Submitted:
13 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diagnostic Criteria of Liver Steatosis along with Shift of Terminology
2.1. Refining the Diagnosis of MASLD
2.1.1. Adults
- -
-
MASLD (i.e., liver steatosis, at least one cardiometabolic risk factor, no other discernible cause, no alcohol consumption or a weekly consumption ≤140 g in females and ≤210 g in males). In adult populations, the initial diagnosis of SLD requires the exact analysis of cardiometabolic criteria to reach the final diagnosis of MASLD or MetALD. The abnormalities are the following:
- Body mass index (BMI) ≥25 kg/m2 (23 kg/m2 for Asians) or waist circumference >94 cm (males), >80 cm (females) or ethnicity adjusted;
- Fasting serum glucose ≥100 mg/dL or 2-hrs post-load glucose levels ≥140 mg/dL) or HbA1c ≥5.7% or T2DM or treatment for T2DM
- Blood pressure ≥130/85 mmHg or specific antihypertensive treatment
- Plasma triglycerides ≥150 mg/dL or lipid-lowering treatment
- Plasma HDL-cholesterol ≤40 mg/dL (males), ≤50 mg/dL (females), or lipid-lowering treatment.
- -
- MetALD, i.e., an overlap of MASLD and ALD, i.e., liver steatosis, no other discernible cause, and intermediate weekly alcohol consumption of 140-350 g in females and 210-420g in males with a continuum ranging from MASLD-predominant to ALD-predominant types.
- -
- ALD, i.e., liver steatosis with weekly alcohol consumption >350 g in females and ≥ 420g in males)
- -
- Specific etiology SLD (i.e., drug-induced liver injury DILI, monogenic diseases such as lysosomal acid lipase deficiency LALD, Wilson disease, hypobetalipoproteinemia, inborn error of metabolism, and miscellaneous such as Hepatitis C virus, malnutrition, celiac disease).
- -
- Cryptogenic SLD, a label prone to re-classification in the future, as long as further diagnostic entities are reached. Importantly, the diagnosis of SLD must be re-assessed periodically to rule out incoming findings suggesting novel diagnoses.
2.1.2. Children and Adolescents
- Overweight/obesity – i.e., BMI ≥85th percentile for age/sex (BMI Z-score ≥+1) or waist circumference >95th percentile (values may vary by ethnicity or race);
- Prediabetes/diabetes testified by fasting serum glucose ≥100 mg/dL or random serum glucose ≥200 mg/dL or 2-hour oral glucose tolerance test ≥140 mg/dL or HbA1c ≥5.7% or established diagnosis of T2DM or specific treatment for T2DM.
- Hypertension testified by blood pressure (BP) ≥130/80 mmHg for age ≥13 years; for age <13 years, BP ≥95th percentile or ≥130/80 mmHg (whichever is lower) or use of antihypertensive treatment.
- Hypertriglyceridemia with triglyceride ≥100 mg/dL for age <10 years or triglyceride ≥150 mg/dL for age ≥10 years or lipid-lowering treatment
- Low cholesterol HDL, i.e., HDL ≤40 mg/dL or lipid-lowering treatment.
3. MASLD Epidemiology and Natural History
4. MASLD Pathogenesis and Molecular Aspects
5. Challenging Diagnosis of MASLD in Children and Adolescents
5.1. Clinical Aspects
5.2. Screening
6. T2DM and MASLD
6.1. Interconnections
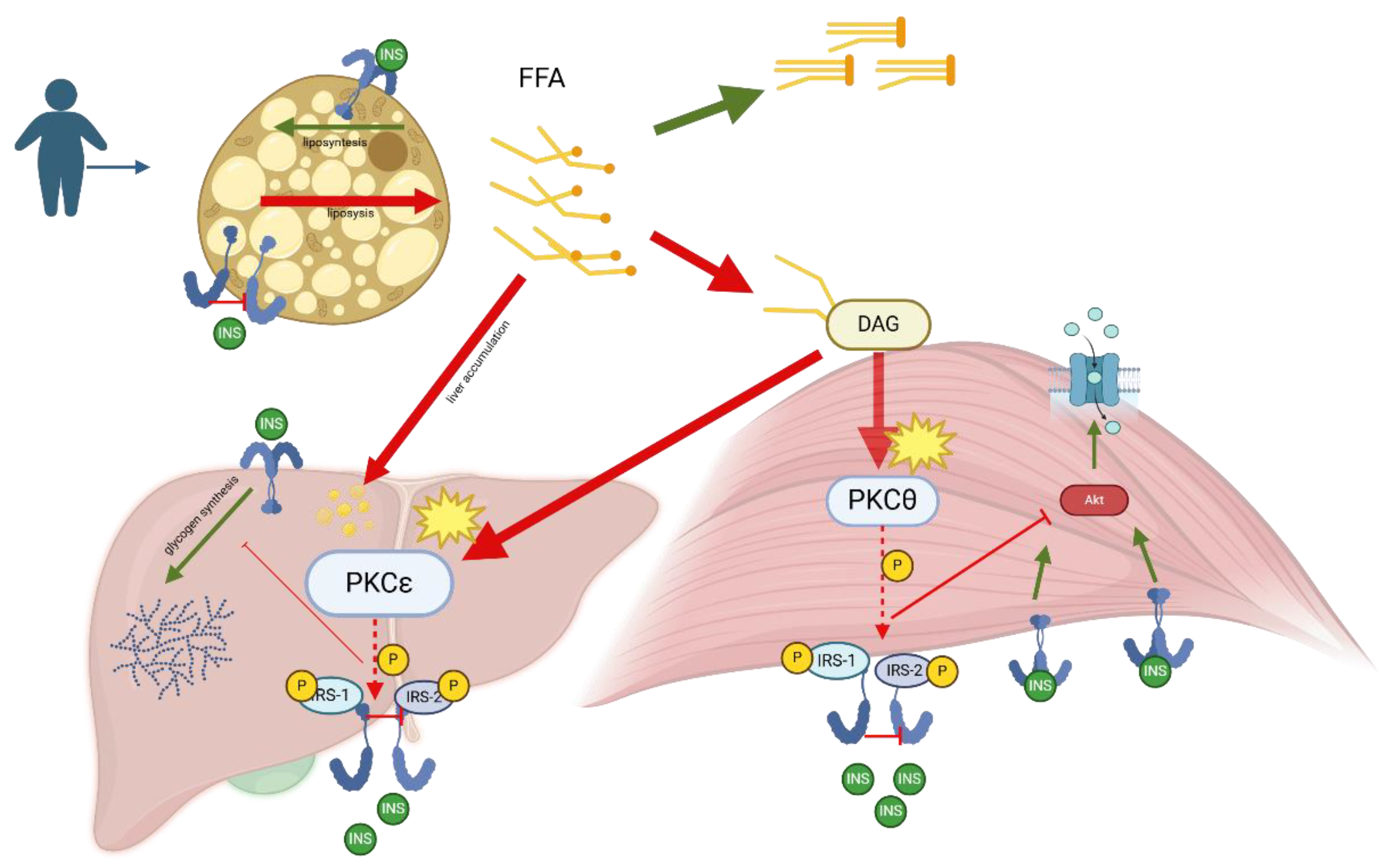
6.2. Follow-up Implications
6.3. Therapeutic Aspects
7. Conclusions
References
- Jebeile, H.; Kelly, A.S.; O'Malley, G.; Baur, L.A. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Wasniewska, M.; Pepe, G.; Aversa, T.; Bellone, S.; de Sanctis, L.; Di Bonito, P.; Faienza, M.F.; Improda, N.; Licenziati, M.R.; Maffeis, C.; et al. Skeptical Look at the Clinical Implication of Metabolic Syndrome in Childhood Obesity. Children (Basel, Switzerland) 2023, 10. [Google Scholar] [CrossRef]
- Di Bonito, P.; Di Sessa, A.; Licenziati, M.R.; Corica, D.; Wasniewska, M.; Umano, G.R.; Morandi, A.; Maffeis, C.; Faienza, M.F.; Mozzillo, E.; et al. Is Metabolic Syndrome Useful for Identifying Youths with Obesity at Risk for NAFLD? Children (Basel, Switzerland) 2023, 10. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Bonfrate, L.; Stella, A.; Garruti, G.; Lamont, J.T. Metabolic dysfunction-associated gallstone disease: expecting more from critical care manifestations. Internal and emergency medicine 2023, 18, 1897–1918. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza'ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Parekh, V.V.; Gabriel, C.L.; Bracy, D.P.; Marks-Shulman, P.A.; Tamboli, R.A.; Kim, S.; Mendez-Fernandez, Y.V.; Besra, G.S.; Lomenick, J.P.; et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, E1143–1152. [Google Scholar] [CrossRef]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 5133–5138. [Google Scholar] [CrossRef]
- Boulenouar, S.; Michelet, X.; Duquette, D.; Alvarez, D.; Hogan, A.E.; Dold, C.; O'Connor, D.; Stutte, S.; Tavakkoli, A.; Winters, D.; et al. Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis through Targeted Cytotoxicity. Immunity 2017, 46, 273–286. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Marusic, M.; Paic, M.; Knobloch, M.; Liberati Prso, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can J Gastroenterol Hepatol 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic proceedings. Mayo Clinic 1980, 55, 434–438. [Google Scholar]
- Yip, T.C.; Vilar-Gomez, E.; Petta, S.; Yilmaz, Y.; Wong, G.L.; Adams, L.A.; de Ledinghen, V.; Sookoian, S.; Wong, V.W. Geographical similarity and differences in the burden and genetic predisposition of NAFLD. Hepatology 2023, 77, 1404–1427. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Paik, J.M.; Kabbara, K.; Eberly, K.E.; Younossi, Y.; Henry, L.; Younossi, Z.M. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology 2022, 75, 1204–1217. [Google Scholar] [CrossRef]
- Portincasa, P. NAFLD, MAFLD, and beyond: one or several acronyms for better comprehension and patient care. Internal and emergency medicine 2023, 18, 993–1006. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus, P. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014 e1991. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. The lancet. Gastroenterology & hepatology 2022, 7, 388–390. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Baffy, G. Metabolic dysfunction-associated steatotic liver disease: Evolution of the final terminology. European journal of internal medicine 2024, 124, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Boursier, J.; Fibrosis, A.G.f.t.S.o.L. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. Journal of hepatology 2024, 80, e51–e52. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Lai, J.C.; Wong, G.L.; Wong, V.W.; Yip, T.C. Can we use old NAFLD data under the new MASLD definition? Journal of hepatology 2024, 80, e54–e56. [Google Scholar] [CrossRef]
- Lee, C.M.; Yoon, E.L.; Kim, M.; Kang, B.K.; Cho, S.; Nah, E.H.; Jun, D.W. Prevalence, distribution, and hepatic fibrosis burden of the different subtypes of steatotic liver disease in primary care settings. Hepatology 2024, 79, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: updated naming and diagnosis criteria for fatty liver disease. Journal of lipid research 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Di Ciaula, A. Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. International journal of molecular sciences 2024, 25, 5640. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Utz-Melere, M.; Targa-Ferreira, C.; Lessa-Horta, B.; Epifanio, M.; Mouzaki, M.; Mattos, A.A. Non-Alcoholic Fatty Liver Disease in Children and Adolescents: Lifestyle Change - a Systematic Review and Meta-Analysis. Annals of hepatology 2018, 17, 345–354. [Google Scholar] [CrossRef]
- Patel, K.R.; White, F.V.; Deutsch, G.H. Hepatic steatosis is prevalent in stillborns delivered to women with diabetes mellitus. Journal of pediatric gastroenterology and nutrition 2015, 60, 152–158. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Kinugasa, A.; Tsunamoto, K.; Furukawa, N.; Sawada, T.; Kusunoki, T.; Shimada, N. Fatty liver and its fibrous changes found in simple obesity of children. Journal of pediatric gastroenterology and nutrition 1984, 3, 408–414. [Google Scholar] [CrossRef]
- Wiegand, S.; Keller, K.M.; Robl, M.; L'Allemand, D.; Reinehr, T.; Widhalm, K.; Holl, R.W.; Group, A.P.-S.; the German Competence Network, A. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. International journal of obesity 2010, 34, 1468–1474. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 2013, 162, 496–500 e491. [Google Scholar] [CrossRef]
- Mischel, A.K.; Liao, Z.; Cao, F.; Dunn, W.; Lo, J.C.; Newton, K.P.; Goyal, N.P.; Yu, E.L.; Schwimmer, J.B. Prevalence of Elevated ALT in Adolescents in the US 2011-2018. Journal of pediatric gastroenterology and nutrition 2023, 77, 103–109. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PloS one 2015, 10, e0140908. [Google Scholar] [CrossRef]
- Awai, H.I.; Newton, K.P.; Sirlin, C.B.; Behling, C.; Schwimmer, J.B. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol 2014, 12, 765–773. [Google Scholar] [CrossRef]
- Ciardullo, S.; Monti, T.; Perseghin, G. Prevalence of Liver Steatosis and Fibrosis Detected by Transient Elastography in Adolescents in the 2017-2018 National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol 2021, 19, 384–390 e381. [Google Scholar] [CrossRef]
- Liu, J.; Mu, C.; Li, K.; Luo, H.; Liu, Y.; Li, Z. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Children and Adolescents: Systematic Review and Meta-Analysis. International journal of public health 2021, 66, 1604371. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Pantangi, V.; Azam, M.; Salomao, M.; Iuga, A.C.; Lefkowitch, J.H.; Gill, J.; Morotti, R.; Lavine, J.E.; Mencin, A.A. Pediatric Nonalcoholic Fatty Liver Disease in New York City: An Autopsy Study. J Pediatr 2018, 200, 174–180. [Google Scholar] [CrossRef]
- Montinaro, F.; Busby, G.B.; Pascali, V.L.; Myers, S.; Hellenthal, G.; Capelli, C. Unravelling the hidden ancestry of American admixed populations. Nat Commun 2015, 6, 6596. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. Metabolic-Dysfunction-Associated Steatotic Liver Disease-Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2021, 137, 111315. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jablkowski, M.; Szemraj, J.; Czarny, P. The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. International journal of molecular sciences 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Comprehensive Physiology 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Tu, W.; Liu, J.; Tian, D. Hepatocytes: A key role in liver inflammation. Front Immunol 2022, 13, 1083780. [Google Scholar] [CrossRef] [PubMed]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab 2020, 42, 101092. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Mani, A. Dysregulation of Lipid and Glucose Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Fotbolcu, H.; Zorlu, E. Nonalcoholic fatty liver disease as a multi-systemic disease. World journal of gastroenterology : WJG 2016, 22, 4079–4090. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Speiser, P.W.; Rudolf, M.C.; Anhalt, H.; Camacho-Hubner, C.; Chiarelli, F.; Eliakim, A.; Freemark, M.; Gruters, A.; Hershkovitz, E.; Iughetti, L.; et al. Childhood obesity. The Journal of clinical endocrinology and metabolism 2005, 90, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, A.D.; Perez-Atayde, A.R.; Graeme-Cook, F.; Higgins, L.; Lavine, J.E. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr 1995, 127, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Roberts, E.A. Nonalcoholic steatohepatitis in children. Journal of pediatric gastroenterology and nutrition 2000, 30, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Phatak, U.P.; Pashankar, D.S. Obesity and gastrointestinal disorders in children. Journal of pediatric gastroenterology and nutrition 2015, 60, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Molleston, J.P.; Schwimmer, J.B.; Yates, K.P.; Murray, K.F.; Cummings, O.W.; Lavine, J.E.; Brunt, E.M.; Scheimann, A.O.; Unalp-Arida, A.; Network, N.C.R. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr 2014, 164, 707–713 e703. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Barlow, S.E.; Quiros-Tejeira, R.E.; Scheimann, A.; Skelton, J.; Suskind, D.; Tsai, P.; Uko, V.; Warolin, J.P.; Xanthakos, S.A.; et al. Childhood obesity for pediatric gastroenterologists. Journal of pediatric gastroenterology and nutrition 2013, 56, 99–109. [Google Scholar] [CrossRef]
- Franzese, A.; Vajro, P.; Argenziano, A.; Puzziello, A.; Iannucci, M.P.; Saviano, M.C.; Brunetti, F.; Rubino, A. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci 1997, 42, 1428–1432. [Google Scholar] [CrossRef]
- Tazawa, Y.; Noguchi, H.; Nishinomiya, F.; Takada, G. Serum alanine aminotransferase activity in obese children. Acta paediatrica 1997, 86, 238–241. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). Journal of pediatric gastroenterology and nutrition 2017, 64, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Newton, K.P.; Awai, H.I.; Choi, L.J.; Garcia, M.A.; Ellis, L.L.; Vanderwall, K.; Fontanesi, J. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Alimentary pharmacology & therapeutics 2013, 38, 1267–1277. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B.; Molleston, J.P.; Scheimann, A.O.; Murray, K.F.; Abrams, S.H.; Rosenthal, P.; Sanyal, A.J.; Robuck, P.R.; Brunt, E.M.; et al. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemporary clinical trials 2010, 31, 62–70. [Google Scholar] [CrossRef]
- Colantonio, D.A.; Kyriakopoulou, L.; Chan, M.K.; Daly, C.H.; Brinc, D.; Venner, A.A.; Pasic, M.D.; Armbruster, D.; Adeli, K. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012, 58, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Alkhouri, N.; Carter-Kent, C.; Monti, L.; Devito, R.; Lopez, R.; Feldstein, A.E.; Nobili, V. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. Journal of pediatric gastroenterology and nutrition 2011, 53, 190–195. [Google Scholar] [CrossRef]
- Bohte, A.E.; Koot, B.G.; van der Baan-Slootweg, O.H.; van Werven, J.R.; Bipat, S.; Nederveen, A.J.; Jansen, P.L.; Benninga, M.A.; Stoker, J. US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology 2012, 262, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Draijer, L.G.; Feddouli, S.; Bohte, A.E.; Vd Baan Slootweg, O.; Pels Rijcken, T.H.; Benninga, M.A.; Stoker, J.; Koot, B.G.P. Comparison of diagnostic accuracy of screening tests ALT and ultrasound for pediatric non-alcoholic fatty liver disease. European journal of pediatrics 2019, 178, 863–870. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Noureddin, M.; Bernstein, D.; Kwo, P.; Russo, M.; Shiffman, M.L.; Younes, Z.; Abdelmalek, M. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. The American journal of gastroenterology 2021, 116, 254–262. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Middleton, M.S.; Behling, C.; Newton, K.P.; Awai, H.I.; Paiz, M.N.; Lam, J.; Hooker, J.C.; Hamilton, G.; Fontanesi, J. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 2015, 61, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. Journal of pediatric gastroenterology and nutrition 2012, 54, 700–713. [Google Scholar] [CrossRef]
- Ezaizi, Y.; Kabbany, M.N.; Conjeevaram Selvakumar, P.K.; Sarmini, M.T.; Singh, A.; Lopez, R.; Nobili, V.; Alkhouri, N. Comparison between non-alcoholic fatty liver disease screening guidelines in children and adolescents. JHEP Rep 2019, 1, 259–264. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Dunn, W.; Norman, G.J.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C.B. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010, 138, 1357–1364 1364 e1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Bussler, S.; Vogel, M.; Pietzner, D.; Harms, K.; Buzek, T.; Penke, M.; Handel, N.; Korner, A.; Baumann, U.; Kiess, W.; et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase): Effects of age, sex, body mass index, and pubertal stage. Hepatology 2018, 68, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Cohen, A.; Konforte, D.; Binesh-Marvasti, T.; Colantonio, D.A.; Adeli, K. Validity of establishing pediatric reference intervals based on hospital patient data: a comparison of the modified Hoffmann approach to CALIPER reference intervals obtained in healthy children. Clinical biochemistry 2014, 47, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.S.; Sokol, R.J.; Capocelli, K.E.; Pan, Z.; Sullivan, J.S.; Robbins, K.; Halbower, A.C. Obstructive sleep apnea and hypoxemia are associated with advanced liver histology in pediatric nonalcoholic fatty liver disease. J Pediatr 2014, 164, 699–706 e691. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E.; Oji, S.; Mufti, A.R.; Browning, J.D.; Parikh, N.D.; Odewole, M.; Mayo, H.; Singal, A.G. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018, 16, 198–210 e192. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Gramlich, T.; Matteoni, C.A.; Boparai, N.; McCullough, A.J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004, 2, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Riekki, H.; Aitokari, L.; Kivela, L.; Lahti, S.; Hiltunen, P.; Vuorela, N.; Huhtala, H.; Lakka, T.A.; Kurppa, K. Prevalence and associated factors of metabolic-associated fatty liver disease in overweight Finnish children and adolescents. Front Endocrinol (Lausanne) 2023, 14, 1090344. [Google Scholar] [CrossRef]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Kobayashi, M.; Saitoh, S.; Sezaki, H.; Akuta, N.; et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. The American journal of gastroenterology 2012, 107, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Zeitler, P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Licenziati, M.R.; Corica, D.; Wasniewska, M.G.; Di Sessa, A.; Del Giudice, E.M.; Morandi, A.; Maffeis, C.; Faienza, M.F.; Mozzillo, E.; et al. Phenotypes of prediabetes and metabolic risk in Caucasian youths with overweight or obesity. Journal of endocrinological investigation 2022, 45, 1719–1727. [Google Scholar] [CrossRef]
- Laffel, L.; Chang, N.; Grey, M.; Hale, D.; Higgins, L.; Hirst, K.; Izquierdo, R.; Larkin, M.; Macha, C.; Pham, T.; et al. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatric diabetes 2012, 13, 369–375. [Google Scholar] [CrossRef]
- Shah, A.S.; Zeitler, P.S.; Wong, J.; Pena, A.S.; Wicklow, B.; Arslanian, S.; Chang, N.; Fu, J.; Dabadghao, P.; Pinhas-Hamiel, O.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatric diabetes 2022, 23, 872–902. [Google Scholar] [CrossRef]
- Nobili, V.; Mantovani, A.; Cianfarani, S.; Alisi, A.; Mosca, A.; Sartorelli, M.R.; Maffeis, C.; Loomba, R.; Byrne, C.D.; Targher, G. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. Journal of hepatology 2019, 71, 802–810. [Google Scholar] [CrossRef]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr 2016, 170, e161971. [Google Scholar] [CrossRef]
- Bardugo, A.; Bendor, C.D.; Zucker, I.; Lutski, M.; Cukierman-Yaffe, T.; Derazne, E.; Mosenzon, O.; Tzur, D.; Beer, Z.; Pinhas-Hamiel, O.; et al. Adolescent Nonalcoholic Fatty Liver Disease and Type 2 Diabetes in Young Adulthood. The Journal of clinical endocrinology and metabolism 2021, 106, e34–e44. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- D'Adamo, E.; Cali, A.M.; Weiss, R.; Santoro, N.; Pierpont, B.; Northrup, V.; Caprio, S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes care 2010, 33, 1817–1822. [Google Scholar] [CrossRef]
- Cali, A.M.; De Oliveira, A.M.; Kim, H.; Chen, S.; Reyes-Mugica, M.; Escalera, S.; Dziura, J.; Taksali, S.E.; Kursawe, R.; Shaw, M.; et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology 2009, 49, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- En Li Cho, E.; Ang, C.Z.; Quek, J.; Fu, C.E.; Lim, L.K.E.; Heng, Z.E.Q.; Tan, D.J.H.; Lim, W.H.; Yong, J.N.; Zeng, R.; et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut 2023, 72, 2138–2148. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. Journal of hepatology 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Xanthakos, S.A.; Lavine, J.E.; Yates, K.P.; Schwimmer, J.B.; Molleston, J.P.; Rosenthal, P.; Murray, K.F.; Vos, M.B.; Jain, A.K.; Scheimann, A.O.; et al. Progression of Fatty Liver Disease in Children Receiving Standard of Care Lifestyle Advice. Gastroenterology 2020, 159, 1731–1751 e1710. [Google Scholar] [CrossRef] [PubMed]
- Safar Zadeh, E.; Lungu, A.O.; Cochran, E.K.; Brown, R.J.; Ghany, M.G.; Heller, T.; Kleiner, D.E.; Gorden, P. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. Journal of hepatology 2013, 59, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Sivaramakrishnan, G.; Sequeira, R.P.; Elamin, A. Pharmacological interventions for non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Postgrad Med J 2018, 94, 556–565. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.; Scorletti, E.; Mantzoros, C.S.; Targher, G. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: An updated systematic review of randomized controlled trials. Diabetes & metabolism 2020, 46, 427–441. [Google Scholar]
- Nobili, V.; Manco, M.; Ciampalini, P.; Alisi, A.; Devito, R.; Bugianesi, E.; Marcellini, M.; Marchesini, G. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clinical therapeutics 2008, 30, 1168–1176. [Google Scholar] [CrossRef]
- Nobili, V.; Manco, M.; Devito, R.; Di Ciommo, V.; Comparcola, D.; Sartorelli, M.R.; Piemonte, F.; Marcellini, M.; Angulo, P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 2008, 48, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism: clinical and experimental 2017, 68, 119–132. [Google Scholar] [CrossRef]
- Spahis, S.; Alvarez, F.; Ahmed, N.; Dubois, J.; Jalbout, R.; Paganelli, M.; Grzywacz, K.; Delvin, E.; Peretti, N.; Levy, E. Non-alcoholic fatty liver disease severity and metabolic complications in obese children: impact of omega-3 fatty acids. J Nutr Biochem 2018, 58, 28–36. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Della Corte, C.; Rise, P.; Galli, C.; Agostoni, C.; Bedogni, G. Docosahexaenoic acid for the treatment of fatty liver: randomised controlled trial in children. Nutrition, metabolism, and cardiovascular diseases : NMCD 2013, 23, 1066–1070. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Lavine, J.E.; Wilson, L.A.; Neuschwander-Tetri, B.A.; Xanthakos, S.A.; Kohli, R.; Barlow, S.E.; Vos, M.B.; Karpen, S.J.; Molleston, J.P.; et al. In Children With Nonalcoholic Fatty Liver Disease, Cysteamine Bitartrate Delayed Release Improves Liver Enzymes but Does Not Reduce Disease Activity Scores. Gastroenterology 2016, 151, 1141–1154 e1149. [Google Scholar] [CrossRef]
- Della Corte, C.; Carpino, G.; De Vito, R.; De Stefanis, C.; Alisi, A.; Cianfarani, S.; Overi, D.; Mosca, A.; Stronati, L.; Cucchiara, S.; et al. Docosahexanoic Acid Plus Vitamin D Treatment Improves Features of NAFLD in Children with Serum Vitamin D Deficiency: Results from a Single Centre Trial. PloS one 2016, 11, e0168216. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liang, S.; Ma, J.; Xiao, Y. Effect of growth hormone therapy on liver enzyme and other cardiometabolic risk factors in boys with obesity and nonalcoholic fatty liver disease. BMC endocrine disorders 2022, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig Liver Dis 2021, 53, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; Abdelhai, D.; Shawky, D. Vitamin D and nonalcoholic fatty liver disease in children: a randomized controlled clinical trial. European journal of pediatrics 2022, 181, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Van Natta, M.L.; Blondet, N.M.; Dasarathy, S.; Fishbein, M.; Hertel, P.; Jain, A.K.; Karpen, S.J.; Lavine, J.E.; Mohammad, S.; et al. Randomized placebo-controlled trial of losartan for pediatric NAFLD. Hepatology 2022, 76, 429–444. [Google Scholar] [CrossRef]
- Saneian, H.; Khalilian, L.; Heidari-Beni, M.; Khademian, M.; Famouri, F.; Nasri, P.; Hassanzadeh, A.; Kelishadi, R. Effect of l-carnitine supplementation on children and adolescents with nonalcoholic fatty liver disease (NAFLD): a randomized, triple-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab 2021, 34, 897–904. [Google Scholar] [CrossRef]
- Goyal, N.P.; Mencin, A.; Newton, K.P.; Durelle, J.; Carrier, C.; Ugalde-Nicalo, P.; Noel, B.; Mouton, J.; Vargas, D.; Magrez, D.; et al. An Open Label, Randomized, Multicenter Study of Elafibranor in Children With Nonalcoholic Steatohepatitis. Journal of pediatric gastroenterology and nutrition 2023, 77, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Namazi, M.J.; Rezasoltani, M.; Kheirkhah, D.; Rajabi, M.; Sharif, A.; Taghavi Ardakani, A.; Raygan, F.; Assareh, A.A.; Sharif, M.R. Effects of Zinc Supplementation on Inflammatory Status and Nonalcoholic Steatohepatitis in Overweight or Obese Children: a Randomized Clinical Trial. Biological trace element research 2024, 202, 3496–3503. [Google Scholar] [CrossRef]
- Zahmatkesh, A.; Sohouli, M.H.; Shojaie, S.; Rohani, P. The effect of orlistat in the treatment of non-alcoholic fatty liver in adolescents with overweight and obese. European journal of pediatrics 2024, 183, 1173–1182. [Google Scholar] [CrossRef]
| Phases | ClinicalTrials.gov | Start Date of Trial (Year Month-Day) | Drug | Molecular Mechanism (Target) | Patients | Main Findings | Adverse Effect | References |
|---|---|---|---|---|---|---|---|---|
| II | NCT02201160 | 2009-01-01 | n-3 PUFA | anti-inflammatory, insulin metabolism regulator | NAFLD young male | FLI, ALT, and ALT/AST ratio reduction, lipid profile, and carotid intima-media thickness improvement | No | [97] |
| I-II | NCT00885313 | 2009-03-01 | Docosahexanoic Acid | anti-inflammatory | NAFLD children | improvement in liver steatosis by US | No | [98] |
| II-III | NCT01529268 | 2012-06-01 | cysteamine bitartrate | activators of PPARα | children with NAFLD activity scores ≥ 4 | AST, ALT, and lobular inflammation reductions | No | [99] |
| III | NCT02098317 | 2014-01-01 | Docosahexanoic Acid + Vitamin D | anti-inflammatory, immunity regulation | children and adolescents biopsy-proven NAFLD | improvement in IR, lipid profile, ALT, and NAFLD activity score | No | [100] |
| II | ChiCTRIPR-17011267 | 2017-03-01 | rhGH | stimulation of growth, IGF-1 production | NAFLD and obese boys | reduction in liver enzymes, CRP, BMI, LDL-C. Increase in HDL-C | No | [101] |
| III | NCT02842567 | 2017-04-01 | hydroxytyrosol + VitE | antioxidant, anti-inflammatory | children and adolescents biopsy-proven NASH | increase IL-10 | No | [102] |
| III | PACTR201710002634203 | 2017-10-19 | Vit D | anti-inflammatory and insulin-sensitizing effects | children with biopsy-proven NAFLD | improvement in hepatic steatosis, liver enzymes, cholesterol | No | [103] |
| III | NCT03467217 | 2018-10-02 | Losartan | angiotensin II receptor blocker | histologic NAFLD adolescents NAFLD activity score ≥ 3, and (ALT) ≥ 50 U/l. | Reduction of ALT | No | [104] |
| II | IRCT20170628034786N2 | 2019-01-16 | l-carnitine | Transport of fatty acids into mitochondria | NAFLD children | No impact on liver enzymes | No | [105] |
| II | NCT04165343 | 2020-02-01 | Elafibranor | Dual PPARα/δ agonist | NASH Children | ALT reduction | No | [106] |
| III | IRCT20200531047614N1 | 2020-09-01 | elemental zinc | anti-inflammatory and antioxidant | NASH overweight or obese children and adolescents | ALT, CRP reduction, HDL-cholesterolincrease | No | [107] |
| II | IRCT20220409054467N2 | 2022-05-13 | Orlistat | inhibiting pancreatic lipase, reducing the absorption of dietary fats | NAFLD amd overweight/obese adolescents | improvement in liver enzymes, steatosis, glucose/lipid metabolism | greasy stools, sleep problems, weakness, headache | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





