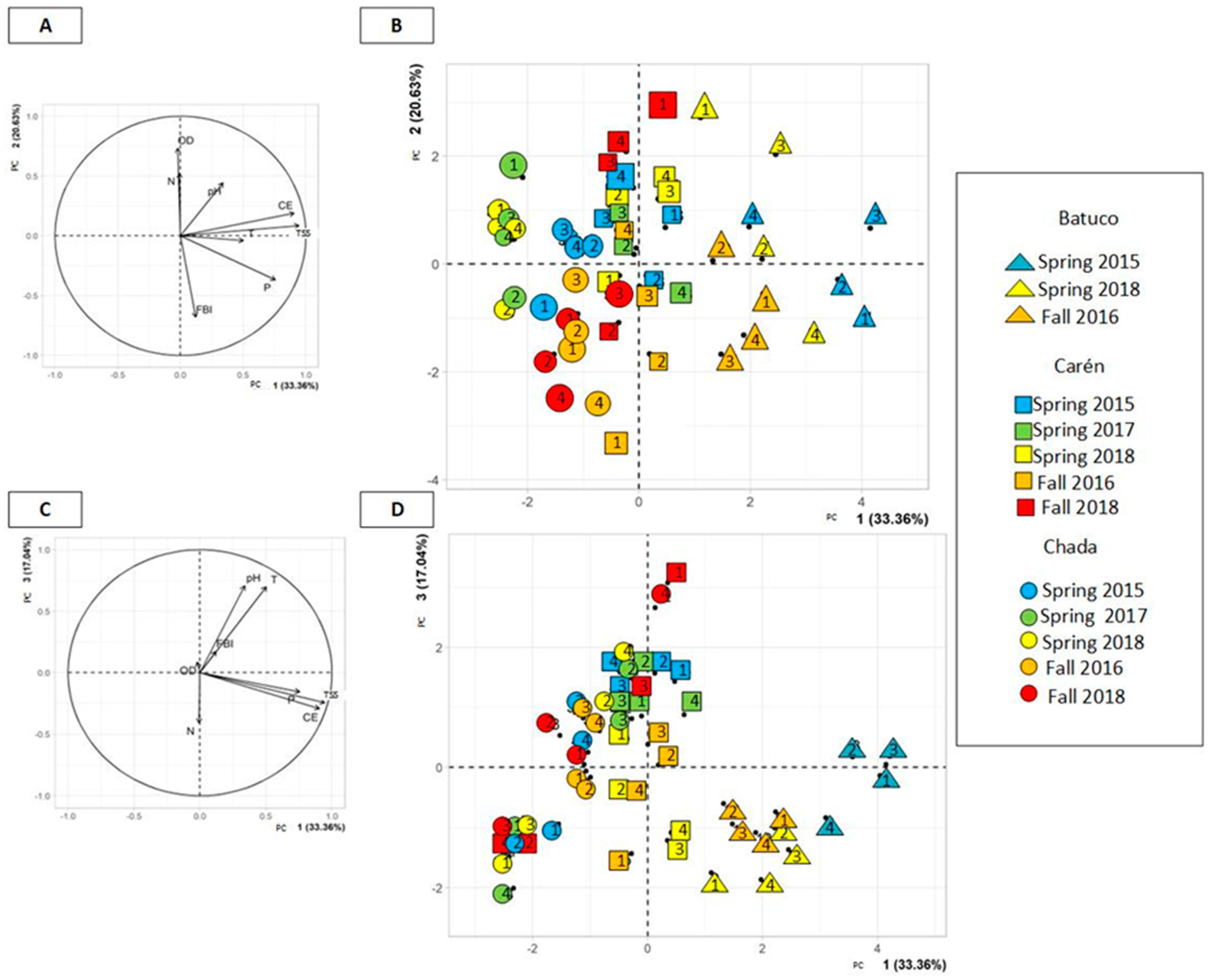
2.5. Principal Component Analysis (PCA) of Physicochemical and Biological Variables
3. Results
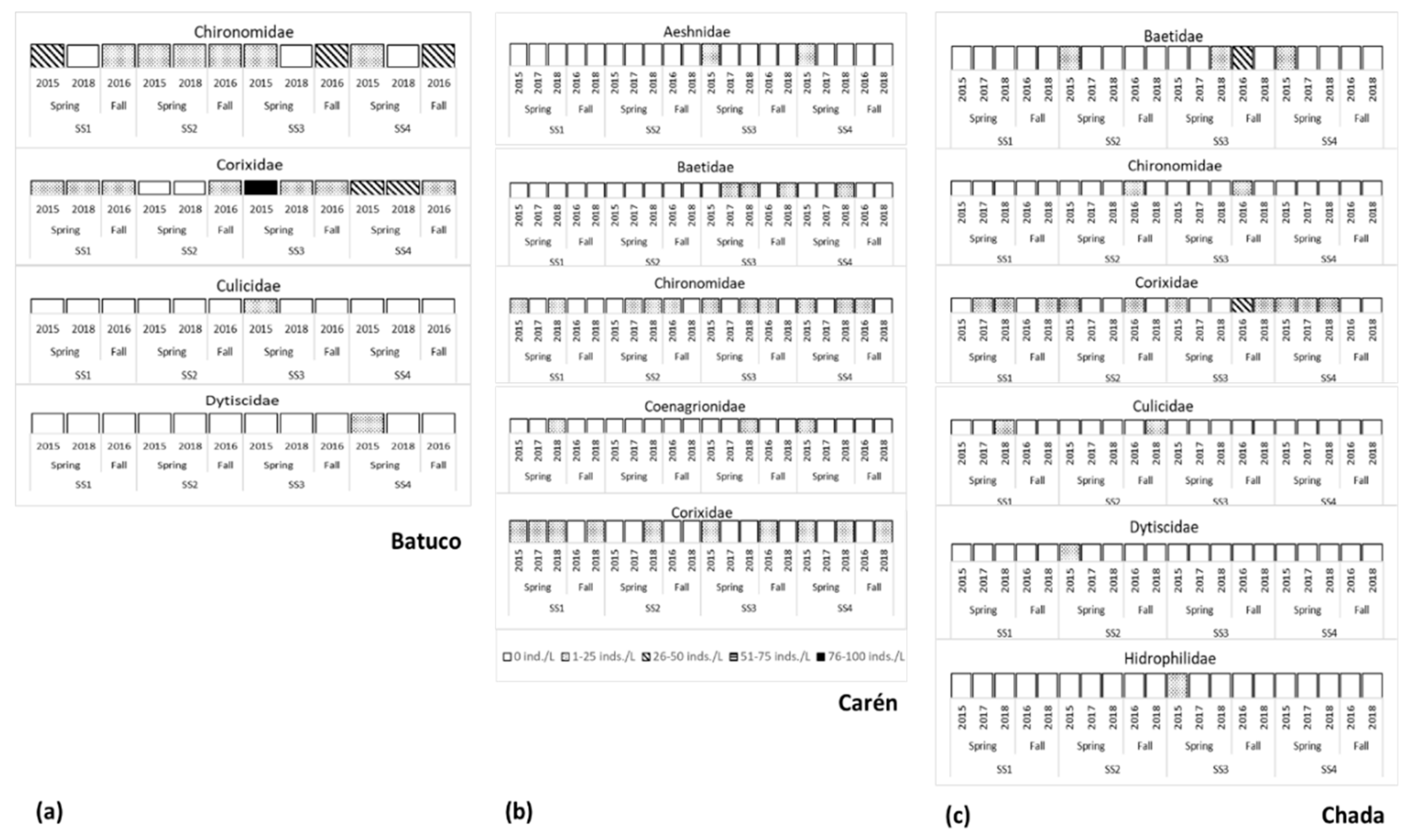
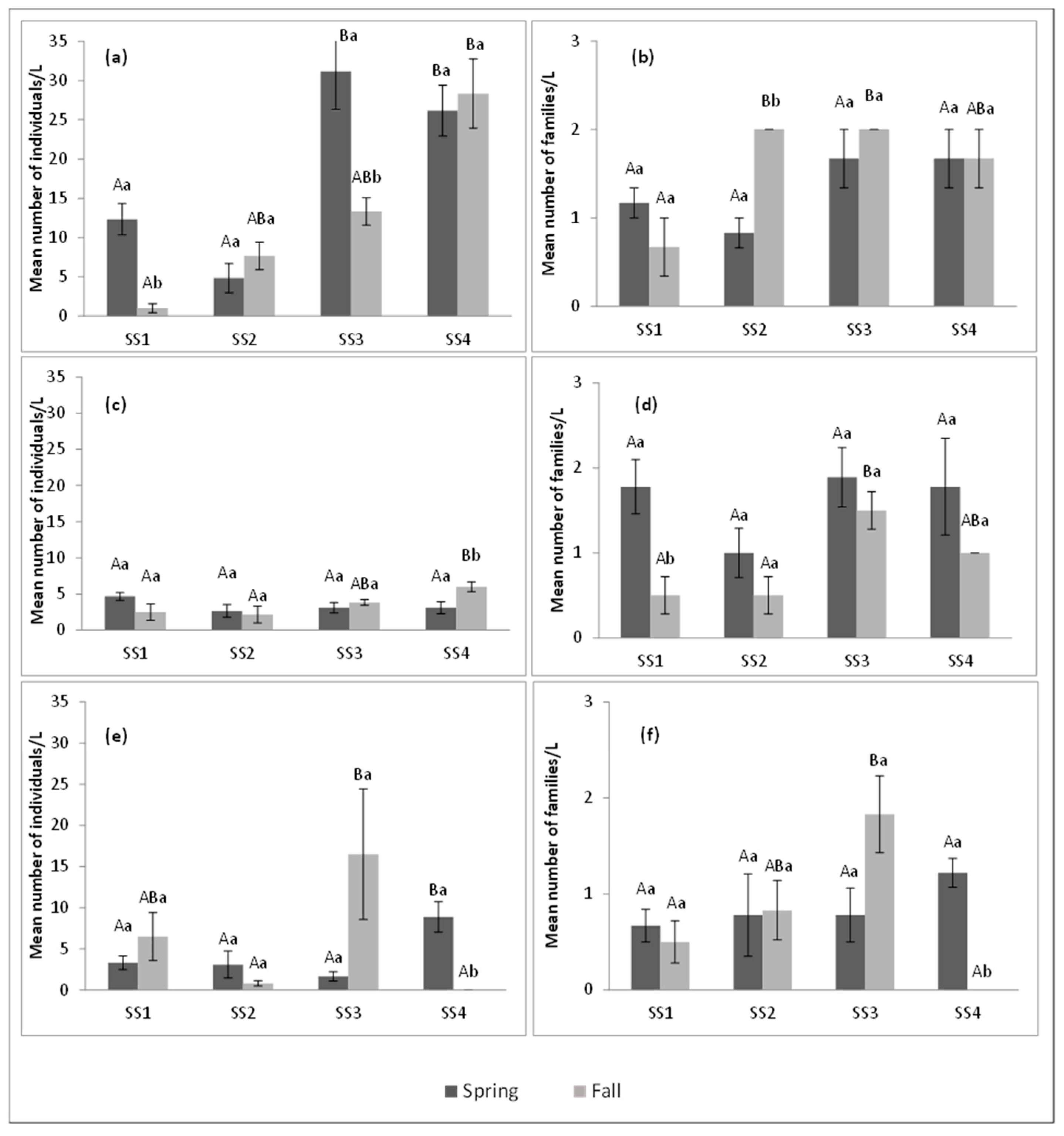
3.1. Abundance and Richness of Aquatic Insect Family Assemblages
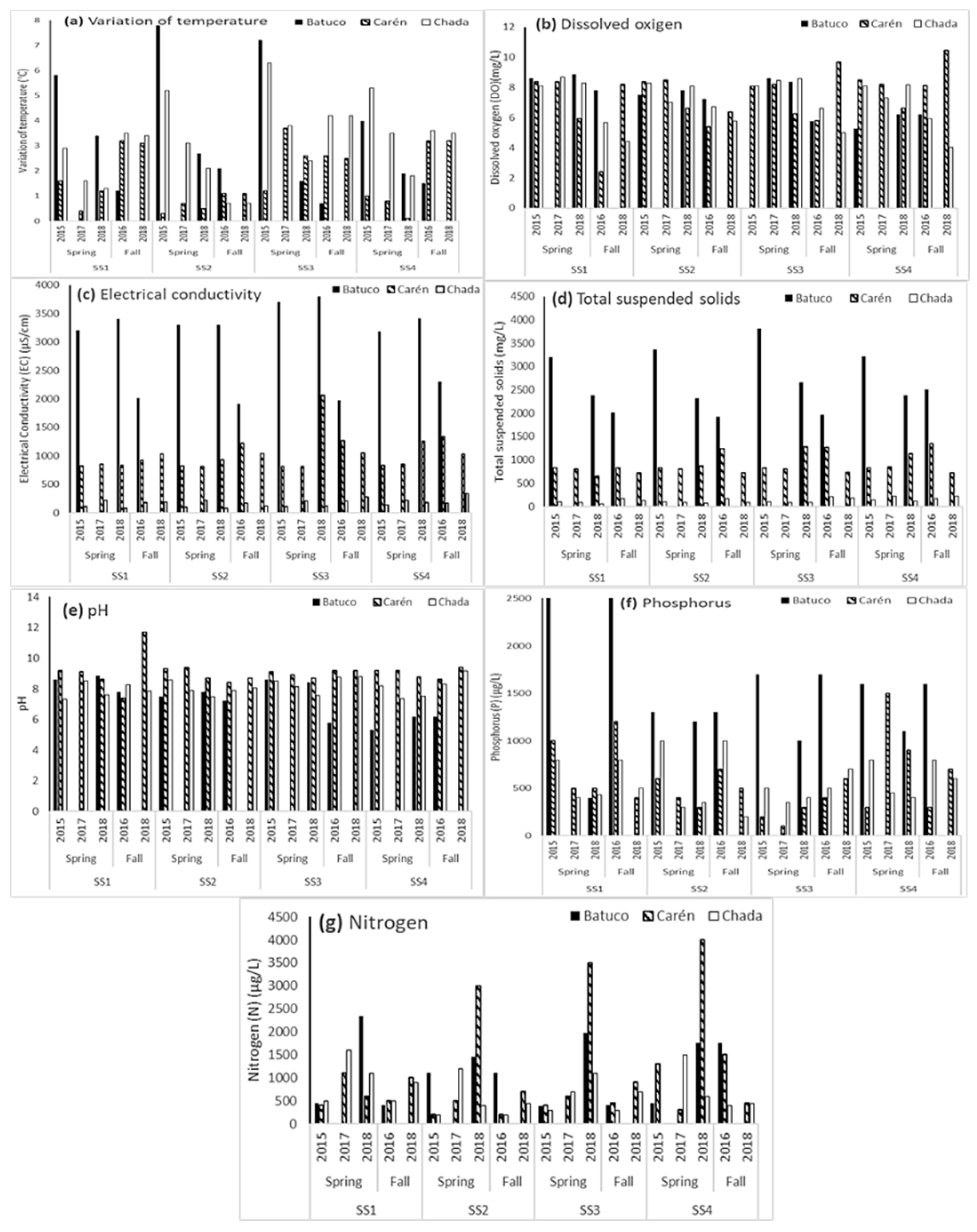
3.2. Water Quality from Physicochemical and Biological Variables
3.3. Principal Component Analysis (PCA)
4. Discussion
4.1. Analysis by Water Body
4.1.1. Batuco
4.1.2. Carén
4.1.3. Chada
4.2. Global Analysis of the Three Water Bodies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Formetta, G.; Marra, F.; Dallan, E.; Zaramella, M.; Borga, M. Differential orographic impact on sub-hourly, hourly, and daily extreme precipitation. Advances in Water Resources 2022, 159, 104084. [Google Scholar] [CrossRef]
- Tesser-Obregón, C. El agua y los territorios hídricos en la Región Metropolitana de Santiago de Chile. Casos de estudio: Tiltil, Valle de Mallarauco y San Pedro de Melipilla. Estudios Geográficos 2013, 74, 255–285. [Google Scholar] [CrossRef]
- Barría, P.; Chadwick, C.; Ocampo-Melgar, A.; Galleguillos, M.; Garreaud, R.; et al. Water management or megadrought: what caused the Chilean Aculeo Lake drying? Regional Environmental Change 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Bruskotter, J.; Vucetich, J.A.; Dietsch, A.; Slagle, K.; Brooks; Nelson, M. Conservationists’ moral obligations toward wildlife: Values and identity promote conservation conflict. Biological Conservation 2019, 240, 108296. [Google Scholar] [CrossRef]
- Schultz, P. W.; Gouveia, V.; Cameron, L.; Tankha, G.; Schmuck, P.; Franěk, M. Values and their relationship to environmental concern and conservation behavior. Journal of Cross-Cultural Psychology 2005, 36, 457–475. [Google Scholar] [CrossRef]
- Rey, O.; Eizaguirre, C.; Angers, B.; Baltazar-Soares, M.; Sagonas, K.; Prunier, J.G.; Blanchet, S. Linking epigenetics and biological conservation: Towards a conservation epigenetics perspective. Functional Ecology 2020, 34, 414–427. [Google Scholar] [CrossRef]
- INN Chile (Instituto Nacional de Normalización Chile). Norma Chilena Oficial NCh 1333. Of. 78 modificada en 1987 “Requisitos de calidad de agua para diferentes usos”; INN Chile: Santiago, Chile, 1987. [Google Scholar]
- CONAMA (Comisión Nacional del Medio Ambiente, Chile). Guía para el establecimiento de las normas secundarias de calidad ambiental para aguas continentales superficiales y marinas; CONAMA: Santiago, Chile, 2004. [Google Scholar]
- Figueroa, R.; Soria, M.; Beltrán, M.; Correa-Araneda, J. Estudio de comunidades biológicas como bioindicadores de calidad de agua. In Estudio de comunidades biológicas como bioindicadores de calidad de agua; Chatata, B., Talavera, C., Villasante, F., Eds.; Universidad Nacional de San Agustín-CONCYTEC: Arequipa, Perú, 2016; pp. 23–34. [Google Scholar]
- Alvarado-Orellana, A.; Huerta-Fuentes, A.; Palma-Muñoz, A.; Rodríguez-Tobar, S. Variación estacional de la diversidad de coleópteros epigeos en la Laguna Carén (Santiago, Chile). Revista Colombiana Entomología 2018, 44, 266–272. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as bioindicator: A hidden gem for environmental monitoring. Frontiers in Environmental Science 2023, 11, 1146052. [Google Scholar] [CrossRef]
- Figueroa, R.; Palma, A.; Ruiz, V.; Niell, X. Análisis comparativo de índices bióticos utilizados en la evaluación de la calidad de las aguas en un río mediterráneo de Chile: río Chillán, VIII Región. Revista Chilena de Historia Natural 2007, 80, 225–242. [Google Scholar] [CrossRef]
- Gobierno de Chile. 2011. Definición contenida, Guía para la Conservación y Seguimiento Ambiental de Humedales Andinos. Available online: https://bibliotecadigital.ciren.cl/server/api/core/bitstreams/45a24e5b-1b80-476d-9660-a01d14e971c7/content (accessed on 8th August 2024).
- Figueroa, R. Calidad ambiental de la cuenca hidrográfica del río Chillán, VIII Región, Chile. Ph.D. Thesis, Universidad de Málaga, Málaga, España, 2004. Dialnet. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=217023 (accessed on 8th August 2024).
- Laino-Guanes, R.M.; Bello-Mendoza, R.; González-Espinosa, M.; Ramírez-Marcial, N.; Jiménez-Otárola, F.; Musálem-Castillejos, K. Concentración de metales en agua y sedimentos de la cuenca alta del río Grijalva, frontera México-Guatemala. Tecnología y Ciencias del Agua 2015, 6, 61–74. [Google Scholar]
- Segnini, S.; Correa, I.; Chacón, M. Evaluación de la calidad del agua de ríos en los Andes venezolanos usando el índice biótico BMWP. In Enfoques y Temáticas en Entomología; Arrivillaga, J.C., El Souki, M., Herrera, B., Eds.; Sociedad Venezolana de Entomología: Caracas, Venezuela, 2009; pp. 217–254. [Google Scholar]
- Hinselhoff, W.L. Rapid field assessment of organic pollution with a family-level biotic index. Journal of the North American Benthological Society 1988, 7, 65–68. [Google Scholar]
- Badaracco, C.; Huerta, A.; Palma, A. Assemblage of riparian epigeal coleopteran of the Batuco wetland (Metropolitan Region, Chile). Gayana 2021, 85, 46–54. [Google Scholar]
- Universidad de Chile. 2017. Parque Carén: Pionera planta piloto de alimentos saludables inició su construcción. Available online: https://www.uchile.cl/noticias/137460/pionera-planta-piloto-de-alimentos-saludables-inicia-su-construccion (accessed on 8th August 2024).
- Universidad de Chile. 2024. Parque Carén-Universidad de Chile. Available online: https://caren.uchile.cl/presentacion/#parqueConclusions (accessed on 8th August 2024).
- Gobernación Provincial de Maipo. 2015. Tranque de Chada, riego y vida para la zona. Available online: www.gobernacionmaipo.gov.cl/noticias/tranque-de-chada-riego-y-vida-parala-zona/ (accessed on 8th August 2024).
- Camousseight, A. Estado de conocimiento de los Ephemeroptera de Chile. Gayana (Concepción) 2006, 70, 50–56. [Google Scholar] [CrossRef]
- Thyssen, P.J. Keys for Identification of Immature Insects. In Current Concepts in Forensic Entomology; Amendt, J., M. Goff, Campobasso, C., Grassberger, M., Eds.; Springer: Dordrecht, Netherlands, 2009; pp. 25–42. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat versión 2020; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina; Available online: http://www.infostat.com.ar (accessed on 8th August 2024).
- MOP-DGA (Ministerio de Obras Públicas-Dirección General de Aguas). Valores de los puntajes de FBI modificados para Chile; MOP-DGA: Santiago, Chile, 2010. [Google Scholar]
- INN Chile. Norma Chilena Oficial de Calidad de Agua NCh 411/1-4; INN Chile: Santiago, Chile, 1996. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Newall, P.; Tiller, D. Derivation of nutrient guidelines for streams in Victoria, Australia. Environmental Monitoring and Assessment 2002, 74, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Mellado, C. Caracterización hídrica y gestión ambiental del Humedal Batuco. Master Thesis, Universidad de Chile, Santiago, Chile, 2008. [Google Scholar]
- Gesam Consultores Ambientales. Conservación, monitoreo y manejo para el humedal de Batuco, Línea de Base Ambiental; Gesam Consultores Ambientales: Santiago, Chile, 2018. [Google Scholar]
- The Nature Conservancy. 2018. Plan de manejo, Laguna de Batuco. Elaborado para Fundación San Carlos de Batuco. Available online: www.fsancarlos.cl/wp-content/uploads/2021/01/Plan-de-Manejo-Laguna-Batuco.pdf (accessed on 8th August 2024).
- MOP-DGA. Decreto 53; MOP-DGA: Santiago, Chile, 2020. [Google Scholar]
- SMA (Superintendencia del Medio Ambiente - Gobierno de Chile). Normas secundarias de calidad ambiental para la protección de las aguas de la cuenca del Río Maipo. Informe Técnico de Cumplimiento de Normas de Calidad del Agua; SMA: Santiago, Chile, 2020. [Google Scholar]
- Instituto Nacional de Investigación de Recursos Naturales (Chile). Suelos. Descripciones. Proyecto Aerofotogramétrico Chile/OEA/BID; IREN-CORFO: Santiago, Chile, 1964. [Google Scholar]
- Sandoval, J. El riego en Chile; Dirección de Obras Hidráulicas (DOH Chile) - Ministerio de Obras Públicas (MOP): Santiago, Chile, 2003. [Google Scholar]
- California Waterboard. (no date). Folleto informativo conductividad eléctrica/salinidad. Folleto Informativo 3.1.3.0. Available online: https://www.waterboards.ca.gov/water_issues/programs/swamp/docs/cwt/guidance/3130sp.pdf (accessed on 8th August 2024).
- Posada, E.; Mojica, D.; Pino, N.; Bustamante, C.; Monzón, A. Establecimiento de índices de calidad ambiental de ríos con bases en el comportamiento del oxígeno disuelto y de la temperatura. Aplicación al caso del río Medellín, en el Valle de Aburrá en Colombia. Dyna 2013, 80, 192–200. [Google Scholar]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Research 2015, 77, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E. Eutrophication. In Water Quality; Boyd, C.E., Ed.; Springer: Cham, Switzerland, 2020; pp. 311–322. [Google Scholar]
- Carrera, D.; Crisanto, T.; Maya, M. Relación entre la composición química inorgánica del agua, la precipitación y la evaporación. Enfoque UTE 2015, 6, 25–34. [Google Scholar] [CrossRef]
- IEE (Instituto Espacial Ecuatoriano). 2013. Memoria Técnica Cantón Chone Proyecto: “Generación de Geoinformación para la Gestión del Territorio a Nivel Nacional, Escala 1: 25 000” Geomorfología, 2013. Available online: http://www.ideportal.iee.gob.ec/ (accessed on 8th August 2024).
- Carrera-Villacrés, D.; Guerrón-Varela, E.; Cajas-Morales, L.; González-Farinango, T.; Guamán-Pineda, E.; Velarde-Salazar, P. Relación de temperatura, pH y CE en la variación de concentración de fosfatos en el Río Grande, Cantón Chone. Congreso de Ciencia y Tecnología ESPE 2018, 13, 37–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




